Introduction
Rhinoplasty is one of the most commonly performed
plastic/cosmetic surgeries worldwide (1-4).
Rhinoplasty is a repositioning technique of the nasal skeleton and
soft tissues to improve function and/or appearance (1-4).
Rhinoplasty includes septoplasty (1,2,5),
septorhinoplasty (6), nasal
reconstruction (7,8), nasal dorsum augmentation (1,2,9,10),
turbinoplasty (1,2), and nasal tip plasty/modification
(1,2,4,9,10).
Rhinoplasty frequently uses autologous cartilaginous grafts,
homologous cartilaginous grafts and/or alloplastic/artificial
grafts to correct contour deformities and restore structural
support (1,3,4).
Autologous cartilages are harvested from the 6-8th rib, nose,
and/or ear of patients themselves (1,5,7-16)
and homologous ones are usually composed of cadaveric rib (5,11,16).
Surgical techniques and management for rhinoplasty have
progressively improved (1,2,9).
Nevertheless, unexpected events or complications can occur
(1-3,9-11),
and revision rhinoplasty with removal of implanted cartilage may be
required (9,14,16).
Such events or complications include nasal
deformity/deviation/asymmetry, infection, skin necrosis,
bleeding/hematoma and vestibular stenosis (1,2,9).
Pathological examination of the removed cartilaginous grafts can
provide useful information to surgeons. Previous investigations
regarding cartilage implantation have focused on cartilaginous
viability and stability in human (13-15)
or animal models (17-20)
although they also have described calcification/ossification
(13,18,19),
vascularization or granulation-fibrosis (13-15,17-19),
chondrocytic cloning (14), and
mild lymphoid infiltration (15)
in implanted cartilages. To date, the detailed histopathological
features/alterations of implanted cartilages remain poorly
understood although cartilaginous changes are frequently mentioned
in osteoarthritic conditions, rheumatoid arthritis, and some
inherited diseases (21-23).
In this study, we examined surgically removed cartilages that had
been implanted during a previous rhinoplasty, and attempted to
define their histopathological features/changes. To the best of our
knowledge, the present study is the first to describe the detailed
histopathological findings of implanted human cartilages in a
relatively larger series.
Materials and methods
Ethics approval
The present study was a retrospective study
performed according to the principles of the Declaration of
Helsinki, and was approved by the Ethical Review Board of the
National Defense Medical College (No. 4804; April 27, 2023).
Patients, removed cartilaginous
grafts, and clinicopathological investigation
Hematoxylin and eosin (H&E)-stained, Masson
trichrome (MT)-stained, periodic acid-Schiff (PAS)-stained, and
elastica van Gieson (EVG)-stained glass slides of 83 removed
cartilaginous grafts were available from Ginza Sumirenohana Clinic
and were examined for pathology. All grafts were surgically removed
from 42 patients (2 men and 40 women), with a median age of 28.0
years (range, 21-47 years), during correction/revision rhinoplasty
at Ginza Sumirenohana Clinic, Tokyo, Japan, between January 2016
and March 2023. All specimens were fixed with 10-20% buffered
formalin, paraffin-embedded, and routinely processed. Clinical
information including time interval between implantation and graft
removal, termed implantation time interval (ITI), was available
from the request forms for pathological examination, and
additionally obtained from the attending physician at the clinic.
According to previous investigations (17,20),
we defined chondrocytic viability as nucleated cell count (%)
within chondrocytic lacunae. The organized rate of chondroid matrix
was calculated as the replacement-fibrotic areas (%) within
possible initial chondroid areas. Pathological changes were graded
as follows: 0, none; 1, mild; 2, mild to moderate; 3, moderate; 4,
moderate to marked; and 5, marked. Histological assessment was
performed using an Olympus BX51 microscope (Olympus, Tokyo,
Japan).
Statistical analysis
We analyzed the clinicopathological differences
between cartilaginous types using the chi-square test, Fisher's
exact test, Mann-Whitney U-test with or without Bonferroni
correction, and Kruskal-Wallis H-test. Statistical
significance was set at P<0.05. To compare data among ≥3
cartilage types, Kruskal-Wallis H-test analysis was applied
first. If there was a significant difference of variables between
them, we further analyzed difference between two cartilage types
using Mann-Whitney U-test with Bonferroni correction.
Results
Clinical findings and histological
type of removed cartilaginous grafts
Table I shows a
summary of clinical findings. ITI ranged from 0.3 to 132 months
(median, 18.0 months). Main complaints/reasons for surgery included
nasal deformity (24 patients), nasal discomfort (15 patients), and
skin thinning at the implantation site (10 patients). Based on
histological characteristics (7,24-26),
removed cartilages were divided into articular/costal hyaline
cartilages, nasal (hyaline) cartilages (NCs), and elastic
cartilages. Additional clinical information concluded that
articular/costal hyaline cartilages corresponded to costal
cartilages (CCs) composed of autologous CCs (ACCs) and irradiated
homologous CCs (IHCCs). NCs were subdivided into autologous NCs
(ANCs) and irradiated homologous NCs (IHNCs), and all ECs
corresponded to autologous ear cartilages (ECs). CCs were usually
triangular or diced, and contained evenly distributed
chondrocytes/lacunae (Fig. 1A and
B). NCs and ECs were frequently
elongated and slightly curved (Fig.
1F and K), and were sometimes
fragmented. Chondrocytes/chondrocytic lacunae in NCs and ECs were
sparse centrally and relatively dense peripherally (Fig. 1G and L). Scattered empty chondrocyte lacunae
indicated chondrocyte necrosis. On H&E-stained sections,
chondroid matrix in CCs and NCs was slightly eosinophilic and
homogeneous, whereas that in ECs was brightly red (Fig. 1C, H and M).
MT stain highlighted unstained chondrocytic lacunae, with NC
chondrocyte lacunae somewhat larger than those of CCs and ECs
(Fig. 1D, I and N).
ECs were characterized by a dense elastic meshwork (Fig. 1O). CCs and NCs lacked this dense
elastic meshwork, but had occasional EVG-positive fine deposits
(Fig. 1E and J). Thirteen grafts were composed of ≥2
different types of cartilages and/or artificial grafts (combined
grafts). Consequently, a total of 83 grafts were divided into 14
ACCs only, 16 IHCCs only, 24 ANCs only, 2 IHNCs only, 14 ECs only,
and 13 combined grafts (Table
II). We examined 16 ACCs (14 ACCs only and 2 ACCs in combined
grafts), 18 IHCCs (16 IHCCs only and 2 IHCCs in combined grafts),
33 ANCs (24 ANCs only and 9 ANCs in combined grafts), 2 IHNCs only,
and 23 ECs (14 ECs only and 9 ECs in combined grafts).
