Introduction
Periodontitis is a chronic and progressive
inflammatory disease that affects the supporting tissues of the
periodontium and primarily results from the invasion of specific
microorganisms. This microbial intrusion into the periodontal
tissues ultimately leads to tooth loosening and loss (1,2).
Periodontitis is a risk factor for various systemic diseases,
including diabetes mellitus and coronary heart disease (3), which has a prevalence of 45-50%
overall, with the severe form affecting 11.2% of the general
population (4). Consequently,
there has been a growing focus on the prevention and treatment of
periodontitis.
The advent of tissue engineering techniques has
opened new avenues for addressing periodontitis (5,6).
Tissue engineering involves three crucial elements: Seed cells,
growth factors and scaffolding materials, with seed cells as the
core components (7). In 2004, Seo
et al (8) first isolated
multipotent stem cells from the periodontal ligament, confirming
the multipotential differentiation, robust self-renewal and
self-repair capacity of human periodontal ligament cells (hPDLCs).
These cells can differentiate into various cell types under
specific conditions, thereby facilitating true periodontal tissue
regeneration. Additionally, hPDLCs exhibit low immunogenicity,
post-implantation stability, adaptability to the implant
environment and minimal harm to the body (9), making them the most promising stem
cell populations for regenerative periodontal therapy. Currently,
hPDLCs are the most widely used seed cells for periodontal tissue
engineering. However, the inflammatory microenvironment caused by
cytokines, such as TNF-α and IL-1, limits the proliferation and
osteogenic capacity of hPDLCs (10). Therefore, targeted antagonism of
TNF-α or its receptors is crucial for reducing inflammatory factors
and enhancing the proliferation and osteogenesis of hPDLCs.
Progranulin (PGRN), also known as pro-epithelin, 88
kDa glycoprotein (GP88) or PC cell-derived growth factor and
granulin-epithelin precursor (11), is a growth factor encoded by the
granulin precursor (GRN) gene with anti-inflammatory
and osteogenic effects. It plays pivotal roles in various
physiological and pathological processes such as early embryonic
development, inflammation (12,13),
wound healing (14), tumorigenesis
and neurological disorders. In inflammatory response, PGRN inhibits
neutrophil activation and proteolytic enzyme secretion by
antagonizing TNF-α. In the context of anti-inflammatory mechanisms,
the macrophage-derived factor secretory leukocyte protease
inhibitor interacts with the inner domain of PGRN, providing
protection against cleaving enzymes, such as proteinase 3 and
elastase (15-17).
Furthermore, studies have revealed that recombinant PGRN proteins
and TNF-α competitively bind to tumor necrosis factor receptor
(TNFR), leading to the antagonism of TNF-α and inhibition of the
inflammatory response (17,18).
PGRN can induce mesenchymal stem cells to
differentiate into cartilage. As a major downstream molecule of
bone morphogenetic protein 2 (BMP2) (19), PGRN activates ERK1/2 signaling and
the transcription factor JunB, which play significant roles in
cartilage formation. Additionally, PGRN knockout mice exhibit
dwarfism and severe skeletal defects, emphasizing the essential
role of PGRN in skeletal development (20-22).
Periodontal regeneration involves recruiting
endogenous stem cells to the defect site and using bioactive
factors with anti-inflammatory and tissue-repair properties to
enhance stem cell proliferation and differentiation. In contrast to
TNF-α inhibitors that directly stimulate osteogenic differentiation
(23), PGRN acts by antagonizing
TNF-α and serving as a downstream protein of BMP2, promoting the
osteogenic differentiation of cells. However, the direct use of
exogenous growth factors presents challenges, such as high cost,
complex protein extraction, short in vivo half-life,
susceptibility to degradation by proteases and limited
functionality (24,25). This often necessitates the use of
large quantities of recombinant proteins, leading to increased
economic costs and potentially excessive dosages as a side
effect.
To address these challenges, researchers have
proposed gene therapy, which relies on effective gene transfection
and expression methods, as an innovative approach for periodontal
regeneration (26). Viral vectors,
particularly lentiviral or adenoviral vectors, are highly efficient
and safe delivery vehicles for transferring exogenous genes to
target cells (27,28). By transfecting target cells with
these vectors, specific genes can be overexpressed or knocked down,
enabling cells to proliferate and undergo osteogenic
differentiation at the genetic level. Consequently, the present
study hypothesized that lentivirus-mediated GRN could
promote the proliferation and osteogenesis of periodontal ligament
cells and aimed to construct a human periodontal cell line stably
overexpressing GRN using the lentiviral method.
Subsequently, it focused on assessing the effect of GRN
overexpression on the proliferation and osteogenic capacity of
hPDLCs, offering a theoretical foundation for advancing the
understanding of periodontal regeneration.
Materials and methods
Isolation, culture and identification
of hPDLCs
The study protocol was approved by the Ethics
Committee of Lanzhou University School of Stomatology (approval no.
LZUKQ-2019-045). A 28-years-old volunteer with good oral health
donated four intact caries-free premolars that were extracted due
to orthodontic treatment and used for PDLCs isolation; written
informed consent was obtained from the patient. Extracted teeth
were placed in α-MEM (Gibco; Thermo Fisher Scientific, Inc.)
containing 2% penicillin-streptomycin solution (PS; Gibco; Thermo
Fisher Scientific, Inc.) and hPDLCs extraction was completed within
2 h. The teeth were rinsed from root to crown with PBS containing
10% double antibody and the periodontal membrane tissue from the
middle 1/3 of the isolated root was scraped and collected in a
tube, which was added to 1 ml of α-MEM and then centrifuged at 100
x g for 5 min at 4˚C. After discarding the supernatant, collagenase
type I (Gibco; Thermo Fisher Scientific, Inc.) was added and the
tissue was digested for 15 min in an incubator at 37˚C. Following
centrifugation at 100 x g for 5 min at 4˚C, trypsin was aspirated
and 5 ml of complete medium [1% PS; 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) and 89% α-MEM] was added. The cells were cultured
for 3-5 days in a cell culture incubator at 37˚C and the cell
culture flask was removed to observe cell growth under an optical
electron microscope (magnification, x40). The medium was changed
every 2 days after cell adhesion to the flask and these cells were
recorded as primary cells (P0) (19).
Finally, the third hPDLCs passage (P3)
exhibiting well-grown were seeded into six-well plates and cultured
in an incubator for 1 day. After fixation with 4% paraformaldehyde
for 10 min at 37˚C, 0.1% Triton X-100 (Millipore Sigma) was added
and incubated for 20 min at room temperature. Cells were washed
three times with PBS and each well was treated with 300 µl of 5%
BSA blocking solution and allowed to stand for 1 h at room
temperature. After aspirating the liquid, anti-vimentin (1:1,000
dilution; rabbit; cat. no. ab137321; Abcam) and anti-keratin
(1:1,000 dilution; cat. no. ab8068 mouse; Abcam) antibodies were
added and incubated overnight at 4˚C. Following antibody removal,
the cells were rinsed three times with TBST (0.1% Tween-20) and the
mouse anti-rabbit IgG (HRP) secondary antibody (1:5,000 dilution;
cat. no. ab99697; Abcam) was added and incubated at room
temperature for 1 h in the dark. Following TBST rinsing, cell
nuclei were stained with DAPI at room temperature for 10 min.
