Superficial mucoceles (SM) are small, translucent,
intra- or subepithelial vesicles affecting the oral mucosa. Their
formation typically results from injuries or chronic and recurrent
inflammation. These lesions tend to be recurrent and cause
discomfort to the patients (1).
Cemiplimab is a highly potent human monoclonal antibody that
targets programmed death 1 (PD-1) (2). Cemiplimab treatment is available
under the national drug program ‘B.125 Treatment of patients with
advanced squamous cell carcinoma of the skin with cemiplimab’ for
the treatment of patients with metastatic or locally advanced
squamous cell carcinoma of the skin, who are not eligible for
radical surgery or radiotherapy. To the best of our knowledge,
previously published literature reviews have detailed a total of 99
cases of Stevens-Johnson Syndrome (SJS)/toxic epidermal necrolysis
(TEN) after immunotherapy (3-58).
The present manuscript reported the case of an adult male diagnosed
with squamous cell carcinoma and treated with cemiplimab, who
developed SJS, which led to SM after 5 weeks of SJS diagnosis. To
enhance the current understanding of the occurrence of these rare
adverse effects, a review of the literature was conducted, which
focused on the incidence of oral mucositis (OM) and the development
of severe adverse effects such as SJS/TEN, during oncological
treatments. The present report aimed to offer valuable insights to
be used by clinicians for the effective understanding and
management of this uncommon condition. Table I presents definitions of different
forms of oral mucosal damage during oncological treatment.
The present report focused on various types of
anticancer therapies and their associated side effects. The Medline
database (http://www.ncbi.nlm.nih.gov/pubmed) was searched using
the following search terms: ‘atezolizumab’ or ‘avelumab’ or
‘camrelizumab’ or ‘cemiplimab’ or ‘dostarlimab’ or ‘durvalumab’ or
‘ipilimumab’ or ‘nivolumab’ or ‘pembrolizumab’ or ‘penpulimab’ or
‘relatlimab’ or ‘retifanlimab’ or ‘serplulimab’ or ‘sintilimab’ or
‘tislelizumab’ or ‘toripalimab’ or ‘tremelimumab’ or ‘EGFR
inhibitor’ or ‘cetuximab’ or ‘panitumumab’ or ‘afatinib’ or
‘osimertinib’ or ‘vandetanib’ or ‘apatinib mesylate’ or ‘gefitinib’
or ‘erlotinib’ or ‘dacomitinib’ or ‘lapatinib’ or ‘angiogenesis
inhibitors’ or ‘sorafenib’ or ‘sunitinib’ or ‘cabozantinib’ or
‘bevacizumab’ or ‘pazopanib’ or ‘axitinib’ or ‘lenalidomide’ or
‘BRAF inhibitor’ or ‘vemurafenib’ or ‘dabrafenib’ or ‘KIT and
BCR-ABL inhibitors’ or ‘imatinib’ or ‘ALK inhibitors’ or
‘crizotinib’ or ‘rituximab’ or ‘bortezomib’ or ‘enfortumab vedotin’
or ‘brentuximab vedotin’ or ‘mTOR’ or ‘sirolimus’ or ‘everolimus’
or ‘temsirolimus’ or ‘alpelisib’ or ‘BTK inhibitor’ or ‘ibrutinib’
or ‘BCL2 inhibitor’ or ‘venetoclax’ and ‘Stevens Johnson syndrome’
