Introduction
Persistent and recurrent hematuria is a urologic
emergency event which is often difficult to be addressed and is
related to significant morbidity and mortality. Several etiologic
factors predispose to lower urinary tract hematuria with trauma and
urogenital malignancy being the most frequently encountered
conditions (1-3).
Copious amounts of normal saline irrigations may be required to
remove blood clots and maintain a macroscopically clear bladder
outflow. Other measures to control intractable hematuria include
cystoscopic evacuation of the bladder with
electrocautery/coagulation of the bleeding vessels, silver nitrate
or alum solution irrigation or, if available, bladder
overdistention with a Helmstein balloon (1). Should conservative measures fail,
open surgical procedures may be undertaken for ligation of the
internal iliac arteries or even salvage extirpation of the bleeding
organs. The latter methods, however, are frequently associated with
unacceptable high morbidity and mortality rates (3,4). In
1974, Hald and Mygind (5) were the
first to describe efficient control of massive hematuria originated
from the bladder with methods of arterial embolization. Since then,
treatment based on selective or superselective arterial embolism is
gradually gaining popularity amongst physicians for the management
of urinary tract hemorrhage (2,3,6-8).
In the present study, the effective control of hemorrhage is
reported due to a locally advanced/metastatic hormone refractory
adenocarcinoma of the prostate in a patient, which was difficult to
be treated.
Case report
A 79-year old Caucasian male was admitted to the