Subsequently, the DAPI solution was discarded, the cells were
washed three times with PBS, the slides were mounted and the entire
cell area was observed under an inverted fluorescence microscope
(magnification, x20). Based on immunofluorescence staining, protein
staining characteristics of hPDLCs were therefore observed in 15
random fields of view at x20 magnification.
Lentiviral construction of a stable
periodontal cell line overexpressing pLV-GRN
Primers were designed according to the gene sequence
of GRN in NCBI (NM_002087.4), as shown in Table I. The Xho1 restriction site
and protective bases were added to the forward primer and the
Nhe1 restriction site, HA tags and protective bases were
added to the reverse primer. All components were added following
the manufacturer's instructions for the PrimerSTAR Max kit
(Invitrogen; Thermo Fisher Scientific, Inc.). Specific PCR
amplification of the coding sequence of the GRN gene was
conducted, followed by nucleic acid electrophoresis detection and
the target bands were excised and recovered. The PCR products of
GRN and pLV-puro plasmid (Addgene, Inc.) underwent double
digestion with the restriction enzymes Xho1 (New England
BioLabs, Inc.) and Nhe1 (New England BioLabs, Inc.) The gel
mixture was incubated at 50-60˚C for 10 min, according to the
instructions for the GeneJET gel extraction kit (Thermo Fisher
Scientific, Inc.). The gel was recovered, ligated with T4 ligase
(New England BioLabs, Inc.) and the ligated product was combined
with DH5α (Invitrogen; Thermo Fisher Scientific, Inc.) receptor
cells for transformation. The plasmid was extracted using the
TIANprep Mini Plasmid Kit (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. The plasmid was
identified by double digestion with Xho1 and Nhe1 and
then sent to Tsingke Biotechnology Co., Ltd. for sequencing.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene | Primer (5'-3') | Accession
number |
|---|
| GRN
forward |
CCCTCGAGGCCACCATGTGGACCCTGGTGAGCTG | NM_002087.4 |
| GRN
reverse |
GGTGCTAGCTTAAGCGTAGTCTGGGACGTCGTATGGGTACAGCAGCTGTC | |
| ALP
forward |
CCACGTCTTCACATTTGGTG | NM_000478.6 |
| ALP
reverse |
AGACTGCGCCTGGTAGTTGT | |
| Runx2
forward |
CACTATCCAGCCACCTTTAC | NM_001015051.4 |
| Runx2
reverse |
CACTCTGGCTTTGGGAAGAG | |
| OPN
forward |
TGAAACGAGTCAGCTGGATG | NM_000582.3 |
| OPN
reverse |
TGAAATTCATGGCTGTGGAA | |
| β-actin
forward |
GAAACTACCTTCAACTCCATC | NM_001101.5 |
| β-actin
reverse |
CTAGAAGCATTTGCGGTGGAC | |
293T cells (Lanzhou Veterinary Research Institute,
Chinese Academy of Agricultural Sciences, China) were cultured in
T25 culture flasks. Upon reaching 80% confluence, cell transfection
was performed using the jetPRIME Transfection Reagent Kit
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions. A total of 300 µl of jetPRIME buffer
was added to a sterile EP tube and combined with 6 µg of pLV-puro
or pLV-GRN plasmid, 4 µg of psPAX2 plasmid (Addgene, Inc.) and 2 µg
of pMD2.G plasmid (Addgene, Inc.). To this mixture, 12 µl of
jetPRIME reagent was added, thoroughly mixed and left at room
temperature for 15 min. The resulting solution was added dropwise
to a 293T cell culture flask, followed by the addition of 5 ml of
complete medium. The medium was changed after 12 and 36 h later,
the supernatant was aspirated, centrifuged at 100 x g for 5 min at
4˚C, filtered through a 0.22 µm filter, collected into a centrifuge
tube, aliquoted into 1 ml EP tubes and stored at -80˚C (29).
Finally, P3 hPDLCs were divided into
hPDLCs, pLV-puro and pLV-GRN groups and inoculated into T25 culture
flasks (n=3). The medium was changed when the cell confluence
reached 70-80% and then 1 ml of the collected pLV-puro or pLV-GRN
lentiviral plasmids was added dropwise to the corresponding flasks,
mixed and cultured for 24 h at 37˚C. Based on the resistance
screening concentration determined by the previous research, the
final concentration of 5 µg/ml polybrene (Gibco; Thermo Fisher
Scientific, Inc.) was added to the culture medium. After 3-4 days,
when, upon microscopic investigation, most cells in the blank group
appeared to have disintegrated and died, the polybrene
concentration was halved and screening continued. After ~7 days,
when all cells in the blank group had died, the cells that remained
adherent to the wall in the PLV-puro and pLV-GRN groups were
successfully infected and continued to be cultured and passaged.
The experiment for constructing an hPDLCs line overexpressing
GRN using lentivirus was repeated at least three times.
Reverse transcription-quantitative
(RT-q) PCR of the mRNA expression level of GRN
After the confluence of cells reached 90%, total RNA
was extracted from P5 cells in the hPDLCs group,
pLV-puro group and pLV-GRN group, followed by reverse transcription
into cDNA using the PrimerScript RT Master Mix Reverse
Transcription Kit (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Gene primers were
designed with β-actin serving as an internal reference (Table I). qPCR amplification was performed
using 2X SYBR Green QPCR Master Mix (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. PCR
conditions were as follows: 95˚C for 5 min, followed by 30 cycles
at 95˚C for 30 sec, 58˚C for 20 sec and 72˚C for 20 sec. Each
experiment was repeated in three sets (n=3). Following the
completion of the reaction, melting curves were analyzed and the
data were processed based on 2-ΔΔCq and the experiment
was repeated three times (30,31).
Western blot analysis of GRN protein
expression
Total protein from P5 cells in the
hPDLCs, pLV-puro and pLV-GRN groups was extracted (Laemmli buffer
2x; cat. no. S3401; MilliporeSigma) and protein quantification was
performed using a BCA kit (n=3) following the manufacturer's
instructions. The cells were evenly scraped with a cell scraper and
the mixed solution was transferred to a 2 ml EP tube and
ultrasonically lysed for 2 min and centrifuged at 16,000 x g for 10
min at 4˚C. The supernatant was added to a new 2 ml EP tube.
Meanwhile, 5x loading Buffer (Thermo Fisher Scientific, Inc.) was
added in a ratio of 1:4 and was denatured at 95˚C for 10 min, and
finally the protein was stored at -20˚C. The pre-thawed protein was
added to the electrophoresis tank (10% SDS-PAGE) at 12 µl per well
and electrophoresed for 2.5 h (80 V 30 min, 120 V 120 min; n=3).
Afterward, the membrane was transferred in the order of
sponge-filter paper gel-PVDF membrane-filter paper-sponge, and the
program was set to 2,000 mA for 120 min. Then, the PVDF membrane
was placed in 15 ml of 5% skimmed milk powder and blocked on a
shaker for 3 h. After blocking, the desired gene bands were cut
into diluted anti-GRN (1:1,000 dilution; rabbit; cat. no. ab191211;
Abcam) and β-actin (1:1,000 dilution; rabbit; cat. no. ab8227;
Abcam) at 4˚C overnight. The next day, the strip was removed and
washed three times with 15 ml TBST (0.1% Tween-20) for 10 min each
time. After washing, the strip was placed in the goat anti-rabbit
(IgG) secondary antibody HRP (1:5,000 dilution; cat. no. ab6721;
Abcam) and incubated for 1 h at room temperature on a shaker. Then,
the strip was washed three times with 15 ml TBST for 10 min each
time. Finally, after adding the developer solution to the stripes,
the image could be exposed and saved by the exposure instrument.