or ‘SJS’ or ‘toxic epidermal necrolysis’ or ‘TEN’ or ‘oral
mucositis’ or ‘stomatitis’ or ‘superficial mucoceles’. All relevant
publications were selected and their references were further
checked and researched for any additional undetected published
cases (V.C, M.S, A.P). Primary case reports, case series, reviews
and reports from clinical trials were included. A total of 8,379
potentially eligible articles were selected. Articles that were not
written in English and did not contain the full manuscript were
excluded, which left 7,812 studies. After the removal of irrelevant
articles and duplicate manuscripts based on titles, abstracts and
full articles, a total of 97 articles were included in the present
review (V.C). A flowchart on the selection and evaluation of
scientific articles is provided in Fig. S1.
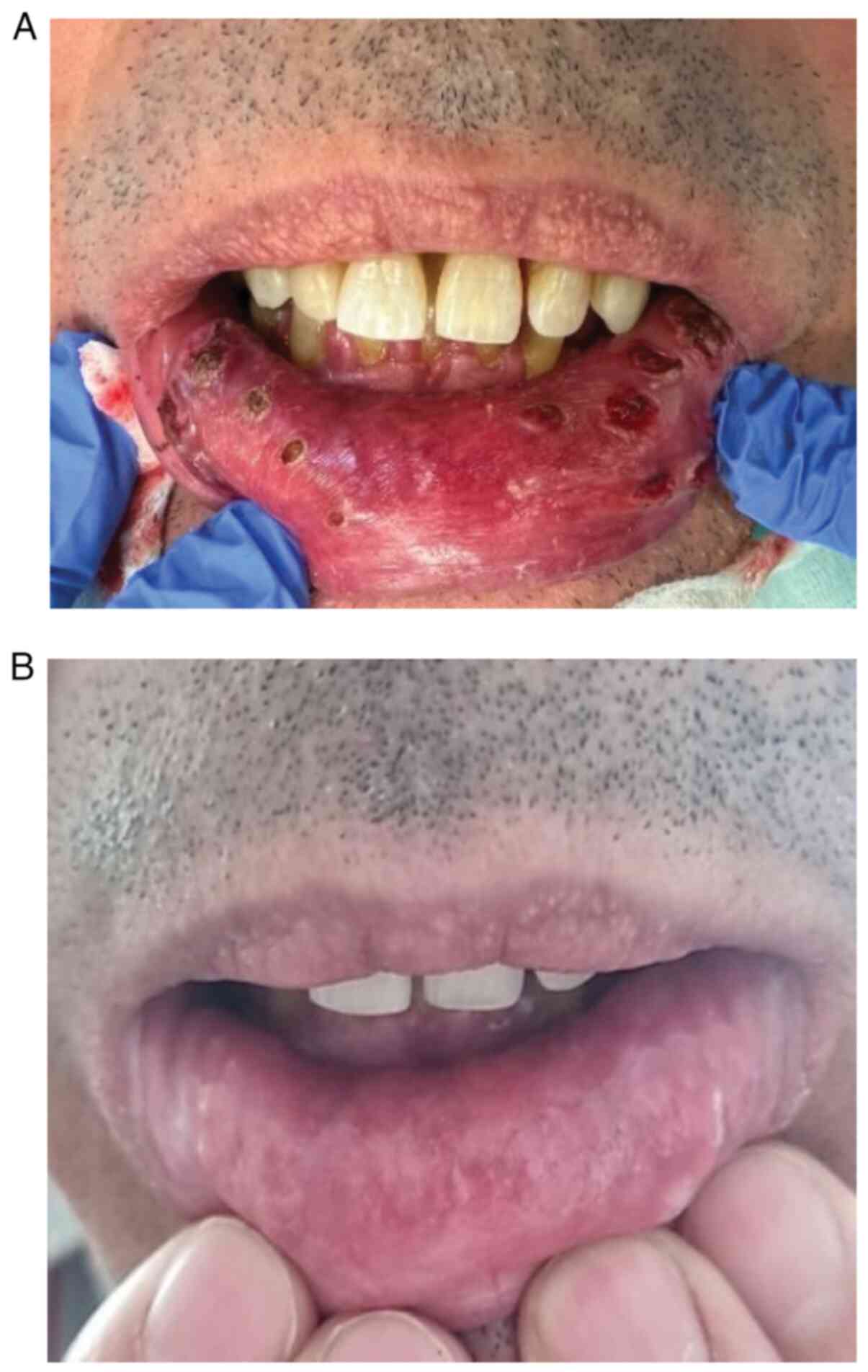
A 56-year-old male undergoing immunotherapy for
squamous cell carcinoma of the right lower leg with metastases to
the lungs and right inguinal lymph nodes presented to the
Department of Dermatology, Military Institute of Medicine-National
Research Institute, Central Clinical Hospital Ministry of Defense
in Warsaw in November 2023. At the time of referral to the
Dermatology Clinic, the patient's cancer stage was assessed as
stable disease according to the Response Evaluation Criteria in
Solid Tumours (RECIST) (59). The
patient had undertaken 16 courses of treatment with cemiplimab 350
mg every 3 weeks beginning in December 2022. The patient reported
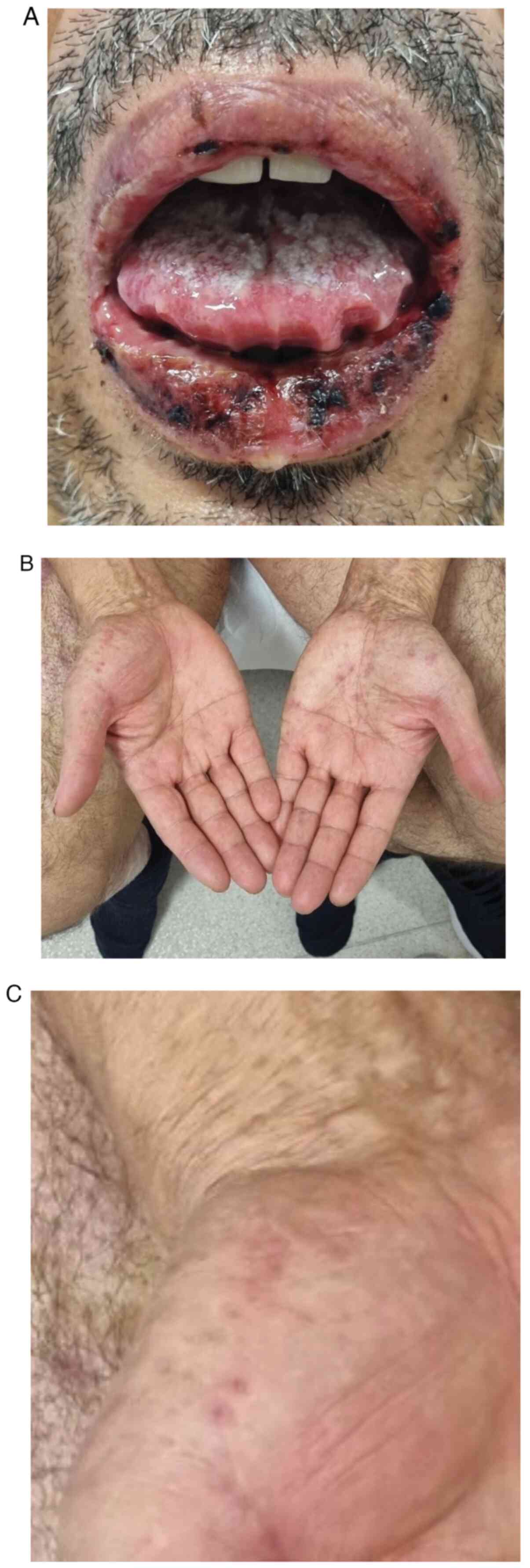
to the Dermatology Clinic due to blistering of the mucous membrane
and epidermis throughout the lips and painful erosions of the oral
mucosa (Fig. 1A). The severity of
the OM was rated as G3 on the Common Terminology Criteria for
Adverse Events (CTCAE) scale (Table
II) (60). Furthermore, the
presence of erythema multiforme (EM) lesions was observed on the
palmar skin of the hands (Fig. 1B
and C). The percentage of body
surface area affected was estimated as 2%. The skin changes were
accompanied by an increase in inflammatory markers: C-reactive
protein (8.3 mg/dl; normal range, 0-0.5 mg/dl), procalcitonin (0.11
ng/ml; normal range, ≤0.046 ng/ml) and erythrocyte sedimentation
rate (72 mm/h; normal range, 0-8 mm/h). Laboratory tests
demonstrated no other abnormalities, such as elevations in liver
enzymes or eosinophilia. Based on the clinical symptoms and
following the exclusion of the potential influence of other drugs,
the patient was diagnosed with SJS induced by immunotherapy (G3 on
the CTCAE scale). This adverse event developed 12 months after the
initiation of cemiplimab as a sole complication of this treatment
regime. Skin lesions were not preceded by pruritus, skin pain or
rashes. During hospitalization, intravenous dexamethasone at a dose