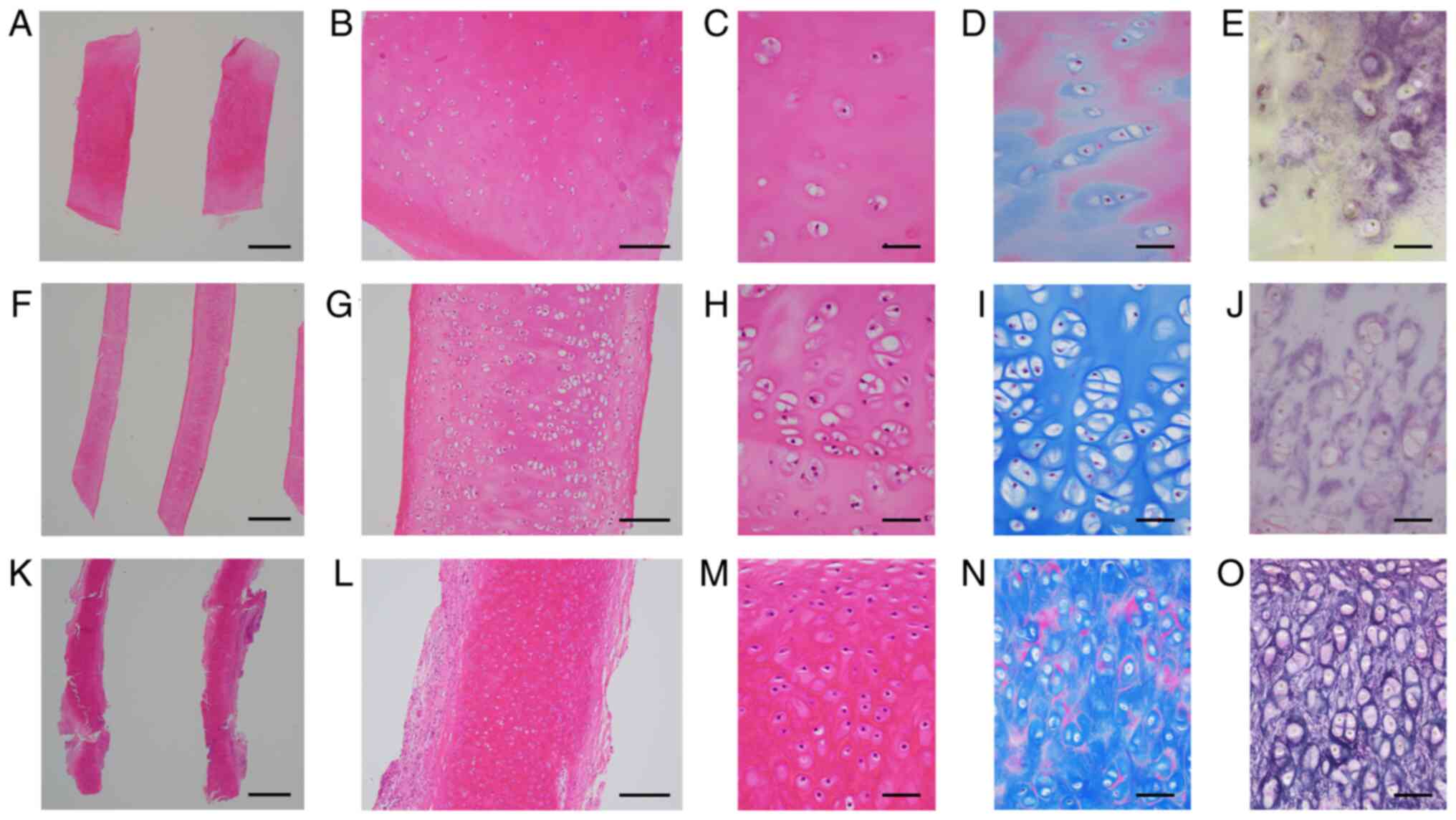 | Figure 1Typical examples of removed CC (A-E),
NC (F-J) and EC (K-O). (A) CC was triangular, and NC (F) and EC (K)
exhibited relatively slender and slightly curved features. (A, F
and K) Scale bar, 1 mm; H&E staining. CC (B) contained evenly
distributed chondrocytes, and NC (G) and EC (L) exhibited centrally
scarce and peripherally dense chondrocytes. (B, G and L) Scale bar,
200 µm; H&E staining. The chondroid matrix in (C) CC and (H) NC
was slightly eosinophilic and homogenous, whereas that in EC (M)
was brightly reddish. (C, H and M) Scale bar, 50 µm; H&E
staining. Masson trichrome staining highlighted unstained
chondrocytic lacunae, which were somewhat larger in NC (I) than CC
(D) and EC (N). (D, I and N) Scale bar, 50 µm; Masson trichrome
staining. EVG staining revealed a dense elastic meshwork around
lacunae in EC (O), which was different from the occasional,
EVG-positive fine deposits in CC (E) and NC (J). (E, J and O) Scale
bar, 50 µm; EVG staining. CC, costal cartilage; EC, ear cartilage;
EVG, Elastica van Gieson; NC, nasal cartilage. |
 | Table IClinical findings in 42 patients who
underwent removal of cartilaginous grafts. |
Table I
Clinical findings in 42 patients who
underwent removal of cartilaginous grafts.
| Variable | Value |
|---|
| Age range, years
(median) | 21-47 (28.0) |
| Sex, n
(male/female) | 2/40 |
| Range of time
interval between implantation and the removal of grafts, months
(median) | 0.3-132.0
(18.0) |
| Complaints/reasons
for correction/revision rhinoplasty, n (%)a | |
|
Nasal
deformity | 24 (57.1) |
|
Nasal
discomfort | 15 (35.7) |
|
Skin
thinning at implantation site | 10 (23.8) |
|
Restriction
on laughing movement | 3 (7.1) |
|
Suspicious
infection at implantation site | 2 (4.8) |
|
Nasal cavity
hematoma | 1 (2.4) |
 | Table IIHistological type of 83 cartilaginous
graft specimens removed from 42 patients. |
Table II
Histological type of 83 cartilaginous
graft specimens removed from 42 patients.
| Cartilaginous graft
type | Number |
|---|
| Costal hyaline
cartilage only | 30 |
|
ACC
only | 14 |
|
IHCC
only | 16 |
| Nasal hyaline
cartilage only | 26 |
|
ANC
only | 24 |
|
IHNC
only | 2 |
| Autologous ear
elastic cartilage only | 14 |
| Combined graft | 13 |
|
ANC +
EC | 3 |
|
ANC +
artificial graft | 3 |
|
EC +
artificial graft | 3 |
|
IHCC + ANC +
EC | 2 |
|
ACC +
ANC | 1 |
|
ACC +
EC | 1 |
Histopathological features of removed
cartilaginous grafts
Table III
summarizes clinicopathological features of the cartilaginous
grafts. Granulation-fibrosis surrounded 11 ACCs, 9 IHCCs, 24 ANCs,
and 22 ECs. Mild lymphoid infiltration was found in 5 ACCs, 6
IHCCs, 11 ANCs, and 7 ECs. Neutrophilia, suggesting bacterial
infection, was not found in any removed cartilages.
 | Table IIIClinicopathological findings of 83
cartilaginous graftsa surgically removed from 42
patients. |
Table III
Clinicopathological findings of 83
cartilaginous graftsa surgically removed from 42
patients.