The whole process of western blot experiment was replicated for
three times. ImageJ 2.2.0-beta6 software (National Institutes of
Health) was used to determine the protein grayscale values and the
experiment was repeated three times (32).
MTT assay of cell proliferative
capacity
P2 hPDLCs were digested with 0.25%
trypsin, counted and inoculated into 96-well plates at a density of
1x104 cells/ml and 100 µl per well with three replicates
per group (n=3). Culturing was conducted for 1-7 days at a constant
temperature of 37˚C with 5% CO2. After aspirating the
medium, 10 µl of MTT labeling reagent (Abcam) was added to each
well. Subsequently, 100 µl of lysis buffer was added to each well
after continuous culture for 4 h and then mixed thoroughly on a
shaker and the absorbance (OD) value at 450 nm was determined with
a spectrophotometer. Each experiment was performed in triplicate
(33).
Alizarin Red staining
P6 cells from the hPDLCs, pLV-puro and
pLV-GRN groups were inoculated into 35 mm2 dishes, with
three replicates per group (n=3). After the cells adhered to the
surface, 2 ml of osteogenic induction solution was added and
continuous culturing was performed for 21 days with the solution
changed every 2 days. On the 7th, 14th and 21st day, the medium was
aspirated and washed with PBS for three times and 300 µl of 4%
paraformaldehyde was added to each group of cells for 30 min at
room temperature. After aspirating the paraformaldehyde, the cells
were washed three times with PBS and 1 ml of alizarin red solution
(Procell Life Science & Technology Co., Ltd.) was added to each
group of cells, allowed to stand for 20 min and observed the entire
cell area under an optical electron microscope (magnification, x4)
(19).
Determination of alkaline phosphatase
(ALP) activity
Cells from the hPDLCs, pLV-puro and pLV-GRN groups
were seeded into 96-well plates after 7, 14 and 21 days of
induction and ALP activity was assessed using an ALP activity assay
kit (Procell Life Science & Technology Co., Ltd.) according to
the manufacturer's instructions. The absorbance (OD) of each well
was measured at 405 nm using a spectrophotometer and a standard
curve was constructed. The ALP activity of each cell group was
calculated using an enzyme activity assay. Each experiment was
performed in triplicate (34).
RT-qPCR determination of mRNA
expression levels of osteogenesis-related genes
Total After the confluence of cells reached 90%, RNA
was extracted from cells in the hPDLCs group, pLV-puro group and
pLV-GRN group after induction for 7, 14 and 21 days, with three
replicates per group (n=3) and reverse transcribed into cDNA using
the PrimerScript RT Master Mix Reverse Transcription Kit (Thermo
Fisher Scientific, Inc.) following the manufacturer's instructions.
PCR conditions were as follows: 95˚C for 5 min, followed by 30
cycles at 95˚C for 30 sec, 58˚C for 20 sec and 72˚C for 20 sec.
Primers for osteogenesis-related genes, including ALP, runt-related
transcription factor 2 (Runx2) and osteopontin (OPN), are listed in
Table I. qPCR amplification was
performed according to the instructions for 2X SYBR Green QPCR
Master Mix. Each experiment for each gene was conducted in
triplicate, with β-actin serving as an internal reference. The
relative mRNA expression of each gene was calculated according to
2-ΔΔCq (30,31).
Western blot determination of
osteogenesis-related protein expression levels
Total protein was extracted from cells in each group
following osteogenic induction for 7, 14 and 21 d, with three
replicates per group (n=3). Protein quantification was conducted
using the BCA kit according to the manufacturer's instructions.
Western blot analysis was performed using GADPH as an internal
reference to assess the expression of osteogenesis-related
proteins, including OPN (1:400 dilution; rabbit; cat. no. ab8448;
Abcam), Runx-2 (1:200 dilution; rabbit; cat. no. ab114133; Abcam)
and GADPH (1:1,000 dilution; rabbit; cat. no. ab263962; Abcam). The
specific method was the same as that in Western blot analysis of
GRN protein expression. ImageJ 2.2.0-beta6 software (National
Institutes of Health) was used to determine the grayscale values of
proteins (32).
Statistical analysis
SPSS Statistics for Windows (version 26.0; IBM
Corp.) was used for the statistical analysis. One-way ANOVA and
two-way ANOVA followed by Tukey's post hoc test were used to
analyze the differences. P<0.05 was considered to indicate a
statistically significant difference.
Results
Culture and identification of primary
hPDLCs
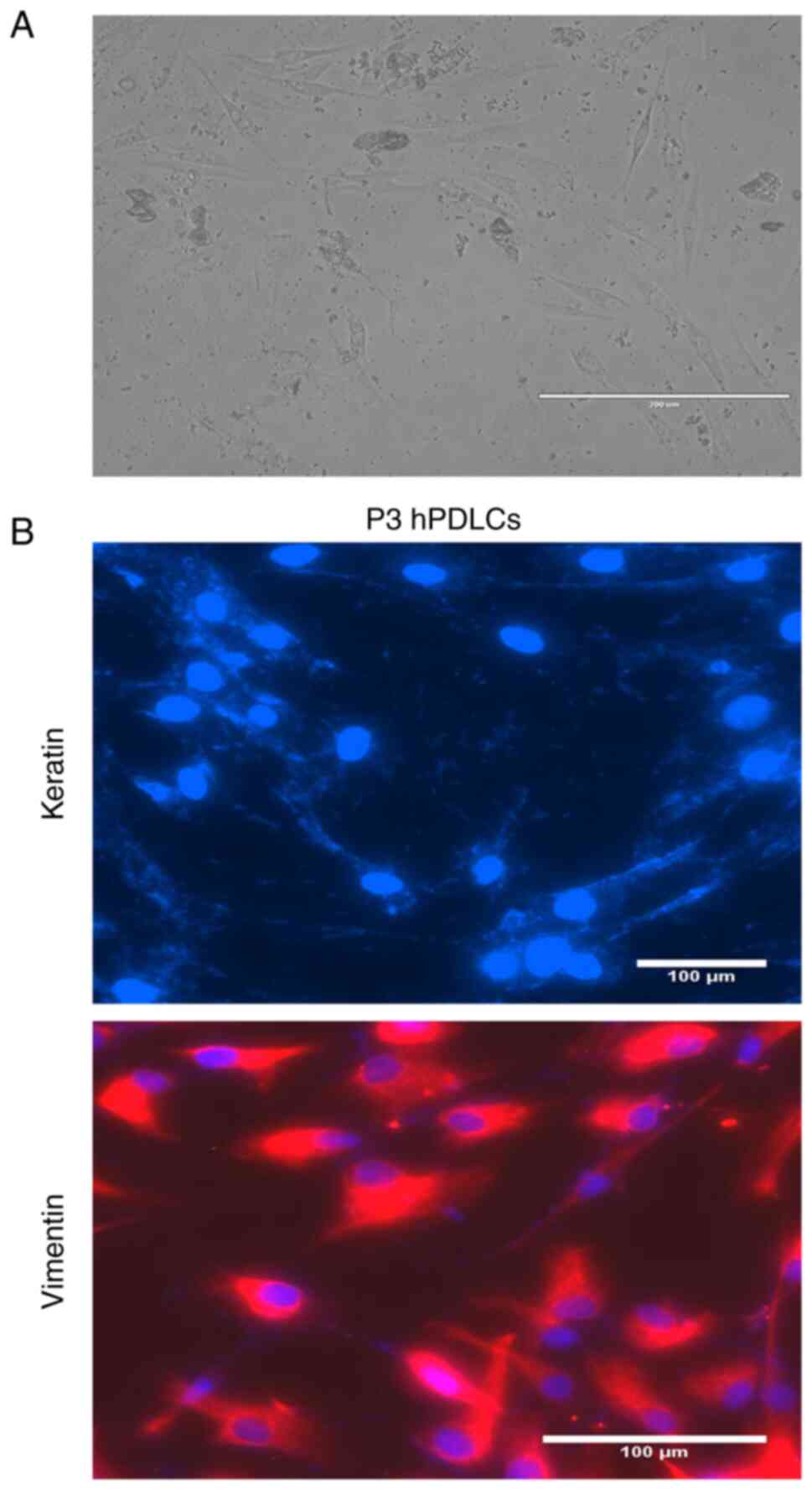
After 5 days of culture, black clusters of
periodontal ligament tissue were observed under an inverted
microscope. Cells could be observed expanding around the tissue
mass, which were spindle or spindle-shaped, uniform in shape and
full of cytoplasm (Fig. 1A). The
immunofluorescence staining of P3 hPDLCs was negative,
indicating that the extracted cells were not contaminated with
epithelial cells (Fig. 1B).