of 12 mg was administered and was gradually reduced after clinical
improvement of the patient's symptoms was shown. Additional
laboratory tests showed no circulating antinuclear antibodies or
pemphigus/pemphigoid antibodies using indirect immunofluorescence
tests. The histopathological examination was waived due to the
typical clinical manifestation of SJS, such as targetoid skin
lesions, classic oral mucosal involvement, the course of the
disease and also partially due to the negative result obtained
using indirect immunofluorescence (61) (cat. no. FA 1501-1005) with a
dilution of 1:10 for anti-pemphigus and anti-pemphigoid antibodies
on the substrates of the company Euroimmun Polska Sp. zo.o. using
the TITERPLANE technique to standardize immunological analysis. The
patient continued the treatment at home, which began with
prednisone (30 mg/day) and the dose was gradually decreased over a
3-week period.
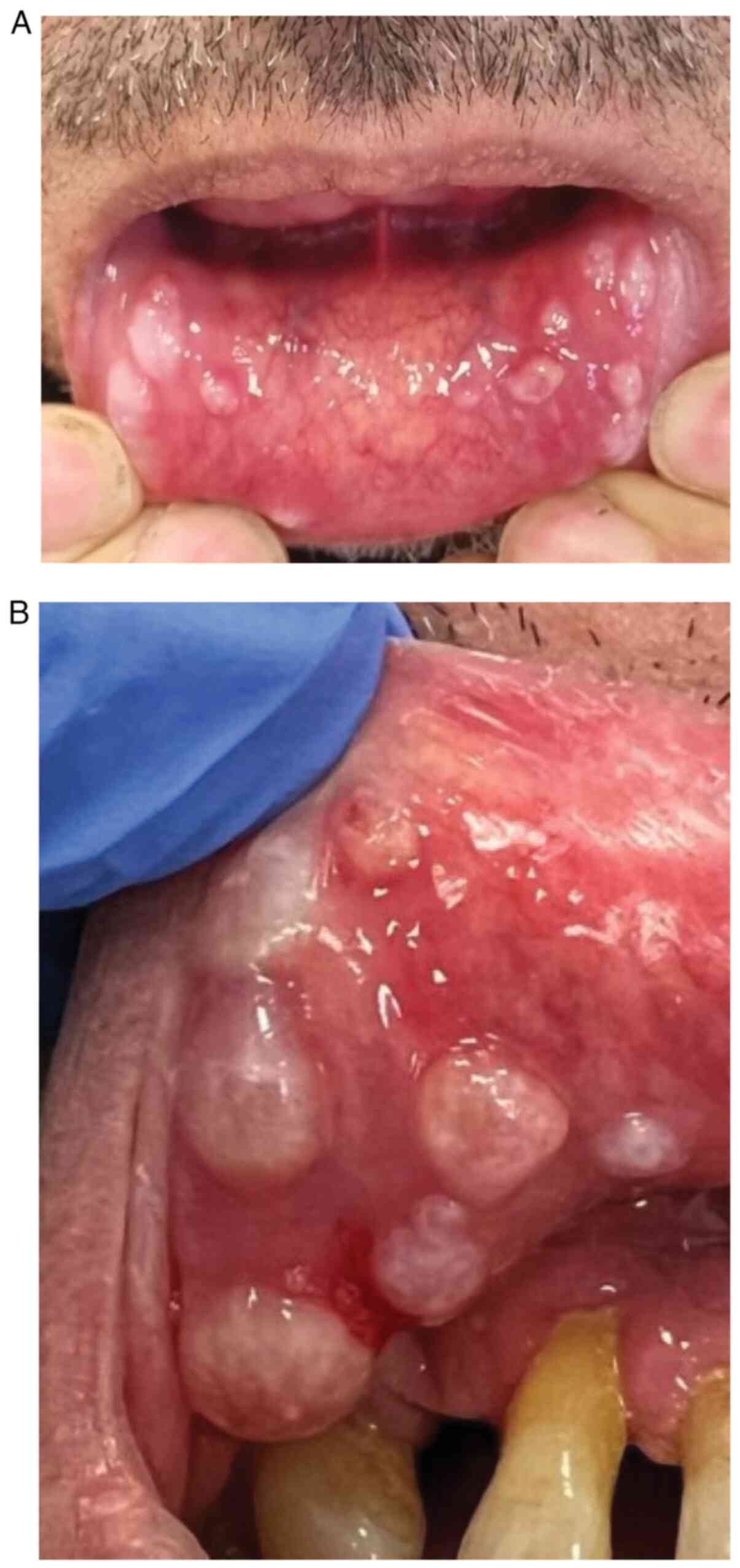
During a follow-up visit at 5 weeks after
hospitalization, numerous whitish cysts, 2-4 mm in diameter, were
found on the inner surface of the lower lip (Fig. 2A and B), and single cysts were present on the
lateral surfaces of the cheek mucosa. The lesions caused discomfort
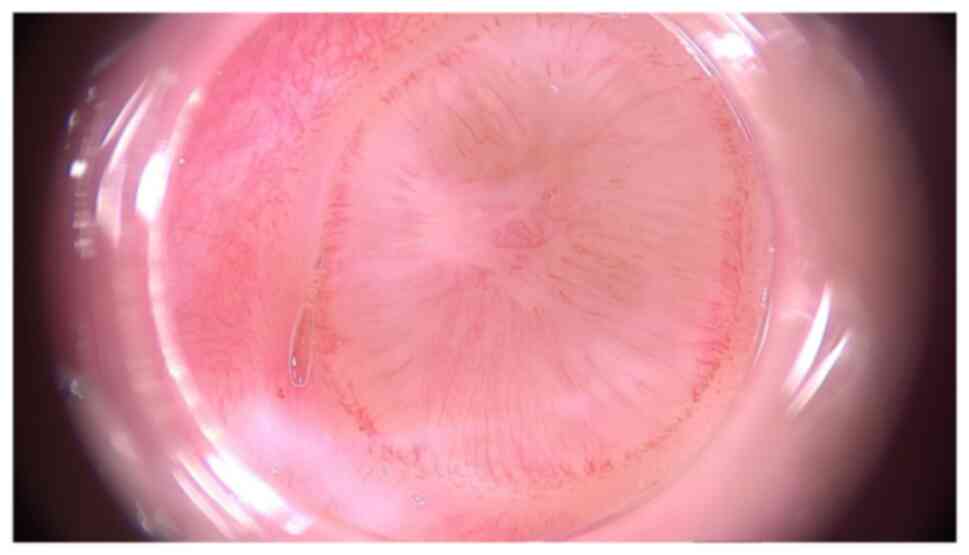
for the patient during speaking or eating. The videodermoscopic
examination was performed with a Fotofinder.Universe 2.0.41.19
videodermoscope (FotoFinder Systems GmbH), having a magnification
of 20x (Fig. 3) and showed nodular
lesions with an opalescent surface, surrounded by a halo. Inside
the lesion, hairpin vessels were arranged radially and polymorphic
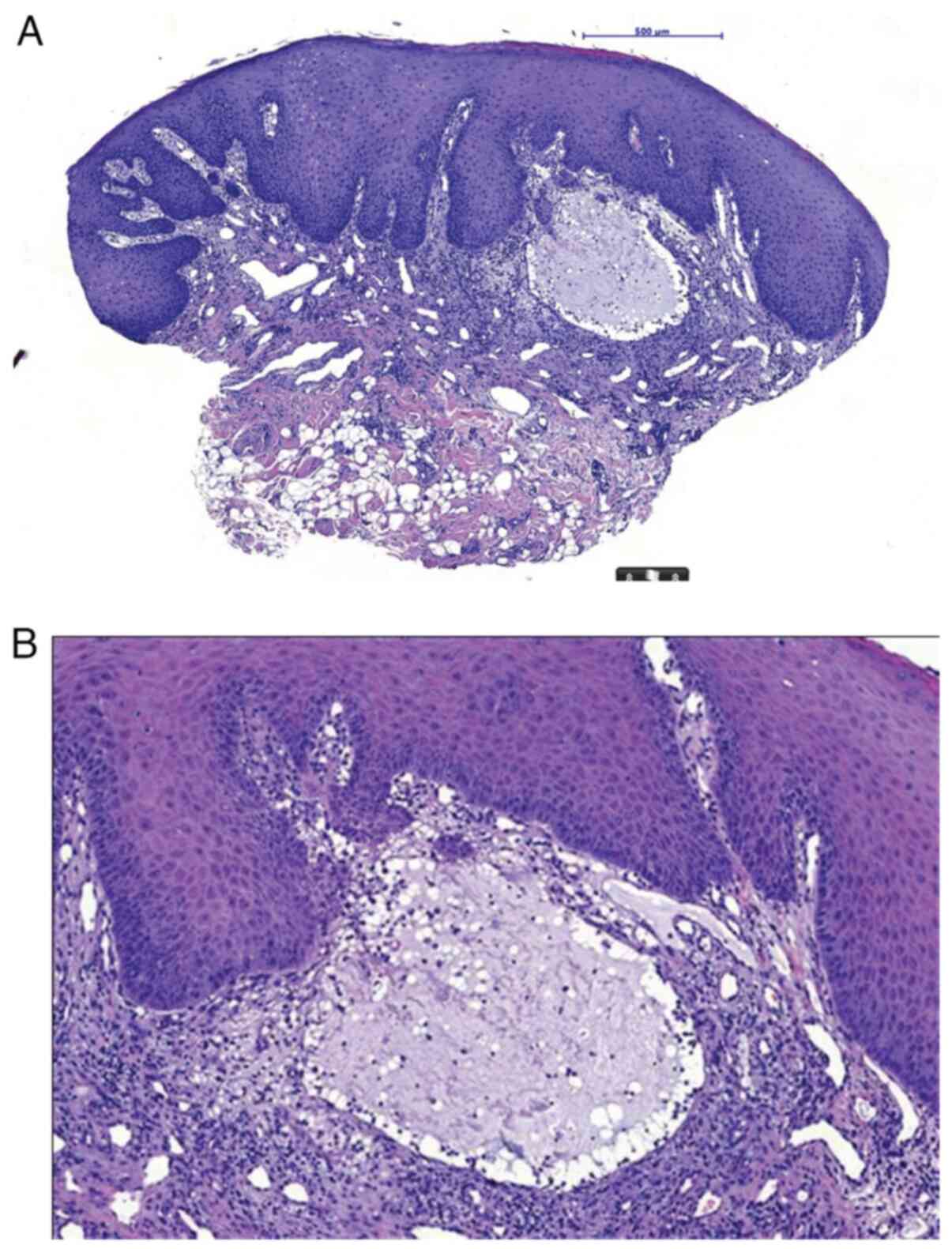
vessels on the periphery of lesions were observed. A punch biopsy
was taken from persistent small erosions within the hypertrophic
mucosal epithelium. A superficial stasis cyst, SM, was diagnosed
based on histopathological examination (histopathology report no.
464/24) (Fig. 4A and B).
The Plasma IQ device (Berger & Kraft Medical)
was used to treat the cysts. The procedure was performed under
local anesthesia with 1% lignocaine solution. A satisfactory
therapeutic and aesthetic outcome was achieved directly following
the procedure (Fig. 5A). However,
after 2 weeks, a number of lesions began to reoccur on the lower
lip. The patient was treated with electrocoagulation, which was
successful (Fig. 5B; the image was
taken 2 weeks after electrocoagulation). Due to the diagnosis of
SJS, the immunotherapy was permanently discontinued. From the SJS
episode (November 2023) until the time of manuscript submission
(October 2024), the patient remained under oncological supervision
with 4-monthly follow-up and no further therapy was introduced due
to the partial response obtained according to the RECIST criteria.
