Introduction
Animal experiments have supported innovative
advances in anticancer drug development. In particular, as there
are no alternative methods, experimental models evaluating the
safety and efficacy of anticancer drugs using carcinoma-bearing
animals are highly valued as preclinical trial data (1). In the traditional model, the cell
line-derived tumor xenograft model, in which cultured human tumor
cell lines are transplanted into immunodeficient mice, has been
frequently used. However, the patient-derived tumor xenograft
(PDTX) model, in which patient-derived tumor tissue is transplanted
subcutaneously or orthotopically into immunocompromised mice
(NOD-SCID mice), provides an environment that more closely
resembles the tumor microenvironment in the patient's body
(2). The main concerns in this
PDTX model are the effects of immunodeficiency in the NOD-SCID
mouse model and the loss of human tumor stromal cells, including
cancer-associated fibroblasts (CAFs), with PDTX tumor passaging
into next-generation mice.
In the conventional PDTX model, researchers have
frequently used commercially available collagen-type IV-based
Matrigel as a scaffold to improve the viability of resected and
PDTX tumors (3-6).
Matrigel contains a variety of cell-derived growth factors as well
as extracellular matrix proteins, including collagen IV. In a PDTX
model of colorectal cancer liver metastasis (CRC-LM) using
Matrigel, it has been reported that tumor stroma derived from human
cells were replaced early by mouse cells; however, the histological
morphology and cellular function of the tumor tissues were
maintained (7,8). However, Matrigel contains factors
derived from mouse cells (9) and
is not an animal-free component. Therefore, it is possible that an
inflammatory or immune response may be induced in the transplanted
tumor by the animal component. Such unintended reactions may make
the obtained data difficult to interpret.
Tumor tissue contains a complex tumor
microenvironment composed of cancer cells, other diverse cells, and
extracellular factors in the tumor stroma. The tumor stroma has a
heterogeneous composition encompassing not only CAFs and immune
cells but also extracellular matrix and other diverse factors that
contribute to resistance to anticancer therapy and increased cancer
grade (10,11). In addition, tumor stroma serves as
a niche for cancer stem cells to maintain their stemness
(tumorigenic potential, self-renewal, resistance to
therapy/apoptosis) (12). The
extracellular matrix of the cancer stroma is mainly composed of
collagen, fibronectin, hyaluronic acid, tenascins, proteoglycans,
and matrix metalloproteinases. This study focused on collagen type
I, which is particularly abundant (13).
The recombinant protein based on human collagen type
I (RCPhC1), the focus of this study, is an innovative experimental
scaffold material synthesized under animal component-free
conditions (14), which is
abundant in the tumor stroma and is important for tumor growth.
However, the contribution of RCPhC1 as a scaffold to the
maintenance of the human tumor microenvironment in the PDTX model
has yet to be investigated.
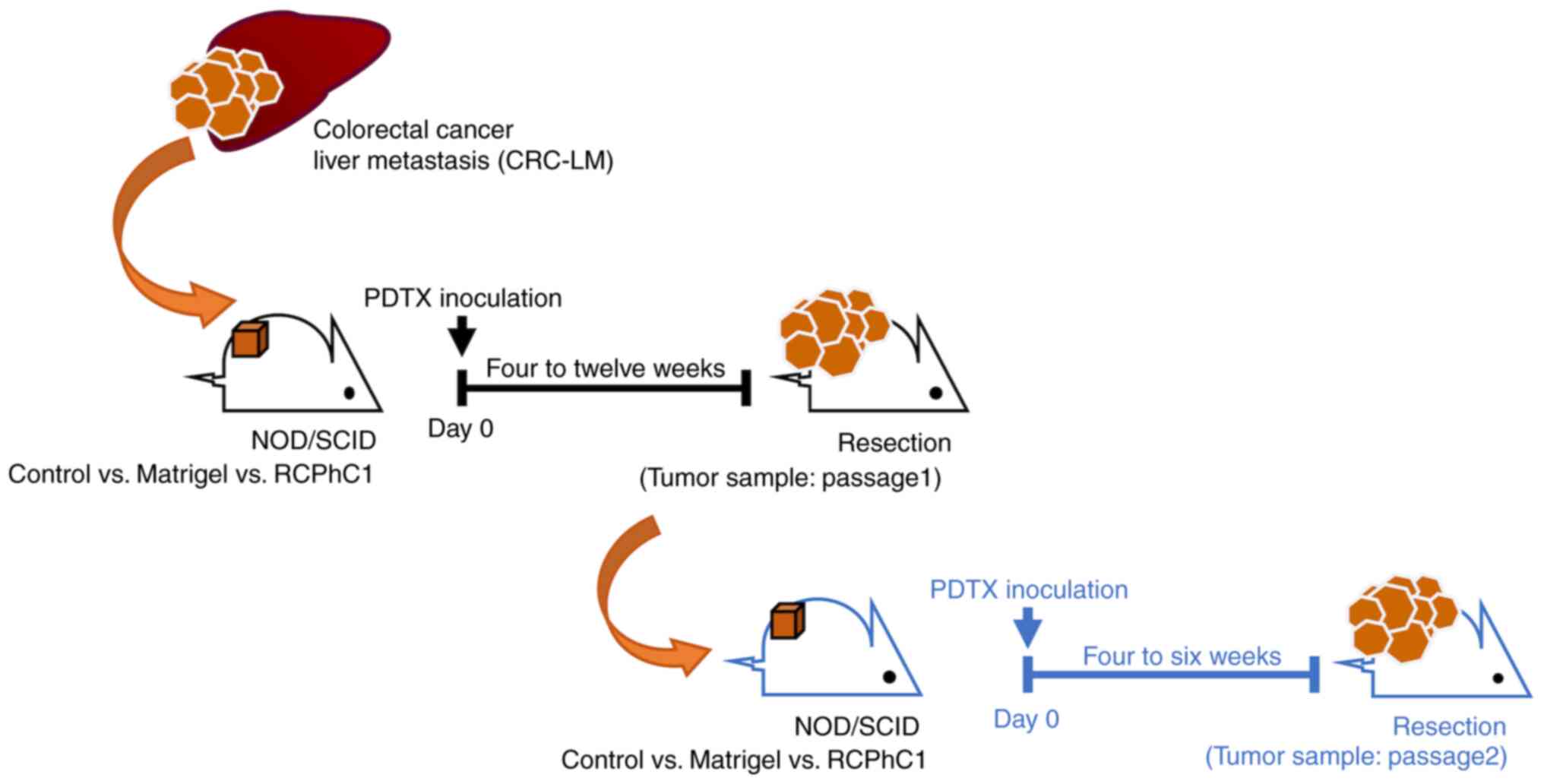
Consequently, we established PDTX models using
resected CRC-LM specimens and compared the number of tumor stromal
human and mouse cells using control (no scaffold), Matrigel, and
RChC1 as scaffold material for resected and post-transplant tumors
(Passage 1 and Passage 2). Lamin B, a major component of most
mammalian cells, predominantly localizes at the nuclear periphery
(15). This protein is highly
conserved across species, including humans and mice.