Positive vimentin staining indicated that the cells were derived
from the mesenchyme (Fig. 1B). The
cells were characterized as hPDLCs based on immunofluorescence
staining.
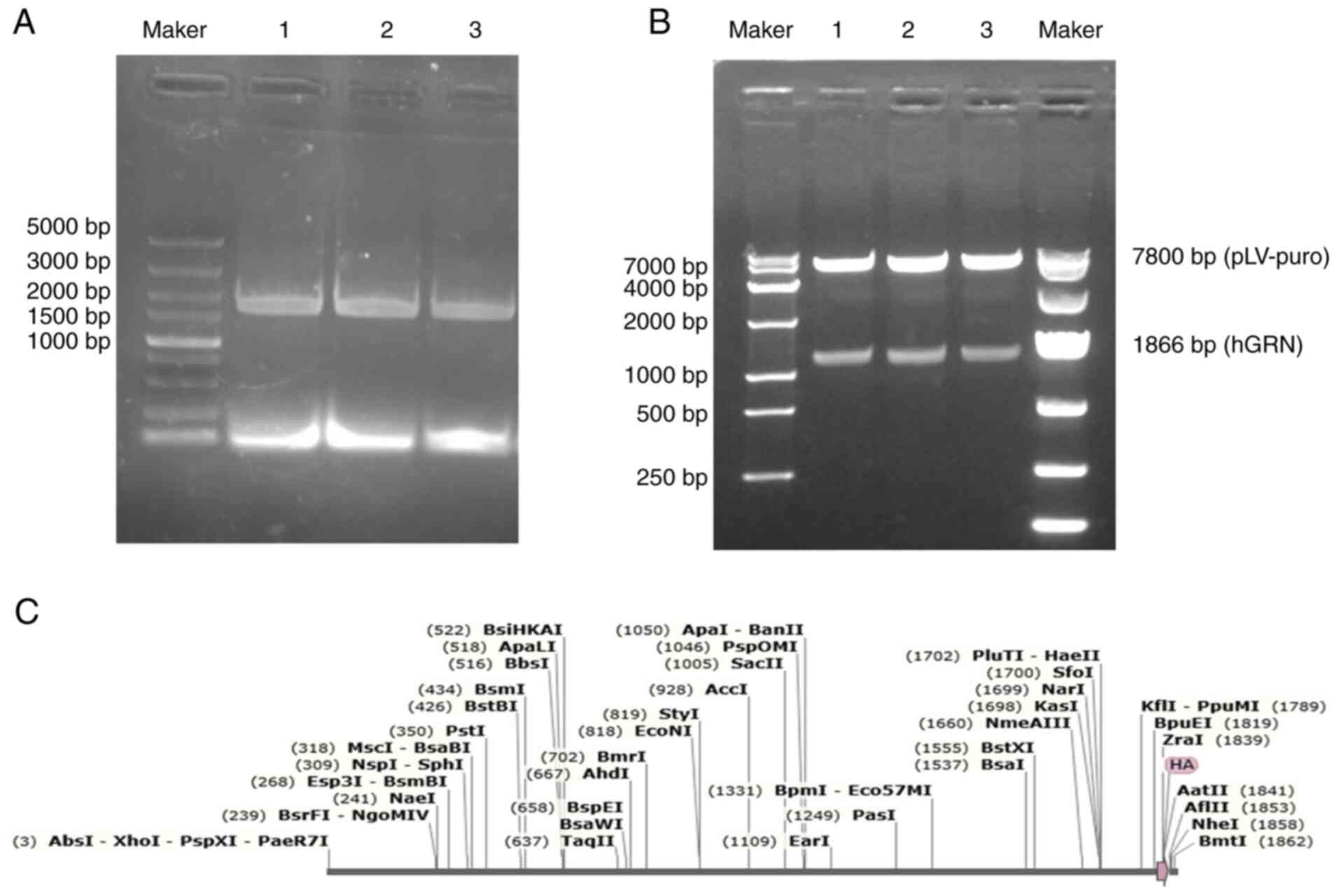
Identification of pLV-GRN recombinant
plasmid by double digestion
The electrophoresis results showed a distinct band
at ~1,866 bp (Fig. 2A). Following
double digestion of the homologous recombinant plasmid pLV-GRN with
XhoI and NheI, electrophoresis displayed clear bands
at ~1,866 and 7,800 bp (Fig. 2B),
which were consistent with the size of the 1,866 bp GRN gene
along with its HA tag. The sequencing results were aligned with the
cDNA sequence of the GRN gene (NM_002087.4) in the NCBI
database (Fig. 2C). The sequence
demonstrated 100% homology with the target sequence, confirming the
successful construction of the recombinant plasmid.