| | Costal cartilage
(n=34a) | Nasal cartilage
(n=35a) | |
|---|
| Variable | ACC (n=16) | IHCC (n=18) | ANC (n=33) | IHNC (n=2) | Ear cartilage
(n=23a) |
|---|
| Patients, n | 11 | 15 | 18 | 1 | 18 |
| Age range, years
(median) | 22-47 (25.0) | 21-45 (29.0) | 21-47 (28.5) | 35 | 21-47 (30.5) |
| Sex, n
(male/female) | 1/10 | 0/15 | 1/17 | 0/1 | 0/18 |
| ITIb range, months (median) | 1.0-35.0
(12.0) | 0.8-119.0
(9.0) | 0.3-72.0
(13.0) | 12.0 | 1.0-132.0
(11.5) |
| Histopathological
findings | | | | | |
|
Presence of
viable chondrocytes, n (%) | 16 (100.0) | 3 (16.7) | 33 (100.0) | 2 (100.0) | 20 (87.0) |
|
Range of
chondrocytic viability, % (median) | 21.5-60.0
(35.9) | 0-10.1 (0.0) | 9.0-63.5
(41.3) | 24.8-26.6
(25.8) | 0-50.0 (21.4) |
|
Organization
(replacement fibrosis) of chondroid matrix, n (%) | 16 (100.0) | 16 (88.9) | 22 (62.9) | 2 (100.0) | 20 (87.0) |
|
Range of
organized rate of cartilaginous matrix, % (median) | 0.1-11.0 (2.5) | 0-24.0 (1.4) | 0-22.0 (0.9) | 0.1 (0.1) | 0-50.0 (2.0) |
|
Granulation-fibrosis
surrounding cartilaginous grafts, n (%) | 11 (68.8) | 9 (50.0) | 24 (72.7) | 1 (50.0) | 22 (95.7) |
|
Possible
transition between chondrocytes and fibroblasts, n (%) | 6 (37.5) | 0 (0.0) | 3 (9.1) | 0 (0.0) | 1 (4.3) |
|
Grading
score of enlarged chondrocytic lacunae, n (0/1/2/3/4/5)
(median) | 2/2/2/4/5/1
(3.0) | 0/3/1/8/4/2
(3.0) | 2/13/9/6/2/1
(2.0) | 0/0/0/0/2/0
(4.0) | 7/13/2/1/0/0
(1.0) |
|
Ossification,
n (%) | 2 (12.5) | 2 (11.1) | 5 (15.1) | 0 (0.0) | 0 (0.0) |
|
Lymphoid
infiltration, n (%) | 5 (31.3) | 6 (33.3) | 11 (33.3) | 0 (0.0) | 7 (30.4) |
|
Focal and
minimal EVG-positive elastic fibersc, n (%) | 8 (50.0) | 6 (33.3) | 0 (0.0) | 0 (0.0) | NA |
|
LFN-like
bodiesd, n (%) | 15 (93.8) | 14 (77.8) | 3 (9.1) | 0 (0.0) | 5 (21.8) |
|
Grading
score of LFN-like bodiesd, n (0/1/2/3/4/5) (median) | 1/7/3/3/2/0
(1.5) | 4/9/1/1/2/1
(1.0) | 30/3/0/0/0/0
(0.0) | 2/0/0/0/0/0
(0.0) | 18/4/0/1/0/0
(0.0) |
|
Multinucleated
histiocytes/foreign body reaction, n (%) | 1 (6.3) | 1 (5.6) | 0 (0.0) | 0 (0.0) | 1 (4.3) |
|
Histopathological
features of viable chondrocytes | | | | | |
|
Grading
score of cloning features, n (0/1/2/3/4/5) (median) | 2/1/8/2/3/0
(2.0) | 18/0/0/0/0/0
(0.0) | 0/16/10/5/1/1
(2.0) | 0/0/0/2/0/0
(3.0) | 10/11/2/0/0/0
(1.0) |
|
Grading
score of binucleated chondrocytes, n (0/1/2/3/4/5) (median) | 1/10/1/0/4/0
(1.0) | 18/0/0/0/0/0
(0) | 3/19/8/3/0/0
(1.0) | 0/0/2/0/0/0
(2.0) | 5/5/1/5/7/0
(3.0) |
|
Trinucleated
chondrocytes, n (%) | 0 (0.0) | 0 (0.0) | 8 (24.2) | 2 (100.0) | 2 (8.7) |
|
Vacuolar
changes, n (%) | 15 (93.8) | 0 (0.0) | 22 (66.7) | 2 (100.0) | 16 (70.0) |
|
Grading
score of vacuolar changes, n (0/1/2/3/4/5) (median) | 1/4/1/3/5/2
(23.0) | 18/0/0/0/0/0
(0.0) | 11/18/4/0/0/0
(1.0) | 0/2/0/0/0/0
(1.0) | 7/12/3/1/0/0
(1.0) |
|
Transition
between LFN-like bodiesd and chondrocytic vacuoles, n
(%) | 2 (12.5) | 0 (0.0) | 1 (3.0) | 0 (0.0) | 0 (0.0) |
CCs
All 16 ACCs contained viable chondrocytes. IHCCs
were mostly necrotic and 3 IHCCs had a few viable chondrocytes.
Median chondrocytic viability was 35.9% in ACCs and 0.0% in IHCCs.
Chondroid matrix was partially organized in all ACCs and in 16
IHCCs (Fig. 2A and B). The median organized rate was 2.5% in
ACCs and 1.4% in IHCCs. Organizing fibroblasts were not stained
with PAS, whereas chondrocytes were PAS-positive. In 6 ACCs,
PAS-positive spindle chondrocytes and PAS-negative fibroblastic
spindle cells were intermingled near the organized areas,
indicating a possible transition between chondrocytes and
fibroblasts (Fig. 2C and D). The median grading score of enlarged
chondrocytic lacunae of both ACCs and IHCCs was 3.0. Newly
developed minimal elastic fibers were found (Fig. 2E) in 8 ACCs and 6 IHCCs.
Eosinophilic serpiginous membranous bodies (Fig. 2F) were identified in 15 ACCs and 14
IHCCs. These bodies were red with MT and PAS stains (Fig. 2F, inset), mimicking lipomembranous