Immunohistochemical staining of Lamin B1 has been reported as a
valuable tool for distinguishing between human and mouse cells
(16). Given its conserved nature,
Lamin B1 is a characteristic marker that provides evidence of
cellular origin between human and mouse cells. Therefore, we
selected the immunohistochemical detected of the Lamin B to
identify human-derived stromal cells in the xenograft tumors,
mainly including mouse stromal components.
Materials and methods
Patient information
Three patients with colorectal cancer liver
metastasis (CRC-LM) who had undergone surgery at Gunma University
Hospital between 2019 and 2021 were included in the study. None of
the patients had received preoperative radiation or chemotherapy.
Three patients with colorectal cancer liver metastasis (CRC-LM) who
had undergone surgery at Gunma University Hospital between 2019 and
2021 were included in the study. The patients consisted of a
37-year-old male (PDTX-9), a 72-year-old female (PDTX-10), and a
66-year-old male (PDTX-12). All patients provided written informed
consent for our study in accordance with institutional guidelines
and the principles of the Declaration of Helsinki, and the study
was approved by the Institutional Review Board for Clinical
Research of Gunma University Hospital (approval no.
HS2018-261).
Preparation of hydrogels composed of
animal-component-free RCPhC1
The solution of RCPhC1 at a concentration of 46.5
mg/ml and the solution of Tetra-PEG-OSu at 37.5 mg/ml were
separately prepared using 50 mM phosphate-buffered saline (PBS).
The preparation involved the following steps: 300 microliters of
the filtered PEG solution were added to 300 microliters of the
RCPhC1 solution in a 1:1 ratio. The combined solution was then
vortexed for about ten seconds. Finally, the RCPhC1 hydrogel
mixture was left to undergo gelation by heating with a water bath
at 37˚C to facilitate the crosslinking reaction.
Patient-derived human tumor xenograft
models of colorectal liver metastasis
All animal experiments were approved by the
Institutional Animal Care and Ethics Committee of Gunma University
(approval no. 18-024). Male NOD-SCID mice aged four weeks (CLEA
Japan Int., Tokyo, Japan) were purchased and housed in the animal
facility of Gunma University under standard conditions (12-h
light/dark cycle, food and water provided ad libitum). All
animal experiments complied with Gunma University guidelines for
the care and use of laboratory animals. Mice were monitored once or
twice a week for clinical signs of morbidity, including but not
limited to rapid weight loss, severe lethargy, difficulty
breathing, impaired mobility, or tumor burden exceeding 2,000
mm3. One PDX12 passage2 control mouse reached the
endpoint and was sacrificed. Animals reaching these endpoints were
humanely euthanized using a cervical dislocation under deep
anesthesia (5% isoflurane) in accordance with ethical
guidelines.
Fresh CRC-LM samples were obtained from patients at
the time of surgery at Gunma University Hospital. The tissues were
immediately transferred to ice in a DMEM medium (FUJIFILM Wako Pure
Chemical Corporation, Osaka, Japan), supplemented with 1%
penicillin/streptomycin (FUJIFILM Wako Pure Chemical Corporation)
and amphotericin B (Gibco, Invitrogen, Paisley, UK). The tissues
were cut into pieces of 5x5x5 mm using sterile surgical instruments
and quickly grafted subcutaneously into the flank of NOD-SCID mice
(Passage 1 generation: P1), embedding without scaffold or with 200
µl Matrigel™ (Corning, NY, USA) or RCPhC1. Matrigel was
used at 2 dilutions in PBS cell suspension and later implanted with
small tumor pieces in the mice subcutaneously. Upon growth, tumor
size (mm) was measured once or twice a week in two dimensions using
a slide caliper. Tumor volume was calculated using the equation
(width x width x length)/2. When each xenografted tumor volume grew
to >2,000 mm3, tumors were harvested and put in
transportation media for either direct propagation into a further
generation (Passage 2 generation: P2) or father analyses.
Multicolor immunofluorescence
staining
Paraffin-embedded blocks were cut into four µm-thick
sections and mounted on glass slides. Sections were deparaffinized
in xylene and dehydrated in alcohol. After rehydration through a
graded series of ethanol treatments, antigen retrieval was
performed using an Immunosaver (Nisshin EM, Tokyo, Japan) at
98-100˚C for 45 min. The endogenous peroxidase activity was
inhibited by incubation with 0.3%
H2O2/methanol for 30 min at room temperature.
