Introduction
Spontaneous intracerebral haemorrhage (ICH) results
from small arterial rupture, associated with hypertension and
excessive salt intake, Inflammation, infection, and dysregulation
of the brain-gut axis further exacerbate the progression of
cerebral small vessel disease (1).
A sudden rise in blood pressure can lead to hematoma formation,
rapid progression of the disease and poor prognosis, as well as
high rates of disability and mortality, The disability rate can be
as high as 73%, with a mortality rate ranging from 6.5 to 19.6%,
and a 30-day mortality rate reaching up to 50, resulting in a
serious social and economic burden (2). In China, cerebral hemorrhage accounts
for 30-55% of cases of stroke, which is higher than the 8-15% in
Western populations (3,4). The pathophysiological changes that
ensue after hematoma formation mainly include neurological function
impairment and brain tissue injuries that are caused by the toxic
effects of hematoma, which can lead to cerebral vascular
permeability changes, cerebral edema and increased intracranial
pressure (ICP) and, in severe cases, cerebral hernia (5). Therefore, timely hematoma removal is
able to reduce the complications and improve prognosis (6). Craniotomy is the definitive
intervention for completely evacuating hematomas and achieving
optimal hemostatic outcomes. This surgical approach is particularly
recommended for patients at high risk for brain herniation, serving
as the preferred method in these critical cases (7). However, clinical trials [for example,
surgical treatment of intracerebral hemorrhage (ICH) phase I and II
trials] have shown that craniotomy fails to significantly improve
neurological prognosis or reduce mortality (8). Preliminary results from the minimally
invasive surgery (MIS) with thrombolysis in ICH evacuation (MISTIE)
II and III trials have suggested that MIS in combination with
alteplase therapy may be advantageous in certain patients with
cerebral hemorrhage, who have been revealed to have reduced
mortality rates and improved prognoses (9). The results of the MISTIE II trial
showed that administering MIS plus recombinant tissue-type
plasminogen activator led to a favorable prognosis in patients who
achieved a residual hematoma <10 ml or clot clearance >3%
(10); >84% of patients
achieved the treatment goals of residual hematoma <15 ml or clot
clearance >70%. For patients within 24-48 h of symptom onset,
with haematoma volumes <50 ml, and in the absence of
contraindications, precise puncture localisation and appropriate
intrahaematoma thrombolytic agents are recommended, Puncturing
along the long axis of the neural fibres reduces nerve fibre
damage. Choosing a puncture along the long axis of the cerebral
haematoma, with side holes in the drainage tube, enhances haematoma
evacuation, emphasizing the importance of optimizing patient
selection and drain placement to improve hematoma evacuation
efficiency and surgical success (11,12).
Currently, MIS techniques for the treatment of
spontaneous ICH internationally include computed tomography
(CT)-guided real-time aspiration, stereotactic-guided aspiration
and endoscopic surgery (13).
Traditional stereotactic surgery requires the use of a stereotactic
head frame for precise positioning, with the patient's head being
immobilized in the head frame, and fixation pins are inserted into
the skull. However, techniques such as frameless stereotactic and
image-guided navigation systems offer alternatives to traditional
methods (14). Moreover, CT-guided
real-time aspiration may result in the exposure of the operator to
radiation, while the use of neuroendoscopic equipment, frameless
navigation systems and robotic surgical instruments are expensive
and difficult to obtain in primary hospitals in developing
countries (15). Uneven economic
and technological development in these regions makes it difficult
to popularize minimally invasive ICH removal techniques. Currently,
hard-channel (YL-1 type) puncture and drainage is mainly used in
primary hospitals in mainland China, although the employment of
this method may result in more damage being caused to normal brain
tissues, inaccurate localization or the emergence of complications,
such as increased incidence of intracranial infections and seizures
(16).
Considering the information available at present,
the treatment for ICH remains challenging and requires a balancing
act, taking into consideration the cost, precision, safety and
accessibility of the technique concerned. Advanced technologies,
such as neuroendoscopy and frameless stereotactic systems, offer
precise and minimally invasive options, although their use is
limited at present by high costs, technical complexity and the
difficulty of use in resource-limited settings (17,18).
On the other hand, simpler methods such as hard-channel drainage,
although more readily available, are associated with compromised
precision and patient safety. Therefore, there is an urgent need to
develop cost-effective, minimally invasive alternatives to ensure
surgical accuracy and patient safety, especially in
resource-limited settings. In the future the development of
portable imaging technology, simplified neuroendoscopy and
affordable navigation systems (19) should improve the accuracy of
hematoma drainage, reduce congenital injuries and improve the
long-term neurological prognosis of patients with ICH, thereby
providing more cost-effective, minimally invasive treatment options
for ICH in resource-limited areas.
In the current study, preliminary results with a
novel laser-guided localization technique integrated with
soft-channel MIS for the management of cerebral hemorrhage are
presented. This new approach was evaluated in comparison with the
conventional YL-1 puncture method to determine its clinical
efficacy concerning hematoma evacuation, puncture precision and
postoperative outcomes. The present study focused on the
development of advanced stereotactic surgical techniques aimed at
enhancing the accuracy and safety of minimally invasive
interventions for ICH and evaluated whether laser localization
combined with soft-channel MIS is effective and safe in the
treatment of cerebral hemorrhage.
Materials and methods
Patients, ethics approval and
consent
The present study was retrospectively registered
with the China Clinical Trial Registration Center (ChiCTR) under
the registration number ChiCTR2400094351, with registration
completed on 20th December 2024. The present prospective, partially
randomized, controlled study included 60 patients aged ≥45 years.