fat necrosis (LFN) (27,28). The median grading score of these
LFN-like bodies was 1.5 in ACCs and 1.0 in IHCCs. Chondrocytic
cloning, binucleated chondrocytes, and chondrocytic vacuolar
changes (Fig. 2G) were found in
14, 15, and 15 ACCs, respectively, and the median grading score was
2.0, 1.0, and 3.0, respectively. A histological transition between
chondrocytic vacuoles and LFN-like bodies was occasionally observed
in 2 ACCs (Fig. 2H). In IHCCs,
chondrocytic cloning, binucleated chondrocytes, and chondrocytic
vacuoles were not observed. Trinucleated chondrocytes were not
found in any of the CCs.
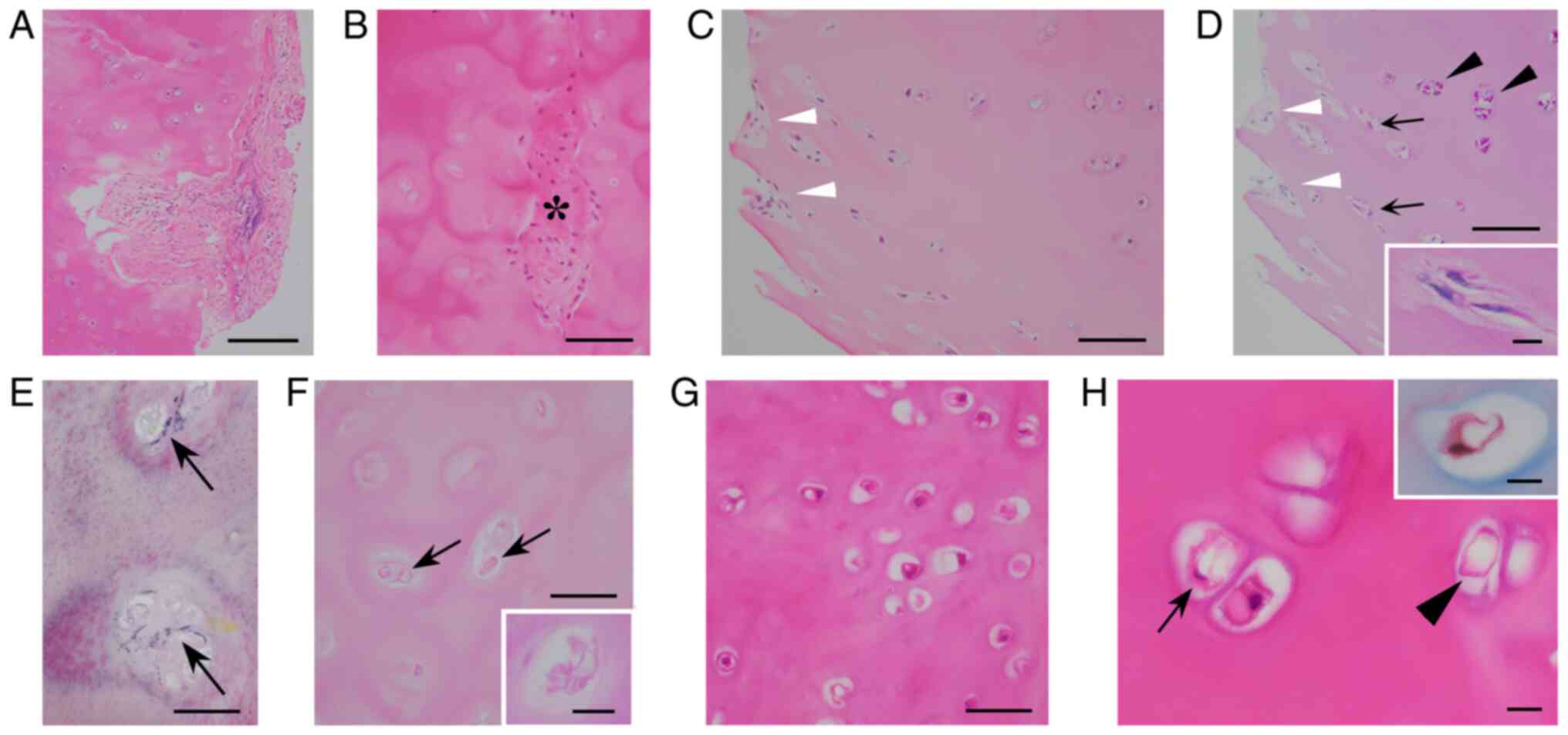 | Figure 2Histopathology of removed CC. (A)
Partially organized ACC (scale bar, 200 µm; H&E staining). (B)
Patchy organized areas (*) containing fibroblasts within massively
necrotic IHCC (scale bar, 100 µm; H&E staining). (C and D)
H&E-stained ACC showing (C) fibroblasts in scattered organized
areas (white arrowheads). (D) These fibroblasts were negative for
PAS staining (white arrowheads) and were intermingled with
PAS-positive spindle chondrocytes (arrows), near PAS-positive
rounded chondrocytes (black arrowheads). The inset in (D) shows
high-power views of PAS-positive spindled chondrocytes. These
features implied a possible transition of chondrocytes into
fibroblasts. (C) Scale bar, 100 µm; H&E staining. (D) Scale
bar, 100 µm; PAS staining. (D) Inset scale bar, 10 µm; PAS
staining. (E) EVG-positive minimal elastic fibers (arrows)
surrounding chondrocyte lacunae in IHCC (scale bar, 50 µm; EVG
staining. (F) H&E-stained IHCC exhibiting eosinophilic
membranous bodies (arrows). The inset shows a high-power view of
PAS-positive crenulated, serpiginous membranous changes, mimicking
LFN (scale bar, 50 µm; H&E staining; inset scale bar, 10 µm,
PAS staining). (G) Chondrocytic vacuolar changes (scale bar, 50 µm;
H&E staining). (H) Intermingled LFN-like body (arrowhead) and
vacuolar chondrocyte (arrow). The vacuolar chondrocyte was stained
red with Masson trichrome (inset), closely resembling an LFN-like
body. These findings suggested a transition between chondrocytic
vacuoles and LFN-like bodies (scale bar, 10 µm; H&E staining;
inset scale bar, 10 µm; Masson trichrome staining). ACC, autologous
CC; CC, costal cartilage; EVG, Elastica van Gieson; IHCC,
irradiated homologous CC; LFN, lipomembranous fat necrosis; PAS,
periodic acid-Schiff. |
NCs. All 35 NCs contained viable
chondrocytes. Median chondrocytic viability was 38.8% in ANCs and
25.8% in IHNCs, with no significant difference between them
(P=0.105; Mann-Whitney U-test without Bonferroni
correction). Median organized rate was 0.9% in ANCs and 0.01% in
IHNCs. Organizing fibroblasts invaded chondroid matrix in a
striated (Fig. 3A) or nested
(Fig. 3B) fashion. There was a
possible transition between PAS-positive chondrocytes and
fibroblasts in 3 ANCs (Fig. 3C and
D). In 1 ANC, there was a fibrous
nodule containing many PAS-positive spindle cells (Fig. 3E), suggesting fibrocartilaginous
metaplasia of ANC. The median grading score of enlarged
chondrocytic lacunae was 2.0 in ANCs and 4.0 in IHNC. Elastic
fibers were not observed in NCs. LFN-like bodies were found in 3
ANCs, but not in IHNCs. The median grading scores of chondrocytic
cloning and binucleated chondrocytes in ANCs were 2.0 and 1.0,
respectively, and those in IHNCs were 3.0 and 2.0, respectively.
Trinucleated chondrocytes were observed in 8 ANCs (Fig. 3F) and in 2 IHNCs. The median
grading score of chondrocytic vacuolar changes in both ANC and IHNC
was 1.0. Chondrocytic vacuoles and LFN-like bodies were focally
intermingled in 1ANC (Fig.
3G).
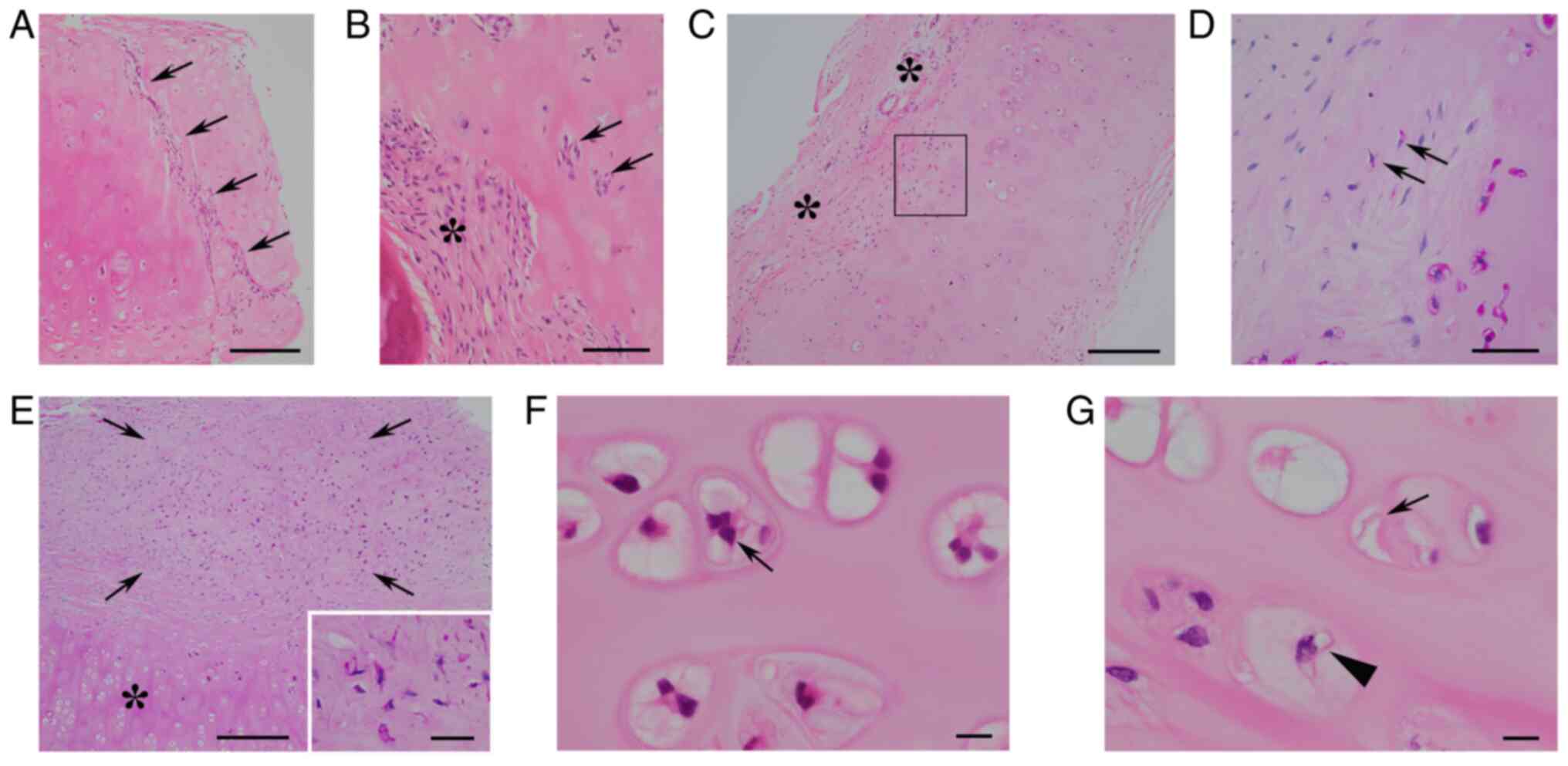 | Figure 3Histopathology of removed ANC. (A)
Organizing fibroblasts invading the chondroid matrix in a striated
fashion (arrows; scale bar, 200 µm; H&E staining). (B) Large
organized chondroid matrix (*) and small organized areas containing
nested fibroblasts (arrows) (scale bar, 50 µm; H&E staining).