Nonspecific binding sites were blocked by incubation with Protein
Block Serum Free Reagent for 30 min, and the sections were
incubated overnight at 4˚C with the primary antibodies against
mouse Lamin B1 (Sartorius Stedim Biotech, Gottingen, Germany,
HS-404 003, rabbit polyclonal antibody, 1:800 dilution), human
Lamin B1 (Sartorius Stedim Biotech, HS-404 013, rabbit polyclonal
antibody, 1:400 dilution), and human/mouse α-SMA (Sigma Aldrich,
MO, USA, A2547, mouse mAb, 1:800 dilution). Multiplex covalent
labeling (human Lamin B1: Opal 690 Fluorophore, OP-001006) (mouse
Lamin B1 and α-SMA: Opal 520 Fluorophore, OP-001001) with tyramide
signal amplification (Akoya Biosciences, MA, USA) was performed
according to the manufacturer's protocol. All sections were
counterstained with DAPI, and after washing in PBS, the sections
were mounted onto glass slides with a SlowFade™ Gold
Antifade mountant (Thermo Fisher Scientific, Waltham, USA). All
slides were examined under an All-in-One BZ-X710 fluorescence
microscope (KEYENCE Corporation, Osaka, Japan).
Evaluation of stromal cell origin in
PDTX tumors: human and mouse specific Lamin B staining
Human and mouse-specific Lamin B antibodies have
been found useful in identifying the cell species origin in the
histological samples (16). Eight
fields from each sample were taken under a 40x objective lens.
Human or mouse lamin B positive cells were manually counted from
each PDTX tissue. The total number of stromal cells counted in the
tumor tissue of each PDTX was calculated.
Cell lines
The mouse NIH-3T3 fibroblast cell was purchased from
the JCRB Cell Bank (Osaka, Japan). The cell lines were cultured in
DMEM (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan)
supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin/streptomycin. All cells were maintained at 37˚C in an
atmosphere of humidified air with 5% CO2.
Invasion assays
The membrane of upper chamber (Corning Life
Sciences, Tewksbury, MA, USA) was coated with PBS (control), 100 µl
Matrigel (x5 PBS dilution) or 100 µl RCPhC1 hydrogels (x2.5 PBS
dilution), and 90 µl was aspirated. The upper chamber was then
solidified at 37˚C for 30 min. The gel-coated chamber (8.0 µm pore
size) with polyethylene terephthalate membranes were hydrated with
a serum-free medium at 37˚C for 30 min. After removing the
serum-free media, the upper chamber was set in the new well of the
24-well plate. NIH-3T3 cells (2x105 cells/well) were
seeded in the upper chamber with 500 µl of FBS-free medium. The
lower chamber was filled with 750 µl of complete medium with 20%
FBS, and the plate was then incubated at 37˚C in a humidified
atmosphere with 5% CO2. After 72 h of incubation, the
invaded cells were fixed and stained with Diff-Quik (Sysmex
Corporation, Kobe, Japan). After staining, the cells that had
invaded through the scaffold gels and pores in the lower membrane
were counted under a microscope. Ten randomly selected fields were
evaluated under an All-in-One BZ-X710 microscope (KEYENCE
Corporation, Osaka, Japan).
Statistical analyses
All statistical analyses were performed using
GraphPad Prism version 10.0 (GraphPad Software Inc., California,
USA). Data for stained cell number in three groups were analyzed
with Kruskal-Wallis test. When the results of the Kruskal-Wallis
test were significant, Steel-Dwass multiple comparison tests were
used to assess differences in stained cell number among each group.
Differences were considered significant at P<0.05.
Results
Human-derived cells were maintained in
PDTX tissues embedded in RCPhC1 hydrogels
In this study, we planned to transplant human CRC-LM
tissue into NOD-SCID mice to establish PDTX. We established and
passaged PDTX without scaffold or with Matrigel or RCPhC1 hydrogels
to compare the scaffold materials used for transplantation
(Fig. 1). The largest tumor
diameters and volumes in each PDTX model were observed as follows:
PDTX-9 passage 1 (20.5 mm, 1,835.1 mm³), PDTX-9 passage 2 (15.2 mm,
706.3 mm³), PDTX-10 passage 1 (17.2 mm, 1,513.7 mm³), PDTX-10
passage 2 (17.9 mm, 1,119.3 mm³), PDTX-12 passage 1 (23.4 mm,
1,858.4 mm³), and PDTX-12 passage 2 (22.7 mm, 2,236.5 mm³). The
RCPhC1 hydrogel scaffold did not increase PDTX tumor growth or
Ki67-positive tumor cells compared to the control and Matrigel
(Fig. S1A and B).
Using multicolor immunofluorescence analysis, we
evaluated the expression positivity of human Lamin B as red and
mouse Lamin B as green in resected CRC-LM tissues and PDTX tumors
(Passage 1 and Passage 2) (Fig.
2A). The number of human Lamin B-positive cells in RCPhC1
hydrogels was significantly higher than that in the controls and
Matrigel at both passage 1 and passage 2 PDTX tumors (Fig. 2B). However, the number of mouse
Lamin B-positive cells in RCPhC1 hydrogels was significantly lower
than that in controls at all passage 1 PDTX tumors and passage two
in PDTX-9 and ten tumors (Fig.
2B). The number of mouse Lamin B-positive cells in RCPhC1
hydrogels was significantly lower than that in Matrigel at passage
one in PDTX-10 and 12 tumors and passage 2 in PDTX-9 and 12 tumors
(Fig. 2B). These data indicated
that the RCPhC1 scaffold preserved the human-derived cells in the
PDTX tumor microenvironments during the passage process.