In case of disease progression, radiotherapy or surgical treatment
should be recommended.
The present literature review summarized all cases
of SJS, TEN and SJS/TEN in patients undergoing cancer therapy
reported to date (Table III).
These are several rare side effects that can occur when using
anticancer therapies and, to date, 99 cases of SJS/TEN have been
described following immunotherapy (3-58).
The clinical presentation of the disease is initially a
morbilliform rash, followed by the development of targetoid
lesions, epidermal detachment and mucous membrane ulcerations
(62). The median time between the
start of immune checkpoint inhibitor (ICI) therapy and the onset of
SJS/TEN ranged from 1 day to 3 years. For patients with SJS, the
median time to onset was 5.8 weeks and the average time was 13.8
weeks. For patients with TEN, the median time to onset was 4.0
weeks and the average time was 11.3 weeks. Several cases of TEN
after termination of treatment have also been previously reported
(3). Various possible mechanisms
have been described to explain the induction of SJS/TEN in patients
treated with ICIs. It has been suggested that cutaneous
immune-related side effects may represent a pathogenic immune
response against the microbiota. Another hypothesis suggests that
ICI therapy increases the patient's sensitivity to other drugs or
active agents (3). Histopathology
of SJS/TEN-like reactions shows epidermal necrolysis associated
with a change in the vacuolar interface, cleavage along the
dermal-epidermal junction and subepidermal lymphocytes.
Leukocytoclastic vasculitis may occur and infiltration of CD8+
T-lymphocytes, and increased programmed cell death ligand 1 (PD-L1)
expression may be a response to lymphocyte overactivity induced by
anti-PD1 drugs (62). Activated
cytotoxic T-cells trigger apoptosis of PD-L1-expressing
keratinocytes. In addition, ICI-induced SJS/TEN-like reactions show
a similar gene expression profile to classic SJS/TEN (62). Type IVc hypersensitivity, as well
as co-stimulatory factor amplification and regulatory T-cell
dysfunction, may also be involved in the pathogenesis of
SJS/TEN-like reactions (63). In
addition, lower patient age was significantly associated with
poorer outcomes for patients with extensive disease, which differs
from standard TEN, where a patient age of >40 years is
associated with higher mortality (3). Further drugs that cause SJS/TEN-like
reactions are enfortumab vedotin (60 cases) (64) and imatinib (20 cases) (4). Severe cutaneous adverse reactions to
enfortumab vedotin may result from the expression of nectin-4 in
epidermal keratinocytes and skin appendages (65). Isolated cases have been described
with other drug therapies. The mortality rate for ICI-induced SJS
was 4%, while it was 35% for ICI-induced TEN (3). The mortality rate of patients with
SJS/TEN induced by targeted anticancer therapies and
immunotherapies was 17.86% (5).
In addition, the present manuscript summarized the
incidence of one of the most common adverse effects of anticancer
therapy, which is OM or stomatitis, depending on the treatment
administered (Table III).
Factors such as smoking, age, female sex, poor oral hygiene and
previous antineoplastic therapy may increase the risk of
mucotoxicity (5,6). OM or stomatitis occurs commonly as a
complication of head and neck radiochemotherapy - in 80-90% of
patients (66,67), in 80% of patients treated with head
and neck radiotherapy (68) and as
a complication of hematopoetic stem cell transplantation/graft
vs. host disease in 60-85% of patients (67,69).
In patients treated with chemotherapy, the development of OM is
dependent on the type of tumor and the chemotherapeutic used and
develops in 14.4-81.3% of these patients (7). OM or stomatitis are less frequently
developed in patients who undergo targeted therapies, such as mTOR
(30% of patients) (70), and is
more common in patients treated with everolimus (67%) (6), angiogenesis inhibitors (7-29%)
(71) and anti-EGFR therapy (15%)
(67). In the anti-EGFR-treated
group, it is significantly more common to experience these side
effects when using tyrosine kinase inhibitors, such as dacomitinib
and afatinib (40%), compared with monoclonal antibodies such as
cetuximab (7%) and panitumumab (5%) (71). Furthermore, these serious adverse
effects are rarely reported in patients treated with immunotherapy
(1.5-5%), and severe OM was reported in just 0.2% of these patients
(6,7). Mucosal involvement was reported in
65% of patients with ICI-induced TEN and was significantly
associated with an increased risk of mortality (3). The median onset of mucositis after
immunotherapy is 21 weeks (72).
The pathogenesis of OM formation during ICI treatment has not yet
been thoroughly investigated. The infiltration of normal tissues,
including the oral mucosa, with activated T-cells catalyzes a local
cellular immune response (6).
Histopathological examination of OM during ICI treatment shows
patchy or florid lichenoid interface dermatitis in the upper lamina
propria and mainly a CD4/CD8-positive band-like T-cell infiltrate
(70). Cases of mucous membrane
pemphigoid and oral lichen planus-like reactions induced by
immunotherapy have also been reported (72). To date, 3 cases of mucosal toxicity
after vemurafenib treatment have been described in the literature,
whereas no reports were found for patients treated with dabrafenib
(73-75).
SM is a rare disease. Based on the review of the
current literature, 10 patients (1.2%) developed SM after
radiotherapy (76) and 26 patients
developed SM during HSCT/GVHD (8,77-86).
To date, just two cases of SM occurring after immunotherapy have
been described in the literature (5,8). Of
these cases, 1 patient was treated with pembrolizumab and initially
developed lichenoid lesions in the oral cavity, followed by the
occurrence of SM (8). The second
patient was treated surgically, with chemotherapy, corticosteroids
and radiotherapy before immunotherapy (5). To date, no case has been reported of
a patient who developed SJS after immunotherapy, followed by SM, to
the best of our knowledge. Based on the currently available
literature, it could be suggested that the chronic persistence of
autoreactive lymphocytes and increased levels of pro-inflammatory
cytokines in tissues after SJS may be responsible for
intraepithelial blisters and cyst formation in vacuolar interface
dermatitis and the development of SM, as a result of chronic
inflammation. The true prevalence of SM remains undetermined, but
is likely to be underreported. To the best of our knowledge, there
have been no reports of patients developing SM when they were
treated with other oncological therapies.
OM is one of the adverse effects of anticancer
therapy, typically manifesting as extensive erosions or ulcers of
the oral cavity, accompanied by pain and causing difficulties
swallowing, which can negatively affect the patient's quality of
life (87). OM in patients treated
with immunotherapy is often recurrent, severe or chronic, and may
persist for months after discontinuation of the treatment.
Immunotherapy may cause various types of side effects in the oral
cavity, which include lichen planus, bullous lichen planus, bullous
pemphigoid, erythema multiforme, Stevens-Johnson syndrome and toxic
epidermal necrolysis (3,8).
Previous reports detailed the dermoscopy of classic
mucocele, which was located deeper compared with the patient of the
present study (89,90). Hence, the videodermoscopic image of
SM presented in the current manuscript, to the best of our
knowledge, was the first report of such a case and differs from an
image of mucocele and other abnormalities of the oral mucosa.
Mucoscopy is a safe, non-invasive method that helps to
differentiate between various mucocutaneous disorders such as
pemphigus vulgaris or lichen planus (91,92).
A previous report by Rather et al (89) described mucoceles located
submucosally, which were deeper compared with the intra- or
subepithelial locations observed with SM. SM can be misdiagnosed as
bullous lichen planus, recurrent aphtous stomatitis, pemphigus
vulgaris, discoid lupus erythematosus, verrucae, mucous membrane
pemphigoid or herpetic lesions (1,89).
To date, no effective treatment protocol has been proposed for SM.
In asymptomatic cases, observation is sufficient. In cases of
functional impairment and irritation, it is necessary to remove the
minor gland by surgical excision, laser vaporization with a
neodymium-doped yttrium aluminum garnet laser, laser ablation using
CO2 or cryosurgery (1,93).