Emergency Department of Konstantopouleio-Patision General Hospital
of Nea Ionia (Nea Ionia, Greece) in April 2024 due to a 4-day
persistent bloody discharge from the urethra, weakness and
dizziness. The patient was pale with prolonged capillary refilling
time. The physical examination also revealed tachycardia,
hypotension and suprapubic discomfort. Urinary ultrasonography
revealed an overdistended bladder with hyperechoic content and the
total blood count (TBC) revealed low levels of hematocrit (HCT) and
hemoglobin (HGB) (22% and 7.1 g/dl, respectively). The patient was
suffering from prostate cancer (PCa) for the last 7 years; however,
a year ago the disease progressed to hormone-resistant with
metastases (in pelvic lymph nodes, bones and lung). Due to an
obstructive acute kidney injury which occurred 6 months ago and
following the failure of placement double-j-stents, the patient
underwent urinary deviation with bilateral nephrostomy and
therefore the free voiding urine volume was negligible. The patient
was treated with a luteinizing hormone-releasing hormone-antagonist
and abiraterone acetate plus prednisone, with dabigatran 150 mg bid
(for atrial fibrillation and coronary heart disease) and he also
suffered from mild ulcerative colitis for which 5-aminosalicylic
acid was provided on demand.
The patient was hospitalized in the Urology
Department for 2 weeks overall. A 22F, 3-way urethral catheter was
placed, and blood clots were removed with a syringe. Continuous
normal saline intravesical irrigation was initiated and tranexamic
acid was administered intravenously along with red blood cell