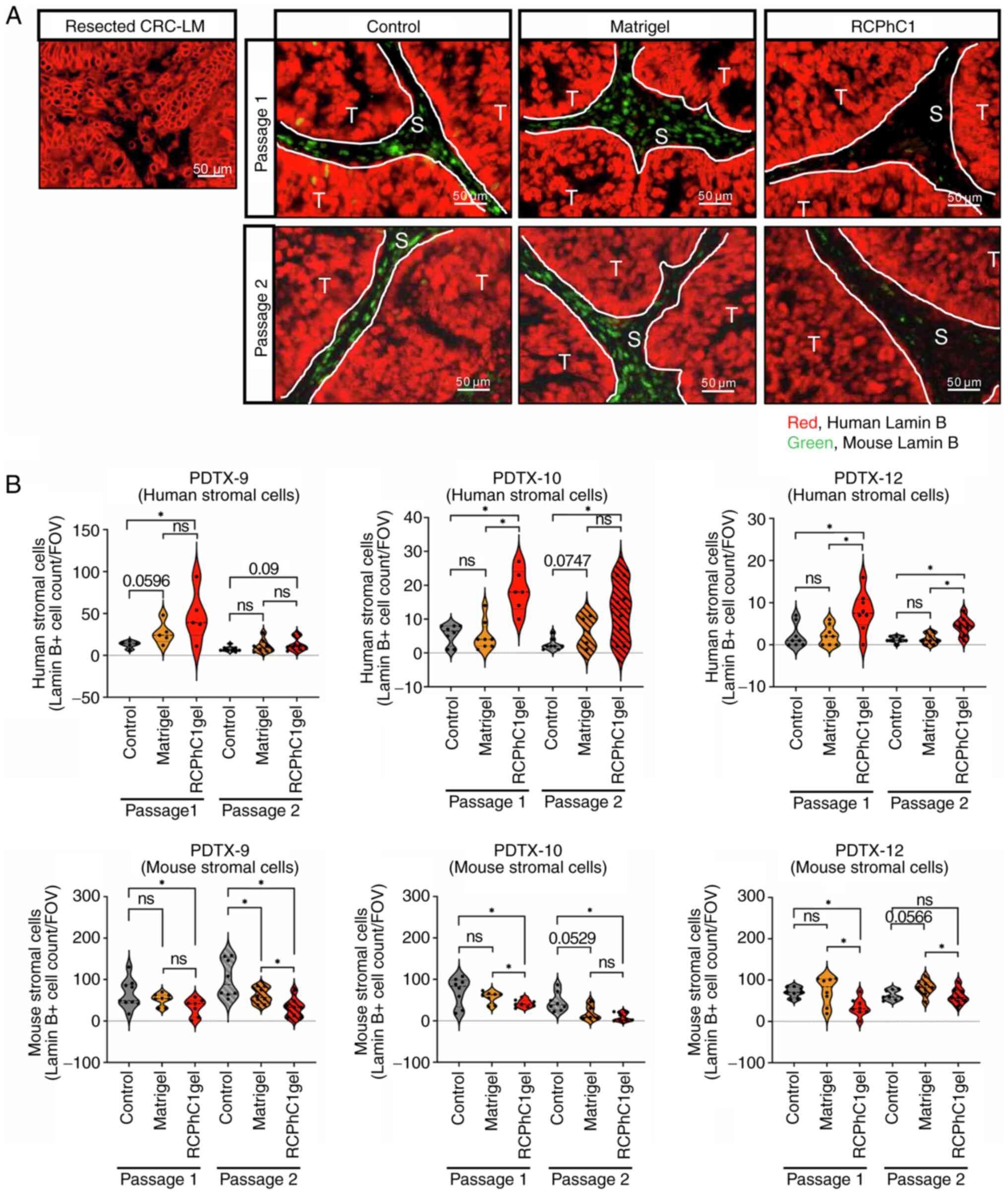 | Figure 2Immunofluorescence staining pattern of
human or mouse-specific Lamin B in PDTX tumors. (A)
Immunofluorescence analysis of human Lamin B (red) and mouse Lamin
B (green) in the control, Matrigel, and RCPhC1 hydrogels group from
PDTX-9 tumor tissues (passage1 and passage2). Scale bar, 50 µm
(original magnification, x20). Left upper panel: human CRC-LM
sample was used as positive control for human Lamin B (red) and
negative control for mouse Lamin B (green). (B) Count data of mouse
Lamin B and human Lamin B positive cells in the control (gray),
Matrigel (yellow) and RCPhC1 hydrogels (red) group from tumor
tissues of PDTX-9, 10, and 12 (passage1 and passage2). The black
dots in the violin plots indicate mean cell number in groups.
*P<0.05. ns, not significant. Lamin B-positive cell
counts were analyzed independently in the control, Matrigel, and
RCPhC1 gel groups of each passage using the Kruskal-Wallis test.
When the Kruskal-Wallis test results were significant, Steel-Dwass
multiple comparison tests were used to assess differences in each
group. CRC-LM, colorectal cancer liver metastases; PDTX,
patient-derived tumor xenograft; RCPhC1, recombinant protein based
on human collagen type I; T, tumor cells; S, stromal cells; FOV,
field of view. |
Additionally, we did not observe the distant
metastasis in our CRC-LM subcutaneous PDTX models. A previous study
reported that CRC PDTX tumors transplanted subcutaneously do not
form distant metastases, however, do form metastases when
transplanted into the colon (17).
On the other hand, it has been reported that PDTX tumors of lung
cancer in this study form distant metastases (18). The difference in the metastatic
potential of such PDTX tumors may depend on not only the PDTX
injection site but also tumor types.
