Introduction
Phyllodes tumors (PTs) are rare (0.3-1% of all
primary breast tumors) fibroepithelial breast neoplasms that carry
a risk of recurrence and metastasis (1,2). PTs
are classified into benign, borderline and malignant-grade disease
according to histopathological criteria of stromal cellularity and
atypia, mitotic count, stromal overgrowth and the nature of the
tumor border (3,4). Histopathological grading is important
for prognostication and management: Recurrence rates increase with
higher tumor grades [10-17, 14-25 and 23-30% for benign, borderline
and malignant PTs, respectively (3,4)];
benign tumors only very rarely metastasize (3); and malignant tumors may benefit from
adjuvant therapy such as postoperative radiotherapy, although the
optimal management remains uncertain (5). Metastasis of PTs heralds a usually
dismal prognosis and death from the disease (6), but in almost all cases, metastasis
only occurs when the primary tumor is graded as malignant (3,6).
Although complete excision is the gold standard
treatment for PT and complete excision with an adequate margin
reduces the subsequent recurrence risk, it is still being debated
what constitutes an adequate margin to reduce the risk of
recurrence (5). Other
clinicopathological features, such as cellular atypia, mitotic rate
and stromal overgrowth, have similarly been examined as predictive
factors for recurrence, with conflicting results (3,5).
Other protein biomarkers such as matrix metalloproteinase (MMP)-14,
Six-1, PAX3, FoxC2, TWIST, C-X-C chemokine receptor 4, vascular
endothelial growth factor, stromal Yes-associated protein, cellular
E-cadherin and CD10 have been associated with recurrent PT, while
others have not [e.g., epidermal growth factor receptor (EGFR),
human EGFR 2, membranous E-cadherin; MMP-1, -2, -7, -9, -11 and
-13; and tissue inhibitor of metalloproteinase (TIMP)-1, -2 and -3]
(7). There are still no definitive
markers of recurrence or other outcomes such as survival to guide
management, despite the persistent risk of recurrence, even in
patients with benign disease. Although benign disease does not
usually result in clinically serious sequelae, it would still be
helpful to identify all individuals at risk of recurrence, even
those with benign disease, to tailor surveillance strategies
(8).
Ki67 is a nuclear antigen that is expressed in all
phases of the cell cycle (G1, S, G2), and it is a reliable and
widely used immunohistochemical biomarker of proliferation in
routine histopathological practice, including in breast cancer
(9). Although practice varies,
Ki67 is routinely used as a prognostic biomarker in invasive ductal
carcinoma, and the Italian Association of Medical Oncology,
European Group on Tumor Markers, European Society for Medical
Oncology and the National Institute for Health and Care Excellence
either recommend or advise considering Ki67 measurement for
prognostication in patients with breast cancer (10). Furthermore, the European Society
for Medical Oncology supports the use of Ki-67 expression as a
predictive biomarker of response to neoadjuvant chemotherapy
(10). Similarly, Ki67 is
prognostic and used routinely in patients with other tumors, such
as in pancreatic neuroendocrine neoplasia, where it predicts both
recurrence and metastasis (11-13).
While the mitotic index only reflects cells in the M-phase, Ki67
detects other cells at different stages of proliferation (or
arrest) and therefore provides different information about
proliferation in the histopathological snapshot of tumor biology
captured in a tumor section (9).
In PT, stromal expression of Ki67 has been reported to be 5-25% in
benign tumors and 15-100% in malignant tumors, and this association
with the grade has been reported in several studies (14). However, whether Ki67 has prognostic
significance in PT remains uncertain.
Therefore, in the present study, a systematic review
and meta-analysis were performed to examine whether Ki67 is
associated with adverse clinical outcomes, particularly recurrence,
in patients with PT.
Methods
This meta-analysis is reported according to the 2020
Preferred Reporting Items for Systematic reviews and Meta-Analyses
statement (15).
Eligibility criteria
The Population, Intervention, Comparison, Outcomes
and Study (PICOS) criteria for inclusion and exclusion were as
follows: P (participants): Studies of uni- or bilateral PTs of the
breast; I and C (intervention and control): Studies in which
Ki67/MIB1 was measured in PTs by immunohistochemistry, where MIB1
describes the commonly used monoclonal antibody clone targeting the
Ki67 antigen; O (outcome): Studies that included the local
recurrence rate or overall survival (OS) rate were included; S
(study type): Research articles published prior to July 11, 2024
were included. Any review papers, conference abstracts,
meta-analyses, editorial/comment papers and case reports were
excluded from the study.