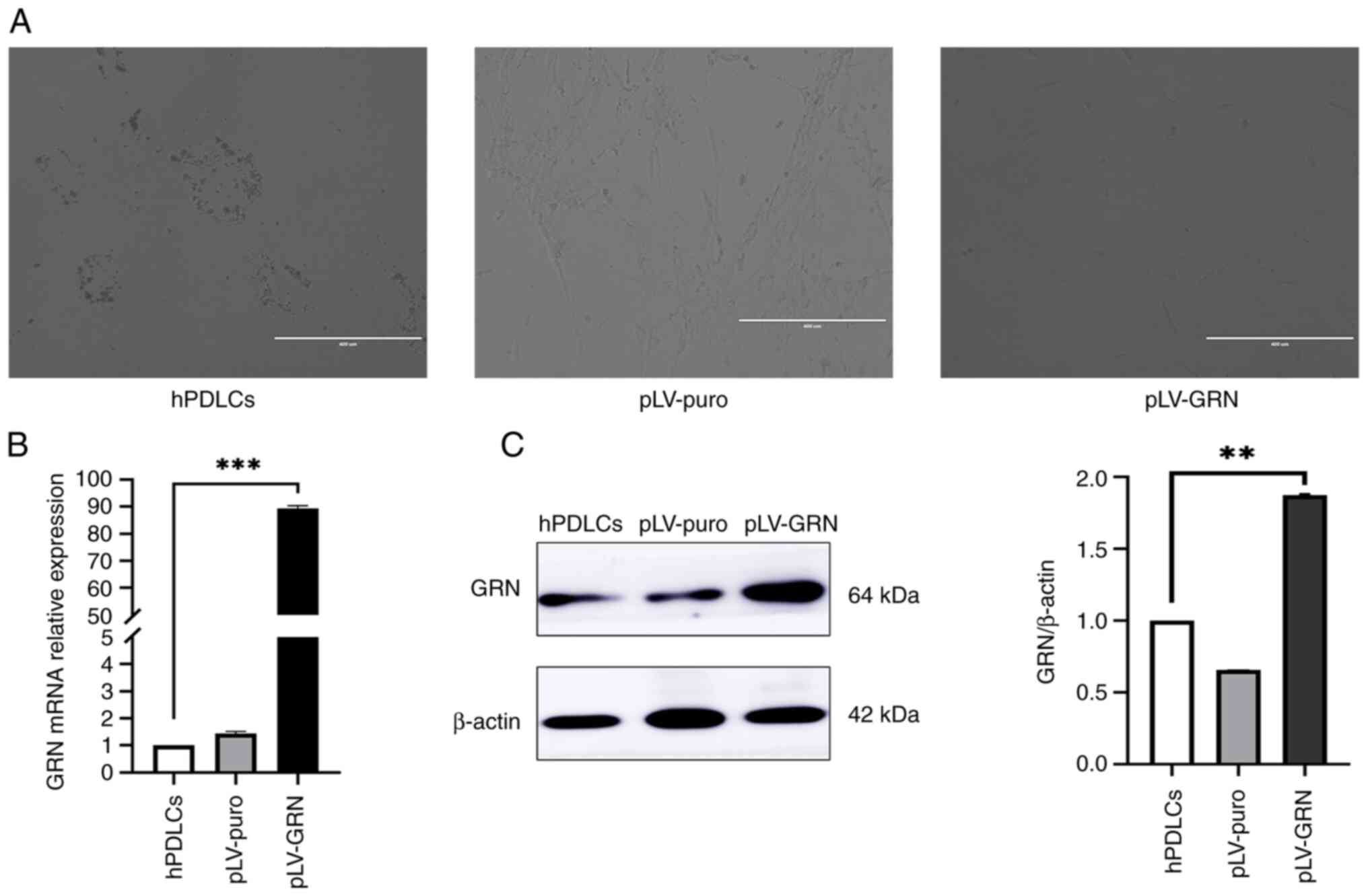
Screening results of hPDLCs
overexpressing GRN
After periodontal ligament cells were infected with
the lentivirus for 24 h (Fig. 3A),
the cell morphology in the hPDLCs, pLV-puro and pLV-GRN groups
remained unchanged, maintaining a radial or spiral arrangement.
After 7 days of puromycin resistance screening, microscopic
examination revealed numerous cell fragments in the hPDLCs group,
indicating the loss of intact cell morphology. Varying degrees of
cell disintegration and death were observed in the pLV-puro and
pLV-GRN groups. However, a small number of hPDLCs with surface
adherence persisted, exhibiting an unaltered long spindle or
fusiform shape. At this stage, cells with normal morphology, clear
edges and continued adherence observed under the microscope were
identified as hPDLCs that were successfully infected with the
lentiviral vector.
RT-qPCR and western blot analysis of
GRN expression
RT-qPCR results revealed robust expression of the
GRN gene in the pLV-GRN group, which was 80-90 times higher
than that in the hPDLCs and pLV-puro groups (P<0.01; Fig. 3B).
With β-actin as the internal reference, western
blotting results demonstrated the expression of HA-tagged protein,
indicating strong PGRN expression in periodontal cells transfected
with GRN. Protein expression assays showed a significant
enhancement in PGRN protein expression in the pLV-GRN group, which
was ~1.8 times higher than that in the hPDLCs and pLV-puro groups
(P<0.05; Fig. 3C).
Comparison of cell proliferation
capacity
The MTT assay results indicated a similar increase
in the cell proliferation rate for all groups cultured for 12-72 h.
The hPDLCs group did not differ significantly from the pLV-puro
group; however, the cell proliferation capacity of the pLV-GRN
group was significantly higher (Fig.
4A; P<0.05).
 | Figure 4The effects of GRN on the
proliferation and osteogenesis of hPDLCs. (A) MTT assay for
determination of cell proliferation capacity. (B) ALP activity
assay results (**P<0.05, ***P<0.01, ns,
not significant). (C) Alizarin red staining results (magnification,
x4; mineralized nodules are visible at the white arrows); (a, b and
c) PDLCs group, pLV-puro group and pLV-GRN group after 7 days of
osteogenic induction respectively; (d, e and f) PDLCs group,
pLV-puro group and pLV-GRN group after 14 days of osteogenic
induction respectively; (g, h and i) PDLCs group, pLV-puro group
and pLV-GRN group after 21 days of osteogenic induction,
respectively. GRN, granulin precursor gene; hPDLCs, human
periodontal ligament cells; RT-qPCR, reverse
transcription-quantitative PCR; ALP, alkaline phosphatase; pLV,
lentivirus recombinant plasmid. |
ALP activity assay
The ALP activity assay results revealed that the ALP
activity of the hPDLCs, pLV-puro and pLV-GRN groups peaked at 14
days. The ALP activity of the pLV-GRN group was significantly
higher than that of the other two groups (P<0.01) and
subsequently declined but remained higher than that of the hPDLCs
and pLV-puro groups (P<0.05). No significant differences were
observed between the hPDLCs and pLV-puro groups (Fig. 4B).
Alizarin red staining
At 7 days after osteogenic induction, no obvious
orange-red mineralized nodules were observed in the hPDLCs
(Fig. 4Ca), pLV-puro (Fig. 4Cb), or pLV-GRN (Fig. 4Cc) groups. After osteogenic
induction for 14 days, orange-red mineralized nodules were observed
in the hPDLCs (Fig. 4Cd), pLV-puro
(Fig. 4Ce) and pLV-GRN (Fig. 4Cf) groups. The pLV-GRN group
exhibited more mineralized nodules than the other two groups. After
21 days of osteogenic induction, the pLV-GRN group (Fig. 4Ci) produced more orange-red
mineralized nodules than the hPDLCs (Fig. 4Cg) and pLV-puro groups (Fig. 4Ch) and there was no significant
difference between the hPDLCs (Fig.
4Cg) and pLV-puro groups (Fig.
4Ch).