transfusion. Dabigatran was temporarily withdrawn and bridged 2
days later with low molecular weight heparin 4,000 bid. The
conservative treatment lasted for 4 days; however, hematuria
reappeared every time bladder irrigation ceased. Computed
tomography and urography revealed a large prostate lesion invading
the bladder neck and a pelvic lymph-node block in the absence of
blood extravasation. Subsequently, diagnostic urethrocystoscopy
under anesthesia was performed. Active vascular bleeding could not
be detected; however, only diffuse hemorrhage originating from the
prostatic tumor and bladder neck were present. Due to the late
stage of the disease, these two anatomic structures were fused and
could be recognized as separate structures. Bipolar coagulation was
performed without any significant improvement. Subsequently, the
patient was referred to an interventional radiologist and he was
informed on the option of superselective arterial embolization
(SAE) as well as its advantages and limitations. Following signing
the relevant consent form, the patient underwent bilateral
embolization of the prostatic arteries and of the inferior cystic
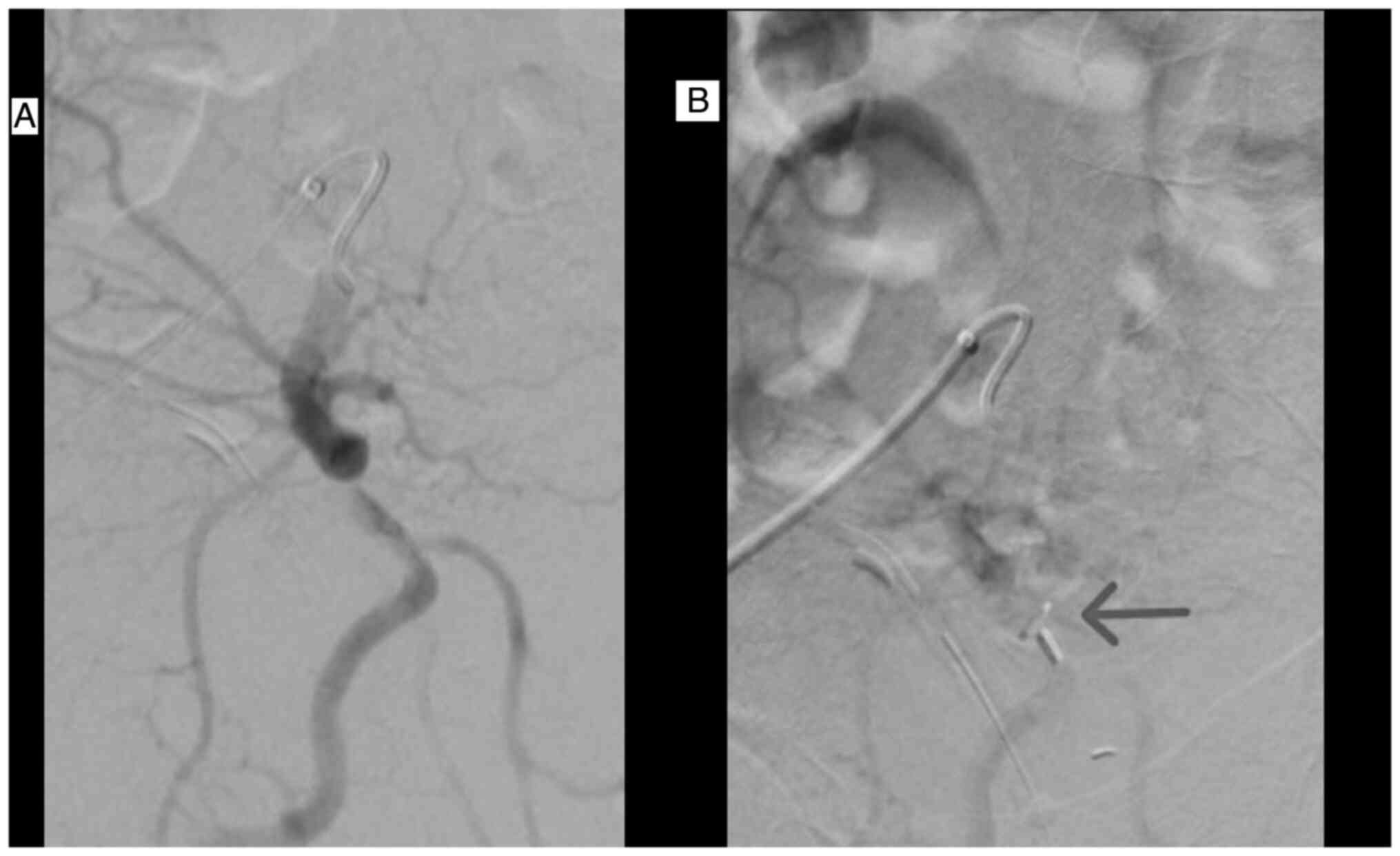
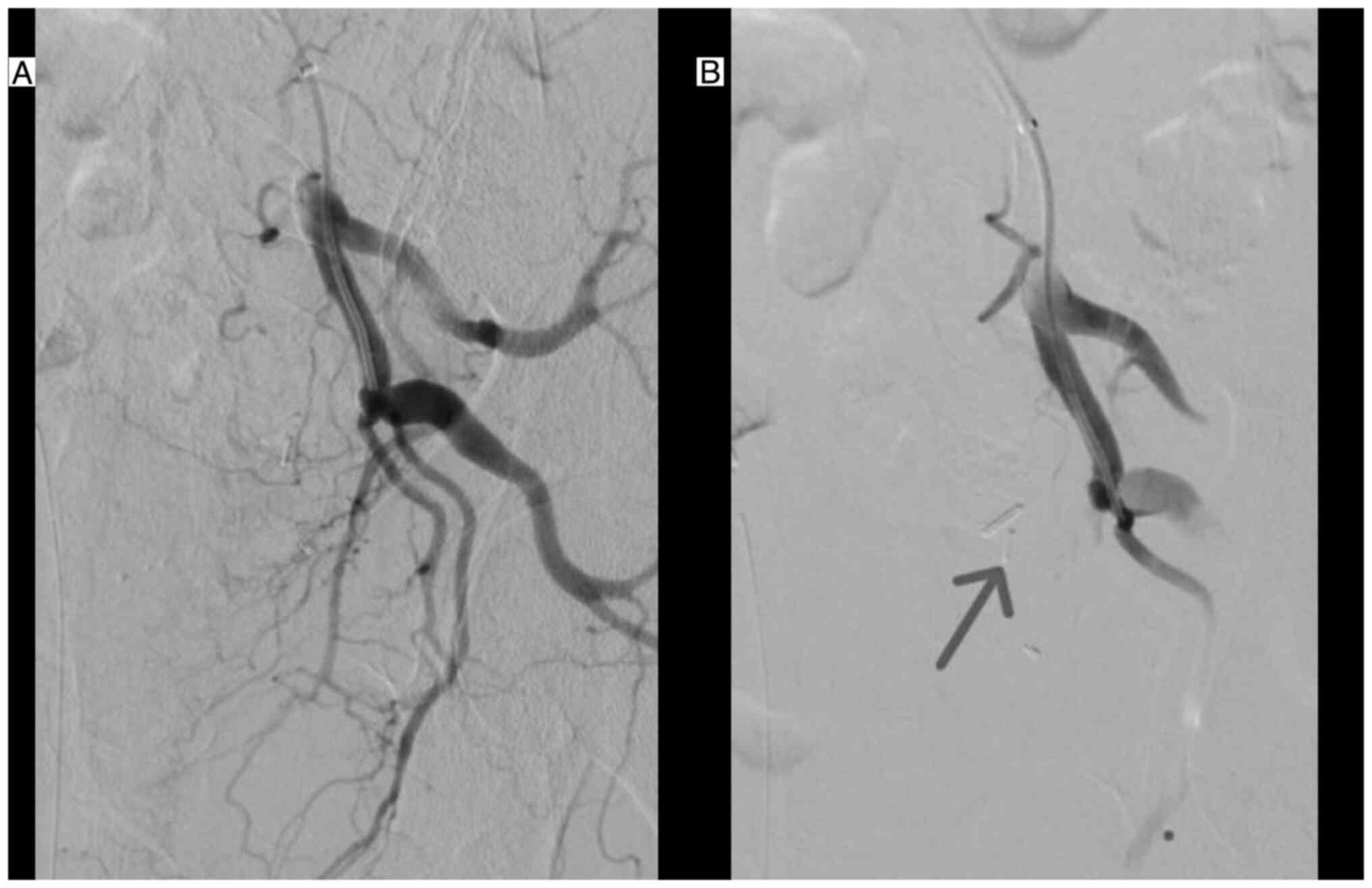
arteries with microparticles 100-300 µm and 300-500 µm (Figs. 1 and 2), while keeping the heparin delivery.
With the exception of lower abdominal discomfort which lasted 3
days and treatment with on demand paracetamol administration, the
convalescence period was uneventful, and hematuria did not occur on
the 2nd day following SAE. Dabigatran was re-administered at the
5th post-procedural day and the catheter was removed 5 days later.