Information sources and search
strategy
The PubMed/MEDLINE (https://pubmed.ncbi.nlm.nih.gov), Web of Science
(available via https://clarivate.com), Scopus
(https://www.scopus.com/), Embase (https://www.embase.com) and Cochrane Library databases
(https://www.cochranelibrary.com) were
searched for reports meeting the inclusion criteria published
before July 11, 2024. The following search strategies were used for
each database: PubMed/MEDLINE: (phyllodes) AND (breast) AND ((Ki67)
OR (proliferation)) AND ((survival) OR (recurrence) OR (outcome));
Web of Science: (phyllodes) AND (breast) AND ((Ki67) OR
(proliferation)) AND (outcome); Scopus: TITLE-ABS-KEY (phyllodes)
AND TITLE-ABS-KEY (breast) AND (TITLE-ABS-KEY (outcome) OR
TITLE-ABS-KEY (survival) OR TITLE-ABS-KEY (recurrence)) AND
(TITLE-ABS-KEY (Ki67) OR TITLE-ABS-KEY (proliferation)); Cochrane:
Phyllodes tumor; Embase (1974 to 2024 July 11): (phyllodes and
breast and (Ki67 or proliferation) and (survival or recurrence or
outcome)).mp. (mp=title, abstract, heading word, drug trade name,
original title, device manufacturer, drug manufacturer, device
trade name, keyword heading word, floating subheading word,
candidate term word).
Selection, data collection and data
items
A total of two reviewers worked independently to
screen the titles and abstracts of all literature retrieved from
the database searches. After screening, two reviewers independently
collected data from each report and input the data items (study
country, outcome types, follow-up period, number of recurrences
according to Ki67 status, clinicopathological characteristics and
total number of cases) into a data entry sheet. These sheets were
then compared and combined.
Risk of bias assessment
The Newcastle-Ottawa scale (NOS) (16) was used to assess the quality of
prognostic/predictive value of studies with scores converted to
Agency for Healthcare Research and Quality (AHRQ) standards, i.e.,
good quality: 3 or 4 stars in the selection domain AND 1 or 2 stars
in the comparability domain AND 2 or 3 stars in the
outcome/exposure domain; fair quality: 2 stars in the selection
domain AND 1 or 2 stars in the comparability domain AND 2 or 3
stars in the outcome/exposure domain; and poor quality: 0 or 1 star
in the selection domain OR 0 stars in the comparability domain OR 0
or 1 stars in the outcome/exposure domain.
Statistical analysis
Analyses were performed in JASP v0.19 for Apple
Silicon (17). The log odds ratio
(OR) with 95% confidence intervals (CI) was used to compare
dichotomous variables, i.e., proportions of local and distant
recurrences/deaths in patients with PTs with a Ki67 index >10 or
>11.2%. Thresholds of 10 and 11.2% were selected, as these were
the thresholds used in the selected studies and were deemed
sufficiently close to not unduly affect the analysis. Homogeneity
of effect sizes was assessed with the test of residual
heterogeneity (Cochran's Q) test and I2 calculations.
Despite the effect sizes being homogeneous (test of residual
heterogeneity Q-test P=0.18), a random-effects (DerSimonian-Laird)
model was most appropriate for meta-analysis to account for the
inevitable heterogeneity for intervention effects among multiple
studies from different groups and geographical regions. Publication
bias was assessed using a funnel plot and Egger's test. P<0.05
was considered to indicate statistical significance.