The study group (n=30) underwent laser-guided minimally invasive
surgery in combination with a soft-channel approach at the
affiliated Nanchuan Hospital of Chongqing Medical University
between May 2022 and October 2023. The control group (n=30)
received YL-1 hard-channel puncture and aspiration for
intracerebral hemorrhage. Ethics approval for the present study was
obtained from the Ethics Committee of Nanchuan Hospital of
Chongqing Medical University (approval no. YXYJ-2022-013;
Chongqing, China), and all patients provided written informed
consent prior. The study sample (n=60 patients) was randomly
divided into two groups (study group and control group) using a
fully randomized numerical table method. All procedures were
performed by the same experienced neurosurgeon within 6-24 h
following the onset of ICH. The inclusion criteria were as follows:
i) The first diagnosis of the patient was CT-confirmed, single-site
ICH; ii) the age of the patient was between 45-85 years; iii) the
patient had a supratentorial hematoma volume (HV) of 30-65 ml with
a midline shift of >5 mm; iv) the cerebellar HV was in the range
of 10-30 ml with brainstem compression; v) the vital signs were
stable (blood Pressure: 140-160/80-90 mmHg; heart Rate: 60-100
beats per min; respiratory Rate: 12-18 breaths per min, Oxygen
Saturation: maintained above 95%, Temperature: 36.5-37.5˚C); vi)
the oxygen saturation level was ≥90%, as measured by finger pulse
oximetry; vii) the Glasgow Coma Scale (GCS) score (20) was ≥8 points, and viii) patients
with seizures treated with sodium valproate. The exclusion criteria
were as follows: i) Cerebral herniation (such as dilated pupils)
was present; and ii) the patient was diagnosed with ischemic stroke
leading to hemorrhage, neoplastic stroke, abnormal vascular
structures (including arteriovenous malformations and aneurysms) or
coagulation abnormalities, as demonstrated by routine CT
angiography (CTA) on admission. However, the duration of bleeding
(from stroke onset to surgery) was not used as an exclusion
criterion.
Materials and equipment
For the study group, a laser localizer
[second-generation laser-positioning instrument (Hunan Zhuoshi
Chuangsi Technology Co., Ltd.) approved by the National Medical
Products Administration, China] was employed. The program ‘DuRofi
CT baseline simulator’ was used, whereas the disposable catheter
and disposable 10- or 12-gauge cerebral drainage puncture devices
were purchased from Shandong Baiduoan Medical Equipment Co., Ltd.
For the control group, a YL-1 puncture needle was obtained from
Beijing Wantefu Medical Devices Co., Ltd.
Unlike traditional stereotactic systems, the novel
laser localizer eliminates the need for a secondary CT or MRI scan
for accurate localization. It utilizes a high-precision
laser-guided coordinate mapping system to minimize targeting
errors. Additionally, the use of a disposable guide during both
preoperative planning and intraoperative localization reduces the
risk of infection by avoiding repeated manipulations. This design
enhances precision and safety while streamlining the localization
process.
Technical parameters and working
principles. Technical parameters
Table I presents
the technical parameters of the laser positioner used in the
present study.
 | Table ISpecific technical parameters of the
laser positioner. |
Table I
Specific technical parameters of the
laser positioner.
| Technical
parameter |
Value/classification |
|---|
| Model no. | DRF-M-01 |
| Marking range | 200x250x90 mm |
| Scale
resolution | 1 mm |
| Maximum laser
power | Red laser, 635±5
nm; green laser, 520±5 nm |
| Number of
lasers | 6 |
| Laser type | 130 degree one-line
laser line width of 1 mm |
| Laser safety
classification | Class 2M |
| Laser safety
requirements | Avoid using optical
instruments to observe the laser |
| Temperature | -25˚ to +40˚C |
| Relative
humidity | ≤90% |
| Atmospheric
pressure | 500-1,060 hPa |
| Operating
conditions | Normal ambient
temperature, 5-40˚C; relative humidity, 30-75%; atmospheric
pressure, 700-1,060 hPa |
| Power supply | 3.7 V, 70 mAh;
charge/discharge 1C; with protection board |
| Dimensions, length
x width x height | 510x255x450 mm |
| Weight | 11 kg |
| Other security
requirements | |
|
Type of
protection against electric shock | Internal power
supply type |
|
Degree of
protection against electric shock | Type B application
section |
|
Degree of
protection against incoming fluids | Not applicable |
|
Degree of
safety when used in the presence of flammable anesthetic gases
mixed with air or flammable anesthetic gases mixed with oxygen or
nitrous oxide | AP/APG type |
|
Mode of
operation | Continuous |
|
Rated
voltage and frequency of the equipment | DC3.7V |
|
Application
section for protection against defibrillation discharge
effects | No |
|
Signal input
and signal output section | No |
|
Installation
of equipment | Non-permanent |
|
Electromagnetic
compatibility | Group 1 Class
B |
Working principles. The core principle of the
laser-positioning system is based on the three-dimensional (3D)
geometric axiom, which states that if two non-overlapping planes
share a common point, there exists exactly one straight line
passing through that point. When the longitudinal and lateral laser
axes are aligned within a single plane targeting the objective, the
positioning device ensures that the intersection of the two laser
planes passes through both the target point and any entry point
within the space (21). This
principle is utilized in neurosurgical planning to achieve precise
localization based on cranial CT imaging data. By passing the
relevant data [anatomical landmarks (bilateral external auditory
canal, lens) and hematoma centre target data on cranial CT scan
films] through the ‘DuRofi CT baseline simulator’ (an application
designed for baseline simulation), the system accurately identifies
and marks the projection of intracranial targets onto the patient's
surface anatomy. This enables precise preoperative localization of
intracranial targets, thereby providing a crucial reference for
cranial surgeries.
Data collection and analysis
The study group consisted of 21 male and 9 female
patients with a mean age of 64.80±10.56 years and a mean
supratentorial HV of 41.32±10.91 ml, mean cerebellar HV of 16.78
ml. There were 6 cases of supratentorial HV (20.00%), 23 cases of
basal ganglia and thalamus HV (76.66%), 11 cases of ventricular
system effusion (36.66%) and 1 case (3.33%) of cerebellar HV.
Occurrence of ventricular system effusion in 11 cases (36.66%) was
associated with hemorrhage in the basal ganglia and thalamus
regions.
The control group comprised of 19 male and 11 female
patients with a mean age of 64.30±9.77 years, a mean supratentorial
HV of 45.99±3.40 ml and a mean cerebellar HV of 17.53±4.50 ml.
There were 4 cases of lobar HV (13.33%), 23 cases of basal ganglia
and thalamus HV (76.66%), 3 cases of cerebellar HV (10.00%) and 8
cases of ventricular HV (26.66%). After the haematoma stabilised (6
h post-haemorrhage), all patients underwent repeat CT scans to
assess any changes in haematoma volume prior to surgery.
