Introduction
Periodontal disease is one of the most common oral
disorders caused by an imbalance between dysbiosis of dental plaque
biofilm and host defense (1,2).
Chronic inflammatory responses induced by periodontal pathogens
lead to the destruction of gingiva and alveolar bone (3), thereby increasing the risk for tooth
loss in patients with periodontal diseases, which subsequently
impairs mastication and nutrient intake (4). Furthermore, a recent study using the
dental data repository has reported that patients with periodontal
diseases exhibit a higher risk for systematic disorders such as
bronchitis, diabetes, and hypertension (5). Therefore, preventing periodontal
disease is not only crucial for addressing oral health issues but
also for maintaining systemic quality of life and health.
Antimicrobial peptides are ubiquitous host defense
substances against pathogenic infection, and possess a broad
antimicrobial spectrum (6). In
addition, antimicrobial peptides are important in diverse functions
such as neutralization of virulence factors derived from pathogens
and immunomodulation (6). In the
oral environment, antimicrobial peptides are secreted by oral
epithelial cells, salivary glands, and neutrophils (7,8). In
particular, epithelial antimicrobial peptides, such as β-defensin
family and cathelicidin, play the pivotal role in the first line of
host defense, as gingival epithelium is in close proximity to
dental plaque that resides around the tooth and root surfaces
(9). Therefore, promoting the
production of endogenous antimicrobial peptides to achieve enhanced
antimicrobial defense is considered to be one of the valuable
strategies for combating periodontal infections.
Aged garlic extract (AGE) is one of the garlic
(Allium sativum L.)-derived products manufactured by soaking
garlic in a water-ethanol mixture for more than 10 months (10). Clinical trials on patients with
mild to moderate periodontitis reported that daily intake of AGE at
2,400 mg/day for 4 and 18 months improved gingival bleeding index
(11) and probing pocket depth
(12). Furthermore, we have shown
that AGE and its sulfur constituents suppressed the tumor necrosis
factor-α-induced intracellular adhesion molecule-1 and
interleukin-6 in human gingival epithelial cell line Ca9-22 cells
(13). In addition, it was found
that S-1-propenyl-L-cysteine, a major sulfur bioactive
compound in AGE, inhibited the Porphyromonas
gingivalis-derived lipopolysaccharide-induced matrix
metalloproteinase-1 in human gingival fibroblast cell line HGF-1
cells (14). More recently, it was
reported that feeding of AGE (18 mg/kg/day) to Beagle dogs with
mild gingivitis for 8 weeks resulted in the improvement of gingival
index score and halitosis, that is possibly due to an increase in
salivary antimicrobial peptide, cathelicidin (15). Taken together, these results
suggest that the therapeutic effects of AGE on periodontal disease
involve not only its anti-inflammatory action but also
antimicrobial action mediated by the production of antimicrobial
peptides. In this study, we investigated whether AGE influences the
production of antimicrobial peptides in mouse gingiva, which would
help prevent the onset or progression of periodontal disease.
Materials and methods
Reagents
All chemicals were purchased from FUJIFILM Wako Pure
Chemical Corporation unless stated otherwise. A canonical Wnt
signaling pathway specific inhibitor LF3 and a glycogen synthase
kinase-3 (GSK-3) specific inhibitor 6-bromoindirubin-3'-oxime (BIO)
were from Cayman Chemical (Ann Arbor). For Western blotting, the
primary antibodies against β-defensin 4 (BS60360, Bioworld
Technology, St. Louis Park, MN, USA) and β-actin (PM053-7, MBL Life
Science), and the secondary antibodies, horse radish peroxidase
(HRP)-conjugated against mouse (#7076S, Cell Signaling Technology)
and rabbit (#7074S, Cell Signaling Technology) were used. A Mouse
Beta-defensin 4 ELISA kit was obtained from BT LAB.
Preparation of AGE
AGE powder was prepared as previously described
(16). The powder was dissolved in
deionized water (DW) to obtain the AGE stock solution (20 mg/ml).
The stock solution was stored at -20˚C until use.
Animals and treatment
Five weeks old male ddY mice were purchased from
Japan SLC Inc. (Hamamatsu, Shizuoka, Japan) and kept at 23±3˚C and
50±10% humidity, under a 12 h light-dark cycle in the animal
facility at Wakunaga Pharmaceutical Co., Ltd. Food (CE-2; CLEA
Japan Inc.) and water were provided ad libitum. Mice were
allowed to acclimate for 1 week, and then at 6 weeks of age,
randomly divided into the DW-treated (control) group and the
AGE-treated group. The control and AGE-treated groups were given DW
and AGE, respectively, by oral gavage administration (10 ml/kg body
weight) using a disposable feeding needle (Fuchigami). We used the
dose 2 g/kg/day of AGE that has been shown to be safe and
sufficiently effective in our previous studies (17-19).
Gingival tissues were dissected out after the mice were euthanized
by exsanguination under anesthesia with 2.5% isoflurane for
induction and maintenance. Animal experiments were approved by the
Wakunaga Pharmaceutical Company Institutional Animal Care and Use
Committee (approval no. 360).
Cell culture
Mouse gingival epithelial GE1 cells (RCB1709, RIKEN
Bioresource Research Center) were cultured in Minimum Essential
Medium alpha (MEMα) with 10% fetal bovine serum,
penicillin-streptomycin (x1), and 10 ng/ml recombinant murine
epidermal growth factor (PeproTech) at 37˚C and 5% CO2
in a humidified atmosphere. GE1 cells were seeded at a density of
13,000 cells/cm2 and grown to confluent monolayer. After
confluency, the culture medium was replaced by fresh MEMα with 1%
fetal bovine serum, penicillin-streptomycin (x1), and 10 ng/ml
recombinant murine epidermal growth factor (20).
Reverse transcription-quantitative PCR
analysis
Total RNA was extracted from mouse gingiva with acid
guanidinium thiocyanate-phenol-chloroform extraction using RNAiso
plus (Takara Bio Inc.). Complementary DNA was synthesized from
total RNA using a PrimeScript RT reagent kit with a genomic DNA
Eraser (RR047A, Takara Bio Inc.), and amplified on a CFX96
real-time PCR detection system (Bio-Rad Laboratories) with KAPA
SYBR fast qPCR master mix (KAPA Biosystems). The PCR primers
(Integrated DNA Technologies, Inc.) are listed in Table I. The fold change in the mRNA level
relative to β-actin was calculated based on the ΔΔCt method
(21).