Results
Study selection
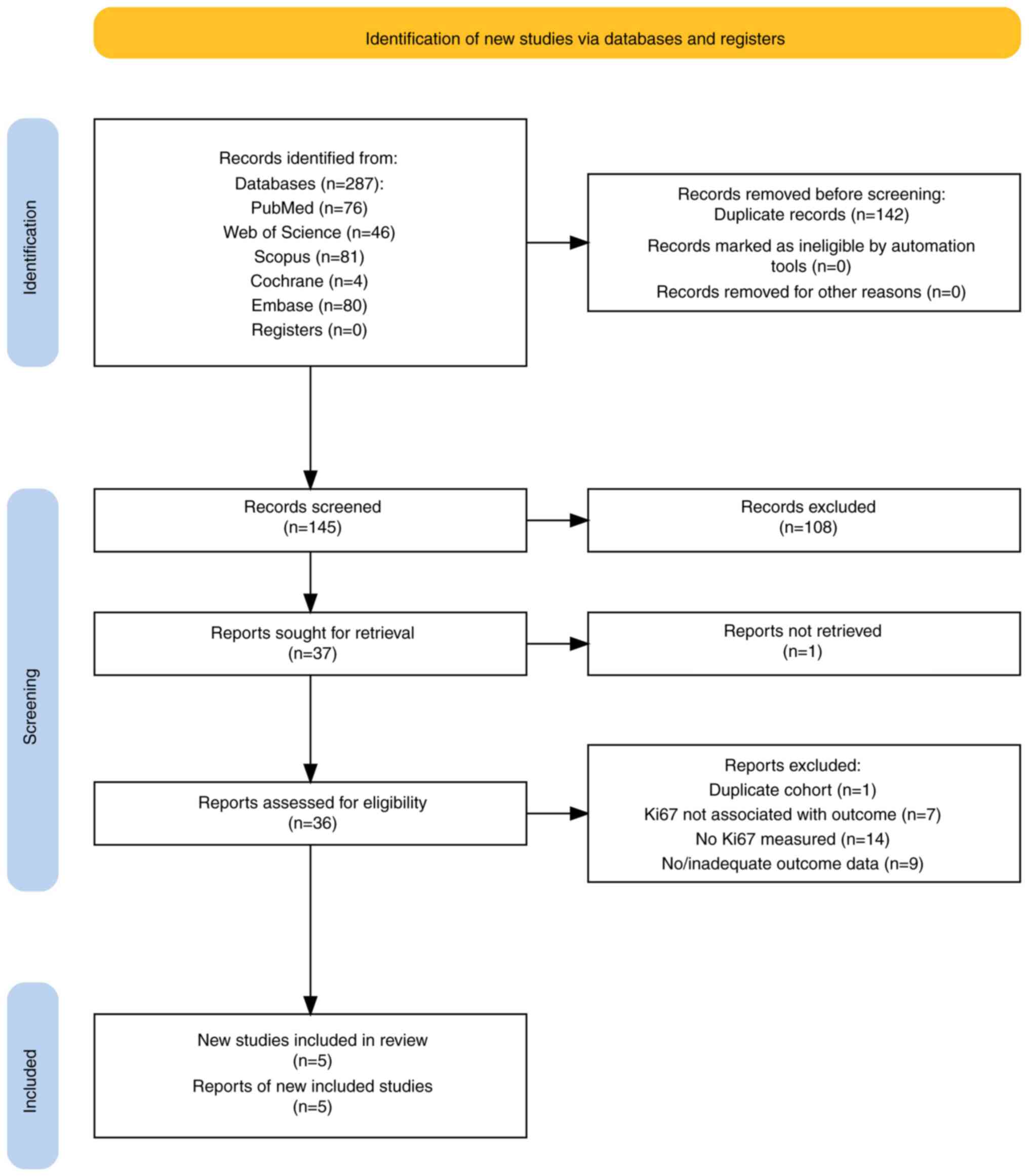
The flow chart of the study selection process is
shown in Fig. 1. Of 287 records
identified in five databases, 145 were screened after removal of
duplicates. Of these, 108 were excluded for not meeting the
inclusion criteria. One report could not be retrieved from a source
in China. Of 36 full reports assessed for eligibility, markers of
proliferation (but not Ki67) were recorded, and in seven papers,
Ki67 was not associated with the outcome of interest. Furthermore,
nine papers did not record outcome data. Two studies (18,19)
were authored by the same research team and published in the same
year and, on closer examination of the cohorts, they were found to
be nearly identical apart from a few more benign PTs in one of the
cohorts. Therefore, the study with the larger cohort was selected
for inclusion (18). After review,
five reports were included in the final analysis (18,20-23).