Surgery is a more invasive and painful method, which causes
discomfort to the patient, particularly those with multiple and
recurrent lesions. In addition, a patient who has undergone severe
adverse reactions may potentially be traumatized and subsequent
oral procedures after extensive ulcerations may be emotionally
challenging. Heguedusch et al (8) reported that topical clobetasol
propionate at a dose of 0.05% was effective for the treatment of SM
associated with GVHD, radio-induced mucositis, erythematous lupus
and oral lichenoid lesion associated with pembrolizumab.
The patient in the present study was treated using
dermosurgical technology with Plasma IQ (Berger & Kraft
Medical). The device is certified by the Food and Drink
Administration for the removal and coagulation of skin lesions.
During the treatment, plasma beams are precisely delivered to the
skin using thin needle electrodes, generating microthermal zones
that lead to the pyrolysis of tissues in the treatment area,
without damaging the surrounding tissues. The recovery period and
tissue regeneration after the procedure ranges from 5-7 days
(94). In the present study, the
plasma device and electrocoagulation were used because of their
high efficacy and low risk of scarring. These devices are more
cost-effective, readily available and comfortable to use in the
oral cavity because of their small size in contrast to larger laser
heads (95). The use of this
method allows for achieving a satisfactory aesthetic outcome with a
short recovery period. However, it was still necessary to treat the
recurring lesions. The recurrence of SM may be the result of the
lichenoid inflammation damaging the salivary gland ducts (1). SM recurrence could also be due to
scar formation and the incomplete removal of adjoining minor
salivary glands (91). In the
present study, recurrence after 2 weeks was potentially associated
with persistent chronic inflammation in the tissue. As a preventive
measure, patients should be closely monitored after severe
cutaneous adverse reactions and educated about possible side
effects.
Immunotherapy is an increasingly common treatment
method used in oncology; however, it is associated with the
possibility of the occurrence of various types of acute
immune-related toxicities. Cutaneous adverse events can occur both
during or after the cessation of treatment. These complications may
persist chronically in ~20% of patients. Therefore, knowledge of
these complications is crucial for effective patient care, early
diagnosis, selection of adequate treatment and further
dermatological supervision. The main concern for the management of
these patients is to manage symptoms effectively to allow the
patients to continue their oncological treatment. To the best of
our knowledge, this is the first published report of SJS secondary
to cemiplimab describing SM structures and the application of a
plasma device for SM removal. Oral mucosal as an adverse effect is
much more frequently reported during ICI treatment than during
targeted therapies (6,67). The rapid development of oncology
therapies and their use in a rising number of patients will
increase the number of reported new complications that will require
the advancement of new management methods.
Not applicable.
Funding: No funding was received.
The data generated in the present study may be
requested from the corresponding author.
MS, VC and AP conceptualized and designed the study
and drafted the manuscript. MS, VC and AP participated in data
collection and analysis. MS, VC, KP and WO interpreted the data and
revised the manuscript. VC, AP, MS, PT and AC treated the patient.
JK conducted the analysis of the pathological results. VC, AP, MS,
JK, PT, AC and WO confirm the authenticity of all the raw data. All
authors read and approved the final version of the manuscript.
Not applicable.
Written informed consent was obtained from the
patient for publication of this case report and accompanying
images.
The authors declare that they have no competing
interests.
|
1
|
Pandarathodiyil AK and Sivapathasundharam
B: Diagnostic challenges of superficial mucoceles: An update. J
Oral Maxillofac Pathol. 27:616–621. 2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hober C, Fredeau L, Pham-Ledard A, Boubaya
M, Herms F, Celerier P, Aubin F, Beneton N, Dinulescu M, Jannic A,
et al: Cemiplimab for Locally Advanced and Metastatic Cutaneous
Squamous-Cell Carcinomas: Real-Life Experience from the French
CAREPI Study Group. Cancers (Basel). 13(3547)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bray ER, Lin RR, Li JN, Elgart GW, Elman
SA and Maderal AD: Immune checkpoint inhibitor associated epidermal
necrosis, beyond SJS and TEN: A review of 98 cases. Arch Dermatol
Res. 316(233)2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lin M, Gong T, Ruan S, Lv X, Chen R, Su X,
Cheng B and Ji C: Emerging insights into stevens-johnson syndrome
and toxic epidermal necrolysis induced by immune checkpoint
inhibitor and tumor-targeted therapy. J Inflamm Res. 17:2337–2351.
2024.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Amy DPB, Shalabi A, Finfter O, Birenzweig
Y and Zadik Y: Severe chronic nonlichenoid oral mucositis in
pembrolizumab-treated patients: New cases and a review of the
literature. Immunotherapy. 12:777–784. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Elad S, Yarom N, Zadik Y, Kuten-Shorrer M
and Sonis ST: The broadening scope of oral mucositis and oral
ulcerative mucosal toxicities of anticancer therapies. CA Cancer J
Clin. 72:57–77. 2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Srivastava A, Nogueras-Gonzalez GM, Geng
Y, Singh J, Myers JN, Li Y and Chambers MS: Oral toxicities
associated with immune checkpoint inhibitors: Meta-analyses of
clinical trials. J Immunother Precis Oncol. 7:24–40.
2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Heguedusch D, Tomo S, Almeida OP and Alves
FA: Superficial mucoceles in cancer patients: A retrospective
series from a Stomatology unit. Med Oral Patol Oral Cir Bucal.
28:e562–e566. 2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Salati M, Pifferi M, Baldessari C,
Bertolini F, Tomasello C, Cascinu S and Barbieri F: Stevens-Johnson
syndrome during nivolumab treatment of NSCLC. Ann Oncol.
29:283–284. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gracia-Cazaña T, Padgett E, Calderero V
and Oncins R: Nivolumab-associated Stevens-Johnson syndrome in a
patient with lung cancer. Dermatol Online J.
27(13030/qt2897t6dq)2021.PubMed/NCBI
|
|
11
|
Pîrlog CF, Paroșanu AI, Slavu CO, Olaru M,
Popa AM, Iaciu C, Niță I, Moțatu P, Horia C, Manolescu LSC and
Nițipir C: Nivolumab hypersensitivity reactions a Myth or reality
in solid tumors-a systematic review of the literature. Curr Oncol.
29:9428–9436. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shah KM, Rancour EA, Al-Omari A and
Rahnama-Moghadam S: Striking enhancement at the site of radiation
for nivolumab-induced Stevens-Johnson syndrome. Dermatol Online J.
24(13030/qt97g3t63v)2018.PubMed/NCBI
|
|
13
|
Dasanu CA: Late-onset Stevens-Johnson
syndrome due to nivolumab use for hepatocellular carcinoma. J Oncol
Pharm Pract. 25:2052–2055. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ito J, Fujimoto D, Nakamura A, Nagano T,
Uehara K, Imai Y and Tomii K: Aprepitant for refractory
nivolumab-induced pruritus. Lung Cancer. 109:58–61. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chirasuthat P and Chayavichitsilp P:
Atezolizumab-Induced stevens-johnson syndrome in a patient with
non-small cell lung carcinoma. Case Rep Dermatol. 10:198–202.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hammond S, Olsson-Brown A, Gardner J,
Thomson P, Ali SE, Jolly C, Carr D, Ressel L, Pirmohamed M and
Naisbitt D: T cell mediated hypersensitivity to previously
tolerated iodinated contrast media precipitated by introduction of
atezolizumab. J Immunother Cancer. 9(e002521)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Saw S, Lee HY and Ng QS:
Pembrolizumab-induced Stevens-Johnson syndrome in non-melanoma
patients. Eur J Cancer. 81:237–239. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liniker E, Menzies AM, Kong BY, Cooper A,
Ramanujam S, Lo S, Kefford RF, Fogarty GB, Guminski A, Wang TW, et
al: Activity and safety of radiotherapy with anti-PD-1 drug therapy
in patients with metastatic melanoma. Oncoimmunology.
5(e1214788)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sandhu M, Kc B, Bhandari J, Gambhir HS and
Farah R: Pembrolizumab-associated stevens-johnson syndrome in a
patient with metastatic non-small cell lung cancer: A case report.