Preoperative CT angiography (CTA) was performed to exclude
aneurysms or arteriovenous malformations. Postoperative CT scans
were conducted to confirm the position of the drainage catheter and
evaluate residual haematoma. Before catheter removal, follow-up CT
was performed to determine whether removal was indicated. A final
CT scan was carried out on the first day after catheter removal to
document residual haematoma volume. The efficacy and safety of the
new stereotactic aspiration were subsequently evaluated. Both the
stable volume and the final HV were calculated on the basis of the
CT data. Hematoma volume was calculated using the ABC/2 formula
(22). Subsequently, the mean of
the two evaluated volumes was calculated; if the difference was
>2 ml, a third radiologist adjudicated on the results.
The following data were collected and analyzed: The
GCS score both on admission and on day 1 after extubation, the
preoperative HV, time from onset to surgery, (from the start of
bleeding to the start of surgery), the surgery time, the drainage
time, the percentage of hematoma cleared, the GCS score on day 7
postoperatively, the GCS score at discharge and the modified Rankin
Scale (mRS) score (23) at 6
months post-discharge. The mRS scores, surgery-associated
complications (hemorrhage, hydrocephalus, intracranial gas and
scalp necrosis) and postoperative systemic complications
(pneumonia, seizures, electrolyte disorders, renal insufficiency,
gastrointestinal symptoms and cardiac failure) were collected and
analyzed. In addition, the patients were monitored for infection,
intracranial gas accumulation and scalp necrosis. The percentage
thrombus clearance was used to assess hematoma clearance, and this
was defined as the reduction in bleeding divided by the
preoperative HV. Favorable and unfavorable outcomes were defined as
mRS scores of 0-2 and 3-5, respectively.
Surgical treatment options. Study
group
After appropriate preoperative preparations (Blood
tests, CT scans, hair removal), the attending physician selected
the midpoint of the external auditory canal for the bilateral lens,
the window value of the lens, the midpoint of the external auditory
canal and the level at which the target point was located using the
‘DuRofi CT baseline simulator’ applet. The external auditory canal
on the affected side was selected as the zero point, and the CT
scanning baseline plane as well as the target point level through
the zero point were then acquired. Four points of the baseline
plane on the patient's body surface (target point) were then
marked. The baseline plane was then matched to the frame of the
Durofi laser, and the laser was subsequently moved to the target
level. Based on the coordinates of the target point at that level,
the laser at the target level was moved to align with the target
point. The laser was then rotated through the puncture point, and
the intersection of the two laser planes in space represented the
direction of the puncture. The direction was recorded and the depth
was determined, using a ‘Du Rofi’ disposable guide plate. The body
surface of the disposable guide plate was marked to indicate the
puncture point, and the positioning device was moved away to end
the positioning. The preoperative positioning information was
reproduced using a sterile guide plate to guide the surgical
direction. A 3-cm incision was made into the scalp, the skull was
drilled into at a depth of 0.5 cm, and a 0.2-0.3-cm incision was
made into the dura mater following hemostasis of the bone margins.
To minimize the risk of malposition during the operation, the
puncture entry point was chosen on the forehead, 3 cm from the
midline. The catheter was subsequently inserted through a
subcutaneous tunnel (5 cm in length), reducing the risk of
infection and ensuring secure fixation. The cortex was then
electrocauterized to test the resistance of the drainage tubes,
ensure a smooth puncture, and minimize additional injury. Under the
guidance of the introducer, a 10- or 12-gauge cerebral drainage
puncture device was guided to the depth of the target point, and
then moved forward by 0.5 cm to ensure that the lateral hole of the
drain was centered on the target point. The drainage tube was then
connected to the 10-ml syringe, and slow aspiration was performed
under negative pressure as the drainage tube rotated. If resistance
was encountered, the force of negative pressure suction was
reduced. Suctioning was stopped when resistance was first
encountered or when hematoma suction had reached one-third of the
total volume, and the drainage tube was retained to facilitate the
subsequent injection of urokinase and the drainage of dissolved
clots. Urokinase (30 thousand units to dissolve blood clots.) was
injected into the hematoma cavity from the heparin cap attached to
the drainage tube 2-3 times a day; the tube was clamped for 2 h,
reopened for 2-4 h, and this procedure was repeated. Clearance of
the hematoma usually lasts 2-3 days and up to 5 days. The drains
are removed after the hematoma is cleared (more than 75%). In
addition, blood pressure, dehydration, hemostasis, and potential
infections were monitored, with provision of enteral or parenteral
nutrition, prevention of deep vein thrombosis, and necessary
rehabilitation. Finally, cranial closure was performed.
Control group. YL-1 disposable puncture
needle was used to remove the hematoma. Based on the preoperative
CT scanning results, a body marking was attached to the affected
temporal scalp, located on the largest slice of the hematoma, i.e.,
this marking was used as the puncture point after routine
disinfection was performed on the scalp. While the patient was
under local anesthesia (lidocaine 100 mg), The distance between the
puncture point and the hematoma was measured by using CT software
and YL-1 puncture needle in the electric drill was driven directly
into the center of the hematoma. The extraction of the core of the
needle, blockage of the puncture opening, connection of the lateral
tube to the drainage tube, suction method and subsequent urokinase
protocol were all performed identically to that described for the
study group.