 | Table IPrimer sequences used for reverse
transcription-quantitative PCR. |
Table I
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene name | Sequence
(5'-3') |
|---|
| Defb1 | Forward: ATT CAA
GCC TCA TCT GTC AGC C |
| | Reverse: TTG TGA
GAA TGC CAA CAC CTG C |
| Defb4 | Forward: GGT GCT
GCT GTC TCC ACT TG |
| | Reverse: TTC ATC
TTG CTG GTT CTT CGT CT |
| Defb14 | Forward: GTA TTC
CTC ATC TTG TTC TTG |
| | Reverse: AAG TAC
AGC ACA CCG GCC AC |
| Cramp | Forward: TGT GAG
GTT CCG AGT GAA GG |
| | Reverse: TGT GCA
CCA GGC TCG TTA C |
| Gapdh | Forward: CCA GCA
AGG ACA CTG AGC AA |
| | Reverse: ATT CAA
GAG AGT AGG GAG GGC T |
Western blotting
Gingival tissues and GE1 cells were lysed in a
radio-immunoprecipitation assay buffer (Merck) with 1X PhosSTOP™
(Sigma-Aldrich) and 1X cOmplete™ protease inhibitor cocktail
(Roche, Basel, Switzerland) to obtain total protein. For nuclear
protein extraction, two extraction buffers were used as follows;
Buffer A, 10 mM HEPES (pH 7.9), 10 mM KCl, 0.1 mM
ethylenediaminetetraacetic acid (EDTA), 1 mM dithiothreitol (DTT),
1X PhosSTOP™ and 1X cOmplete™ protease inhibitor cocktail; Buffer
B, 20 mM HEPES, 400 mM NaCl, 1 mM EDTA, 1 mM DTT, 1X PhosSTOP™ and
1X cOmplete™ protease inhibitor cocktail. GE1 cells were incubated
in Buffer A on ice for 15 min, and then added 1/10 volume of 10%
Nonidet P-40 substitute (Nacalai Tesque, Kyoto, Japan). Lysates
were centrifuged at 20,000 x g for 5 min at 4˚C. The supernatant
was removed, and the resultant pellet was washed twice with Buffer
A. The washed pellet was resuspended in Buffer B, incubated on ice
for 30 min, and subsequently centrifuged at 20,000 x g for 30 min
at 4˚C. After centrifugation, the supernatant was used as the
nuclear protein fraction. Each extracted protein was diluted to a
protein concentration of 1 mg/ml with 4X sample buffer containing
250 mM Tris-HCl (pH 6.8), 8% sodium dodecyl sulfate, 40% glycerol,
2% bromophenol blue, and 400 mM DTT, and then boiled at 98˚C for 5
min. Protein extracts (20 µg) were separated on 4-20% Mini-PROTEAN
TGX™ Gel (Bio-Rad Laboratories) and transferred onto Trans-Blot
Turbo nitrocellulose membranes (Bio-Rad Laboratories) using a
Trans-Blot Turbo Transfer System (Bio-Rad Laboratories). The
membranes were treated with the primary antibody against β-defensin
4 (1:500) or β-actin (1:2,000) overnight at 4˚C, and then the
HRP-conjugated secondary antibody (1:20,000) for 1 h at room
temperature. Immunoreactive proteins were visualized with Armasham
ECL Prime peroxidase solution (Cytiva) or ImmunoStar™ LD by using
ChemiDoc™ MP (Bio-Rad Laboratories). The density of each
immunoreactive band was analyzed using Band/Peak Quantification
Tool in ImageJ 1.54i (22).
Enzyme-linked immunosorbent assay
(ELISA)
Quantification of β-defensin 4 in culture medium was
performed using a Mouse Beta-defensin 4 ELISA kit according to the
manufacturer's protocol. Culture medium was collected and
centrifuged at 13,200 x g for 10 min at 4˚C. The supernatants were
stored at -80˚C until use. The colorimetric absorbance was measured
at a test wavelength of 450 nm using Multiskan GO Microplate
Spectrophotometer (Thermo Scientific).
Proteomics analysis
The lysis of gingival tissues resected from mice
after 6 h treatment with AGE was performed by incubating with
tris(2-carboxyethyl)phosphine hydrochloride for 1 h at 55˚C,
alkylating with iodoacetamide for 30 min at room temperature, and
digesting overnight with Pierce™ Trypsin Protease MS-Grade (Thermo
Fisher Scientific) at a trypsin-protein ratio of 1:50 (w/w).
Phosphorylated peptides were enriched using Titansphere Phos-TiO
Tip (GL Sciences Inc.). Residual detergents and salts in the
samples were removed using a HiPPR Detergent Removal Spin Column
Kit (Thermo Fisher Scientific) and GL-Tip SDB columns (GL Sciences
Inc.), respectively. The clean-up peptides were analyzed on a
Q-Exactive Mass Spectrometer equipped with a Vanquish Neo LC System
(Thermo Fisher Scientific). Phosphorylated peptides were identified
using the Proteome Discoverer software (Thermo Fisher Scientific),
and then compared with the Uniport curated M. musculus
proteome database (release 2023.6). As a result, only ‘Annotated
Sequence’ passing a cut-off of 5% false discovery rate (FDR
Confidence: ‘Medium’) was considered for further analysis.
Functional enrichment analysis was performed by Gene Ontology (GO)
biological process database using GeneCodis 4(23).
Statistical analysis
Data analyses and graphical visualization were
performed using KyPlot Free ver. 6.0.2 (KyensLab Inc.). Data are
expressed as mean ± standard deviation. Unpaired Student's t test,
Welch's t test, Mann-Whitney U test (for 2 groups) or one-way
analysis of variance (for more than 3 groups), followed by post hoc
Dunnett's test or Holm-Bonferroni test, were used to assess
statistical significance. Differences at P<0.05 were considered
statistically significant.