(C) Granulation-fibrosis (*) attached to organizing ANC (scale bar,
200 µm; H&E staining). (D) High-power view of transition zones
between granulation-fibrosis and ANC [square area in (C)] revealing
PAS-positive spindled chondrocytes (arrows) (scale bar, 50 µm; PAS
staining). (E) Fibrocartilaginous nodule (arrows) close to ANC (*),
and high-power views (inset) of fibrocartilaginous nodule showing
numerous PAS-positive spindled chondrocytes (scale bar, 200 µm;
inset scale bar, 30 µm; PAS staining). (F) Binucleated and
trinucleated chondrocytes (arrow) in enlarged lacunae (scale bar,
10 µm; H&E staining). (G) Intermingled eosinophilic membranous
bodies (arrow) and vacuolar chondrocyte (arrowhead) (scale bar, 10
µm; H&E staining). ANC, autologous nasal cartilage; PAS,
periodic acid-Schiff. |
ECs. Median chondrocytic viability and median
organized rate of removed ECs were 21.4 and 2.0%, respectively.
Organized areas consisted of neovascularized fibrous tissues
lacking elastic meshwork (Fig. 4A
and B). Near organized areas in 15
ECs, there were patchy degenerated chondroid areas lacking
collagenous matrix blue stained by MT but preserving the elastic
meshwork (Fig. 4A-C; asterisks).
In 1 EC, there was a possible transition between chondrocytes and
fibroblasts (Fig. 4D and E). The median grading score of enlarged
chondrocytic lacunae was 1.0. LFN-like bodies (Fig. 4F-H) were observed in 5 ECs, and the
median grading score was 0. The median grading scores for
chondrocytic cloning (Fig. 4I) and
binucleated chondrocytes (Fig. 4J)
were 1.0 and 3.0, respectively. Trinucleated chondrocytes (Fig. 4K) were found in 2 ECs. The mean
grading score of chondrocytic vacuolar changes (Fig. 4J) was 1.0. The histological
transition between vacuolar chondrocytes and LFN-like bodies was
unclear in all ECs.
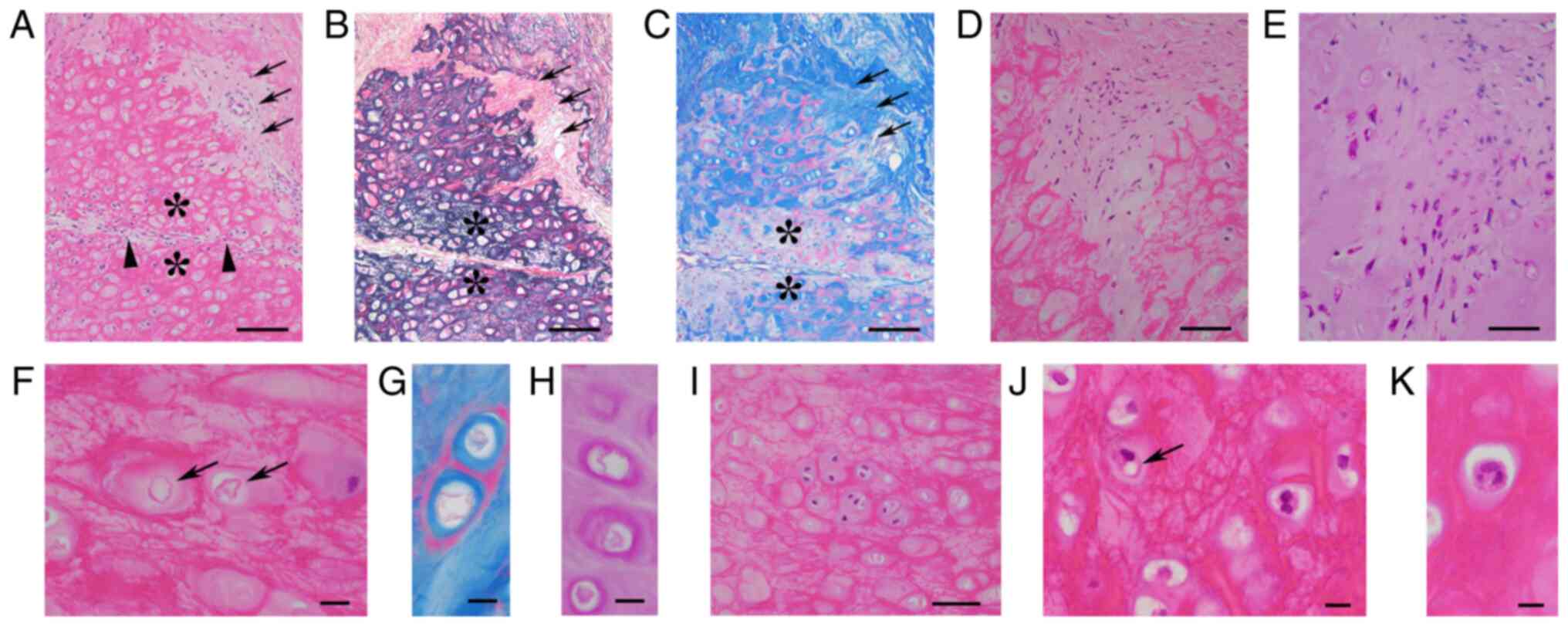 | Figure 4Histopathology of removed ear
cartilage. (A) Organized areas (arrows) lacking an (B) EVG-positive
meshwork (arrows), but preserving (C) Masson trichrome-blue-stained
collagens (arrows). (C) Patchy areas exhibiting depletion of
collagen blue-stained with Masson trichrome (*) but preserving the
(B) EVG-positive meshwork (*) showing no remarkable changes on (A)
H&E-stained section (asterisks) close to striated organized
areas (arrowheads). (A-C) Scale bar, 100 µm. (A) H&E staining.
(B) EVG staining. (C) Masson trichrome staining. (D) Organized
areas containing spindle cells. (E) On a PAS-stained section, these
spindle cells were composed of PAS-positive spindled chondrocytes
and PAS-negative fibroblasts. (D and E) Scale bar, 50 µm. (D)
H&E staining. (E) PAS staining. (F) Scattered eosinophilic
membranous bodies (arrows), stained red with (G) Masson trichrome
and (H) PAS staining. (F-H) Scale bar, 10 µm. (F) H&E staining.
(G) Masson trichrome staining. (H) PAS staining. (I) Chondrocyte
cloning (scale bar, 10 µm; H&E staining). (J) Vacuolar
chondrocyte (arrow) and binucleated chondrocyte (scale bar, 10 µm;
H&E staining). (K) Trinucleated chondrocyte (scale bar, 10 µm;
H&E staining). EVG, Elastica van Gieson; PAS, periodic
acid-Schiff. |
Statistical analysis of
clinicopathological findings of implanted cartilaginous grafts
The incidence of ossification in ACC, IHCC, ANC, and
EC was 12.5, 11.1, 15.1, and 0%, respectively (Table III). There was no significant
difference between them (P=0.549, Fisher's exact test). The
incidence of lymphoid infiltration in ACC, IHCC, ANC, and EC was
31.3, 33.3, 33.3, and 30.4%, respectively (Table III), and there was no significant
difference between them (P=0.996, nxm chi-square test). Fig. 5 shows comparisons of other
clinicopathological findings among cartilaginous types.
Kruskal-Wallis H-test revealed no significant difference of
ITI or organized rate between ACC, IHCC, ANC, and EC (Fig. 5A and C) and no significant difference of
grading score for binucleated chondrocytes between ACC, ANC, and EC
(Fig. 5G). On the other hand,
Kruskal-Wallis H-test demonstrated a significant difference
of grading score for chondrocytic viability, enlarged chondrocytic
lacunae, and LFN-like bodies among ACC, IHCC, ANC, and EC, (all,
P<0.001) and a significant difference of grading score for
chondrocytic cloning and vacuolar change between ACC, ANC, and EC
(both, P<0.001). Chondrocytic viability in IHCC was lower than
that in the others (all, P<0.001), and that in ECs was lower
than that in ACCs and ANCs (both, P<0.001) (Fig. 5B). The grading score of enlarged
chondrocytic lacunae was lower than that of the others (all,
P<0.001) (Fig. 5D). The grading
score of LFN-like bodies in ACCs and IHCCs was higher than that in
ANCs and ECs (Fig. 5E). The
grading score of chondrocytic cloning features in ECs was lower
than that of ACCs and ANCs (both, P<0.001) (Fig. 5F). The grading score of
chondrocytic vacuolar changes in ACCs was significantly higher than
that in ANCs and ECs (both, P<0.001) (Fig. 5H). The higher grading scores of
LFN-like bodies in ACCs were closely associated with higher grading
scores of chondrocytic vacuolar changes (P=0.026), but this was not
found in ANCs or ECs (Table IV).