RCPhC1 hydrogel preserved
human-derived fibroblasts within PDTX tumors during the passage
process
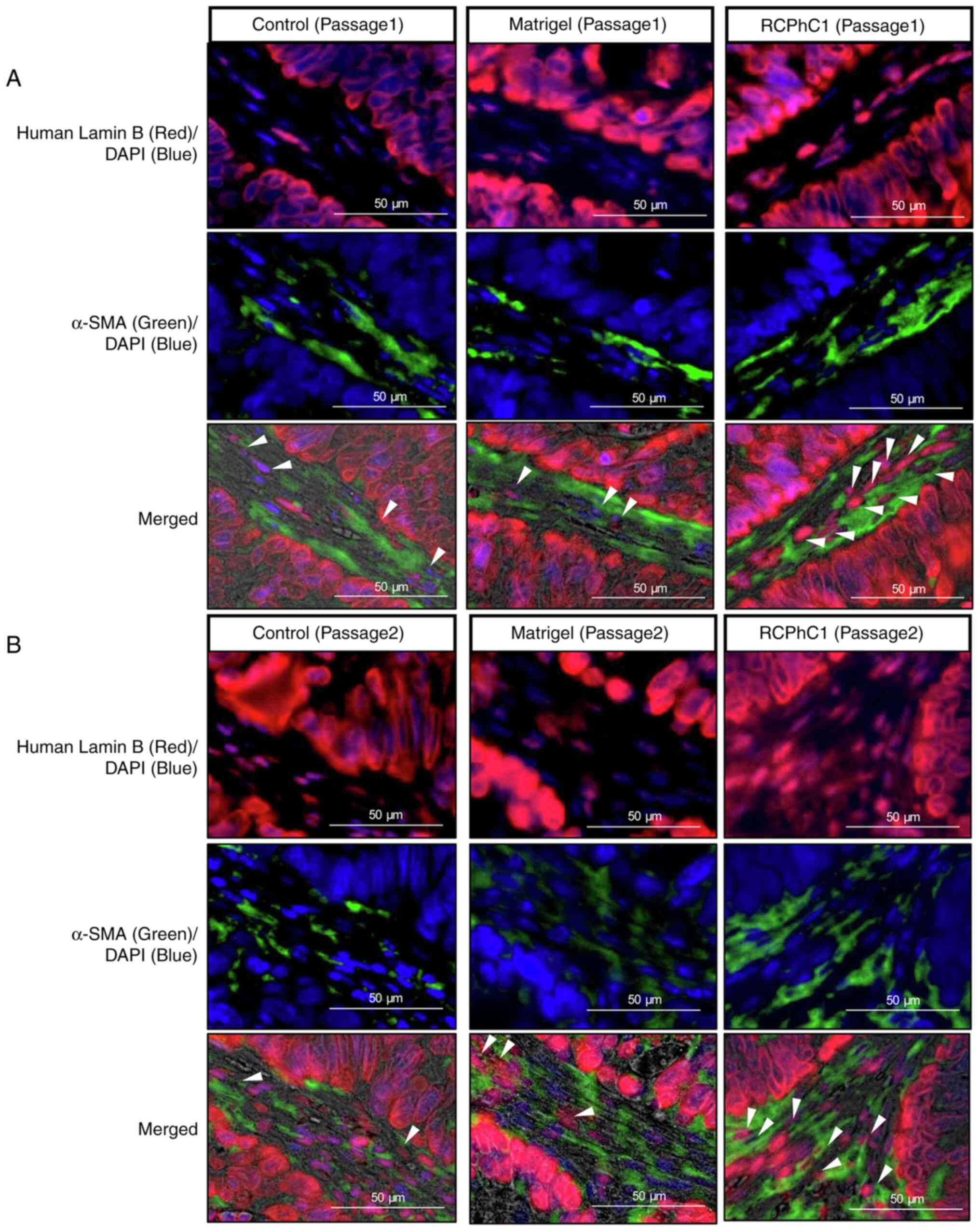
Consequently, human Lamin B and α-SMA
double-positive cells were higher in RCPhC1 hydrogels than the
control and Matrigel, suggesting that human stromal cells were
maintained in PDTX tissue embedded in RCPhC1 hydrogels (Fig. 3A). There was a similar trend in
passage 2 of all PDTX cases (Fig.
3B).
RCPhC1 hydrogels inhibit the invasion
of mouse fibroblast cells
An in vitro invasion assay was performed to
test whether the RCPhC1 hydrogel affected the invasion of mouse
fibroblasts into PDTX tumors. As a result, the number of murine
fibroblast cell line NIH-3T3 cells invading the RCPhC1
hydrogel-coated chamber was significantly lower than that in the
control and Matrigel-coated ones (Fig.
4), suggesting that the RCPhC1 hydrogel can prevent murine
fibroblast invasion into the PDTX tumor microenvironments.