Study characteristics and risk of
bias/quality assessment
The characteristics of the included studies are
shown in Table I. All five studies
were retrospective observational studies and all five scored eight
points on the NOS, equivalent to ‘good’ quality by AHRQ standards.
Although follow-up varied, it was long enough to capture the most
common period of recurrence in all studies (average, 13.8 months)
(24). While all five studies
reported recurrence (local and/or distant) data and two studies
reported OS data (21,23), one study only reported on the
association of Ki67 with the OS (and not recurrence) outcome
(21). A total of three studies
(18,20,22)
applied a Ki67 threshold of >10%, while two studies (21,23)
applied a Ki67 threshold of 11.2%.
 | Table ICharacteristics of the included
studies. Ki67high was defined as >10% for (18,20,22)
and >11.2% for (21,23), as stated in the respective
manuscripts, while an adverse outcome was either a recurrence [in
four studies (18,20,22,23)]
or death from the disease [in one study (21)]. |
Table I
Characteristics of the included
studies. Ki67high was defined as >10% for (18,20,22)
and >11.2% for (21,23), as stated in the respective
manuscripts, while an adverse outcome was either a recurrence [in
four studies (18,20,22,23)]
or death from the disease [in one study (21)].
| Author, year | Country | Total cases | PT types | Age, years | Outcome type(s) | Follow-up period
(range) | Adverse outcome,
n/total Ki67high |
Ki67low | Log OR | NOS (SE) | Quality (AHRQ
scale) | (Refs.) |
|---|
| Chan, 2004 | Taiwan | 63 | i) Benign (n=50); ii)
malignant (n=13) | i) 42.4±14.3; ii)
44.3±9.9 | Recurrence | Mean, 3 (range, 1-15)
years | 4/19 | 3/44 | 1.29 (0.67) | 8 | Good | (20) |
| Kuijper, 2005 | Netherlands | 40 (37 with
follow-up) | i) Benign (n=21); ii)
borderline (n=8); iii) malignant (n=11) | i) 45.5±16.8; ii)
57.9±12.8; iii) 54.3±12.9 | Recurrence | 93 (4-215)
months | 6/14 | 4/23 | 1.27 (0.77) | 8 | Good | (18) |
| Niezabitowski,
2001 | Poland | 117 | i) Benign (n=52); ii)
borderline (n=24); iii) malignant (n=42) | 49 (16-87) | Overall survival | NS | 10/31 | 3/86 | 2.58 (0.70) | 8 | Good | (21) |
| Shpitz, 2002 | Israel | 23 (22 with
follow-up) | i) Benign (n=16); ii)
borderline (n=4); iii) malignant (n=3) | 41 (17-69) | Recurrence | 52 (27-102)
months | 1/8 | 3/14 | -0.65 (1.25) | 8 | Good | (22) |
| Yonemori, 2006 | Japan | 41 | i) Benign (n=20);
ii) borderline (n=5); iii) malignant (n=16) | 47 (22-65) | Recurrence | 42 (1-90)
months | 6/22 | 3/19 | 0.69 (0.79) | 8 | Good | (23) |
Results of individual studies
The pooled data consisted of five studies comprising
280 cases. The overall adverse outcome rate was 15.4% (95% CI,
11.6-20.1%). The adverse outcome rate for the Ki67high
population was 28.7% (95% CI, 20.1-38.6%), while the adverse
outcome rate for the Ki67low population was 9.4% (95%
CI, 5.4-13.5%). The effect sizes of Ki67high scoring
with an adverse outcome, expressed as the log OR together with
their standard errors, are shown in Table I and ranged from -0.65±1.25 to
2.58±0.70.