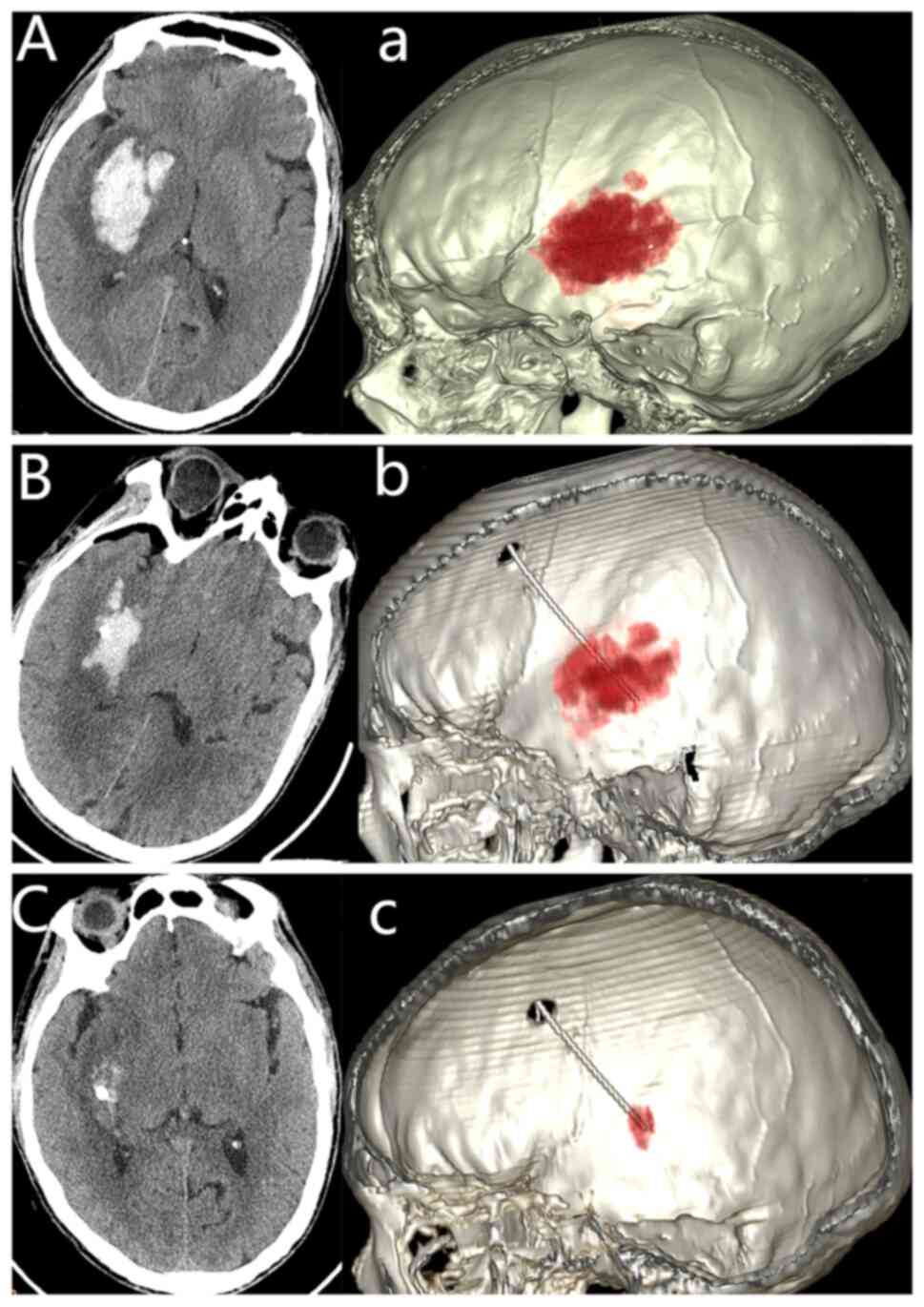
Case presentation from the study group. An
initial CT scan showed a right basal ganglia hemorrhage of 35 ml
(Fig. 1Aa), and a 3D
reconstruction of the hematoma was also performed (Fig. 1). A follow-up CT scan performed 6 h
postoperatively revealed a notable reduction in the volume and
density of the hematoma (Fig. 1B
and b). Another CT scan performed
at 48 h postoperatively (prior to the removal of the catheter from
the hematoma cavity) revealed that the hematoma had largely
resolved (Fig. 1C and c).
Statistical analysis
Statistical analyses were performed using SPSS
version 25.0 (IBM Corp.) or Excel 2019 (Microsoft), and GraphPad
Prism 10.0 software (Dotmatics) was used for visual representation
of the data. Continuous variables are presented as the mean ±
standard deviation (SD) or the median plus interquartile range
(IQR) depending on the distribution of the data. The normality of
data was assessed using the Shapiro-Wilk test. For comparisons
between continuous variables, the independent samples Student's
t-test was used for normally distributed data and the Mann-Whitney
U-test for non-normally distributed data. For the analysis of
paired data (comparisons between preoperative and postoperative
study and control groups), mixed ANOVA followed by Bonferroni post
hoc test was used for data conforming to the normal distribution,
while the Mann-Whitney U test followed by Bonferroni's correction
and the Friedman test followed by Dunn's post hoc test and
Bonferroni's correction were used for data not conforming to the
normal distribution. Categorical variables are presented as
frequencies and percentages, and differences between groups were
analyzed using the χ2 test or Fisher's exact test as
appropriate. P<0.05 was considered to indicate a statistically
significance difference.
Results
Demographic and baseline
characteristics
No statistical differences were identified in terms
of sex, age, the hematoma location, or Surgical operation time
between the study group and the control group (P>0.05; Table II). The distribution of hematoma
locations (supratentorial lobe, basal ganglia and thalamus,
cerebellum, and ventricles) did not reveal significant differences
between the groups (P>0.05). All patients underwent successful
surgical treatment. In the study group, the time from onset of the
symptoms to surgery was 13.20±6.25 h, the Control group was
10.17±5.29, no statistically significant between the two groups
(P>0.05 Table II). The
operation time was 32.20±4.69 min in the study group and 32.40±5.04
min in the control group, although no statistically significant
difference in the operation time was identified between the two
groups (P>0.05).
 | Table IIClinical characteristics of
patients. |
Table II
Clinical characteristics of
patients.