The patient was discharged with HCT and HGB levels of 34% and 10.6
g/dl, respectively. Following a 4-month, follow-up, the TBC
parameters were stable and there was no need for transfusion or
hospitalization. No bloody urethral discharge or local pain
(perineal or suprapubically) were observed. It is important to note
that the prostate specific antigen (PSA) level diminished from 14
to 10 ng/ml.
Discussion
In 1974, two Danish doctors, Hald and Mygind
(5) were the first to control
hematuria with arterial embolization and the method has been
implemented to control cancer-related hematuria (9,10) as
well as non-cancer related bleeding, such as post traumatic
hemorrhage (2).
With regard to the urinary tract, significant
experience with SAE has been acquired in the treatment of benign
prostate hyperplasia symptoms. Pisco et al (11) reported a mid- and long-term success
rate of 82 and 76%, respectively. Among the 630 patients treated
with prostate artery embolization, only 2 experienced major
complications, underscoring the safety of the method (11). A relevant systematic review with
meta-regression analysis reported that SAE is a safe and effective
method to treat prostate hyperplasia-related symptoms although the
authors concluded that the embolization should still be considered
experimental (12).
The body of evidence is limited regarding the
control of PCa-related hematuria with the embolism technique. A
total of ~35 relevant cases in small non-comparative retrograde
case series with mixed population (comprising patients with bladder
and pelvic cancer as well as non-malignant prostatic diseases) have
been reported in the literature (3,6-8,13,14).
However, the long-term success rate in relieving the patient from
hematuria is encouraging. In a case series with 44 patients (15
with PCa), the control of bleeding was achieved in 82% following a
single session while 11% required a second intervention. However,
durable results were experienced by 43% of the patients (3). In another series with 18 cases, 6 of
them presented with hematuria due to PCa and the technical success
was noted in 16 (88%) of the patients. Hemorrhage was terminated
following a maximum of 3 days and it was followed by improvement of
the TBC measures after a mean follow-up of 18 months (8). Nabi et al (6) presented a very small series of 6
cases. Half of the cases presented with PCa. Successful
embolization was performed in 5 cases and the control of hematuria
was maintained following a mean follow-up of 22 months (6).
All reports demonstrated the main advantage of SAE,
which was the minimal invasive nature of the procedure. Therefore,
it is related with low rate of side effects which, if they occur,
they are mostly minor. Nausea, vomiting, fever and abdominal pain
may be experienced by the patients. They are relieved with
conservative measures usually within 2-3 days (8). This constellation of symptoms
resembles the post-embolization syndrome described following
transarterial embolization of hepatocellular carcinoma.
Post-embolization syndrome may be related with tissue necrosis,
hypoxia and release of cytokines into the bloodstream. No specific
methods of treatment exist; the management includes supportive
therapy with analgesics, antiemetics and antipyretics. Recently, it
was reported that the administration of dexamethasone and/or
N-acetylcysteine may also be helpful (15). Liguori et al (3) after a mean of follow-up of 10.5
months, reported a post-embolism syndrome as high as 27% in a
series of 39 cases with urogenital hemorrhage. They also noted
external genitalia swelling in 5% of the cases. Noteworthy, the
mortality rates at 6 and 12 months were as high as 66 and 18%,
respectively (3). Different
studies have reported gluteal or lower abdominal pain, urgency,
frequency and dysuria as potential complications of the method. The
same authors published 1-, 6- and 12-month mortality rates of 0, 42
and 71%, respectively (7). The
high sort-term mortality rates reported in the literature may be
explained by the comorbidities and the frailty of the patients who
underwent embolization, as well as the complexity and burden of
disease that makes any invasive treatment to be accompanied by high
rates of morbidity and mortality. Nevertheless, future comparative
studies may provide an answer regarding the optimal treatment
option depending on the final outcome.
Severe complications, for example tissue necrosis or
sepsis, have not been reported in modern series, which are
attributed to the super-selective nature of the procedure (6). The use of very small unresolvable
particles, as small as 100 µm, such as those used in the present
case renders superselective embolization feasible and efficient.