Results
Effect of daily administration of AGE
on the mRNA and protein level of β-defensin 4 in mouse gingiva
We administered AGE (2 g/kg/day) to mice for 2 weeks
and examined the production of antimicrobial peptides, specifically
β-defensin 1, β-defensin 4, β-defensin 14, and cathelicidin, in
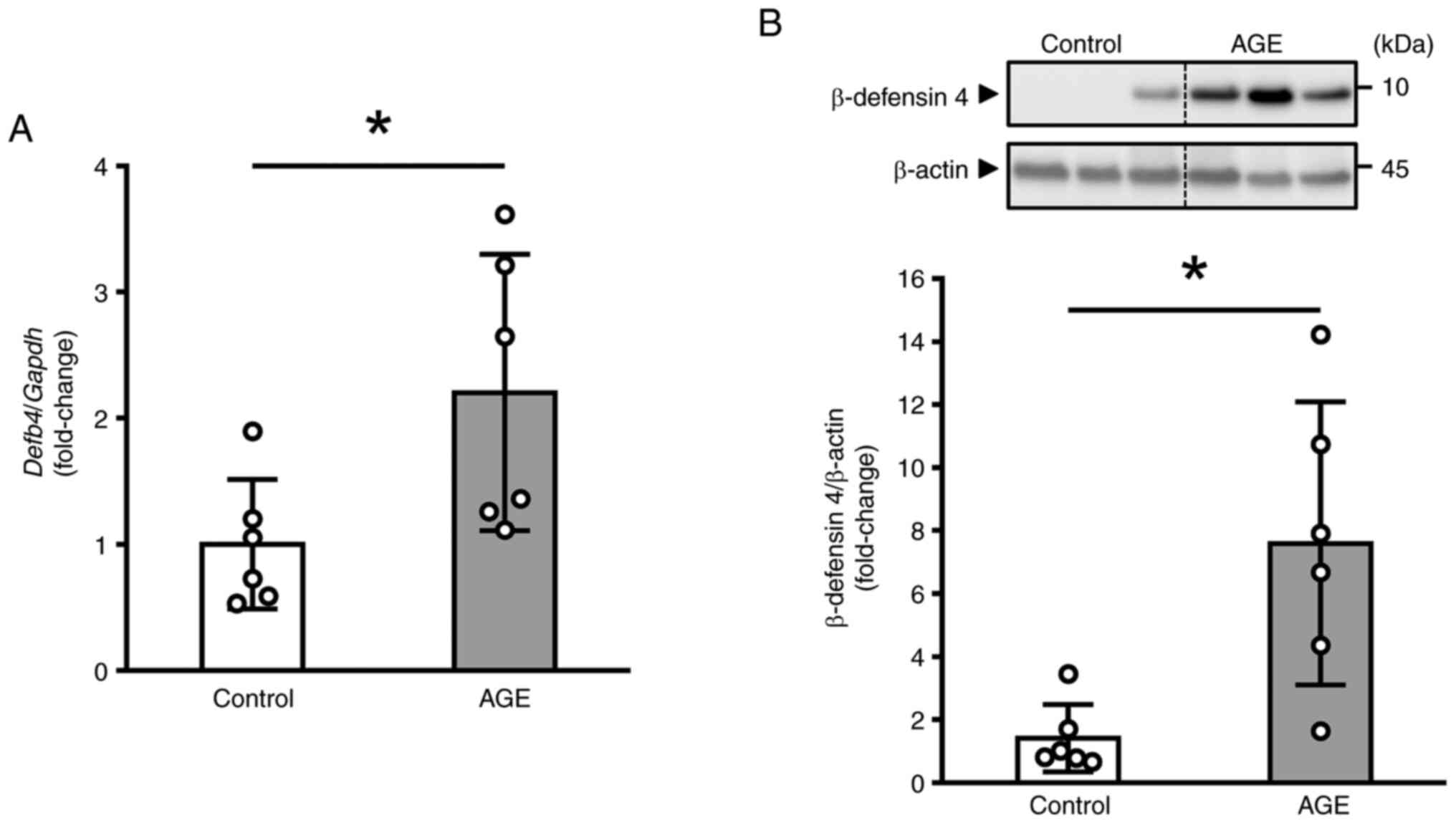
gingiva. As shown in Fig. 1A, the
mRNA level of Defb4 was significantly increased in
AGE-treated mice compared to DW-treated (control) mice. In
contrast, the mRNA level of other epithelial antimicrobial
peptides, including Defb1, Defb14, and Cramp,
remained unchanged (Fig. S1). We
next performed Western blot analysis to examine the effect of AGE
on the protein level of β-defensin 4, and found that AGE induced
the significant increase (Fig.
1B).
Effect of single administration of AGE
on the mRNA and protein level of β-defensin 4 in mouse gingiva
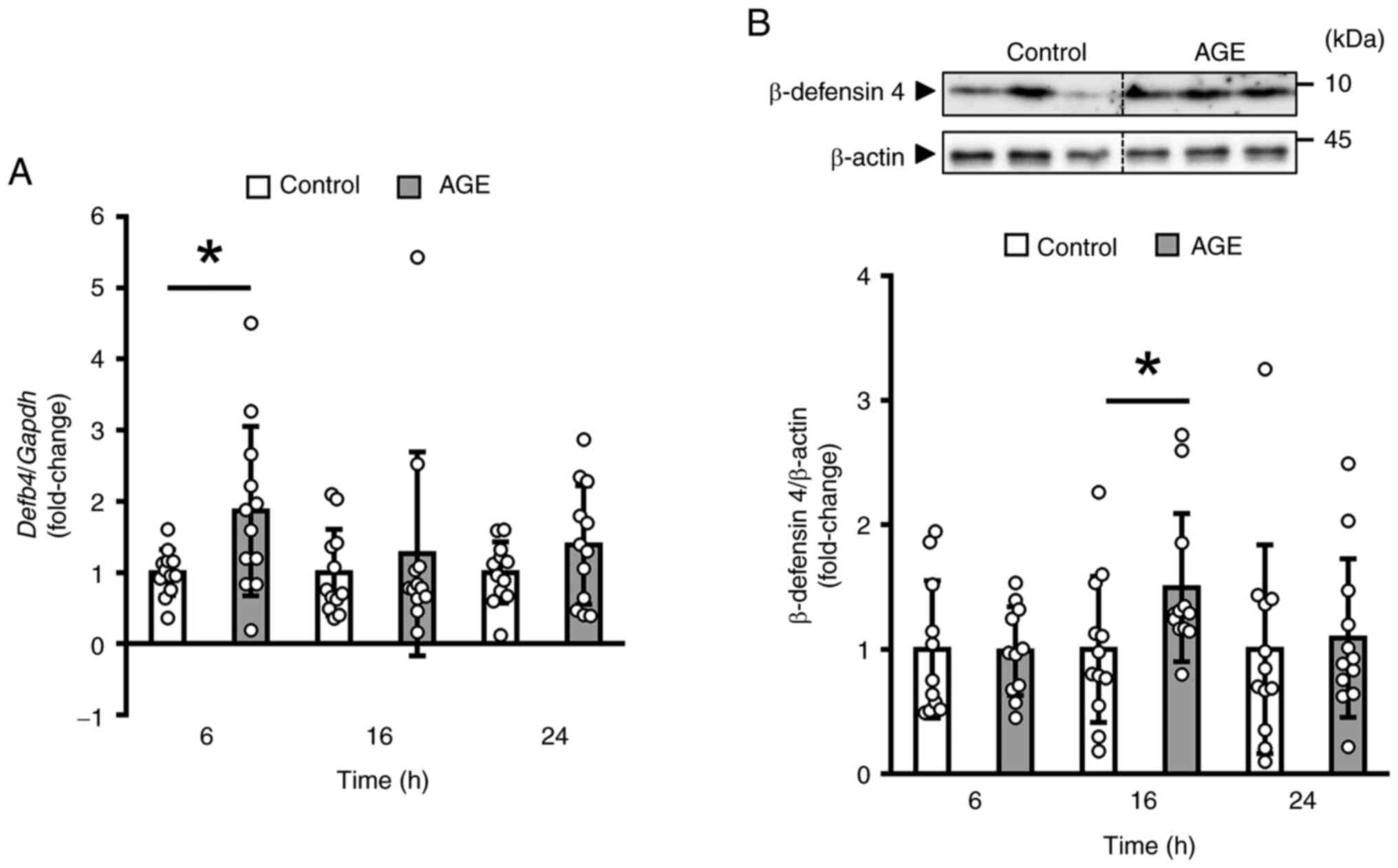
We next gave a single administration of AGE and
examined the change of the β-defensin 4 during 24 h. The data
obtained by reverse transcription-quantitative PCR analysis
indicated that AGE induced a transient and significant increase in
the mRNA level of Defb4 at 6 h (Fig. 2A). In addition, the protein level
of β-defensin 4 was significantly elevated in the AGE group at 16 h
compared to the control group (Fig.