Results of meta-analysis
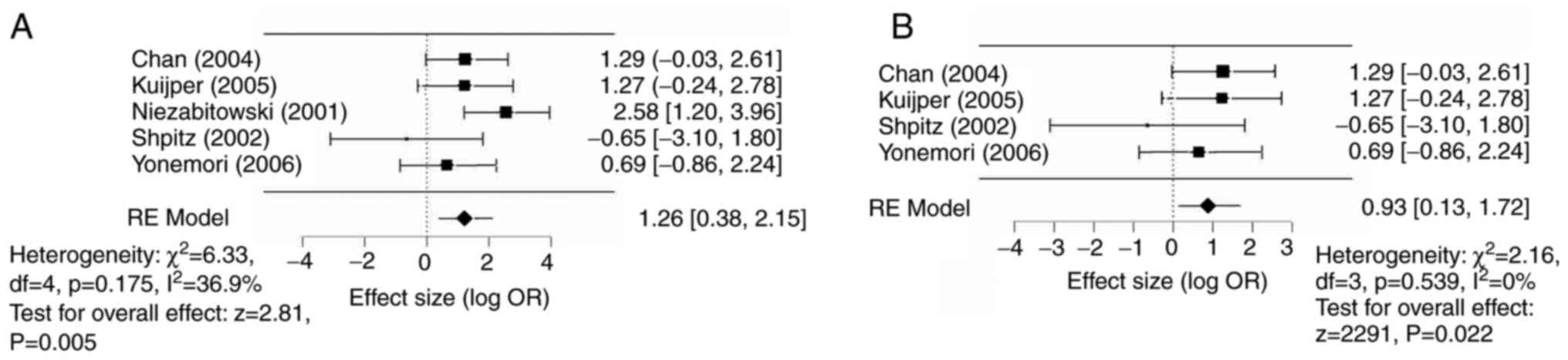
A random-effects (DerSimonian-Laird) model was used
for meta-analysis. Ki67high was associated with an
increased odds of an adverse outcome [log OR, 1.26 (95% CI
0.38-2.15), P=0.005] compared with a Ki67low status
(Fig. 2A). Similarly, when
examining associations with recurrences alone [excluding (21), with an OS endpoint],
Ki67high status was still associated with an increased
odds of local recurrence [log OR 0.93 (95% CI 0.13-1.72),
P<0.02] compared with a Ki67low status (Fig. 2B).
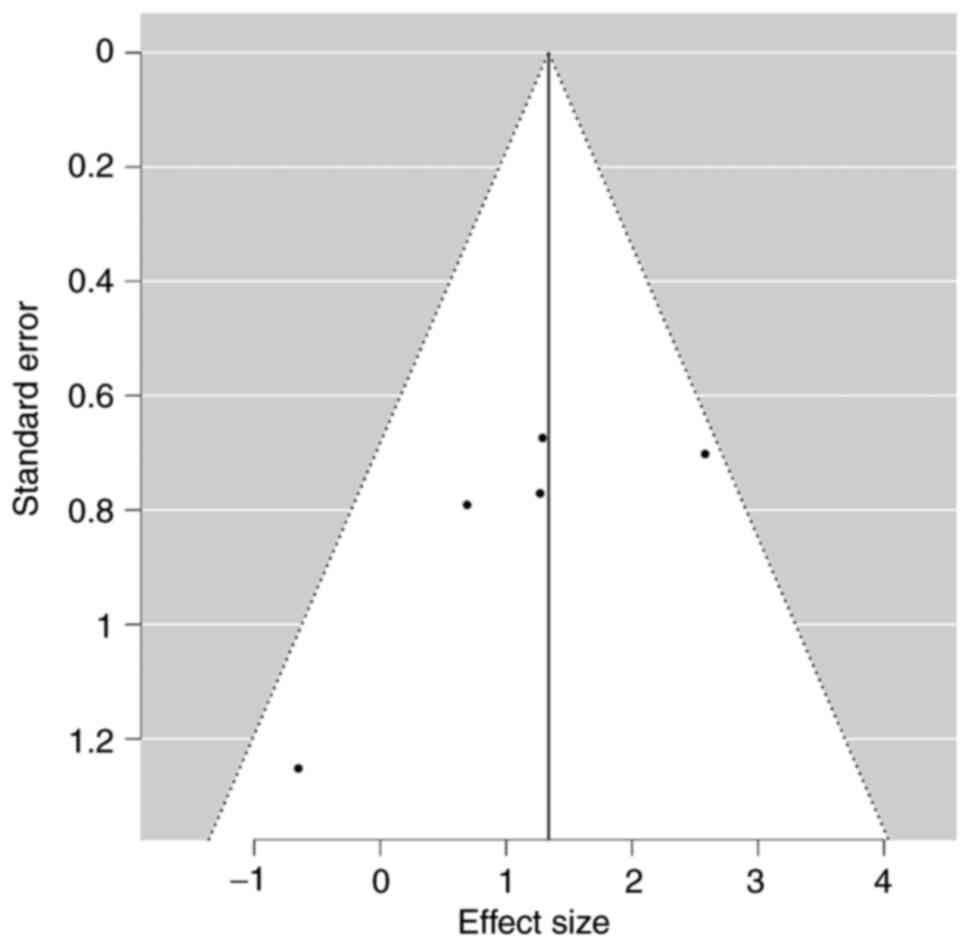
Publication bias
No significant publication bias with respect to
adverse outcomes was noted in the funnel plot (Egger's test P=0.06;
Fig. 3).