For lower urinary tract hemorrhage, the superselective technique
mandates the embolization to be performed distally to the gluteal
artery avoiding complications related to gluteal muscle ischemia
and perineal pain (3). Based on
the experience obtained following treatment of benign prostatic
hyperplasia with SAE it was shown that nanoparticles of larger
sizes (300-500 µm) were equally safe and effective with those that
were smaller in size (100-300 µm). The size of the particles was
determined proportionally by the caliber of the branch that had to
be occluded. Therefore, larger vessels were embolized with larger
particles (16,17).
Previous studies that have compared different means
of embolization are scarce. In one of them it is supported that
liquids and small particles may have more favorable results in
controlling severe bleeding compared with coils. However, this
result was not significant (9);
the ideal agent is yet to be determined. It should also be
considered that the blood supply of the prostate stems from both
sides of internal iliac artery branches. Therefore, bilateral
embolization is recommended for optimal control of prostatic
hemorrhage (3,8).
One of the major advantages of arterial embolization
is that it can be performed under local anesthesia obviating the
potential complications related to general or regional anesthesia
particularly in high risk patients. It is also important that the
withdrawal of antiplatelet and/or antithrombotic agents is not
necessary. In one multicenter study comprising 92 patients with
malignancy-related bleeding of any origin treated with selective
embolization, 2 out of 3 patients (67%) received antithrombotic
agents and 89% of the patients were on antiplatelet or
antithrombotic treatment. Almost 11% received dual antiplatelet
treatment. The clinical success rate was as high as 85% with only
14 patients experiencing re-bleeding (9). In the present case report, the
antiplatelet treatment was withdrawn as one of the first measures
to control bleeding. Due to the risks related with atrial
fibrillation, low molecular weight heparin was administered and it
was continued during the SAE procedure with a favorable outcome.
Therefore, following meticulous consideration of the risks and
benefits, it can be deduced that embolization could be proposed to
patients with cardiovascular disorders as a measure to avoid the
risks of withdrawal of antithrombotic/antiplatelet agents;
concomitantly, it can be used to provide an effective treatment of
severe bleeding.
It is interesting to note that a post-procedural
decline of the serum PSA was recorded. The deprivation of the tumor
from its blood supply may be in part beneficial in tumor control;
however, the duration of this effect and long-term outcomes remain
unknown. Until now, the group of patients with PCa who will
experience benefit, if any, with SAE is unclear and several
questions need to be addressed. For example, it remains unknown
whether patients with low or intermediate-risk disease have an
optimal outcome with SAE compared with those with advanced disease.
In addition, it is not known whether it is preferable to use SAE in
combination with other treatment (radiotherapy or hormonal therapy)
or whether SAE is more efficacious as monotherapy. The combination
of SAE with intraprostatic administration of chemotherapeutic
agent(s), which is similar with the chemoembolization of
hepatocellular cancer, requires further investigation in future
studies.
Recently, unilateral super-selective embolization of
the prostatic artery has been used as focal therapy in 10 patients
with low risk PCa. In total, 40% of the patients had a negative
post-embolization biopsy at 6 months and 30% presented with an
undetectable lesion in multiparametric magnetic resonance imaging.
Moreover, all 10 patients experienced a PSA decline. All cases
except one continued in the active surveillance protocol (18). In a different non-comparative pilot
study based on 9 patients with advanced cancer treated with SAE, a
significant tumor necrosis was noted in only 1 patient (19). In a proof-of concept study from
Switzerland, 12 patients with localized PCa underwent prostate
artery embolization and subsequently were treated with radical
prostatectomy. In the radical prostatectomy specimens, elimination
of the tumor size was recorded; however, none of the patients
experienced complete tumor necrosis (20). However, there are no reports on the
mid- and long-term effect of bilateral embolization in the natural
history of PCa.