2B).
Effect of single administration on the
canonical Wnt signaling pathway in mouse gingiva
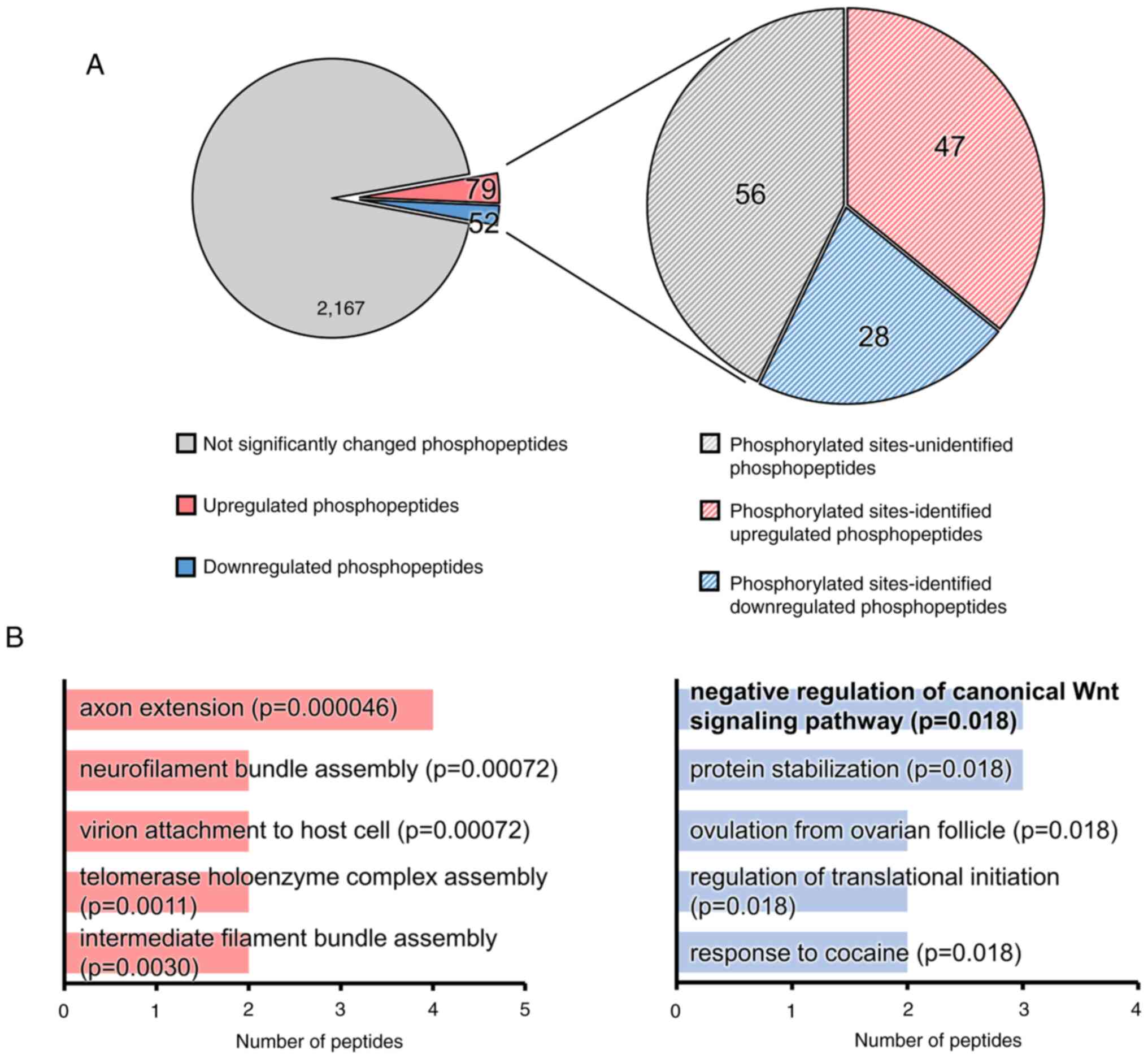
To explore the underlying mechanism of AGE-induced
increase of β-defensin 4, we performed phosphoproteomics analysis
using mouse gingival tissues treated with AGE for 6 h. Our
phosphoproteomics analysis identified a total of 2,298
phosphopeptides, revealing 79 up-regulated and 52 down-regulated
phosphopeptides in the AGE group compared to the control group
(Fig. 3A). In addition, among the
131 phosphopeptides, the phosphorylated amino acid residues were
distinctly defined in 47 up-regulated and 28 down-regulated
phosphopeptides (Fig. 3A). We
subsequently performed GO biological process enrichment analysis of
proteins with differentially phosphorylated peptides in the AGE
group using GeneCodis 4(23). The
top 5 significantly enriched GO terms in the biological process,
based on the number of proteins with up-regulated phosphopeptides
(adjusted P-value <0.05), were axon extension,
neurofilament bundle assembly, virion attachment to host
cell, telomerase holoenzyme complex assembly, and
intermediate filament bundle assembly (Fig. 3B). Similarly, the top 5
significantly enriched GO terms for proteins with down-regulated
phosphopeptides were negative regulation of canonical Wnt
signaling pathway, protein stabilization, ovulation
from ovarian follicle, regulation of translational
initiation, and response to cocaine (Fig. 3B). The GO term ‘negative
regulation of canonical Wnt signaling pathway’ included
proteins that are involved in forkhead box protein O3 (S7),
glycogen synthase kinase-3 alpha (GSK-3α) (Y279), and catenin
delta-1 (S252) (Table II).
Non-targeted proteomics analysis revealed a decrease in axin-1
(adjusted P-value=0.047) among the 125 up-regulated and 73
down-regulated proteins (Fig.