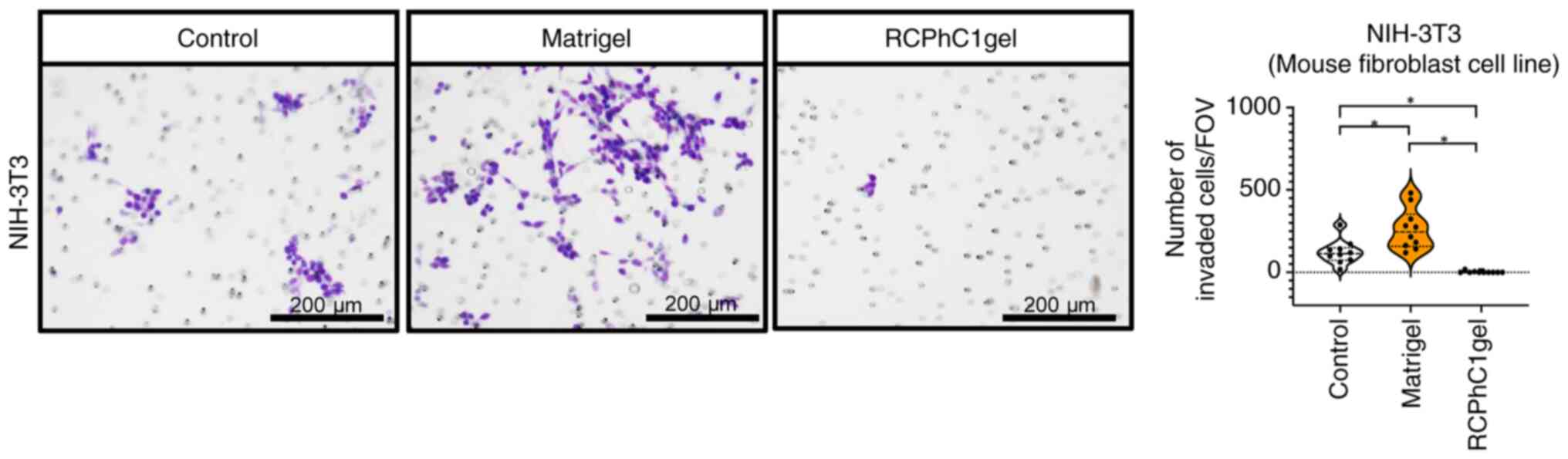 | Figure 4Invasion ability of murine fibroblast
cell line NIH-3T3 against the Matrigel and RCPhC1 hydrogels. Left
panel: invading cell images of NIH-3T3 cells in the control,
Matrigel, and RCPhC1 hydrogels group. Cells were cultured in the
chamber inserts coated with Matrigel or RCPhC1 hydrogels for 72 h
in a serum-free DMEM medium. Cells that invaded the scaffold gel
and migrated into the bottom chamber side with 20% FBS medium were
stained using a Diff-Quik staining kit. Control group indicates the
camber inserts without scaffold gel coating. Scale bar, 200 µm
(original magnification, x10). Right panel: invaded cell counts of
10 fields in the control, Matrigel, and RCPhC1 hydrogels group.
*P<0.05. Invaded cell numbers were analyzed
independently in the control, Matrigel, and RCPhC1 gel groups using
the Kruskal-Wallis test. When the Kruskal-Wallis test was
significant, Steel-Dwass multiple comparison tests were used to
assess differences in each group. RCPhC1, recombinant protein based
on human collagen type I; FOV, field of view. |
Discussion
In this study, we established a PDTX mouse model
using CRC-LM resection specimens from three cases and evaluated the
scaffold-dependent maintenance function of human-derived cells
during the passage process. The results show that patient-derived
tumor tissues embedded with RCPhC1 hydrogels had significantly more
human Lamin B-positive cells and fewer mouse Lamin B cells than
PDTX tumors embedded without scaffolds or with Matrigel. The human
Lamin B-positive cells in PDTX tumors with RCPhC1 hydrogels were
recognized as fibroblasts. Furthermore, in vitro invasion
assays showed that RCPhC1 hydrogels significantly inhibited the
invasion of mouse fibroblast cell lines compared to Matrigel.
This study showed that RCPhC1 hydrogels had an
advantage in maintaining human tumor stromal fibroblasts in the
PDTX model compared to the control and Matrigel groups. Why human
immune and vascular endothelial cells could not be maintained even
with RCPhC1 hydrogels should be considered (Fig. S2). Ghanekar et al (19) attempted direct immunostaining of
human CD31 in three human tumor xenografts, but did not detect
human CD31, leading the authors to conclude that the endothelial
cells in human HCC xenografts were of mouse rather than human
origin. For this reason, we postulated two mechanisms for entering
mouse-derived cells into PDTX tumors: a direct migration/invasion
route from the surroundings and a route via tumor neovasculature
constructed after transplantation. We suggest that immune cells and
vascular endothelial cells were not affected by the scaffold
material because they are shared from the blood via the
neovasculature established by the PDTX tumor (tumors that cannot
construct neovasculature cannot be viable). We also speculate that
migratory fibroblasts infiltrate directly into PDTX tumors from the
periphery of PDTX tumors, rapidly replacing human stromal
fibroblasts with mouse fibroblasts in the control and Matrigel
groups; however, this phenomenon may have been inhibited in the
RCPhC1 group. In fact, in vitro experiments in this study
confirmed that RCPhC1 hydrogels inhibited the invasion of mouse
fibroblasts better than Matrigel. Therefore, we hypothesize that
the RCPhC1 hydrogel physically inhibits the invasion and migration
of mouse fibroblasts. Furthermore, the various factors in the
Matrigel may promote the invasion of host mouse fibroblasts into
the tumors (9). On the other hand,
such stromal fibroblasts in tumor tissues are called CAFs and have
been reported to be associated with the drug sensitivity of tumor
tissues. CAFs also attract attention as a cancer therapeutic target
(20-22).
The RCPhC1 hydrogel, which can maintain human stromal-derived CAFs,
is expected to serve as a scaffold material during passaging in
PDTX models to evaluate the efficacy not only of existing
anti-cancer drug sensitivity but also of future CAF-targeted
therapies.
Our PDTX model used immunocompromised mice, which
makes it impossible to assess tumor-immune cell interactions. To
solve this problem, the humanized PDTX model, in which human
hematopoietic stem cells are transplanted into immunodeficient
mice, has attracted attention recently (1,3,4,6).
This humanized PDTX model mimics the human immune system and
enables the evaluation of the drug efficacy of immunotherapy, which
cannot be evaluated in the conventional xenograft model. It is
expected that a new PDTX model that more closely resembles the
human tumor microenvironment may be established using new scaffold
RCPhC1 hydrogels, which can maintain tumor stroma, in combination
with a humanized PDTX model that maintains human immune cells.
This study had some limitations. Firstly, it
involved only three cases of CRC-LM, and whether the findings can
be generalized to carcinomas other than CRC is unclear. Therefore,
the potential of RCPhC1 hydrogels as a new scaffold material should
be validated in PDTX models using extended sample size and other
tumor types. Secondary, The PTX model using immunodeficient mice
does not accurately reflect the relationship between tumors and
immune cells in the microenvironment of human tumor tissue.