Discussion
As PT is a rare tumor, there are no large,
prospective studies on PTs available. However, previous
retrospective studies have reported that various
clinicopathological factors, including adequacy of resection,
histopathological features and tumor protein expression, are
associated with clinical outcomes in patients with PT (7,8).
Although PTs that clearly lie at the extreme ends of the diagnostic
spectrum, such as those that are difficult to differentiate from
fibroadenomas or those with overt features of malignancy, allow
straightforward clinicopathological correlation and management
planning, the disease still poses pathological and clinical
challenges in practice. The absence of clear prognostic factors
means that there are still no evidence-based guidelines for the
follow-up of patients with PT, and accordingly, certain patients
with benign lesions are subjected to unnecessary routine follow-up
imaging, which may not be required (8). Any indicator of an adverse prognostic
course, particularly in patients with clinically benign lesions,
would be useful to tailor follow-up and spare the costs and
resources associated with surveillance imaging or, conversely, to
divert resources to those at greatest risk of future
recurrence.
This situation inspired the present systematic
review and meta-analysis of the prognostic significance of the Ki67
proliferation marker in patients with PT. Although only five
studies that specifically examined the prognostic value of Ki67 in
patients with PT were identified, the present analysis indicates
that patients with PT with high expression of Ki67, here defined as
>10 or >11.2% expression in tumor stroma cells, have a
significantly increased risk of an adverse outcome, particularly
recurrence. Although the small sizes of the included studies and a
lack of relevant data precluded formal subgroup analysis of the
prognostic significance of Ki67 in patients with benign and
borderline disease alone, the available data appear to suggest that
the association between high Ki67 and recurrence is not limited to
malignant disease. Indeed, all seven recurrences reported by Chan
et al (20) occurred after
the resection of benign primary lesions, four of which had Ki67
indices >10%. In the other included reports, even though some
initially benign primary lesions went on to recur, their Ki67
status was unclear. The present analysis suggests that a Ki67 index
>10% may be an indicator of the need for extra vigilance during
follow-up, regardless of other clinical or histopathological
features. Even though locally recurrent PT often presents
clinically and not through imaging (8,25),
establishing Ki67 as an adverse prognostic indicator could be
helpful for identifying the subset of patients who may benefit from
focused radiological follow-up.
There have been previous attempts to devise
predictive models of the clinical behavior of PT. For instance, Tan
et al (6) developed a
nomogram that included atypia, mitoses, stromal overgrowth and
surgical margin status that predicted recurrence-free survival up
to 10 years, which was subsequently validated in several
independent cohorts from around the world (26-28).
Of note, stromal mitotic activity was usually associated with
recurrence even when the other included parameters were not
(26,27). Given the present findings and that
proliferation as measured by mitosis counts are likely to be
critical factors related to recurrence in PT, there is a need to
re-evaluate the relationship between Ki67 indices and clinical
outcomes to establish whether this simple and cost-effective
ancillary diagnostic test should be integrated into clinical
nomograms, particularly with regard to predicting responses in
benign and borderline tumors.
This meta-analysis has several limitations. As noted
above, only five studies were available for analysis, all of which
were retrospective, and thus, selection bias cannot be excluded.
The sample size in the meta-analysis was relatively small, limiting
the level of evidence. Due to the limited number of available
studies, it was required to combine results for similar but
slightly different Ki67 thresholds (10 and 11.2%), which may have
introduced a certain imprecision into the results. As the different
Ki67 cut-off points were not considered, the optimal threshold for
prognostication remains uncertain. Although the results of the
meta-analysis remained the same when the study reporting OS
outcomes was excluded, it was necessary to combine outcomes to
fully evaluate the small number of available studies. Likewise, it
was not possible to separate local from distant recurrences to
evaluate the impact of the Ki67 index on the risk of distant
recurrence/metastasis. Finally, Ki67 measurements were not
standardized across laboratories, which may have influenced the
results.