Due to the inconclusive early results, researchers
may be reluctant to design and conduct comparative studies between
SAE and standard of care (surgery and radiotherapy) due to
potential risks and the lack of data from reliable national or
population registries, such as the Surveilance, Epidemiology and
End Results database. Large registries may provide valuable
preliminary data for retrograde comparative studies prior to the
design of prospective clinical trials. Not surprisingly, following
review of the site of clinical trial database for research in
humans, it was deduced that studies comparing SAE with other
methods of treatment have not been registered so far (21). Nevertheless, SAE is a new and
promising concept for the treatment of patients with PCa that
warrants further investigation.
The current report presents an unusual case
indicating that the bloody urethral discharge and not hematuria was
the symptom of intractable bleeding. Due to urinary deviation, the
inability of the patient to store urine and void normally caused
difficult diagnosis and treatment of hemorrhage. Bladder
irrigations were applied for various days with insufficient
results. Moreover, the consumption of copious amounts of water to
increase urine output, which is a useful measure to relieve
hematuria in patients with normal vesical storage, could not be
beneficial in the present case. In addition, even if the
conservative measures were initially successful, the deprivation of
lower urinary tract from the urine increased the likelihood of new
blood clot formation following patient discharge, forcing the
patient to readmission. Although the method is considered
experimental, it has been regarded that embolization could be an
option with favorable results and the patient was informed
accordingly. Following a 4-month follow up, the treatment could be
considered successful with regard to maintaining stable HCT and HGB
levels without bloody discharge from the urethra and lack of
readmission.
The results and conclusions of this single case
should be generalized with caution. However, it can be assumed that
this case contributes to the unmet need to determine the role of
embolization in patients with intractable hematuria or lower
urinary tract hemorrhage and particularly when other measures have
failed. It also underscores the requirement of close cooperation
between urologists and interventional radiologists on the basis of
multidisciplinary medical approach targeting optimal results.
In conclusion, SAE is an attractive treatment option
notably in patients with cardiovascular comorbidities, since it is
a minimal invasive procedure. It appears to be an effective
alternative method to control hematuria and lower urinary tract
hemorrhage compared with other invasive procedures, with acceptable
rate of minor complications. The encouraging results and the
survival outcomes warrant further evaluation with comparative
prospective multicenter studies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
DK, PF and VK were major contributors in the writing
of the manuscript, performed the primary treatment and consulted
the patient for the novel treatment. PP, assisted by VK and AF,
were responsible for the novel treatment. AZ, DB and AK analyzed
the patient's data and the literature data. GH and AMK obtained the
medical images. GK, KS, ER and DM advised on the patient's
treatment. DK and PP confirm the authenticity of the raw data. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The treatment methods and the study were approved by
the Ethics Committee of Konstantopouleio-Patision General Hospital,
Nea Ionia, Greece (approval no. 26/09052024) and are in full
compliance with the Declaration of Helsinki. Informed consent was
obtained from the patient before treatment.
Patient consent for publication
Informed consent was obtained for publication of
patient data and associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Choong SK, Walkden M and Kirby R: The
management of intractable haematuria. BJU Int. 86:951–959.
2000.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Somani BK, Nabi G, Thorpe P and McClinton
S: Endovascular control of haemorrhagic urological emergencies: An
observational study. BMC Urol. 6(27)2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Liguori G, Amodeo A, Mucelli FP, Patel H,
Marco D, Belgrano E and Trombetta C: Intractable haematuria:
Long-term results after selective embolization of the internal
iliac arteries. BJU Int. 106:500–503. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Taha DE, Shokeir AA and Aboumarzouk OA:
Selective embolisation for intractable bladder haemorrhages: A
systematic review of the literature. Arab J Urol. 16:197–205.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hald T and Mygind T: Control of
life-threatening vesical hemorrhage by unilateral hypogastric
artery muscle embolization. J Urol. 112:60–63. 1974.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nabi G, Sheikh N, Greene D and Marsh R:
Therapeutic transcatheter arterial embolization in the management
of intractable haemorrhage from pelvic urological malignancies:
Preliminary experience and long-term follow-up. BJU Int.