S2), indicating the involvement of the canonical Wnt
pathway.
 | Table IIChanges in the phosphorylation levels
of proteins related to the canonical Wnt pathway in mouse gingiva
treated with AGE for 6 h. |
Table II
Changes in the phosphorylation levels
of proteins related to the canonical Wnt pathway in mouse gingiva
treated with AGE for 6 h.
| Description | Phosphorylated
site | Log2
ratio (AGE/DW) | Adjusted
P-value |
|---|
| Forkhead box
protein O3 | 1xPhospho [S7] | -6.6439 |
2.38x10-16 |
| Glycogen synthase
kinase-3α | 1xPhospho
[Y279] | -0.7442 |
7.76x10-3 |
| Catenin δ1 | 1xPhospho
[S252] | -0.4170 |
4.66x10-2 |
Effect of AGE on the β-defensin 4
production and Wnt/β-catenin signaling pathway in mouse gingival
epithelial GE1 cells
The proteomics analysis on mouse gingiva suggested
the possible involvement of the canonical Wnt signaling pathway in
AGE-induced β-defensin 4 production. This hypothesis was supported
by two key observations: (1) the
suppressed phosphorylation level of GSK-3α, a negative regulator of
the Wnt/β-catenin pathway (Fig.
3B; Table II), and (2) the decreased protein level of axin-1
(adjusted P-value=0.047), a component of the β-catenin destruction
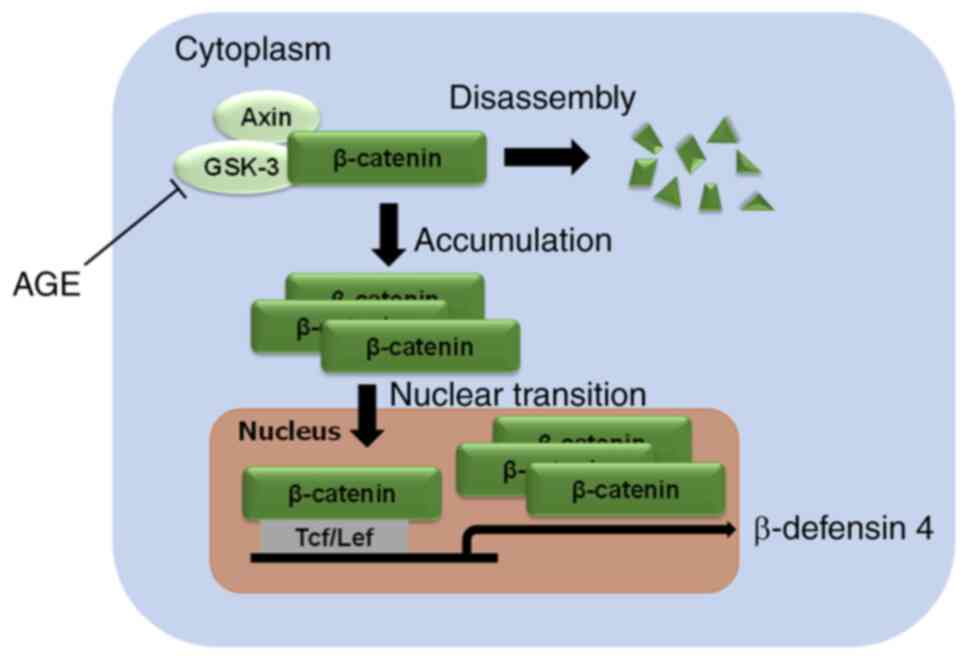
complex along with GSK-3α. Since β-catenin functions as a
transcription factor downstream of this pathway and its
localization to the nucleus is essential for exerting its
transcriptional effects (24), we
investigated the mechanism by which AGE induces β-defensin 4 in
gingiva, using mouse gingival epithelial GE1 cells in culture.
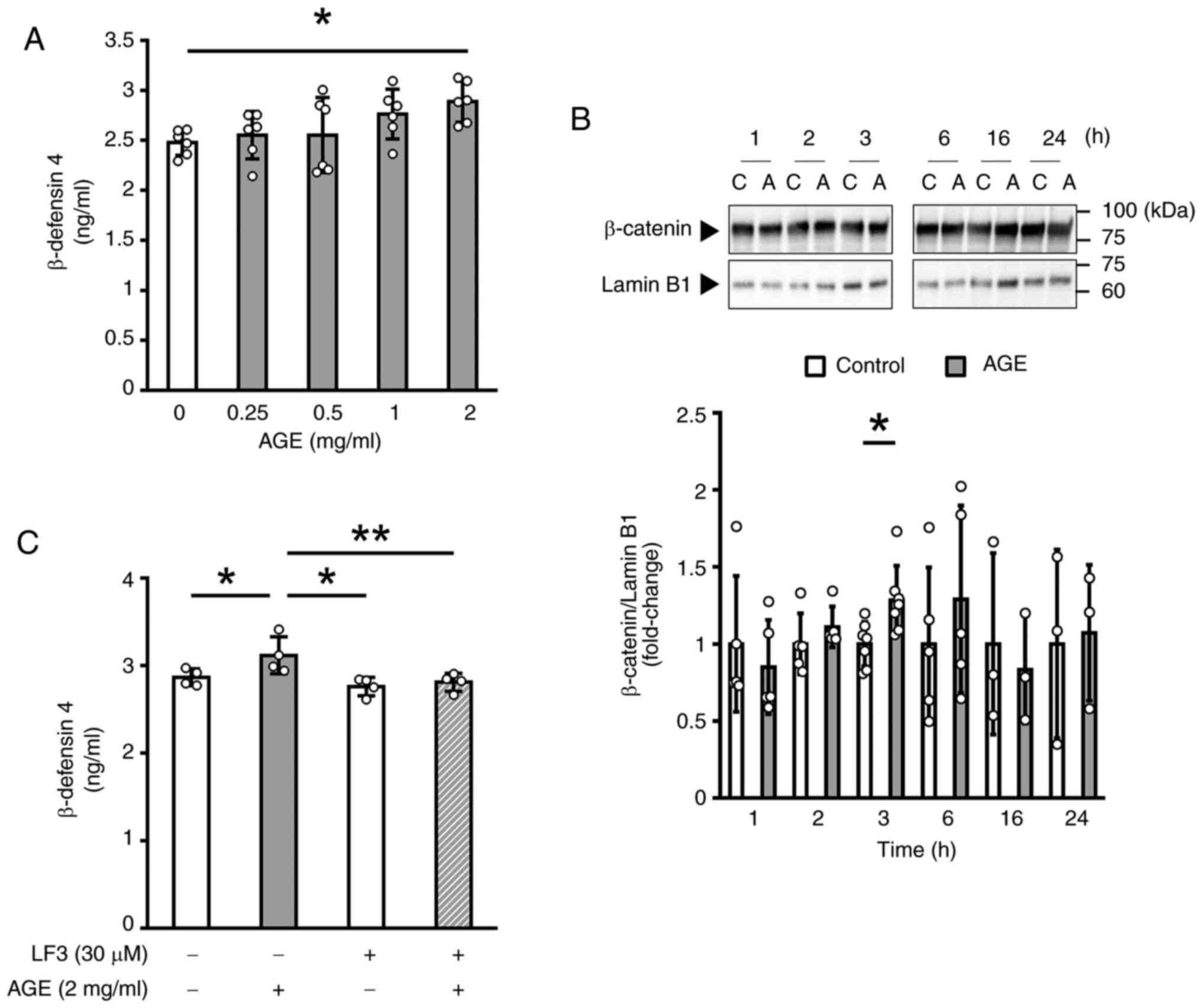
As shown in Fig.