In conclusion, the present study was the first
meta-analysis of the predictive value of Ki67 in PT of the breast
and the results clearly show that a relatively high Ki67 index
(>10%) is associated with recurrence. Given that Ki67 is widely
used in clinical practice, it seems timely to re-evaluate the
prognostic value of the marker in large retrospective cohorts with
long follow-up to firmly establish whether it could contribute to
identifying patients at risk of recurrence, particularly those with
histologically benign disease. Doing so could impact clinical
practice by refining follow-up recommendations based on quality
evidence and sparing low-risk individuals from unnecessary
imaging.
Acknowledgements
The author would like to thank Dr Samah Saharti
(Faculty of Medicine, King Abdulaziz University and King Abdulaziz
University Hospital, Jeddah, KSA), for her valuable contribution in
reviewing and verifying the data.
Funding
Funding: No funding was received.
Availability of data
The data generated in the present study may be
requested from the corresponding author.
Author's contributions
FR was responsible for the conception and design of
the study, data collection, analysis and interpretation of the
data, drafting and revising the manuscript, and approval of the
final version for publication. FR also checked and confirms the
authenticity of the raw data for the pooled analysis.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The author declares that he has no competing
interests.
References
|
1
|
Lissidini G, Mule A, Santoro A, Papa G,
Nicosia L, Cassano E, Ashoor AA, Veronesi P, Pantanowitz L, Hornick
JL and Rossi ED: Malignant phyllodes tumor of the breast: A
systematic review. Pathologica. 114:111–120. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rakha EA and Ellis IO: Phyllodes Tumor of
the Breast. In: Textbook of Uncommon Cancer. Raghavan D, Blanke CD,
Johnson DH, Moots PL, Reaman GH, Rose PG and Sekeres MA (eds).
Wiley-Blackwell, Hoboken, NJ, p. 243-56, 2012.
|
|
3
|
Tan BY, Acs G, Apple SK, Badve S,
Bleiweiss IJ, Brogi E, Calvo JP, Dabbs DJ, Ellis IO, Eusebi V, et
al: Phyllodes tumours of the breast: A consensus review.
Histopathology. 68:5–21. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tan PH, Ellis I, Allison K, Brogi E, Fox
SB, Lakhani S, Lazar AJ, Morris EA, Sahin A, Salgado R, et al: The
2019 World Health Organization classification of tumours of the
breast. Histopathology. 77:181–185. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yu CY, Huang TW and Tam KW: Management of
phyllodes tumor: A systematic review and meta-analysis of
real-world evidence. Int J Surg. 107(106969)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tan PH, Thike AA, Tan WJ, Thu MM, Busmanis
I, Li H, Chay WY and Tan MH: Phyllodes Tumour Network Singapore.
Predicting clinical behaviour of breast phyllodes tumours: A
nomogram based on histological criteria and surgical margins. J
Clin Pathol. 65:69–76. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bogach J, Shakeel S, Wright FC and Hong
NJL: Phyllodes Tumors: A scoping review of the literature. Ann Surg
Oncol. 29:446–459. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lim RS, Cordeiro E, Lau J, Lim A, Roberts
A and Seely J: Phyllodes tumors-the predictors and detection of
recurrence. Can Assoc Radiol J. 72:251–257. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lashen AG, Toss MS, Ghannam SF, Makhlouf
S, Green A, Mongan NP and Rakha E: Expression, assessment and
significance of Ki67 expression in breast cancer: An update. J Clin
Pathol. 76:357–364. 2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Finkelman BS, Zhang H, Hicks DG and Turner
BM: The Evolution of Ki-67 and Breast Carcinoma: Past observations,
present directions, and future considerations. Cancers (Basel).
15(808)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Boninsegna L, Panzuto F, Partelli S,
Capelli P, Delle Fave G, Bettini R, Pederzoli P, Scarpa A and
Falconi M: Malignant pancreatic neuroendocrine tumour: Lymph node
ratio and Ki67 are predictors of recurrence after curative
resections. Eur J Cancer. 48:1608–1615. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
La Rosa S: Diagnostic, prognostic, and
predictive role of Ki67 proliferative index in neuroendocrine and
endocrine neoplasms: Past, present, and future. Endocr Pathol.