Thirdly, comparative studies should be conducted to verify whether
the PDTX model using RCPhC1 hydrogels or the Matrigel model
accurately reflects the drug sensitivity of patients with cancer.
Fourthly, RCPhC1 hydrogels physically palpate the gel itself even
before PDTX tumor regrowth begins (Fig. S1A). This may be problematic for
short-term drug evaluation and for recognizing mice whose
transplanted tissue has failed to grow. Finally, one of the
advantages of RCPhC1 hydrogel is that the gel eliminates the risk
of inducing an inflammatory or immune response in the transplanted
tumor due to the presence of animal components. However, in this
study, we did not directly compare RCPhC1 and other animal-free
scaffolds to evaluate its full potential. Further studies are
needed to assess the performance of RCPhC1 hydrogel in contracts
with other non-animal scaffolds.
This study investigated RCPhC1 hydrogels as new
scaffold materials for tumor engraftment in PDTX mouse models. In
PDTX tissues embedded with RCPhC1 hydrogels, the replacement of
human-derived fibroblasts with mouse-derived fibroblasts was
suppressed compared to that in the controls. Therefore, RCPhC1
hydrogels may be a promising experimental tool for maintaining the
tumor microenvironment in the PDTX model.
Supplementary Material
Tumor growth and proliferation potency
in PDTX tumors embedded by RCPhC1 hydrogels. (A) Average growth
curves of tumor volume in the control (gray line), Matrigel (yellow
line), and RCPhC1 hydrogels (red line) group from tumor tissues of
PDTX-9, 10, and 12 (passage 1 and passage 2). (B) Upper panel:
immunohistochemical staining of human Ki67 in control, Matrigel,
and RCPhC1 hydrogels groups from PDTX-9 tumor tissues (passage 1).
Scale bar, 50 μm. (original magnif ication, x20). Lower
panel: Ki67 positive cell number of eight fields in control,
Matrigel, and RCPhC1 hydrogels groups from PDTX-9 tumor tissues
(passage 1 and passage 2). Human Ki67 positive cell numbers were
analyzed independently in the control, Matrigel, and RCPhC1 gel
groups of each passage using the Kruskal-Wallis test. When the
Kruskal-Wallis test results were significant, Steel-Dwass multiple
comparison tests were used to assess differences in each group. ns,
not significant. Sections were prepared, and endogenous peroxidase
activity and non-specific binding sites were blocked as described
above. Sections from the clinical samples were incubated overnight
at 4˚C with primary antibodies against mouse anti-Human Ki67
(Abcam, ab833, rabbit polyclonal antibody, 1:50 dilution).
Histofine Simple Stain MAX-PO (Multi) Kit (Nichirei, Tokyo, Japan)
was used with the secondary antibody at room temperature for 30
min. The chromogen 3,3'-diaminobenzidine tetrahydrochloride was
applied as a 0.02% solution containing 0.005% H2O2 in 50 mM
ammonium acetate-citrate acid buffer (pH 6.0). Nuclear
counterstaining was performed using Mayer's hematoxylin solution.
Analysis of at least five fields for the cells staining positive
for human Ki67 was performed. The average Ki67 positive cell number
of 1 microscope field of view was used for analysis of microscope
field number. PDTX, patient-derived tumor xenograft; RCPhC1,
recombinant protein based on human collagen type I; FOV, field of
view.
Expression pattern of blood cell and
endothelial markers in tumor stroma of PDTX tumors.
Immunohistochemical staining of human CD45, human CD31, and mouse
Cd31 in the control, Matrigel, and RCPhC1 hydrogel group from
PDTX-9 tumor tissues (passage1). Scale bar, 50 μm. (original
magnification, x20). Sections were prepared and endogenous
peroxidase activity and non-specific binding sites were blocked as
described above. Sections from clinical samples were incubated
overnight at 4˚C with primary antibodies against anti-human CD45
(Ventana, 760-2505, mouse monoclonal antibody, ready to use),
anti-human CD31 (Dako, JC70A, mouse monoclonal antibody, 1:50
dilution), and anti-mouse Cd31 (Cell Signaling Technology, D8V9E,
rabbit monoclonal antibody, 1:100 dilution). Histofine Simple Stain
MAX-PO (Multi) Kit (Nichirei, Tokyo, Japan) was used with the
secondary antibody at room temperature for 30 min. Chromogen
3,3'-diaminobenzidine tetrahydrochloride was applied as a 0.02%
solution containing 0.005% H2O2 in 50 mM ammonium acetate-citrate
acid buffer (pH 6.0). Nuclear counterstaining was performed using
Mayer's hematoxylin solution. Resected human CRC-LM samples were
used as positive control samples for detecting human cells and
negative control for mouse cells. CRC-LM, colorectal cancer liver
metastases; PDTX, patient-derived tumor xenograft; RCPhC1,
recombinant protein based on human collagen type I.
Acknowledgements
The authors would like to thank Ms. Mariko Nakamura
and Ms. Kao Abe (Department of General Surgical Science, Graduate
School of Medicine, Gunma University) for their excellent technical
assistance, and Fujifilm Corporation for their technical assistance
in the preparation of RCPhC1 hydrogels. The authors thank Dr
Navchaa Gombodorj (Division of Integrated Oncology Research, Gunma
University Initiative for Advanced Research, Gunma University) and
Dr Tadashi Handa (Department of Medical Technology and Clinical
Engineering, Faculty of Medical Technology and Clinical
Engineering, Gunma University of Health and Welfare) for useful
discussions and excellent technical assistance for this study.
Funding
Funding: This study was supported by a grant from the Japan
Society for the Promotion of Science (grant no. 22H02912).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HO, RM and KA wrote the manuscript. RM, HO and BEO
collected and analyzed the image data. HO, GeD, TS, TO, RF, SK,
KHo, GaD, KHa, TYa, NI, TI, AW, NK, MT and TYo analyzed and
interpreted the data. HO, RM and TY drafted the manuscript. HO, TY,
KA, HS and KS conceptualized the study. All authors have read and
approved the final version of the manuscript. TYo and KA confirm
the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was performed in compliance with
the principles of The Declaration of Helsinki. All patients were
eligible for our study by the Institutional Review Board for
Clinical Research of Gunma University Hospital (approval no.