4A, treatment with AGE at 2 mg/ml for 24 h significantly
increased the amount of β-defensin 4 in culture medium. We next
examined β-catenin localization to assess the involvement of the
Wnt/β-catenin pathway. We found that β-catenin protein accumulated
within the nucleus of cells treated with AGE (2 mg/ml) for 3 h
(Fig. 4B). To further examine the
involvement of the Wnt/β-catenin pathway, we used two specific
inhibitors of this pathway, LF3 and BIO. Simultaneous treatment of
GE1 cells for 24 h with LF3 (30 µM), a specific inhibitor of
β-catenin on canonical Wnt signaling, significantly suppressed the
AGE-induced increase in the β-defensin 4 protein production
(Fig. 4C). Moreover, treatment
with a GSK-3 specific inhibitor BIO (0.1 and 1 µM) alone resulted
in a statistically significant increase of the β-defensin 4
production (Fig. S3). These
results suggested the involvement of the Wnt/β-catenin pathway in
the β-defensin 4 production induced by AGE in mouse gingival
epithelial cells.
Discussion
Gingival epithelium serves as a mechanical barrier,
protecting the soft and hard tissues of the periodontal structures.
In addition, the gingival epithelium secretes antimicrobial
peptides, mainly β-defensin family that play a significant role in
the innate immune system of periodontal tissues (25). In human, the most
well-characterized β-defensins are β-defensin 1, 2, and 3(26). The production of β-defensin 1 is
essentially constitutive, whereas β-defensin 2 and 3 are inducible
in response to inflammatory stimuli in human gingival keratinocytes
(27). Several functional foods,
such as human milk oligosaccharides (28), Lactobacillus helveticus
SBT2171(29) and green tea
extracts (30), have been shown to
up-regulate inducible human β-defensin 2 and/or 3 in human gingival
epithelial cells.
In this study, we used the ddY strain of mice for
several reasons. The first one is related to its genetic diversity.
It is not as extensive as in humans but is substantially greater
than that of inbred strains such as C57BL/6. Thus, the finding
obtained by this study may be more applicable to humans when we
consider the possible use of AGE for our oral health. The second
one is that in our preliminary studies, ddY strain gave the best
response of β-defensin to AGE treatment among a few strains tested.
Thus this strain serves as a good experimental model to assess the
effect of AGE on β-defensin production.
AGE is reported to be beneficial for patients with
hypertension (31),
atherosclerosis (32), and
metabolic syndrome (33).
Furthermore, recent findings have indicated that AGE suppresses
inflammation and subsequent tissue destruction in the gingiva,
thereby preventing the progression of periodontal disease (11-15).
However, the effects of AGE on the innate immune function of
periodontal tissues remained unclear. The present study
demonstrated that AGE increased the production of mouse β-defensin
4, which is an ortholog to human β-defensin 2(34), in both mouse gingival tissue and
epithelial GE1 cells, suggesting that AGE is capable of bolstering
antimicrobial efficacy in gingival epithelium.
The canonical Wnt pathway, also termed Wnt/β-catenin
signaling, is well-known to contribute to cell fate determination
during developmental processes and tissue homeostasis (24). Recently it was reported that this
pathway is involved in the maintenance of the periodontium and the
progression of periodontal disease (35). Our proteomics analysis showed that
AGE suppressed the phosphorylation of GSK-3α (Y279) as well as the
protein level of axin-1, a well-known component of the destruction
complex of β-catenin (36).
Furthermore, AGE reduced the phosphorylation of forkhead box
protein O3 and catenin delta-1 that also participate in the
degradation of β-catenin (37,38).
Moreover, the present study revealed that AGE increased the protein
level of nuclear β-catenin in GE1 cells. These results suggested
that AGE activates the canonical Wnt pathway in gingival tissue by
inhibiting the degradation of β-catenin.
Wnt3a, an endogenous Wnt agonist, is reported to
elevate the mRNA level of mouse β-defensin 1 in mouse
macrophage-like RAW264.7 cells (39). Moreover, Wang et al
(40) have shown that DEAD-box
Helicase 15 induces α-defensins in Paneth cells through the
Wnt/β-catenin signaling. These findings suggested that activation
of the Wnt pathway is involved in the production of various
defensins. Recently, Chen Y. and Hu Y. have reported that the level
of activated β-catenin is increased in gingiva of Porphyromonas
gingivalis-associated ligature-induced periodontitis model
mice, and that Wnt3a induced the production of tumor necrosis
factor-α (TNF-α) in Raw264.7 cells (41). In the present study, LF3, a
specific inhibitor of β-catenin in the canonical Wnt signaling,
suppressed AGE-induced production of β-defensin 4 in GE1 cells. To
elucidate the relationship between the AGE-induced decrease in the
phosphorylation level of GSK-3α (Y279) and β-defensin 4 production,
we examined the effect of BIO, a GSK-3 specific inhibitor, on the
β-defensin 4 production in GE1 cells, and found that this inhibitor
increased the β-defensin 4 production. These findings suggested
that AGE regulates the production of β-defensin 4 in mouse gingiva
through the activation of the canonical Wnt pathway. We plan to
investigate the involvement of GSK and/or its phosphorylation and
other key molecules by conducting intervention and other
experiments both in vivo and in vitro to elucidate
the mechanism action of AGE.
Periodontal health is linked to the balance of the
oral microbiome, with dysbiosis being a key factor in the onset and
progression of periodontal disease (1,2). Bee
pollen, which is a pollen ball or pellet that is carried by the
honey bee, has been reported to elevate the mRNA level of
β-defensin-2 and -3 and alter the oral microbiota in the oral
cavities of mice (42). It is
possible that AGE helps to maintain the oral microbiome in a
healthy state by increasing the production of antimicrobial
peptides, and thus foster an oral environment less susceptible to
periodontal disease. Further investigation is needed to clarify
whether AGE affects the oral microbiota in periodontal disease
patients and model mice.
In conclusion, our findings showed that AGE can
up-regulate antimicrobial defense potential by promoting the
production of β-defensin 4 via the canonical Wnt signal
transduction pathway in gingiva (Fig.
5). Although more studies in vivo are required to
clarify the role of β-defensin 4 induced by AGE, the present study
suggests that AGE serves as a potential oral supplement for
preventing onset of periodontal disease.
Supplementary Material
Effect of daily administration of AGE
on the mRNA levels of epithelial antimicrobial peptides other than
β-defensin 4 in mouse gingiva. ddY mice were orally administrated
deionized water (control) or AGE (2 g/kg/day) for 2 weeks. Gingival
tissues were analyzed by reverse transcription-quantitative PCR.