A close relationship between the presence of trinucleated
chondrocytes and increasing binucleated chondrocytes was found in
ANCs (P=0.001), but not in ECs (Table
V).
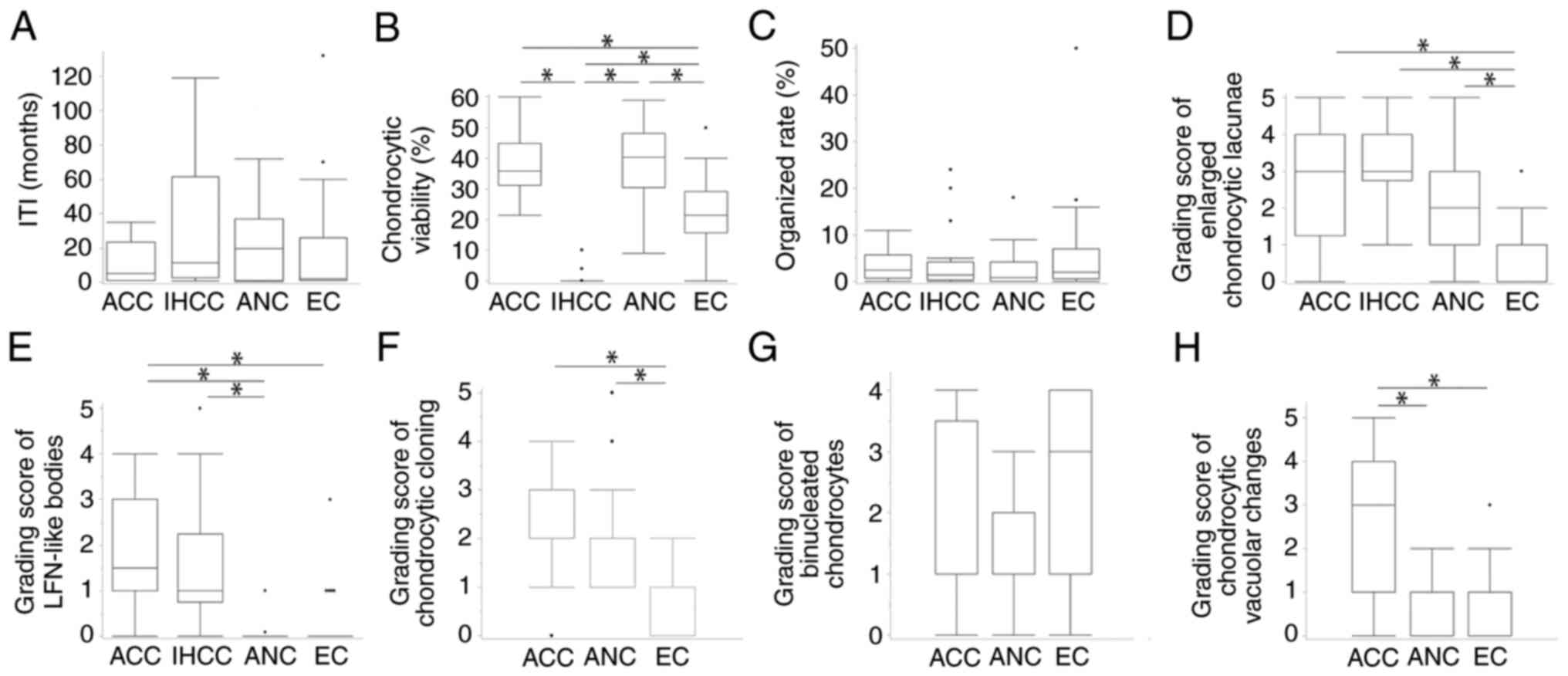 | Figure 5Comparison of clinicopathological
findings among cartilaginous types. (A) There was no significant
difference in the ITI among ACC, IHCC, ANC and EC
(P=0.554)a. (B) Chondrocytic viability in IHCC was
significantly lower than that in ACC, ANC and EC, whereas that in
EC was significantly lower than that in ACC and ANCa,b.
(C) There was no significant difference in the organized rate of
chondroid matrix among ACC, IHCC, ANC and EC (P=0.909)a.
(D) The grading score of enlarged chondrocyte lacunae in EC was
significantly lower than that in ACC, IHCC and ANCa,b.
(E) The grading score of LFN-like bodies in ACC was significantly
higher than that in ANC and EC, and that in IHCC was higher than
that in ANCa,b. (F) The grading score of chondrocyte
cloning in EC was significantly lower than that in ACC and
ANCa,b. (G) There was no significant difference in the
grading score of binucleated chondrocytes among ACC, ANC and EC
(P=0.098)a. (H) The grading score of chondrocytic
vacuolar changes in ACC was significantly higher than that in ANC
and ECa,b. *P<0.05.
aKruskal-Wallis H-test; bMann-Whitney U-test
with Bonferroni correction. ACC, autologous costal cartilage; ANC,
autologous nasal cartilage; EC, ear cartilage; IHCC, irradiated
homologous costal cartilage; ITI, implantation time interval; LFN,
lipomembranous fat necrosis. |
 | Table IVRelationship between eosinophilic
membranous bodies and chondrocytic vacuolar changes in removed
cartilaginous grafts. |
Table IV
Relationship between eosinophilic
membranous bodies and chondrocytic vacuolar changes in removed
cartilaginous grafts.
| | Grading scores of
viable chondrocytic vacuolar changes | |
|---|
| Histopathological
findings | 0-1 | 2-5 | P-value |
|---|
| Grading scores of
LFN-like bodies in ACC, score 0-1/2-5 (n=16) | 5/3 | 0/8 | 0.026a,b |
| Grading scores of
LFN-like bodies in IHCC, score 0-1/2-5 (n=18) | 13/5 | 0/0 | NA |
| LFN-like bodies in
ANC, present/none (n=33) | 2/27 | 1/3 | 0.330a |
| LFN-like bodies in
EC, present/none (n=23) | 3/16 | 1/3 | 0.194a |
 | Table VRelationship between binucleated
chondrocytes and trinucleated chondrocytes. |
Table V
Relationship between binucleated
chondrocytes and trinucleated chondrocytes.
| | Grading scores of
binucleated chondrocytes | |
|---|
| Histopathological
findings | 0-1 | 2-5 | P-value |
|---|
| Trinucleated
chondrocytes in ANC, present/none (n=33) | 1/21 | 7/4 | 0.001a,b |
| Trinucleated
chondrocytes in EC, present/none (n=23) | 3/16 | 1/3 | 0.486a |
Discussion
The present histological examination could
distinguish NCs and elastic cartilages from articular
cartilages/CCs. However, because of close histological
similarities, discrimination of CCs from articular cartilages is
challenging and would require additional clinical information. NCs
are known to be hyaline cartilages containing elastin elements
(24,29). However, in the present NCs, elastic
fibers were not found. On the other hand, in 14 of 34 CCs (32.4%),
possibly newly developed elastic fibers were observed although they
were focal and minimal. Radiation induces chondrocytic necrosis,
which would be a hallmark for irradiated history. Indeed, in this
study, chondrocytic viability of IHCC was significantly lower than
that of ACC. However, there was no significant difference of
chondrocytic viability between IHNCs and ANCs. Sinclair and Walsh
(30) measured chondrocyte numbers
per square millimeter of IHCCs ranging from 591 and 281, which is
higher than chondrocytic viability in the current IHCCs. These
findings would be accounted for by the difference in radiation
dose. In the present study, chondrocyte viability in IHNC was high
and this was attributed to low radiation dose.
Cartilages are nourished by diffusion or permeation
from surrounding tissues or fluid, without direct vascular supply
(19,25,26,31).