| Characteristic | Study group | Control group | P-value |
|---|
| Sex | | | 0.786 |
|
Male | 21 | 19 | |
|
Female | 9 | 11 | |
| Age, years | 64.80±10.56 | 64.30±9.77 | 0.850 |
| Preoperative GCS
score | 10.0 (9.0,
12.0) | 9.0 (8.0,
11.0) | 0.164 |
| Preoperative HV,
ml | 40.85±10.11 | 43.14±13.55 | 0.460 |
| Hematoma location,
n (%) | | | |
|
Supratentorial
lobe of the brain | | | 0.317 |
|
No | 24 (80.00) | 26 (86.67) | |
|
Yes | 6 (20.00) | 4 (13.33) | |
|
Basal
ganglia and thalamus | | | 1.000 |
|
No | 7 (23.33) | 7 (23.33) | |
|
Yes | 23 (76.67) | 23 (76.67) | |
|
Cerebellum | | | 0.157 |
|
No | 29 (96.67) | 27 (90.00) | |
|
Yes | 1 (3.33) | 3 (10.00) | |
|
Ventricles | | | 0.096 |
|
No | 19 (63.33) | 22 (73.33) | |
|
Yes | 11 (36.67) | 8 (26.67) | |
| Time from onset to
surgery, h | 13.20±6.25 | 10.17±5.29 | 0.047 |
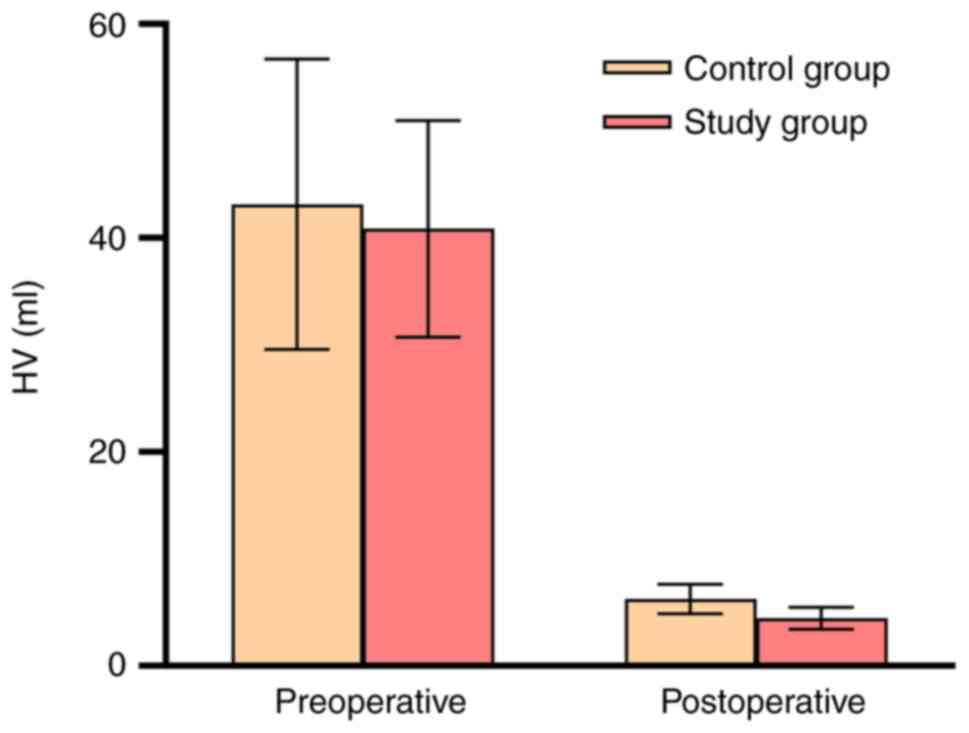
Hematoma and neurological outcomes. An
analysis was conducted to evaluate the differences in HV between
the two groups at preoperative and postoperative (extubation) time
points. The results indicated no significant differences in HV
between the two groups preoperatively (P>0.05; Fig. 2 and Table II). However, both the study and
control group exhibited a substantial reduction in hematoma
residuals postoperatively. Notably, the study group demonstrated
lower HV compared with the control group, which reached statistical
significance (P<0.001; Fig. 2
and Table III). Furthermore,
within-group analyses revealed significant reductions in HV
postoperatively compared with the preoperative time point for both
the control and study groups (P<0.05; Fig. 2).
 | Table IIIComparison of postoperative
characteristics between the two groups. |
Table III
Comparison of postoperative
characteristics between the two groups.
| Characteristic | Study group | Control group | P-value |
|---|
| HV at
extubation |
4.45±1.01a |
6.26±1.38a | <0.001 |
| Positioning and
puncture accuracy, n (%) | | | 0.011 |
|
No | 0 (0.00) | 7 (23.33) | |
|
Yes | 30 (100.00) | 23 (76.67) | |
| Operating time,
min | 32.20±4.69 | 32.40±5.04 | 0.874 |
| Drainage tube
retention time, h | 40.57±8.24 | 56.80±14.40 | <0.001 |
| Hematoma clearance,
% | 88.72±2.82 | 84.50±4.26 | <0.001 |
| Rebleeding rate, n
(%) | | | 0.326 |
|
No | 29 (96.67) | 28 (93.33) | |
|
Yes | 1 (3.33) | 2 (6.67) | |
| GCS score at 7 days
postoperatively | 13.0 (12.0,
14.0)a | 12.0 (11.0,
13.0)a | 0.017 |
| GCS score at
discharge | 15.0 (15.0,
15.0)a,b | 15.0 (14.0,