The graphs show the mRNA levels of (A) Defb1, (B)
Defb14 and (C) Cramp normalized to those of
Gapdh. Data are presented as the mean ± standard deviation
(n=6). AGE, aged garlic extract.
Enrichment analysis of the AGE-induced
differentially phosphorylated peptides in mouse gingiva. ddY mice
were orally administrated deionized water (control) or AGE (2
g/kg). After 6 h, gingival tissues were analyzed using
phosphoproteomics. (A) Pie chart showing the number of
differentially phosphorylated peptides in the AGE group compared
with the control group. The adjusted P-value threshold was set to
<0.05. (B) Graphs showing the top 5 enriched Gene Ontology
biological process terms for upregulated (left) and downregulated
proteins (right). The adjusted P-value threshold was set to
<0.05. AGE, aged garlic extract.
Effect of a GSK-3 inhibitor on
β-defensin 4 production in GE1 cells. The cells were treated with
BIO, a GSK-3 inhibitor, at the indicated concentrations (0.1.10
μM) for 24 h. The amount of β-defensin 4 protein secreted
into the culture medium was determined using an ELISA. The graph
shows the concentration of β-defensin 4 in the medium. Data are
presented as the mean ± standard deviation (n=3).
*P<0.05 (Holm-Bonferroni test). BIO,
6-bromoindirubin-3’-oxime; GSK-3, glycogen synthase kinase-3.
Acknowledgements
The authors would like to thank Dr Takami Oka
(Wakunaga Pharmaceutical Co., Ltd., Akitakata, Hiroshima, Japan)
for his helpful advice, encouragement and critical reading of the
manuscript.
Funding
Funding: Wakunaga Pharmaceutical Co., Ltd., provided the funding
for the present study.
Availability of data and materials
The mass spectrometry immunopeptidomics and
proteomics data generated in the present study may be found in the
ProteomeXchange Consortium (43)
via the jPOSTrepo partner repository (44) under the accession numbers PXD053155
and PXD053156 for ProteomeXchange, and JPST002964 and JSPT002963
for jPOSTrepo or at the following URLs: https://proteomecentral.proteomexchange.org/cgi/GetDataset?ID=PXD053155,
https://proteomecentral.proteomexchange.org/cgi/GetDataset?ID=PXD053156,
https://repository.jpostdb.org/entry/JPST002963.0
and https://repository.jpostdb.org/entry/JPST002964.0. All
other data generated in the present study may be requested from the
corresponding author.
Authors' contributions
HN and MO designed the experimental procedures. HN
performed animal experiments. DF and HN performed cell experiments,
reverse transcription-quantitative PCR and western blot analysis.
HN performed the proteomics analysis and ELISA. DF and HN performed
data analysis. DF, HN and MO confirm the authenticity of all the
raw data. MO validated the results to ensure accuracy. DF and HN
created graphical representations of the data. HN wrote the
original draft. DF and MO reviewed and edited the manuscript. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Animal care and experiments were performed in
accordance with the guidelines for the care and use of laboratory
animals of the Wakunaga Pharmaceutical Co., Ltd., and animal
experiments were reviewed and approved by the Wakunaga
Pharmaceutical Company Institutional Animal Care and Use Committee
(approval no. 360; Akitakata, Japan).
Patient consent for publication
Not applicable.
Competing interests
All the authors are employees of Wakunaga
Pharmaceutical Co., Ltd., who provided funding for this study.
References
|
1
|
Janakiram C and Dye BA: A public health
approach for prevention of periodontal disease. Periodontol.
84:202–214. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lamont RJ, Koo H and Hajishengallis G: The
oral microbiota: Dynamic communities and host interactions. Nat Rev
Microbiol. 16:745–759. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kurgan S and Kantarci A: Molecular basis
for immunohistochemical and inflammatory changes during progression
of gingivitis to periodontitis. Periodontol. 76:51–67.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tonetti MS, Jepsen S, Jin L and
Otomo-Corgel J: Impact of the global burden of periodontal diseases
on health, nutrition and wellbeing of mankind: A call for global
action. J Clin Periodontol. 44:456–462. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chatzopoulos GS, Jiang Z, Marka N and
Wolff LF: Periodontal disease, tooth loss, and systemic conditions:
An exploratory study. Int Dent J. 74:207–215. 2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Prasad SV, Fiedoruk K, Daniluk T, Piktel E
and Bucki R: Expression and function of host defense peptides at
inflammation sites. Int J Mol Sci. 21(104)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Johnstone KF and Herzberg MC:
Antimicrobial peptides: Defending the mucosal epithelial barrier.
Front Oral Health. 3(958480)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pedersen AM and Belstrøm D: The role of
natural salivary defences in maintaining a healthy oral microbiota.
J Dent. 80 (Suppl 1):S3–S12. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hans M and Hans VM: Epithelial
antimicrobial peptides: Guardian of the oral cavity. Int J Pept.
2014(370297)2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kodera Y, Kurita M, Nakamoto M and
Matsutomo T: Chemistry of aged garlic: Diversity of constituents in
aged garlic extract and their production mechanisms via the
combination of chemical and enzymatic reactions. Exp Ther Med.
19:1574–1584. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zini A, Mann J, Mazor S and Vered Y: The
efficacy of aged garlic extract on gingivitis-a randomized clinical
trial. J Clin Dent. 29:52–56. 2018.PubMed/NCBI
|
|
12
|
Zini A, Mann J, Mazor S and Vered Y:
Beneficial effect of aged garlic extract on periodontitis: A
randomized controlled double-blind clinical study. J Clin Biochem
Nutr. 67:297–301. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ohtani M and Nishimura T:
Sulfur-containing amino acids in aged garlic extract inhibit
inflammation in human gingival epithelial cells by suppressing
intercellular adhesion molecule-1 expression and IL-6 secretion.
Biomed Rep. 12:99–108. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nango H and Ohtani M:
S-1-propenyl-L-cysteine suppresses lipopolysaccharide-induced
expression of matrix metalloproteinase-1 through inhibition of
tumor necrosis factor-α converting enzyme-epidermal growth factor
receptor axis in human gingival fibroblasts. PLoS One.