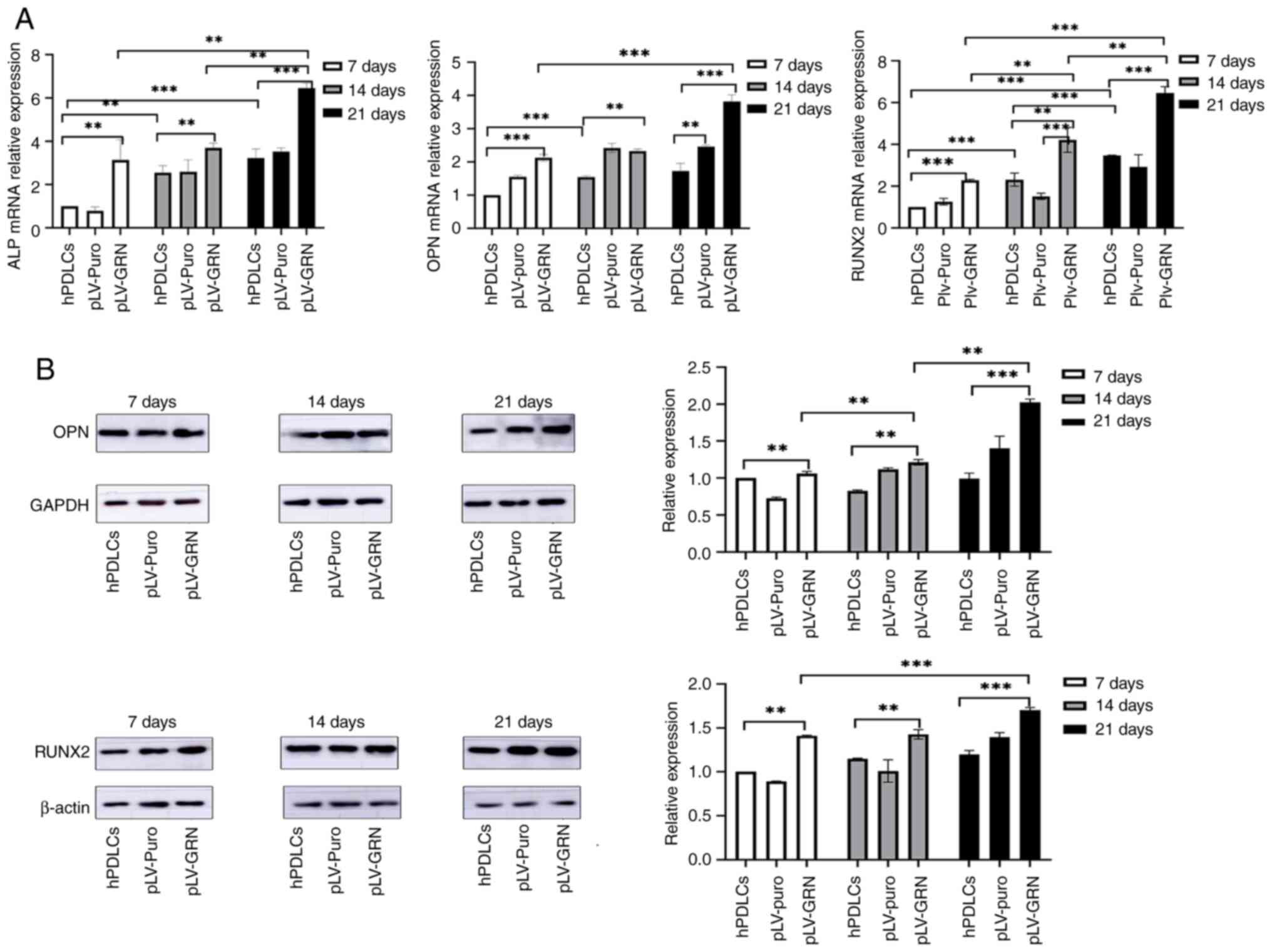
RT-qPCR analysis of
osteogenesis-related genes
RT-qPCR results demonstrated that after 7, 14 and 21
days of osteogenic induction, the mRNA expression levels of
osteogenesis-related genes, including ALP and Runx2, in the pLV-GRN
group were 2-3 times higher than those in the hPDLCs and pLV-puro
groups (P<0.05). The mRNA expression level of the
osteogenesis-related gene OPN was ~1.5 times higher in the pLV-GRN
group than in the hPDLCs and pLV-puro groups (P<0.05; Fig. 5A).
Western blot analysis of
osteogenesis-related protein expression
Western blotting results indicated that the
expression level of the osteogenic protein Runx-2 in the pLV-GRN
group was ~1.5-fold higher than that in the hPDLCs and pLV-puro
groups at 7, 14 and 21 days of osteogenic induction (P<0.05).
The expression level of the osteogenic protein OPN in the pLV-GRN
group did not differ significantly from that in the hPDLCs and
pLV-puro groups on days 7 and 14 of osteogenic induction. However,
on day 21 of osteogenic induction, the expression level of OPN in
the pLV-GRN group was ~2-fold higher than that in the hPDLCs and
pLV-puro groups (P<0.05; Fig.
5B).
Discussion
Periodontitis, characterized by chronic inflammation
affecting the supporting periodontal tissues, poses a significant
threat, leading to the progressive destruction of the alveolar bone
and potential tooth loss in severe cases. Current clinical
treatments primarily focus on addressing etiological factors and
controlling inflammation through scaling and root planning.
However, these methods failed to achieve comprehensive periodontal
regeneration. Therefore, exploring effective strategies for
complete periodontal regeneration is imperative for managing
periodontitis. Tissue engineering techniques, which are gaining
prominence in domestic and international research, offer a
promising avenue for advancing periodontal regeneration (10).
hPDLCs, which are multipotent cells that can
differentiate into fibroblasts, osteoblasts and cementoblasts, have
emerged as the ideal seed cells for periodontal tissue engineering
(10). In the present study,
primary hPDLCs were successfully isolated and cultured from teeth
extracted during orthodontic procedures and from third molars.
Immunofluorescent staining for keratin and vimentin confirmed the
mesenchymal origin of the cells, ensuring stable biological
characteristics following passaging. Microscopic examination
revealed a consistent fusiform or long-spindle morphology,
indicating a robust growth status suitable for subsequent
investigation (35).
The dual protective and regenerative attributes of
PGRN make it a promising target for novel therapies to treat
diseases associated with tissue defects (36). Periodontal regeneration promotes
the proliferation and differentiation of stem cells by recruiting
endogenous stem cells to the defect site and using bioactive
factors with anti-inflammatory and tissue repair effects.
Therefore, the effect of PGRN on hPDLCs is key to promoting
periodontal tissue regeneration (37). While local application of exogenous
PGRN has shown promise in promoting periodontal regeneration
(37), direct administration of
recombinant proteins presents challenges, such as frequent dosing
and high quantities. To address these problems, a stable hPDLCs
cell line overexpressing GRN was successfully constructed using a
lentiviral vector. Western blotting results unequivocally indicated
that the GRN gene was successfully constructed in the
present study using a lentiviral vector and the protein expression
of GRN protein in transfected cells of the pLV-GRN group was
compared with that in the hPDLCs and pLV-puro groups. The RT-qPCR
results demonstrated robust expression of the GRN gene in
the pLV-GRN group, exhibiting a substantial difference from that in
the hPDLCs and pLV-puro groups. Moreover, following passaging, the
morphological consistency of hPDLCs transfected with the GRN
gene, resembling primary cells with a fusiform or long spindle
shape, confirmed the stable effect of GRN on hPDLCs. This
stable research model provides a foundation for exploring the
effects of GRN on the proliferation and osteogenic capacity
of hPDLCs.
Exogenous PGRN has been shown to induce cell
proliferation and increase ALP activity in hPDLCs (37,38).
In the present study, the proliferative capacity of hPDLCs
successfully transfected with pLV-GRN was assessed using MTT and
ALP activity assays. The results revealed that the proliferative
capacity of cells in the pLV-GRN group significantly surpassed that
of cells in both the hPDLCs and pLV-puro groups, underscoring the
role of GRN in promoting hPDLCs proliferation.