A previous investigation (19)
suggested that smaller implanted cartilages are more viable than
larger ones because smaller cartilages can receive richer diffusion
nourishment. Greater chondrocyte viability avoids organization or
absorption of chondroid matrix, which would play a critical role in
the stability of implanted cartilages on rhinoplasty (13-15).
In the current study, however, chondrocytic viability of ECs was
significantly lower than ACCs, ANCs, and ECs, but there was no
significant difference of organized rate among them. Vila et
al (11) also reported no
difference in absorption rate between IHCCs and ACCs. These
findings suggest that the stability of implanted chondroid matrix
does not depend on only chondrocyte viability. Furthermore, the
current study revealed a possible transition between chondrocytes
and fibroblasts. Dedifferentiation or fibroblastic transformation
of chondrocytes can occur in osteoarthritic articular cartilages
(23). These findings indicate a
possibility that viable chondrocytes not only preserve chondroid
matrix but also transform into fibroblasts, inducing organization.
In the present study, such possible transition between chondrocytes
and fibroblasts was not rare (37.5% of ACCs, 9.1% of ANCs, and 4.3%
of ECs). The molecular mechanism regarding the transition of
chondrocytes into a fibroblastic phenotype remains poorly
understood, although the transition is related to decreased gene
expression of SOX9 and COL2A1, suppressed production of
aggrecan, and increased expression of COL1A1 (32,33).
Such transition would lead to a mechanically weak chondroid matrix
(33), possibly resulting in
provoking unexpected degeneration and/or resorption of the
implanted cartilaginous graft. In fact, in one ANC, a possible
fibrocartilaginous metaplasia was found. However, the current study
failed to reveal pathogeneses determining whether chondrocytes
avoid or induce organization or fibrocartilaginous metaplasia. The
association of chondrocyte dedifferentiation with the stability of
implanted cartilages is unclear. To elucidate these points, further
investigations are required.
The current study identified unique LFN-like bodies
in 93.8% of ACCs, 77.8% of IHCCs, 9.1% of ANCs, and 21.8% of ECs.
To our knowledge, LFN-like bodies in cartilages have not been
mentioned previously in any cartilaginous conditions, including
osteoarthritis, rheumatoid arthritis, or metabolic diseases
(21-23).
We initially predicted LFN-like bodies as sclerotic changes of
chondrocytic lacunar ‘capsules’ (25). However, in 2 ACCs and 1 ANC, an
occasional histological transition between LFN-like bodies and
chondrocytic vacuoles was observed. There was a statistically
significant relationship between chondrocytic vacuolar changes and
LFN-like bodies in ACCs. These findings suggest that LFN-like
bodies are a vacuolar change-related necrotic form of chondrocytes
themselves. Chondrocytic vacuolar changes can occur in the
hypertrophic zone of the maturing epiphyseal growth plate (23) and in achondrogenesis (21,23)
and fibrochondrogenesis (23).
Chondrocytic vacuoles in achondrogenesis indicate some metabolic
abnormalities in chondrocytes (21). The presence of implanted
chondrocytic vacuoles may imply similar metabolic
abnormalities.
The current study of implanted cartilages also
detected other histological features, such as enlarged chondrocytic
lacunae, chondrocytic cloning, increased binucleated chondrocytes,
and trinucleated chondrocytes. Enlarged chondrocytic lacunae
represent degenerative changes of chondroid matrix. Chondrocytic
cloning is associated with possible intrinsic cartilaginous repair
or regeneration in osteoarthritic articular cartilages (22,23).
Therefore, both chondrocyte cloning and more binucleated
chondrocytes in implanted cartilages may indicate chondrocytic
reactive proliferation, although binucleated chondrocytes are
common in normal articular (23)
and nasal septal (13) cartilage.
Trinucleated chondrocytes would not be recognized in normal
cartilages, but can occur in atelosteogenesis (23). The presence of trinucleated
chondrocytes in the present ANCs was statistically associated with
more binucleated chondrocytes. However, this tendency was unclear
in ECs and trinucleated chondrocytes were not found in ACCs despite
frequent binucleated chondrocytes. These findings suggest
differences of chondrocytic natures or responses among CC, NC, and
EC. In this study, the grading score of chondrocyte cloning in ECs
was significantly lower than that in ACCs and ANCs. Similarly, the
grading score of enlarged chondrocyte lacunae in ECs was lower than
that in ACCs, IHCCs, and ANCs. These differences would contribute
to the amount of elastic fibers in the chondroid matrix. Elastic
fibers in ECs are more prominent compared with those in CCs and
NCs, and tightly surround the chondrocytes and their lacunae.
Therefore, chondrocytes are less likely to form clones and lacunae
would be less prone to enlarge. The relationship between these
histopathological differences and the outcomes of implanted
cartilages remains unclear in the present study. However, further
studies would provide histopathological markers that predicts
unexpected graft resorption and future weakening of cartilaginous
quality in rhinoplasty.
In 15 ECs, near organized areas, there were patchy
degenerated chondroid areas, where the elastic meshwork was
preserved but collagenous elements were depleted. These degenerated
features may be another pathogenesis that contributed to absorption
of implanted elastic cartilages. Unfortunately, these chondroid
degenerated lesions could not be assessed in detail in this study.
Additional Safranin-O staining detecting the amount of proteoglycan
(13,17,34)
may be useful to evaluate these lesions. Further
immunohistochemical examination using antibodies against
alpha-smooth muscle actin and S-100 protein may provide effective
evidence of transition between fibroblasts and chondrocytes
although paraffin-embedded specimens were not available in the
present study.
Another limitation of the current study is using
symptomatically removed cartilages. Asymptomatic implanted
cartilages were not removed, and their histological changes were
not examined. Therefore, the true incidences of the
histopathological changes in cartilaginous grafts including
asymptomatic ones remain unknown. Histopathological findings of
original cartilaginous grafts at the time of initial rhinoplasty,
such as original graft size, initial chondrocytic viability, and
degrees of chondrocyte cloning, were not available and comparative
analyses were not performed. In addition, the present study
examined not only one cartilage type, but also combined
cartilaginous grafts composed of ≥2 different types of cartilages.
Unknown interrelationships between these complicated cartilages may
occur, and the histopathological data regarding each cartilaginous
type in the present study would be heterogeneous. Furthermore, for
a control study, the surgical pathology files of the Department of
Laboratory Medicine, National Defense Medical College Hospital
(from 2010 to 2023), were searched for cases of surgically removed
CC, NC, and EC. Unfortunately, however, sufficient specimens for a
control study were not available because the excision of these
cartilages was rare in the usual surgical treatment setting.
Nevertheless, we believe that the present study reveals the
detailed histopathological findings of implanted cartilaginous
grafts.
In conclusion, the present study classified removed
cartilaginous grafts into ACCs, IHCCs, ANCs, IHACs, and ECs, and
demonstrated their histopathological changes, including
underrecognized LFN-like chondrocytic necrosis and a possible
transformation of implanted chondrocytes into fibroblasts. We
believe that further collected data of these features could provide
useful information evaluating implanted cartilages not only in
rhinoplasty but also in other body regions.
Acknowledgements
The authors would like to thank Dr Toshiya Yokoyama
(Ginza Sumirenohana Clinic, Tokyo, Japan) for kindly providing the
study specimens and clinical information, and Mr. Yoshimi Shimada
(SKK Soshikikagaku Kenkyujo, Hodogaya, Yokohama, Kanagawa, Japan)
for his assistance with pathology.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are not
publicly available due to the nature of the research in which
participants did not agree for their data to be shared publicly but
may be requested from the corresponding author.
Authors' contributions
SM conceived and designed the study. SM examined
cartilaginous grafts, reviewed previous articles and drafted the
manuscript. AM helped with the examination of cartilaginous grafts
and reviewed most of the reference articles. SO also participated
in the histopathological examination of removed cartilaginous
grafts, provided comments regarding subsequent changes of implanted
cartilages, and edited the manuscript. All authors discussed the
assessment of histopathological findings of implanted cartilages.