Cureus. 15(e41439)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Riano I, Cristancho C and Treadwell T:
Stevens-Johnson syndrome-like reaction after exposure to
pembrolizumab and recombinant zoster vaccine in a patient with
metastatic lung cancer. J Investig Med High Impact Case Rep.
8(2324709620914796)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Machida M, Yamazaki C, Kouda N, Hanai Y,
Sato H, Konda A, Yamagata Y, Itho T and Aisaka H: A case report
involving suppressed nuclear receptor transcription factors 4a1 and
Stevens-Johnson syndrome induced by a single dose of pembrolizumab
and successfully treated with early steroid administration,
resulting in complete remission of stage III lung cancer. J Pharm
Health Care Sci. 8(29)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wu JY, Kang K, Yi J and Yang B:
Pembrolizumab-induced Stevens-Johnson syndrome in advanced squamous
cell carcinoma of the lung: A case report and review of literature.
World J Clin Cases. 10:6110–6118. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lopez M, Hagopian G, Doan L, Lee BJ, Rojek
NW, Smith J, Ou SI, Demirdag YY and Nagasaka M: Osimertinib
tolerance in a patient with Stevens Johnson syndrome during
osimertinib therapy after treatment with pembrolizumab. Allergy
Asthma Clin Immunol. 19(93)2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Haratake N, Tagawa T, Hirai F, Toyokawa G,
Miyazaki R and Maehara Y: Stevens-Johnson syndrome induced by
pembrolizumab in a lung cancer patient. J Thorac Oncol.
13:1798–1799. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gianni C, Bronte G, Delmonte A, Burgio MA,
Andrikou K, Monti M, Menna C, Frassineti GL and Crinò L: Case
Report: Stevens-Johnson Syndrome and Hepatotoxicity Induced by
Osimertinib Sequential to Pembrolizumab in a Patient With
EGFR-Mutated Lung Adenocarcinoma. Front Pharmacol.
12(672233)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Godfrey H, Jedlowski P and Thiede R:
Severe cutaneous adverse reactions associated with the immune
checkpoint inhibitors: A case/non-case analysis using the Food and
Drug Administration Adverse Event Reporting System. Australas J
Dermatol. 65:243–253. 2024.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hwang A, Iskandar A and Dasanu CA:
Stevens-Johnson syndrome manifesting late in the course of
pembrolizumab therapy. J Oncol Pharm Pract. 25:1520–1522.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang J, Zhang P, Xu QY, Zhu YT, Chen W
and Ji C: Pembrolizumab associated Stevens-Johnson syndrome with
porokeratosis in a patient in the setting of primary hepatocellular
carcinoma. Australas J Dermatol. 63:e71–e74. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kamei J, Yokoyama H, Niki T, Suda R,
Sugihara T, Fujisaki A, Ando S, Iwami D and Fujimura T: Complete
response to pembrolizumab for metastatic urothelial carcinoma in
the renal pelvis of allograft kidney. IJU Case Rep. 5:199–202.
2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li X, Lei Y, Liu J, Lin H, Chen K, Yin F,
Wang C and Zhang H: Case report: A successful treatment with immune
checkpoint inhibitors was associated with severe dermatologic
toxicities in a patient with double primary malignancies. Discov
Oncol. 14(146)2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Dika E, Ravaioli GM, Fanti PA, Piraccini
BM, Lambertini M, Chessa MA, Baraldi C, Ribero S, Andrea A, Melotti
B and Patrizi A: Cutaneous adverse effects during ipilimumab
treatment for metastatic melanoma: A prospective study. Eur J
Dermatol. 27:266–270. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Alexandris D, Alevizopoulos N, Gakiopoulou
H, Stavrinou N and Vourlakou C: Cutaneous Stevens Johnson-Toxic
Epidermal Necrolysis Immunotherapy related Toxicities in Lung
Cancer Patients. J Oncol Pharm Pract. 28:1276–1282. 2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Rouyer L, Bursztejn AC, Charbit L, Schmutz
JL and Moawad S: Stevens-Johnson syndrome associated with radiation
recall dermatitis in a patient treated with nivolumab. Eur J
Dermatol. 28:380–381. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ryu S, Jun I, Kim TI, Seo KY and Kim EK:
Pembrolizumab-induced stevens-johnson syndrome with severe ocular
complications. Ocul Immunol Inflamm. 30:1533–1535. 2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Rodríguez-Otero N, Chamorro-Pérez J,
Fernández-Lozano C, Elías-Sáenz I, Berná-Rico E, de Nicolás-Ruanes
B, Meléndez-Gispert MR, Moreno-García Del Real C, Martínez-Botas J,
Cortés-Salgado A and Solano-Solares E: Nivolumab-induced
Stevens-Johnson syndrome: not only due to PD-1 inhibition. J
Allergy Clin Immnol Pract. 11:2936–2938.e1. 2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Goldinger SM, Stieger P, Meier B,
Micaletto S, Contassot E, French LE and Dummer R: Cytotoxic
cutaneous adverse drug reactions during Anti-PD-1 therapy. Clin
Cancer Res. 22:4023–4029. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Pierre AB, Jernigan AM and Castellano T:
SJS/TEN immune-related dermatologic reaction secondary to immune
checkpoint inhibitor pembrolizumab in skin of color. Gynecol Oncol
Rep. 50(101290)2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Robinson S, Saleh J, Curry J and Mudaliar
K: Pembrolizumab-Induced stevens-johnson syndrome/toxic epidermal
necrolysis in a patient with metastatic cervical squamous cell
carcinoma: A case report. Am J Dermatopathol. 42:292–296.
2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Saad E, Adhikari P, Antala D, Abdulrahman
A, Begiashvili V, Mohamed K, Ali E and Zhang Q: Steven-Johnson
Syndrome: A Rare but Serious Adverse Event of Nivolumab Use in a
Patient With Metastatic Gastric Adenocarcinoma. J Med Cases.
13:449–455. 2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Cao J, Li Q, Zhi X, Yang F, Zhu W, Zhou T,
Hou X and Chen D: Pembrolizumab-induced autoimmune Stevens-Johnson
syndrome/toxic epidermal necrolysis with myositis and myocarditis
in a patient with esophagogastric junction carcinoma: A case
report. Transl Cancer Res. 10:3870–3876. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Oguri T, Sasada S, Shimizu S, Shigematsu
R, Tsuchiya Y, Ishioka K, Takahashi S, Oki K, Kimura Y, Seki R, et
al: A case of guillain-barré syndrome and stevens-johnson
syndrome/toxic epidermal necrosis overlap after pembrolizumab
treatment. J Investig Med High Impact Case Rep.
9(23247096211037462)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Maloney NJ, Ravi V, Cheng K, Bach DQ and
Worswick S: Stevens-Johnson syndrome and toxic epidermal
necrolysis-like reactions to checkpoint inhibitors: A systematic
review. Int J Dermatol. 59:e183–e188. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Nayar N, Briscoe K and Fernandez Penas P:
Toxic epidermal necrolysis-like reaction with severe satellite cell
necrosis associated with nivolumab in a patient with ipilimumab
refractory metastatic melanoma. J Immunother. 39:149–152.
2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Vivar KL, Deschaine M, Messina J, Divine
JM, Rabionet A, Patel N, Harrington MA and Seminario-Vidal L:
Epidermal programmed cell death-ligand 1 expression in TEN
associated with nivolumab therapy. J Cutan Pathol. 44:381–384.
2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Koshizuka K, Sakurai D, Sunagane M, Mita
Y, Hamasaki S, Suzuki T, Kikkawa N, Nakano M and Hanazawa T: Toxic
epidermal necrolysis associated with nivolumab treatment for head
and neck cancer. Clin Case Rep. 9:848–852. 2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Pintea I, Petricau C, Dumitrascu D,
Muntean A, Branisteanu DC, Branisteanu DE and Deleanu D:
Hypersensitivity reactions to monoclonal antibodies: Classification
and treatment approach (Review). Exp Ther Med.