92:245–247. 2003.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Alarayedh A, Abdulwahab S and Mubarak M:
Super-selective trans-catheter arterial embolization (TAE) of the
vesical arteries in the management of intractable hematuria
secondary to advanced bladder and prostate cancers. Cureus.
16(e58016)2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Korkmaz M, Şanal B, Aras B, Bozkaya H,
Çınar C, Güneyli S, Gök M, Adam G, Düzgün F and Oran I: The short-
and long-term effectiveness of transcatheter arterial embolization
in patients with intractable hematuria. Diagn Interv Imaging.
97:197–201. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Minici R, Guzzardi G, Venturini M, Fontana
F, Coppola A, Spinetta M, Piacentino F, Pingitore A, Serra R, Costa
D, et al: Transcatheter arterial embolization (TAE) of
cancer-related bleeding. Medicina (Kaunas). 59(1323)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
El-Assmy A and Mohsen T: Internal iliac
artery embolization for the control of severe bladder hemorrhage
secondary to carcinoma: Long-term follow-up.
ScientificWorldJournal. 7:1567–1574. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pisco JM, Bilhim T, Pinheiro LC, Fernandes
L, Pereira J, Costa NV, Duarte M and Oliveira AG: Medium- and
long-term outcome of prostate artery embolization for patients with
benign prostatic hyperplasia: results in 630 patients. J Vasc
Interv Radiol. 27:1115–1122. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shim SR, Kanhai KJ, Ko YM and Kim JH:
Efficacy and safety of prostatic arterial embolization: Systematic
review with meta-analysis and meta-regression. J Urol. 197:465–479.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Delgal A, Cercueil JP, Koutlidis N, Michel
F, Kermarrec I, Mourey E, Cormier L, Krausé D and Loffroy R:
Outcome of transcatheter arterial embolization for bladder and
prostate hemorrhage. J Urol. 183:1947–1953. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rastinehad AR, Caplin DM, Ost MC,
VanderBrink BA, Lobko I, Badlani GH, Weiss GH, Kavoussi LR and
Siegel DN: Selective arterial prostatic embolization (SAPE) for
refractory hematuria of prostatic origin. Urology. 71:181–184.
2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Simasingha N, Tanasoontrarat W, Claimon T
and Sethasine S: Efficacy of dexamethasone and N-acetylcysteine
combination in preventing post-embolization syndrome after
transarterial chemoembolization in hepatocellular carcinoma. World
J Gastroenterol. 29:890–903. 2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gonçalves OM, Carnevale FC, Moreira AM,
Antunes AA, Rodrigues VC and Srougi M: Comparative study using
100-300 versus 300-500 µm microspheres for symptomatic patients due
to enlarged-BPH prostates. Cardiovasc Intervent Radiol.
39:1372–1378. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bhatia A, Maini A and Bhatia S: Prostatic
artery embolization: Technical pearls. Semin Intervent Radiol.
39:555–561. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Frandon J, Bey E, Hamard A, Mohammad H,
Gonzalez S, Greffier J, Chevallier T, de Forges H, Beregi JP and
Droupy S: Early results of unilateral prostatic artery embolization
as a focal therapy in patients with prostate cancer under active
surveillance: Cancer prostate embolisation, a pilot study. J Vasc
Interv Radiol. 32:247–255. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Burkhardt O, Abt D, Hechelhammer L, Kim O,
Omlin A, Schmid HP, Engeler D, Zumstein V and Müllhaupt G:
Prostatic artery embolization in patients with advanced prostate
cancer: A prospective single center pilot study. Cardiovasc
Intervent Radiol. 47:771–782. 2024.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mordasini L, Hechelhammer L, Diener PA,
Diebold J, Mattei A, Engeler D, Müllhaupt G, Kim SK, Schmid HP and
Abt D: Prostatic artery embolization in the treatment of localized
prostate cancer: A bicentric prospective proof-of-concept study of
12 patients. J Vasc Interv Radiol. 29:589–597. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
ClinicalTrials.gov. https://clinicaltrials.gov/search?cond=
prostate%20cancer&term=Embolization&intr=Prostate%20Artery%20Embolization).
|