SM and AM confirm the authenticity of all the data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted according to the
principles of the Declaration of Helsinki, and was approved by the
Ethical Review Board of the National Defense Medical College
(approval no. 4804; April 27, 2023; Tokorozawa, Japan). The present
study was a retrospective study. Patients were not required to give
written informed consent for the present study because the analysis
used anonymous clinicopathological data that were obtained after
each patient agreed to treatment by written consent. The opt-out
method was applied to obtain consent for participation in the
present study using a poster. Oral informed consent of all patients
for participation in the present study was obtained by the
attending physician.
Patient consent for publication
The opt-out method was applied to obtain patient
consent for publication of the present study using a poster. The
poster was approved by the Ethical Review Board of the National
Defense Medical College (approval no. 4804). Oral informed consent
of all patients for publication of the present study was obtained
by the attending physician.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rohrich RJ and Ahmad J: Rhinoplasty. Plast
Reconstr Surg. 128:49e–73e. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fichman M and Piedra Buena IT:
Rhinoplasty. In: StatPearls (Internet). StatPearls Publishing,
Treasure Island, FL, 2023.
|
|
3
|
Dayan E and Rohrich RJ: Developing
consistency in rhinoplasty. Plast Reconstr Surg Glob Open.
8(e2697)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kim JH, Kim GW and Kang WK: Nasal tip
plasty using three-dimensional printed polycaprolactone (Smart
Ball®). Yeungnam Univ J Med. 37:32–39. 2002.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jang YJ (ed): Rhinoplasty and septoplasty.
1st edition. Koonja Publishing, Seoul, 2014.
|
|
6
|
Miyawaki T, Tsumiyama S, Umeda G, Moriyama
W, Mori E, Asaka D, Iimura J and Otori N: Plastic surgery
principles in septorhinoplasty. -Importance of the caudal septum in
the treatment of caudal septal deviationa-. Nihon Bikagaku
Gakkaishi. 57:637–646. 2018.(Japanese).
|
|
7
|
Griffin MF, Premakumar Y, Seifalian AM,
Szarko M and Butler PE: Biomechanical characterization of the human
nasal cartilages; implications for tissue engineering. J Mater Sci
Mater Med. 27(11)2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shah R and Alford EL: Reconstructive
rhinoplasty using cadaver cartilage in relapsing polychondritis.
Proc (Bayl Univ Med Cent). 36:130–131. 2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jin HR and Won TB: Recent advances in
Asian rhinoplasty. Auris Nasus Larynx. 38:157–164. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wright JM, Halsey JN and Rottgers SA:
Dorsal augmentation: A review of current graft options. Eplasty.
23(e4)2023.PubMed/NCBI
|
|
11
|
Vila PM, Jeanpierre LM, Rizzi CJ, Yaeger
LH and Chi JJ: Comparison of autologous vs homologous costal
cartilage grafts in dorsal augmentation rhinoplasty: A systematic
review and meta-analysis. JAMA Otolaryngol Head Neck Surg.
146:347–354. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Resuli AS, Dilber M, Bayar Muluk N and
Cingi C: Septal extension graft use in the treatment of alar
collapse. Eur Rev Med Pharmacol Sci. 27 (2 Suppl):S8–S13.
2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Calvert JW, Brenner K, DaCosta-Iyer M,
Evans GRD and Daniel RK: Histological analysis of human diced
cartilage grafts. Plast Reconstr Surg. 118:230–236. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Manafi A, Kaviani A, Hamedi ZS, Rajabiani
A and Manafi N: Evidence-based efficacy of autologous grated
cartilage in primary and secondary rhinoplasty. World J Plast Surg.
6:137–143. 2017.PubMed/NCBI
|
|
15
|
Lin SI, Hsiao YC, Chang CS, Chen PKT, Chen
JP and Ueng SH: Histology and long-term stability of diced
cartilage graft for revision rhinoplasty in a cleft patient. Plast
Reconstr Surg Glob Open. 4(e763)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bender-Heine AN, Zdilla MJ, Russell ML,
Rickards AA, Holmes JS, Armeni MA and Lambert HW: Optimal costal
cartilage graft selection according to cartilage shape: anatomical
considerations for rhinoplasty. Facial Plast Surg. 33:670–674.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Brenner KA, McConnell MP, Evans GR and
Calvert JW: Survival of diced cartilage grafts: An experimental
study. Plast Reconstr Surg. 117:105–115. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jurk V, Kampmann H, Iblher N, Bannasch H
and Gubisch W: Long-term comparison of rib and ear cartilage grafts
in autologous and allogenic fascia lata: An experimental study in a
white rabbit model. Plast Reconstr Surg. 137:1465–1474.
2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kim SH, Suh JH and Jang YJ:
Histomorphological findings of cartilage and surrounding tissues
according to thickness and manipulations in rabbits. Aesthet Surg
J. 42:NP489–NP500. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Heatley DG, Clary RA, Garner FT and Lusk
RP: Auricular cartilage versus costal cartilage as a grafting
material in experimental laryngotracheal reconstruction.
Laryngoscope. 105 (9 Pt 1):983–987. 1995.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Milgram JW (ed): Radiologic and histologic
pathology of nontumorous diseases of bones and joints. Northbrook
Publishing Co Inc, Northbrook IL, pp21-32, 57-89, 95-112, 1990.
|
|
22
|
Ishida T and Imamura T (eds): Surgical
pathology of non-neoplastic bone and joint diseases. Bunkoudo,
Tokyo, pp48-61, pp226-236, 2003 (in Japanese).
|
|
23
|
Klein MJ, Bonar SF, Freemont T, Vinh TN,
Lopez-Ben R, Siegel HJ and Siegel GP: Non-neoplastic diseases of
bones and joints. In: King DW, Gardner WA, Sobin LH, Stocker JT,
Wagner B (eds) Atlas of nontumor pathology. first series, fascicle
9. American Registry of Pathology, Washington DC, pp1-53, 85-298,
577-767, 2011.
|
|
24
|
Nakajima H: A histological study of the
nasal cartilages in man (Japanese adults). Oto-Rhino-Laryngology
Tokyo. 17:171–202. 1974.(in Japanese).
|
|
25
|
Pawlina W: Histology: a text and atlas:
with correlated cell and molecular biology. 9th edition. Wolters
Kluwer Business, Philadelphia PA, pp228-237, 2024.
|
|
26
|
Maclean F: Joints. In: Mills SE (ed)
Histology for Pathologists. 5th edition. Wolters Kluwer,
Philadelphia PA: pp113-132, 2020.
|
|
27
|
Machinami R: Membranous lipodystrophy-like
changes in ischemic necrosis of the legs. Virchows Arch A Pathol
Anat Histopathol. 399:191–205. 1983.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Matsukuma S, Matsunaga A, Takahashi O and
Ogata S: Lipomembranous fat necrosis: A distinctive and unique
morphology (Review). Exp Ther Med. 24(759)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bos EJ, Pluemeekers M, Helder M, Kuzmin N,
van der Laan K, Groot ML, van Osch G and van Zuijlen P: Structural
and mechanical comparison of human ear, alar, and septal cartilage.
Plast Reconstr Surg Blob Open. 6(e1610)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sinclair S and Walsh WR: Characterization
of costal cartilage allografts. ANZ J Surg. 92:2274–2279.
2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang Y, Wei L, Zeng L, He D and Wei X:
Nutrition and degeneration of articular cartilage. Knee Surg Sports
Traumatol Arthrosc. 21:1751–1762. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cheng T, Maddox NC, Wong AW, Rahnama R and
Kuo AC: Comparison of gene expression patterns in articular
cartilage and dedifferentiated articular chondrocytes. J Orthop
Res. 30:234–245. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hall AC: The role of chondrocyte
morphology and volume in controlling phenotype-Implications for
osteoarthritis, cartilage repair, and cartilage engineering. Curr
Rheumatol Rep. 21(38)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sato K, Moy OJ, Peimer CA, Nakamura T,
Howard C, Ko SH, Lee TC and Nishiwaki Y: An experimental study on
costal osteochondral graft. Osteoarthritis Cartilage. 20:172–183.
2012.PubMed/NCBI View Article : Google Scholar
|



