18(e0284713)2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Takahashi K, Nango H, Ushijima M,
Takashima M, Nakamoto M, Matsutomo T, Jikihara H, Arakawa N, Maki
S, Yabuki A, et al: Therapeutic effect of aged garlic extract on
gingivitis in dogs. Front Vet Sci. 10(1277272)2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ryu K, Ide N, Matsuura H and Itakura Y: N
alpha-(1-deoxy-D-fructos-1-yl)-L-arginine, an antioxidant compound
identified in aged garlic extract. J Nutr. 131:972S–976S.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Matsutomo T, Ushijima M, Kodera Y,
Nakamoto M, Takashima M, Morihara N and Tamura K: Metabolomic study
on the antihypertensive effect of S-1-propenylcysteine in
spontaneously hypertensive rats using liquid chromatography coupled
with quadrupole-Orbitrap mass spectrometry. J Chromatogr B Analyt
Technol Biomed Life Sci. 1046:147–155. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ushijima M, Kunimura K and Suzuki JI: S
-1-Propenylcysteine, a sulfur compound in aged garlic extract,
alleviates cold-induced reduction in peripheral blood flow in rat
via activation of the AMPK/eNOS/NO pathway. Exp Ther Med.
20:2815–2821. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kunimura K, Nakamoto M and Ushijima M:
S-1-propenylcysteine enhances endurance capacity of mice by
stimulating fatty acid metabolism via muscle isoform of carnitine
acyltransferase-1. J Nutr. 154:2707–2716. 2024.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sanui T, Tanaka U, Fukuda T, Toyoda K,
Taketomi T, Atomura R, Yamamichi K and Nishimura F: Mutation of
Spry2 induces proliferation and differentiation of osteoblasts but
inhibits proliferation of gingival epithelial cells. J Cell
Biochem. 116:628–639. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ohgane K and Yoshioka H: Quantification of
gel bands by an image J macro, band/peak quantification tool.
protocols.io, 2019.
|
|
23
|
Garcia-Moreno A, López-Domínguez R,
Villatoro-García JA, Ramirez-Mena A, Aparicio-Puerta E, Hackenberg
M, Pascual-Montano A and Carmona-Saez P: Functional enrichment
analysis of regulatory elements. Biomedicines.
10(590)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang
X, Zhou Z, Shu G and Yin G: Wnt/β-catenin signalling: Function,
biological mechanisms, and therapeutic opportunities. Signal
Transduct Target Ther. 7(3)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gursoy UK and Könönen E: Understanding the
roles of gingival beta-defensins. J Oral Microbiol.
4(4)2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pazgier M, Hoover DM, Yang D, Lu W and
Lubkowski J: Human beta-defensins. Cell Mol Life Sci. 63:1294–1313.
2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Joly S, Organ CC, Johnson GK, McCray PB Jr
and Guthmiller JM: Correlation between beta-defensin expression and
induction profiles in gingival keratinocytes. Mol Immunol.
42:1073–1084. 2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gürsoy UK, Salli K, Söderling E, Gürsoy M,
Hirvonen J and Ouwehand AC: Regulation of hBD-2, hBD-3,
hCAP18/LL37, and proinflammatory cytokine secretion by human milk
oligosaccharides in an organotypic oral mucosal model. Pathogens.
10(739)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kobatake E, Kobayashi R, Kabuki T and
Kurita-Ochiai T: Lactobacillus helveticus SBT2171 upregulates the
expression of β-defensin and ameliorates periodontal disease caused
by Porphyromonas gingivalis. Microbiol Immunol. 63:293–302.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bedran TB, Feghali K, Zhao L, Spolidorio
DM and Grenier D: Green tea extract and its major constituent,
epigallocatechin-3-gallate, induce epithelial beta-defensin
secretion and prevent beta-defensin degradation by Porphyromonas
gingivalis. J Periodontal Res. 49:615–623. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ried K: Garlic lowers blood pressure in
hypertensive subjects, improves arterial stiffness and gut
microbiota: A review and meta-analysis. Exp Ther Med. 19:1472–1478.
2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gruenwald J, Bongartz U, Bothe G and
Uebelhack R: Effects of aged garlic extract on arterial elasticity
in a placebo-controlled clinical trial using EndoPAT™ technology.
Exp Ther Med. 19:1490–1499. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gómez-Arbeláez D, Lahera V, Oubiña P,
Valero-Muñoz M, de Las Heras N, Rodríguez Y, García RG, Camacho PA
and López-Jaramillo P: Aged garlic extract improves adiponectin
levels in subjects with metabolic syndrome: A double-blind,
placebo-controlled, randomized, crossover study. Mediators Inflamm.
2013(285795)2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Röhrl J, Yang D, Oppenheim JJ and Hehlgans
T: Specific binding and chemotactic activity of mBD4 and its
functional orthologue hBD2 to CCR6-expressing cells. J Biol Chem.
285:7028–7034. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
González-Quintanilla D, Abásolo N and
Astudillo P: Wnt signaling in periodontal disease. Front Dent Med.
2(23)2021.
|
|
36
|
Mukherjee A, Dhar N, Stathos M, Schaffer
DV and Kane RS: Understanding how wnt influences destruction
complex activity and β-catenin dynamics. iScience. 6:13–21.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liu H, Yin J, Wang H, Jiang G, Deng M,
Zhang G, Bu X, Cai S, Du J and He Z: FOXO3a modulates WNT/β-catenin
signaling and suppresses epithelial-to-mesenchymal transition in
prostate cancer cells. Cell Signal. 27:510–518. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bareiss S, Kim K and Lu Q:
Delta-catenin/NPRAP: A new member of the glycogen synthase
kinase-3beta signaling complex that promotes beta-catenin turnover
in neurons. J Neurosci Res. 88:2350–2363. 2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chen K, Fu Q, Li D, Wu Y, Sun S and Zhang
X: Wnt3a suppresses Pseudomonas aeruginosa-induced inflammation and
promotes bacterial killing in macrophages. Mol Med Rep.
13:2439–2446. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wang Y, He K, Sheng B, Lei X, Tao W, Zhu
X, Wei Z, Fu R, Wang A, Bai S, et al: The RNA helicase Dhx15
mediates Wnt-induced antimicrobial protein expression in Paneth
cells. Proc Natl Acad Sci USA. 118(e2017432118)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Chen Y and Hu Y: Wnt Signaling activation
in gingival epithelial cells and macrophages of experimental
periodontitis. Dent J (Basel). 11(129)2023.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Khurelchuluun A, Uehara O, Paudel D,
Morikawa T, Kawano Y, Sakata M, Shibata H, Yoshida K, Sato J, Miura
H, et al: Bee pollen diet alters the bacterial flora and
antimicrobial peptides in the oral cavities of mice. Foods.
10(1282)2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Vizcaíno JA, Deutsch EW, Wang R, Csordas
A, Reisinger F, Ríos D, Dianes JA, Sun Z, Farrah T, Bandeira N, et
al: ProteomeXchange provides globally coordinated proteomics data
submission and dissemination. Nat Biotechnol. 32:223–226.
2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Okuda S, Watanabe Y, Moriya Y, Kawano S,
Yamamoto T, Matsumoto M, Takami T, Kobayashi D, Araki N, Yoshizawa
AC, et al: jPOSTrepo: An international standard data repository for
proteomes. Nucleic Acids Res. 45:D1107–D1111. 2017.PubMed/NCBI View Article : Google Scholar
|