Studies have shown that the intricate process of
osteogenic differentiation in hPDLCs involves the orchestration of
various factors, including BMPs, Wnt family proteins and
transcription factors (Runx-2, β-catenin), with BMP2 being a
pivotal inducer in bone formation (5,6,10,39).
PGRN, a downstream target of BMP2, plays a crucial role in inducing
bone formation (19). In the
present study, osteogenic induction spanning 21 days was conducted
on cells from the hPDLCs, pLV-puro and pLV-GRN groups, followed by
Alizarin Red staining on days 7, 14 and 21. Microscopic
observations revealed a higher occurrence of orange-red mineralized
nodules in the pLV-GRN group than in the other two groups. This
observation supported the robust osteogenic-promoting capability of
GRN in hPDLCs at the cellular level.
Runx-2 regulates osteoblast differentiation and
maturation primarily through the Wnt and BMP signaling pathways
(40,41). PGRN promotes the expression of
Runx-2, exerting a pivotal role in the proliferation, maturation
and differentiation of mesenchymal stem cells (19). Additionally, OPN plays a crucial
role in bone metabolism, not only as a vital factor in the
neuron-mediated and endocrine regulation of bone mass but also in
various biological activities (42-44).
ALP, recognized as the principal mineralizing enzyme in
osteogenesis, metabolism and regeneration (45,46),
serves as an early differentiation marker for osteoblasts and a
characteristic indicator for evaluating differentiation toward
osteogenic lineages. In the present study, RT-qPCR results revealed
that the mRNA expression of osteogenesis-related genes, including
OPN, Runx-2 and ALP, in the pLV-GRN group significantly surpassed
that in both the hPDLCs and pLV-puro groups. No significant
differences were observed between the hPDLCs and pLV-puro groups.
Consistent with these findings, western blotting demonstrated
higher protein expression levels of OPN and Runx-2 in the pLV-GRN
group than in the hPDLCs and pLV-puro groups during osteogenic
induction. Moreover, OPN expression in the pLV-GRN group was
notably higher than that in the hPDLCs and pLV-puro group on the
21st day. These results further substantiated that GRN
effectively enhanced the ability of hPDLCs to differentiate into an
osteogenic lineage at both the molecular and protein levels.
The present study unequivocally demonstrated that
GRN possessed a pronounced ability to enhance both the
proliferation and osteogenic differentiation of hPDLCs.
Importantly, it addressed the drawbacks associated with the
frequent administration and high dosage of exogenous GRN
observed in previous studies. Using lentiviral-mediated methods,
hPDLCs cell lines that overexpressed GRN and exhibited
stable and noteworthy osteogenic effects were successfully
constructed. The present study provided a solid theoretical
foundation for future investigations on periodontal
regeneration.
To summarize, the present study successfully
established a stable hPDLCs cell line overexpressing the GRN
gene through the use of a lentiviral vector. The ability of
GRN to promote proliferation and osteogenic differentiation
of hPDLCs was confirmed. Further exploration of this signaling
pathway is needed to comprehensively verify the role of GRN
in promoting the proliferation and osteogenesis of hPDLCs. The
present study not only served as a robust experimental basis for
advancing the understanding of periodontal tissue regeneration but
also charted a novel direction for preventing and treating
periodontal disease.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Research Fund of
Lanzhou University (grant no. 20JR10RA653-ZDKF20210103) and the Key
Research Fund of Gansu Province (grant no. 21YF5GA100).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
XY and RQ conducted the synthesis of recombinant
plasmids and osteogenic differentiation experiments and were major
contributors to writing the manuscript. ZC, DH and XS collected the
clinical samples and experimental data. YS and XH designed the
experiments and reviewed and edited the manuscript. XY and XH
confirm the authenticity of all the raw data. All the authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
Human periodontal ligament cells were isolated and
used in accordance with the ethical standards established in the
Declaration of Helsinki. The present study was approved by the
ethics committee of the School of Stomatology, Lanzhou University
(approval no. LZUKQ-2019-045) and informed consent form was signed
by the patient prior to participation in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Professor Xiangyi He ORCID: 0000-0002-5687-0991.
References
|
1
|
Guzik TJ and Czesnikiewicz-Guzik M:
Mounting pressure of periodontitis. Hypertension. 78:552–554.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sirisereephap K, Maekawa T, Tamura H,
Hiyoshi T, Domon H, Isono T, Terao Y, Maeda T and Tabeta K:
Osteoimmunology in periodontitis: Local proteins and compounds to
alleviate periodontitis. Int J Mol Sci. 23(5540)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Michalowicz BS, Hodges JS, DiAngelis AJ,
Lupo VR, Novak MJ, Ferguson JE, Buchanan W, Bofill J, Papapanou PN,
Mitchell DA, et al: Treatment of periodontal disease and the risk
of preterm birth. N Engl J Med. 335:1885–1894. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chatzaki N, Zekeridou A, Paroz E, Gastaldi
G and Giannopoulou C: Knowledge and practice attitudes regarding
the relationship between diabetes and periodontitis: A survey among
Swiss endocrinologists and general physicians. BMC Prim Care.
24(238)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lee HS, Byun SH, Cho SW and Yang BE: Past,
present, and future of regeneration therapy in oral and periodontal
tissue: A review. Appl Sci. 9(1046)2019.
|
|
6
|
Raveau S and Jordana F: Tissue engineering
and three-dimensional printing in periodontal regeneration: A
literature review. J Clin Med. 9(4008)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tsuchida S and Nakayama T: Periodontal
tissue regeneration therapy using stem cells. Stem Cell Rev Rep.
19:825–826. 2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Seo BM, Miura M, Gronthos S, Bartold PM,
Batouli S, Brahim J, Young M, Robey PG, Wang CY and Shi S:
Investigation of multipotent postnatal stem cells from human
periodontal ligament. Lancet. 364:149–155. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tomokiyo A, Wada N and Maeda H:
Periodontal ligament stem cells: Regenerative potency in
periodontium. Stem Cells Dev. 28:974–985. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhao B, Zhang W, Xiong Y, Zhang Y, Zhang D
and Xu X: Effects of rutin on the oxidative stress, proliferation
and osteogenic differentiation of periodontal ligament stem cells
in LPS-induced inflammatory environment and the underlying
mechanism. J Mol Histol. 51:161–171. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mendsaikhan A, Tooyama I and Walker DG:
Microglial progranulin: Involvement in Alzheimer's disease and
neurodegenerative diseases. Cells. 8(230)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yin FF, Banerjee R, Thomas B, Zhou P, Qian
LP, Jia T, Ma XJ, Ma Y, Iadecola C, Beal MF, et al: Exaggerated
inflammation, impaired host defense, and neuropathology in
progranulin-deficient mice. J Exp Med. 207:117–128. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Saeedi-Boroujeni A, Purrahman D, Shojaeian
A, Poniatowski ŁA, Rafiee F and Mahmoudian-Sani MR: Progranulin
(PGRN) as a regulator of inflammation and a critical factor in the
immunopathogenesis of cardiovascular diseases. J Inflamm (Lond).
20(1)2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
He Z, Ong CH, Halper J and Bateman A:
Progranulin is a mediator of the wound response. Nat Med.