HS2018-261). All patients provided written informed consent for the
present study in accordance with institutional guidelines and the
principles of The Declaration of Helsinki. All animal experiments
were approved by the Institutional Animal Care and Ethics Committee
of Gunma University (approval no. 18-024).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Olson B, Li Y, Lin Y, Liu ET and Patnaik
A: Mouse models for cancer immunotherapy research. Cancer Discov.
8:1358–1365. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
DeRose YS, Wang G, Lin YC, Bernard PS,
Buys SS, Ebbert MT, Factor R, Matsen C, Milash BA, Nelson E, et al:
Tumor grafts derived from women with breast cancer authentically
reflect tumor pathology, growth, metastasis and disease outcomes.
Nat Med. 17:1514–1520. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Tischfield DJ, Ackerman D, Noji M, Chen
JX, Johnson O, Perkons NR, Nadolski GJ, Hunt SJ, Soulen MC, Furth
EE and Gade TP: Establishment of hepatocellular carcinoma
patient-derived xenografts from image-guided percutaneous biopsies.
Sci Rep. 9(10546)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Xiao M, Rebecca VW and Herlyn M: A
melanoma patient-derived xenograft model. J Vis Exp.
(10.3791/59508)2019.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Meehan GR, Scales HE, Osii R, De Niz M,
Lawton JC, Marti M, Garside P, Craig A and Brewer JM: Developing a
xenograft model of human vasculature in the mouse ear pinna. Sci
Rep. 10(2058)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bergamaschi A, Hjortland GO, Triulzi T,
Sørlie T, Johnsen H, Ree AH, Russnes HG, Tronnes S, Maelandsmo GM,
Fodstad O, et al: Molecular profiling and characterization of
luminal-like and basal-like in vivo breast cancer xenograft models.
Mol Oncol. 3:469–482. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Blomme A, Van Simaeys G, Doumont G,
Costanza B, Bellier J, Otaka Y, Sherer F, Lovinfosse P, Boutry S,
Palacios AP, et al: Murine stroma adopts a human-like metabolic
phenotype in the PDX model of colorectal cancer and liver
metastases. Oncogene. 37:1237–1250. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yoshida GJ: Applications of
patient-derived tumor xenograft models and tumor organoids. J
Hematol Oncol. 13(4)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Vukicevic S, Kleinman HK, Luyten FP,
Roberts AB, Roche NS and Reddi AH: Identification of multiple
active growth factors in basement membrane Matrigel suggests
caution in interpretation of cellular activity related to
extracellular matrix components. Exp Cell Res. 202:1–8.
1992.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Junttila MR and de Sauvage FJ: Influence
of tumour micro-environment heterogeneity on therapeutic response.
Nature. 501:346–354. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kobayashi H, Enomoto A, Woods SL, Burt AD,
Takahashi M and Worthley DL: Cancer-associated fibroblasts in
gastrointestinal cancer. Nat Rev Gastroenterol Hepatol. 16:282–295.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Melzer C, von der Ohe J, Lehnert H,
Ungefroren H and Hass R: Cancer stem cell niche models and
contribution by mesenchymal stroma/stem cells. Mol Cancer.
16(28)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nissen NI, Karsdal M and Willumsen N:
Collagens and cancer associated fibroblasts in the reactive stroma
and its relation to cancer biology. J Exp Clin Cancer Res.
38(115)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kamata H, Ashikari-Hada S, Mori Y, Azuma A
and Hata KI: Extemporaneous preparation of injectable and
enzymatically degradable 3D cell culture matrices from an
animal-component-free recombinant protein based on human collagen
type I. Macromol Rapid Commun. 40(e1900127)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kind J, Pagie L, Ortabozkoyun H, Boyle S,
de Vries SS, Janssen H, Amendola M, Nolen LD, Bickmore WA and van
Steensel B: Single-cell dynamics of genome-nuclear lamina
interactions. Cell. 153:178–192. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nomura M, George J, Hashizume C, Saito T,
Ueda Y, Ishigaki Y, Tsuchishima M and Tsutsumi M: Surgical
implantation of human adipose derived stem cells attenuates
experimentally induced hepatic fibrosis in rats. Mol Med.
28(143)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang Y, Lee SH, Wang C, Gao Y, Li J and
Xu W: Establishing metastatic patient-derived xenograft model for
colorectal cancer. Jpn J Clin Oncol. 50:1108–1116. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yang X and Meng G: Establishment of a
non-small-cell lung cancer-liver metastasis patient-derived tumor
xenograft model for the evaluation of patient-tailored
chemotherapy. Biosci Rep. 39(BSR20182082)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ghanekar A, Ahmed S, Chen K and Adeyi O:
Endothelial cells do not arise from tumor-initiating cells in human
hepatocellular carcinoma. BMC Cancer. 13(485)2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
De Jaeghere EA, Denys HG and De Wever O:
Fibroblasts Fuel immune escape in the tumor microenvironment.
Trends Cancer. 5:704–723. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen Y, McAndrews KM and Kalluri R:
Clinical and therapeutic relevance of cancer-associated
fibroblasts. Nat Rev Clin Oncol. 18:792–804. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sahai E, Astsaturov I, Cukierman E,
DeNardo DG, Egeblad M, Evans RM, Fearon D, Greten FR, Hingorani SR,
Hunter T, et al: A framework for advancing our understanding of
cancer-associated fibroblasts. Nat Rev Cancer. 20:174–186.
2020.PubMed/NCBI View Article : Google Scholar
|