22(949)2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Basu P, Tong Y, Hinds BR and Schneider JA:
Nivolumab-induced toxic epidermal necrolysis with retiform purpura.
Br J Dermatol. 183(e32)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Keerty D, Koverzhenko V, Belinc D, LaPorta
K and Haynes E: Immune-Mediated toxic epidermal necrolysis. Cureus.
12(e9587)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kim MC and Khan HN: Nivolumab-Induced
toxic epidermal necrolysis: Rare but fatal complication of immune
checkpoint inhibitor therapy. Cureus. 13(e15017)2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Griffin LL, Cove-Smith L, Alachkar H,
Radford JA, Brooke R and Linton KM: Toxic epidermal necrolysis
(TEN) associated with the use of nivolumab (PD-1 inhibitor) for
lymphoma. JAAD Case Rep. 4:229–231. 2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Gopee NH, Gourley AM, Oliphant TJ and
Hampton PJ: Toxic epidermal necrolysis occurring with immune
checkpoint inhibitors. Dermatol Online J.
26(13030/qt8fc428f6)2020.PubMed/NCBI
|
|
52
|
Gallo Marin B, Oliva R, Kahn B, Borgovan
T, Brooks BE and Massoud CM: Pembrolizumab-induced Toxic Epidermal
Necrolysis in a Patient with Metastatic Esophageal Adenocarcinoma.
R I Med J (2013). 105:34–36. 2022.PubMed/NCBI
|
|
53
|
Neema S, Sathu S, Vasudevan B, Shreshta S,
Bhatt S and K L: Pembrolizumab-induced toxic epidermal necrolysis:
A rare cause of severe adverse drug reaction. Indian J Dermatol
Venereol Leprol. 89:589–591. 2023.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Kian W, Zemel M, Elobra F, Sharb AA,
Levitas D, Assabag Y, Alguayn F, Yakobson A, Rouvinov K and Fuchs
L: Intravenous immunoglobulin efficacy on pembrolizumab induced
severe toxic epidermal necrolysis. Anticancer Drugs. 33:e738–e740.
2022.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Eldani C, Darrigade AS, Beylot-Barry M,
Jullie ML, Ducharme O, Milpied B and Pham-Ledard A: Successful
rechallenge for severe lichenoid drug reaction to pembrolizumab
presenting as ‘toxic epidermal necrolysis-like’. Eur J Dermatol.
32:805–807. 2022.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Borg L, Buhagiar M, La Ferla E, Pisani D,
Said J and Boffa MJ: Pembrolizumab-Induced Toxic Epidermal
Necrolysis. Case Rep Oncol. 15:887–893. 2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Chow KVC, O'Leary C, Paxton-Hall F, Lambie
D and O'Byrne K: Pembrolizumab-induced toxic epidermal necrolysis:
Case report. Oxf Med Case Reports. 2022(omac025)2022.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Cai ZR, Lecours J, Adam JP, Marcil I,
Blais N, Dallaire M, Belisle A and Mathieu A: Toxic epidermal
necrolysis associated with pembrolizumab. J Oncol Pharm Pract.
26:1259–1265. 2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Schwartz LH, Litière S, de Vries E, Ford
R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J,
et al: RECIST 1.1-Update and clarification: From the RECIST
committee. Eur J Cancer. 62:132–137. 2016.PubMed/NCBI View Article : Google Scholar
|
|
60
|
US Department of Health and Human
Services: Common terminology criteria for adverse events (CTCAE)
version 5.0. National Institutes of Health, National Cancer
Institute, 2017.
|
|
61
|
Yang A, Xuan R, Melbourne W, Tran K and
Murrell DF: Validation of the BIOCHIP test for the diagnosis of
bullous pemphigoid, pemphigus vulgaris and pemphigus foliaceus. J
Eur Acad Dermatol Venereol. 34:153–160. 2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Ellis SR, Vierra AT, Millsop JW, Lacouture
ME and Kiuru M: Dermatologic toxicities to immune checkpoint
inhibitor therapy: A review of histopathologic features. J Am Acad
Dermatol. 83:1130–1143. 2020.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Teng YS and Yu S: Molecular mechanisms of
cutaneous immune-related adverse events (irAEs) induced by immune
checkpoint inhibitors. Curr Oncol. 30:6805–6819. 2023.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Reike MJ, Bahlburg H, Brehmer M, Berg S,
Noldus J, Roghmann F, Bach P and Tully KH: Side effects of
drug-antibody conjugates enfortumab-vedotin and
sacituzumab-govitecan in targeted therapy in cancer. Cancer
Epidemiol. 90(102574)2024.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Lacouture ME, Patel AB, Rosenberg JE and
O'Donnell PH: Management of dermatologic events associated with the
Nectin-4-directed antibody-drug conjugate enfortumab vedotin.
Oncologist. 27:e223–e232. 2022.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Xia C, Jiang C, Li W, Wei J, Hong H, Li J,
Feng L, Wei H, Xin H and Chen T: A phase II Randomized clinical
trial and mechanistic studies using improved probiotics to prevent
oral mucositis induced by concurrent radiotherapy and chemotherapy
in nasopharyngeal carcinoma. Front Immunol.
12(618150)2021.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Pulito C, Cristaudo A, Porta C, Zapperi S,
Blandino G, Morrone A and Strano S: Oral mucositis: The hidden side
of cancer therapy. J Exp Clin Cancer Res. 39(210)2020.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Maria OM, Eliopoulos N and Muanza T:
Radiation-Induced oral mucositis. Front Oncol. 7(89)2017.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Radochová V, Šembera M, Slezák R, Heneberk
O and Radocha J: Oral Mucositis association with periodontal
status: A retrospective analysis of 496 patients undergoing
hematopoietic stem cell transplantation. J Clin Med.
10(5790)2021.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Lacouture M and Sibaud V: Toxic side
effects of targeted therapies and immunotherapies affecting the
skin, oral mucosa, hair, and nails. Am J Clin Dermatol. 19 (Suppl
1):S31–S39. 2018.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Vigarios E, Epstein JB and Sibaud V: Oral
mucosal changes induced by anticancer targeted therapies and immune
checkpoint inhibitors. Support Care Cancer. 25:1713–1739.
2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Klein BA, Alves FA, de Santana Rodrigues
Velho J, Vacharotayangul P, Hanna GJ, LeBoeuf NR, Shazib MA, Villa
A, Woo SB, Sroussi H, et al: Oral manifestations of immune-related
adverse events in cancer patients treated with immune checkpoint
inhibitors. Oral Dis. 28:9–22. 2022.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Lloyd-Lavery A, Hodgson T, Coupe N, Bond
S, Shah K, Espinosa O, Payne MJ, Middleton MR and Matin RN: Delayed
oral toxicity from long-term vemurafenib therapy. Br J Dermatol.
174:1159–1160. 2016.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Shephard MK and Lloyd-Lavery A: Resolution
of severe oral mucosal changes related to vemurafenib therapy with
intensive periodontal treatment. Br J Dermatol. 181:639–640.
2019.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Pileri A, Cricca M, Gandolfi L, Misciali
C, Casadei B, Zinzani PL and Patrizi A: Vemurafenib mucosal
side-effect. J Eur Acad Dermatol Venereol. 30:1053–1055.
2016.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Prado-Ribeiro AC, Santos-Silva AR, Faria
KM, Silva WG, Simonato LE, Moutinho K and Brandão TB:
Radiation-related superficial oral mucoceles: An under-recognized
acute toxicity in head and neck cancer patients. Med Oral Patol
Oral Cir Bucal. 23:e518–e523. 2018.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Campana F, Sibaud V, Chauvel A, Boiron JM,
Taieb A and Fricain JC: Recurrent superficial mucoceles associated
with lichenoid disorders. J Oral Maxillofac Surg. 64:1830–1833.
2006.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Demarosi F, Lodi G, Carrassi A and
Sardella A: Superficial oral mucoceles: Description of two cases in
patients with graft-versus-host disease. J Otolaryngol. 36:E76–E78.