34:79–97. 2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yamaguchi T, Fujimori T, Tomita S,
Ichikawa K, Mitomi H, Ohno K, Shida Y and Kato H: Clinical
validation of the gastrointestinal NET grading system: Ki67 index
criteria of the WHO 2010 classification is appropriate to predict
metastasis or recurrence. Diagn Pathol. 8(65)2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tse GM, Niu Y and Shi HJ: Phyllodes tumor
of the breast: An update. Breast Cancer. 17:29–34. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Love J, Selker R, Marsman M, Jamil T,
Dropmann D, Verhagen J, Ly A, Gronau QF, Šmíra M, Epskamp S, et al:
JASP: Graphical statistical software for common statistical
designs. Journal of Statistical Software. 88:1–17. 2019.
|
|
18
|
Kuijper A, de Vos RA, Lagendijk JH, van
der Wall E and van Diest PJ: Progressive deregulation of the cell
cycle with higher tumor grade in the stroma of breast phyllodes
tumors. Am J Clin Pathol. 123:690–698. 2005.PubMed/NCBI
|
|
19
|
Kuijper A, van der Groep P, van der Wall E
and van Diest PJ: Expression of hypoxia-inducible factor 1 alpha
and its downstream targets in fibroepithelial tumors of the breast.
Breast Cancer Res. 7:R808–R818. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Chan YJ, Chen BF, Chang CL, Yang TL and
Fan CC: Expression of p53 protein and Ki-67 antigen in phyllodes
tumor of the breast. J Chin Med Assoc. 67:3–8. 2004.PubMed/NCBI
|
|
21
|
Niezabitowski A, Lackowska B, Rys J,
Kruczak A, Kowalska T, Mitus J, Reinfuss M and Markiewicz D:
Prognostic evaluation of proliferative activity and DNA content in
the phyllodes tumor of the breast: Immunohistochemical and flow
cytometric study of 118 cases. Breast Cancer Res Treat. 65:77–85.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shpitz B, Bomstein Y, Sternberg A, Klein
E, Tiomkin V, Kaufman A, Groisman G and Bernheim J:
Immunoreactivity of p53, Ki-67, and c-erbB-2 in phyllodes tumors of
the breast in correlation with clinical and morphologic features. J
Surg Oncol. 79:86–92. 2002.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yonemori K, Hasegawa T, Shimizu C, Shibata
T, Matsumoto K, Kouno T, Ando M, Katsumata N and Fujiwara Y:
Correlation of p53 and MIB-1 expression with both the systemic
recurrence and survival in cases of phyllodes tumors of the breast.
Pathol Res Pract. 202:705–712. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sain B, Gupta A, Ghose A, Halder S,
Mukherjee V, Bhattacharya S, Mondal RR, Sen AN, Saha B, Roy S and
Boussios S: Clinico-Pathological factors determining recurrence of
phyllodes tumors of the breast: The 25-year experience at a
tertiary cancer centre. J Pers Med. 13(866)2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
McCarthy E, Kavanagh J, O'Donoghue Y,
McCormack E, D'Arcy C and O'Keeffe SA: Phyllodes tumours of the
breast: Radiological presentation, management and follow-up. Br J
Radiol. 87(20140239)2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chng TW, Lee JY, Lee CS, Li H, Tan MH and
Tan PH: Validation of the Singapore nomogram for outcome prediction
in breast phyllodes tumours: An Australian cohort. J Clin Pathol.
69:1124–1126. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Choi JE, Kang SH, Tan PH and Bae YK:
Recurrence prediction for breast phyllodes tumours: Validation of
the Singapore nomogram in Korean women. J Clin Pathol. 75:159–163.
2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nishimura R, Tan PH, Thike AA, Tan MH,
Taira N, Li HH and Ohsumi S: Utility of the Singapore nomogram for
predicting recurrence-free survival in Japanese women with breast
phyllodes tumours. J Clin Pathol. 67:748–750. 2014.PubMed/NCBI View Article : Google Scholar
|