15.0)a,b | 0.015 |
| mRS score at 6
months | 2.0 (2.0, 3.0) | 2.0 (2.0, 3.0) | 0.869 |
| Postoperative
complications, n (%) | | | |
|
Intracranial
infection | | | 0.083 |
|
No | 30 (100.00) | 27 (90.00) | |
|
Yes | 0 (0.00) | 3 (10.00) | |
|
Intracranial
pneumatosis | | | 0.489 |
|
No | 22 (73.33) | 20 (66.67) | |
|
Yes | 8 (26.67) | 10 (33.33) | |
|
Scalp
infection/necrosis | | | 0.326 |
|
No | 30 (100.00) | 29 (96.67) | |
|
Yes | 0 (0.00) | 1 (3.33) | |
|
Seizures | | | 0.326 |
|
No | 28 (93.33) | 27 (90.00) | |
|
Yes | 2 (6.67) | 3 (10.00) | |
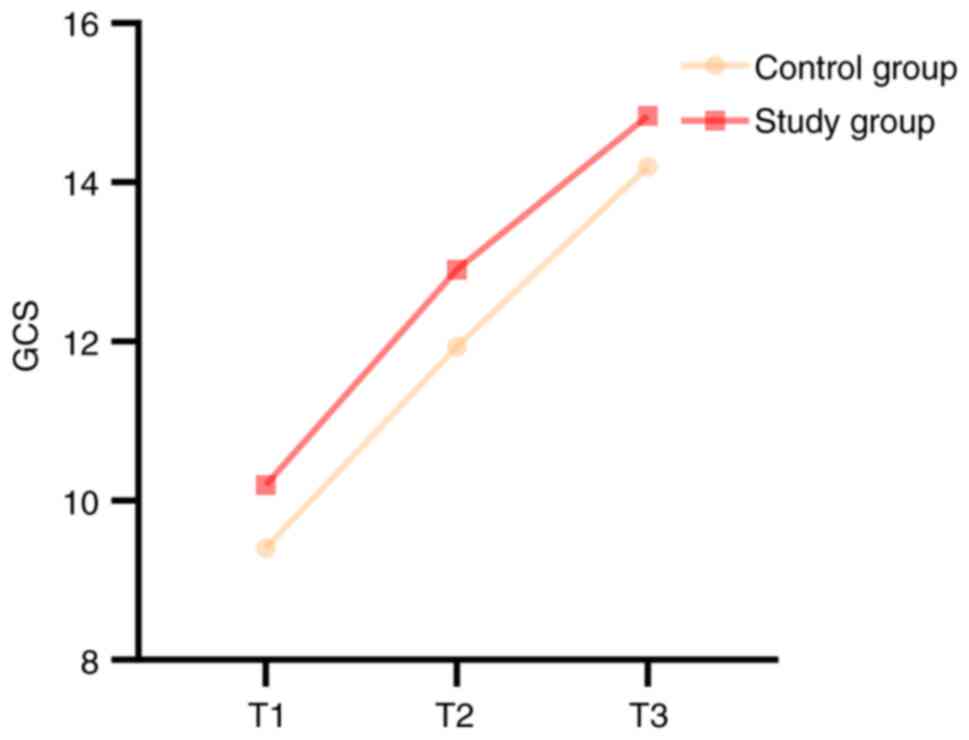
Glasgow coma scale (GCS) scores. The GCS
scores were assessed preoperatively, at 7 days postoperatively and
at discharge (Fig. 3). The results
demonstrated no significant differences in the GCS scores between
the two groups preoperatively (P>0.05; Table II). However, at both the 7-day
postoperative and discharge time points, the study group exhibited
significantly higher GCS scores compared with the control group
(P<0.05; Table III).
The results of within-group comparisons indicated
that the GCS scores at 7 days postoperatively were significantly
higher compared with those preoperatively in both the control and
study groups (P<0.05; Fig. 3
and Table III). Additionally,
the GCS scores at discharge were significantly higher compared with
those at both the preoperative and 7-day postoperative assessments
(P<0.05; Fig. 3 and Table III).
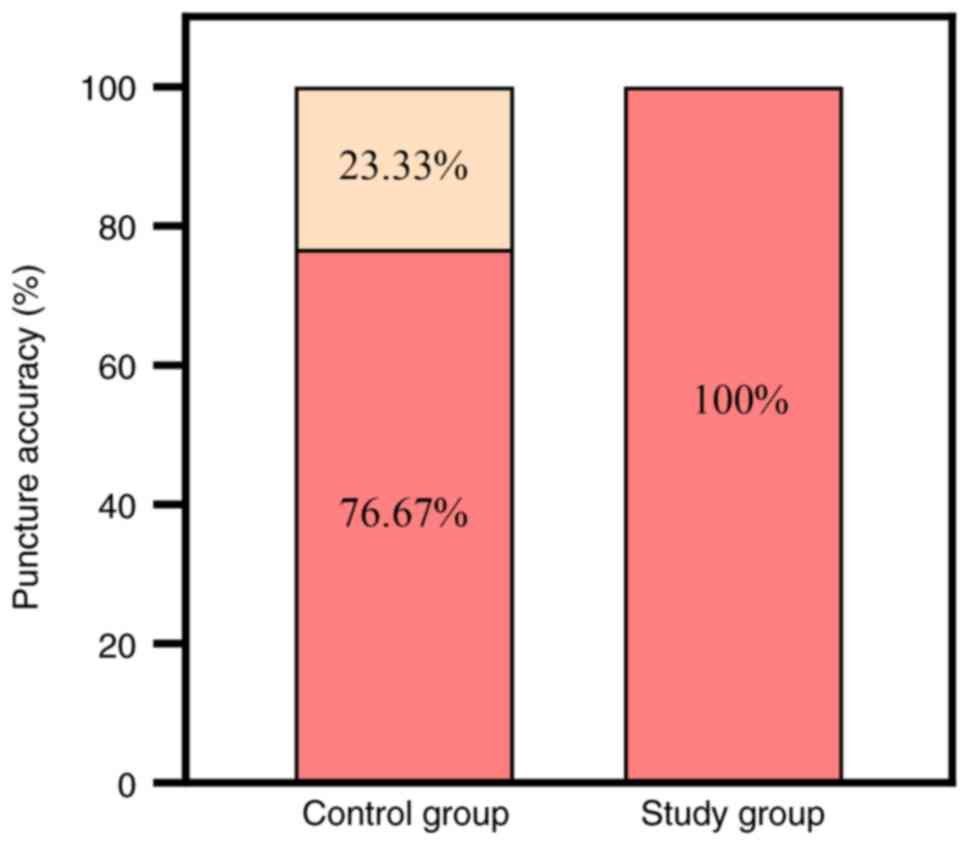
Surgical and postoperative outcomes. The
puncture localization and accuracy rate was 100% (30/30 patients)
in the study group and 76.67% (23/30 patients) in the control
group. The difference between the puncture accuracy rates of the
two groups was found to be statistically significant (P<0.05),
indicating a significant improvement in the study group compared
with the control group (Fig. 4 and
Table III). Postoperatively, the
drainage tube was left in place for 40.57±8.24 h in the study group
and 56.80±14.40 h in the control group. The difference in drain
retention time between the two groups was also found to be
statistically significant (P<0.001), and this was significantly
shorter in the study group compared with the control group
(Table III). In addition, the
residual hematoma in the study group was 4.45±1.01 ml, with a clot
clearance rate of 88.72±2.82%, whereas in the control group the
residual hematoma was 6.25±1.37 ml, and the clot clearance rate
reached 84.50±4.26% (Table III).