2007.PubMed/NCBI
|
|
79
|
Balasubramaniam R, Alawi F and DeRossi S:
Superficial mucoceles in chronic graft-versus-host disease: A case
report and review of the literature. Gen Dent. 57:82–88.
2009.PubMed/NCBI
|
|
80
|
García-F-Villalta MJ, Pascual-López M,
Elices M, Daudén E, García-Diez A and Fraga J: Superficial
mucoceles and lichenoid graft versus host disease: report of three
cases. Acta Derm Venereol. 82:453–455. 2002.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Brooks JK, Schwartz KG and Basile JR:
Superficial mucocele of the ventral tongue: Presentation of a rare
case and literature review. J Oral Maxillofac Surg. 74:1175–1179.
2016.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Masri BA, Perry LM and Stoopler ET:
Palatal superficial mucoceles associated with chronic
graft-versus-host disease. Hematol Transfus Cell Ther.
45(140)2023.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Deutsch A and McLellan BN: Topical
tacrolimus for refractory superficial mucoceles in a patient with
chronic graft versus host disease. JAAD Case Rep. 6:426–427.
2020.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Nicolatou-Galitis O, Kitra V, Van
Vliet-Constantinidou C, Peristeri J, Goussetis E, Petropoulos D and
Grafakos S: The oral manifestations of chronic graft-versus-host
disease (cGVHD) in paediatric allogeneic bone marrow transplant
recipients. J Oral Pathol Med. 30:148–153. 2001.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Zadik Y, Keshet N and Aframian DJ: Oral
superficial mucocele in cancer patients. Oral Oncol. 56:e15–e16.
2016.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Pengpis N, Prueksrisakul T and
Chanswangphuwana C: Clinical characteristics of oral chronic
graft-versus-host disease according to the 2014 National Institutes
of Health (USA) consensus criteria. Med Oral Patol Oral Cir Bucal.
28:e167–e173. 2023.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Elad S, Zadik Y and Yarom N: Oral
complications of nonsurgical cancer therapies. Atlas Oral
Maxillofac Surg. Clin. North Am. 25:133–147. 2017.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Eveson JW: Superficial mucoceles: Pitfall
in clinical and microscopic diagnosis. Oral Surg Oral Med Oral
Pathol. 66:318–322. 1988.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Rather S, Shah AA, Shah FY, Kaur S, Bhat
MA, Reyaz S and Hassan I: Dermoscopy of oral mucosal lesions:
experience from a tertiary care center in north india and review of
literature. Indian Dermatol Online J. 13:346–360. 2022.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Ayhan E, Toprak SF, Kaya Ş and Akkaynak Ş:
Dermoscopy of oral mucocele: Three types of extravasation
mucoceles. Turk J Med Sci. 50:96–102. 2020.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Kumar Jha A, Vinay K, Sławińska M,
Sonthalia S, Sobjanek M, Kamińska-Winciorek G, Errichetti E, Kamat
D, Chatterjee D, Apalla Z, et al: Application of mucous membrane
dermoscopy (mucoscopy) in diagnostics of benign oral
lesions-literature review and preliminary observations from
International Dermoscopy Society study. Dermatol Ther.
34(e14478)2021.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Manfredini M, Pedroni G, Bigi L, Apponi R,
Murri Dello Diago A, Dattola A, Farnetani F and Pellacani G:
Acquired white oral lesions with specific patterns: Oral lichen
planus and lupus erythematosus. Dermatol Pract Concept.
11(e2021074)2021.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Venugopal DC, Warrier SA, S E, H T and
Ramesh P: Superficial Mucocele: A Rare Presentation. Cureus.
13(e18038)2021.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Płatkowska A, Słowińska M, Zalewska J,
Swacha Z, Szumera-Ciećkiewicz A, Wągrodzki M, Patera J,
Łapieńska-Rey K, Lorent M, Ługowska I, et al: Minimally Invasive
plasma device management of multiple benign skin cancers associated
with rare genodermatoses-case series and review of the therapeutic
methods. J Clin Med. 13(4377)2024.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Holcomb JD, Kalhan R and Pilcher B:
Evaluation of skin tissue effects from treatment with a novel
hand-held plasma energy device. J Cosmet Dermatol. 21:1998–2004.
2022.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Bell A and Kasi A: Oral Mucositis. In:
StatPearls [Internet]. StatPearls Publishing, Treasure Island, FL,
2024.
|
|
97
|
National Library of Medicine:
Stomatitides. https://www.ncbi.nlm.nih.gov/medgen/52511.
|
|
98
|
Lee JH, Lee JH, Lee JH, Kim SY and Kim GM:
Case of sunitinib-induced Stevens-Johnson syndrome. J Dermatol.
40:753–754. 2013.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Gronich N, Maman D, Stein N and Saliba W:
Culprit medications and risk factors associated with
stevens-johnson syndrome and toxic epidermal necrolysis:
Population-Based nested case-control study. Am J Clin Dermatol.
23:257–266. 2022.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Vinay K, Yanamandra U, Dogra S, Handa S,
Suri V, Kumari S, Khadwal A, Prakash G, Lad D, Varma S and Malhotra
P: Long-term mucocutaneous adverse effects of imatinib in Indian
chronic myeloid leukemia patients. Int J Dermatol. 57:332–338.
2018.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Yang S, Wu L, Li X, Huang J, Zhong J and
Chen X: Crizotinib-associated toxic epidermal necrolysis in an
ALK-positive advanced NSCLC patient. Mol Clin Oncol. 8:457–459.
2018.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Koizumi T, Fukushima T, Tatai T, Kobayashi
T, Sekiguchi N, Sakamoto A and Sasaki S: Successful treatment of
crizotinib-induced dysgeusia by switching to alectinib in
ALK-positive non-small cell lung cancer. Lung Cancer. 88:112–113.
2015.PubMed/NCBI View Article : Google Scholar
|
|
103
|
European Medicines Agency: ANNEX I:
Summary of product characteristics: MabThera. https://www.ema.europa.eu/en/documents/product-information/mabthera-epar-product-information_en.pdf.
|
|
104
|
Patrizi A, Venturi M, Dika E, Maibach H,
Tacchetti P and Brandi G: Cutaneous adverse reactions linked to
targeted anticancer therapies bortezomib and lenalidomide for
multiple myeloma: New drugs, old side effects. Cutan Ocul Toxicol.
33:1–6. 2014.PubMed/NCBI View Article : Google Scholar
|
|
105
|
European Medicines Agency: ANNEX I:
Summary of product characteristics: velcade. https://www.ema.europa.eu/en/documents/product-information/velcade-epar-product-information_en.pdf.
|
|
106
|
Villa A and Kuten-Shorrer M: Pathogenesis
of Oral Toxicities Associated with Targeted Therapy and
Immunotherapy. Int J Mol Sci. 24(8188)2023.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Kurian CJ, Desai A, Rafferty W and Abou
Hussein AK: Case report: Alpelisib-induced Stevens-Johnson
syndrome. Front Oncol. 12(954027)2022.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Nunnery SE and Mayer IA: Management of
toxicity to isoform α-specific PI3K inhibitors. Ann Oncol. 30
(Suppl_10):x21–x26. 2019.PubMed/NCBI View Article : Google Scholar
|
|
109
|
European Medicines Agency: ANNEX I:
Summary of product characteristics: Piqray. https://www.ema.europa.eu/en/documents/product-information/piqray-epar-product-information_en.pdf.
|
|
110
|
Vigarios E, Beylot-Barry M, Jegou MH,
Oberic L, Ysebaert L and Sibaud V: Dose-limiting stomatitis
associated with ibrutinib therapy: A case series. Br J Haematol.
185:784–788. 2019.PubMed/NCBI View Article : Google Scholar
|
|
111
|
European Medicines Agency: ANNEX I:
Summary of product characteristics: imbruvica. https://www.ema.europa.eu/en/documents/product-information/imbruvica-epar-product-information_en.pdf.
|
|
112
|
European Medicines Agency: ANNEX I:
Summary of product characteristics: venclyxto. https://www.ema.europa.eu/en/documents/product-information/venclyxto-epar-product-information_en.pdf.
|