9:225–229. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Gulluoglu S, Tuysuz EC, Sahin M, Yaltirik
CK, Kuskucu A, Ozkan F, Dalan AB, Sahin F, Ture U and Bayrak OF:
The role of TNF-α in chordoma progression and inflammatory
pathways. Cell Oncol (Dordr). 42:663–677. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Noguchi T, Ebina K, Hirao M, Kawase R,
Ohama T, Yamashita S, Morimoto T, Koizumi K, Kitaguchi K, Matsuoka
H, et al: Progranulin plays crucial roles in preserving bone mass
by inhibiting TNF-α-induced osteoclastogenesis and promoting
osteoblastic differentiation in mice. Biochem Biophys Res Commun.
465:638–643. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Pogonowska M, Poniatowski ŁA, Wawrzyniak
A, Królikowska K and Kalicki B: The role of progranulin (PGRN) in
the modulation of anti-inflammatory response in asthma. Cent Eur J
Immunol. 44:97–101. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wei F, Zhang Y, Jian J, Mundra JJ, Tian Q,
Lin J, Lafaille JJ, Tang W, Zhao W, Yu X and Liu CJ: PGRN protects
against colitis progression in mice in an IL-10 and TNFR2 dependent
manner. Sci Rep. 4(7023)2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Qin R, Cui Z, Zhou H, Guo R, Yao X, Wang
T, Qin X and He X: Effect of lentivirus-mediated BMP2 from
autologous tooth on the proliferative and osteogenic capacity of
human periodontal ligament cells. J Periodont Res. 57:869–879.
2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ding Y, Wei J, Hettinghouse A, Li G, Li X,
Einhorn TA and Liu CJ: Progranulin promotes bone fracture healing
via TNFR pathways in mice with type 2 diabetes mellitus. Ann N Y
Acad Sci. 1490:77–89. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yang Y, Feng N, Liang L, Jiang R, Pan Y,
Geng N, Fan M, Li X and Guo F: Progranulin, a moderator of
estrogen/estrogen receptor α binding, regulates bone homeostasis
through PERK/p-eIF2 signaling pathway. J Mol Med (Berl).
100:1191–1207. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhao Z, Li E, Luo L, Zhao S, Liu L, Wang
J, Kang R and Luo J: A PSCA/PGRN-NF-κB-Integrin-α4 axis promotes
prostate cancer cell adhesion to bone marrow endothelium and
enhances metastatic potential. Mol Cancer Res. 18:501–513.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sfikakis PP and Tsokos GC: Towards the
next generation of anti-TNF drugs. Clin Immunol. 141:231–235.
2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Han CM, Cheng B and Wu P: Writing group of
growth factor guideline on behalf of Chinese Burn Association.
Clinical guideline on topical growth factors for skin wounds. Burns
Trauma. 8(tkaa035)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hefka Blahnova V, Dankova J, Rampichova M
and Filova E: Combinations of growth factors for human mesenchymal
stem cell proliferation and osteogenic differentiation. Bone Joint
Res. 9:412–420. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ridet JL and Privat A: Gene therapy in the
central nervous system direct versus indirect gene delivery. J
Neurosci Res. 42:287–293. 1995.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Milone MC and O'Doherty U: Clinical use of
lentiviral vectors. Leukemia. 32:1529–1541. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Perry C and Rayat ACME: Lentiviral vector
bioprocessing. Viruses. 13(268)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Cui Z, Qin R, Feng J, Liu Y, Zhou X, Qin
X, Li Y, Zhang Z and He X: XBP1s gene of endoplasmic reticulum
stress enhances proliferation and osteogenesis of human periodontal
ligament cells. Tissue Cell. 83(102139)2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Fathi E, Farahzadi R and Charoudeh HN:
L-carnitine contributes to enhancement of neurogenesis from
mesenchymal stem cells through Wnt/β-catenin and PKA pathway. Exp
Biol Med (Maywood). 242:482–486. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bagheri Y, Barati A, Nouraei S, Jalili
Namini N, Bakhshi M, Fathi E and Montazersaheb S: Comparative study
of gavage and intraperitoneal administration of gamma-oryzanol in
alleviation/attenuation in a rat animal model of renal
ischemia/reperfusion-induced injury. Iran J Basic Med Sci.
24:175–183. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kumar P, Nagarajan A and Uchil PD:
Analysis of cell viability by the MTT assay. Cold Spring Harb
Protoc. 2018(pdb.prot095505)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Suga T, Usui M, Onizuka S, Sano K, Sato T,
Nakazawa K, Ariyoshi W, Nishihara T and Nakashima K:
Characterization and study of gene expression profiles of human
periodontal mesenchymal stem cells in spheroid cultures by
transcriptome analysis. Stem Cells Int.
2021(5592804)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Fathi E, Azarbad S, Farahzadi R,
Javanmardi S and Vietor I: Effect of rat bone marrow
derived-mesenchymal stem cells on granulocyte differentiation of
mononuclear cells as preclinical agent in cell based therapy. Curr
Gene Ther. 22:152–161. 2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li L, Jiang H, Chen R, Zhou J, Xiao Y,
Zhang Y and Yan F: Human β-defensin 3 gene modification promotes
the osteogenic differentiation of human periodontal ligament cells
and bone repair in periodontitis. Int J Oral Sci.
12(13)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zheng C, Chen J, Liu S and Jin Y: Stem
cell-based bone and dental regeneration: A view of
microenvironmental modulation. Int J Oral Sci.
11(23)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chen Q, Wu Z and Xie L: Progranulin is
essential for bone homeostasis and immunology. Ann N Y Acad Sci.
1518:58–68. 2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chen Q, Cai J, Li X, Song A, Guo H, Sun Q,
Yang C and Yang P: Progranulin promotes regeneration of
inflammatory periodontal bone defect in rats via anti-inflammation,
osteoclastogenic inhibition, and osteogenic promotion.
Inflammation. 42:221–234. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Sun R, Wang D, Song Y, Li Q, Su P and Pang
Y: Correction: Granulin as an important immune molecule involved in
lamprey tissue repair and regeneration by promoting cell
proliferation and migration. Cell Mol Biol Lett.
27(96)2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Gomathi K, Akshaya N, Srinaath N, Moorthi
A and Selvamurugan N: Regulation of Runx2 by post-translational
modifications in osteoblast differentiation. Life Sci.
245(117389)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Komori T: Regulation of proliferation,
differentiation and functions of osteoblasts by Runx2. Int J Mol
Sci. 20(1694)2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yang J, Ye L, Hui TQ, Yang DM, Huang DM,
Zhou XD, Mao JJ and Wang CL: Bone morphogenetic protein 2-induced
human dental pulp cell differentiation involves p38
mitogen-activated protein kinase-activated canonical WNT pathway.
Int J Oral Sci. 7:95–102. 2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Min KK, Neupane S, Adhikari N, Sohn WJ, An
SY, Kim JY, An CH, Lee Y, Kim YG, Park JW, et al: Effects of
resveratrol on bone-healing capacity in the mouse tooth extraction
socket. J Periodont Res. 55:247–257. 2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zernik J, Twarog K and Upholt WB:
Regulation of alkaline phosphatase and alpha 2(I) procollagen
synthesis during early intramembranous bone formation in the rat
mandible. Differentiation. 44:207–215. 1990.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wang Y, Li X, Zhou X, Wang T, Liu Y, Feng
J, Qin X, Zhang Z, Li Y and He X: Regulation of proliferation and
apoptosis of aging periodontal ligament cells by autophagy-related
gene 7. Mol Biol Rep. 50:6361–6372. 2023.PubMed/NCBI View Article : Google Scholar
|