The difference in hematoma clearance rate between the two groups
was statistically significant (P<0.001), with the study group
exhibiting a significantly improved hematoma clearance rate
compared with the control group. These findings indicated that all
patients in both groups achieved the goals of <10 ml of residual
blood and >70% hematoma clearance. All patients regained
consciousness at discharge, and the median GCS score was 15.0 (IQR,
15.0, 15.0) in the study group and 15.0 (IQR, 14.0, 15.0) in the
control group (Table III). At
the end of the 6-month follow-up period, no patient had died or was
bedridden, and the majority of patients had a favorable
neurological prognosis (mRS score <3). In terms of comparing the
groups, 63.33% (19/30) of the patients in the study group had an
improved prognosis compared with 56.67% (17/30) of the patients in
the control group), and the median mRS score was 2.0 (IQR, 2.0,
3.0) in both groups (P=0.869; Table
III).
Complications and adverse events
Among the patients in the study group, 24 cases of
headache, 21 cases of hemiplegia, 9 cases of aphasia, 8 cases of
intracranial Pneumatosis and 2 cases of Seizures were reported,
while no cases of mild scalp necrosis were reported. In
intracranial pneumatosis, a total of 6 cases of mild intracranial
pneumatosis and 2 cases of moderate pneumatosis were located either
around the hematoma or below the frontal dura mate. In comparison,
among the patients in the control group, 25 cases of headache, 24
cases of hemiplegia, 11 cases of aphasia, 10 cases of intracranial
Pneumatosis, 3 cases of Seizures and 1 case of mild scalp necrosis
were reported. Neither group experienced rebleeding, hydrocephalus,
or cerebral infarction. No intracranial infections occurred in the
treatment group, whereas three cases of intracranial infection were
observed in the control group. Patients with seizures who were
treated with sodium valproate experienced relief of their
symptoms.
Discussion
ICH exerts physical pressure on surrounding
structures, impeding neural signaling and leading to neurological
deficits. Cytotoxic metabolites, the inflammatory response and
blood-brain barrier disruption resulting from ICH have all been
shown to trigger secondary brain injury (24). Early intervention and hematoma
removal are therefore essential to limit secondary damage and
improve prognosis (25). Reducing
ICP, improving cerebral perfusion, removing toxic metabolites and
preventing brain herniation are key to treatment (26,27).
Traditional soft-channel hematoma puncture and drainage are
associated with the risk of making errors and repeated puncture
(28,29), whereas stereotactic drainage can be
accurately localized, although it requires a second CT scan to be
performed, is complicated to operate and the supporting equipment
is expensive, which makes it difficult to be implemented in primary
hospitals (30,31). Craniotomy allows rapid hematoma
removal and hemostasis, although this procedure is prone to
intraoperative hemorrhage and brain tissue injury, also affecting
postoperative recovery due to high trauma. Compared with
craniotomy, neuroendoscopic hematoma debridement is a simpler,
shorter and more straightforward approach; however, this technique
is limited by two-dimensional imaging, high operator-training
requirements, the difficulty in dealing with potentially massive
hemorrhage and high costs, which limits its applicability in less
developed regions (32,33). YL-1 hard-channel aspiration is
mostly used in primary hospitals in China, and although it is an
effective method, it carries the risks of brain tissue damage,
rebleeding, inaccurate localization, infection and epilepsy
(34,35). Despite the promise provided by
precision soft-channel technology in neurosurgery, its application
in resource-limited areas is constrained by the shortage of
resources, facilities and personnel. These areas usually lack both
precise instruments for surgical localization and the necessary
infrastructure and monitoring systems (36). Therefore, there is a need to
develop stereotactic alternatives that are cost-effective, safe and
precise.
The present study investigated a novel approach of
stereotactic aspiration in patients with ICH using laser-guided
localization in combination with soft-channel MIS. The laser
soft-channel MIS combined with urokinase thrombolysis (LAS-MISTIE)
trial (37) concluded that a
higher proportion of patients with a residual HV of <10 ml had
improved clinical outcomes in the experimental group compared with
the hard-channel group. Specifically, ~73.3% of patients with a
residual HV of <10 ml had favorable clinical outcomes. In the
present study, all patients in the study group had a residual HV of
<10 ml, and 63.33% had an mRS score <3 at the end of the
study.
In the study group, the time from onset of the
symptoms to surgery was 13.20±6.25 h, which is consistent with
emergency surgery. The puncture accuracy was higher in the
experimental group compared with the control group. In addition,
drainage tube retention time in the study group was shorter than in
the control group, Theoretically, shorter drain retention times may
reduce the risk of intracranial infection. The design of the
laser-guided puncture path was simply achieved. Through combining
basic CT scanning parameters with the optics of laser technology,
the method allows for flexible selection of puncture points and
directions, avoiding critical brain areas and blood vessels. The
puncture path is aligned with the long axis of the brain's nerve
fibers, and the use of soft-channel material effectively reduces
additional damage to normal brain tissue. The procedure is suited
for treatment of small hematomas in functional areas, the deep
brain and posterior cranial fossa (38).
The anatomic location and size of the hematoma are
key factors in determining the success of surgical removal. MISTIE
offers potential advantages over medical treatment in the
management of supratentorial hemorrhage, including a reduction in
HV and peripheral edema, with minimal damage to healthy brain
tissue. This approach may subsequently reduce mortality (39). Hemorrhage in deep brain structures,
such as the basal ganglia, thalamus and internal capsule, is
associated with high morbidity and poor functional prognosis.
Postoperative neurological deficits are a major concern,
necessitating individualized therapeutic strategies and early
intervention (40).
The present study demonstrated that early removal of
the basal ganglia and internal capsule hematoma protects motor
function, whereas thalamic hemorrhage often results in sensory and
cognitive deficits (41). This
underscores the need for careful balancing of risks and benefits
due to the complexity of the neural networks involved. Treatment of
lobar hemorrhage requires the preservation of cortical function for
optimal postoperative recovery, with procedures tailored to
specific functional areas (42).
For frontal lobe hematomas, particularly those near the motor
cortex, LAS-MISUT facilitates the precise preservation of motor
pathways, enhancing functional outcomes. However, larger hematomas
may limit the feasibility of minimally invasive methods. In the
temporal lobe, LAS-MISUT minimizes cognitive and sensory deficits,
especially near structures such as the hippocampus, but may be less
effective for deeper lesions. In cases of occipital hemorrhage, the
purpose of LAS MISUT is to reduce the risk of damage to the visual
pathways (43-45).
For intraventricular hemorrhage (IVH), which often
leads to hydrocephalus and is exacerbated by inflammatory responses
to blood degradation products, external ventricular drainage (EVD)
remains the primary treatment, particularly in cases with cast-like
hematoma or elevated ICP (46).
While MISTIE techniques, such as endoscopic or stereotactic
hematoma removal, offer precision, they may not fully substitute
for EVD in cases of persistent cerebrospinal fluid flow obstruction
(47). In patients with
moderate-to-large IVH and Severe ventricular cast haematoma EVD
placement alone has been associated with improved survival,
although prognosis remains poor for those with concomitant thalamic
hemorrhage (48).
Regarding posterior cranial fossa hematomas,
spontaneous cerebellar hemorrhages are associated with
hydrocephalus, brainstem compression and posterior fossa
herniation. To reduce mortality, urgent surgical removal of the
hematoma is recommended over conservative treatment, especially if
the hematoma is >3 cm, or if hydrocephalus or brainstem
involvement is present (49). For
larger hematomas, suboccipital craniotomy is recommended, whereas
for smaller hematomas (≤3 cm), the MISTIE technique may be
considered to prevent neurological deterioration (47). Hemorrhages near the brainstem or
fourth ventricle may require more aggressive surgical intervention,
while hemorrhages located more laterally are more amenable to
MISTIE (50). The comparison
between endoscopic or stereotactic aspiration and conventional
suboccipital craniectomy requires further study.
Brainstem hemorrhages pose significant challenges
due to their control over vital functions. Hematomas >1.5 cm
with severe neurological deficits (Respiratory depression,
circulatory instability, profound impairment of consciousness, and
pupil abnormalities) may benefit from early surgical intervention,
but only when the benefits clearly outweigh the risks (51). MISTIE techniques, such as
LAS-MISUT, offer a potential alternative for moderate-sized
hemorrhages (≤1.5 cm) or in patients with contraindications to
conventional surgery. Multidisciplinary management remains
essential.
In addition, the age, comorbidities and initial
bleeding of the patient are key prognostic factors (52). Elderly patients may exhibit
decreased hematoma clearance and postoperative recovery rates due
to poor neuroplasticity, and also present a weaker ability to
recover following brain injury. Furthermore, a larger initial
hemorrhage volume increases the difficulty of clearance, especially
when the hematoma extends to multiple brain regions or important
structures; therefore, individualized treatment plans for high-risk
patients are needed to improve surgical success and long-term
prognosis.
Future research should stratify patients by ICH
subtype to better evaluate the efficacy of MISTIE across different
hemorrhage types. There is a need for more robust clinical trial
data comparing MIS technologies in ICH, as surgeon expertise,
complete hematoma evacuation and reduced rebleeding risks may offer
advantages over individual techniques. Ongoing randomized
controlled trials will provide valuable insights into these
considerations.
From a pathophysiological perspective, minimizing
collateral damage to surrounding tissues is essential for reducing
secondary inflammatory responses, edema and subsequent neuronal
injury (53). The soft-channel MIS
approach offers notable advantages, allowing atraumatic access to
the hematoma with minimal pressure on the adjacent brain tissue. By
contrast, conventional techniques, such as YL-1 puncture, may cause
greater tissue disruption and higher rates of iatrogenic injury.
The present study demonstrated that the integration of laser
guidance with soft-channel technology enhances targeting precision,
promoting more complete hematoma evacuation, reducing residual clot
volume and preventing sustained ICP elevation, thereby lowering the
risk of secondary ischemic injury, cerebral infarction and
hydrocephalus. This approach also accelerates hematoma clearance
and improves neurological recovery, as evidenced by the improved
GCS scores in patients. One of the primary objectives of future
research is to mitigate post-ICH brain injury by addressing key
pathological mechanisms, including inflammation, oxidative stress
and excitotoxicity, which are key drivers of neuronal damage
following ICH) (54).
Additionally, advancements in minimally invasive techniques, such
as laser-guided soft-channel surgery, represent a promising avenue
for enhancing clinical outcomes and warrant further investigation.
Further studies are required, however, to assess long-term outcomes
and broader clinical applicability.
In the present study, the major complication
associated with surgery was intracranial pneumatosis. A total of 6
cases of mild intracranial pneumatosis and 2 cases of moderate
pneumatosis were located either around the hematoma or below the
frontal dura mater. In none of the cases, however, did intracranial
pneumatosis result in consequential complications, such as
increased ICP or nerve fiber damage. A total of 2 cases of seizure
patients treated with sodium valproate were observed, which were
resolved following treatment with the extended-release tablets of
sodium valproate. No cases of postoperative rebleeding,
hydrocephalus, cerebral infarction or intracranial infection were
reported.
To minimize damage to nerve fibers and shorten the
drainage time, the following measures were taken: i) Precise
positioning and stereotactic application using CT data to design a
path through the frontal lobe to bypass the vessel; ii) gentle
suctioning to minimize excessive ICP fluctuations; iii) planned
puncture along the long axis of the hematoma using a porous
soft-access channel to facilitate post-thrombolytic drainage; and
iv) use of a soft-access channel, rather than a rigid needle, to
perform stereotactic aspiration. Previously, the use of a rigid
needle required drilling through the skull with direct entry into
the hematoma cavity, necessitating penetration of the scalp, skull
and dura mater. Furthermore, the high-speed rotation of the rigid
metal needle caused significant additional damage. In addition, the
lack of a 3D view of the hematoma and preoperative surface
localization often resulted in inaccurate targeting, which could
impede hematoma clearance and increase the risk of intracranial
infection and rebleeding, ultimately compromising surgical outcomes
(55).
In conclusion, the present study presented a MIS
technique for the stereotactic treatment of ICH. The results
obtained suggested that the LAS-MISUT technique used is a safe and
effective treatment for ICH, as demonstrated in a cohort-controlled
clinical trial. This method ensures precise puncture, significantly
improves hematoma clearance and yields a favorable prognosis. In
addition, its low cost makes it suitable for patients with ICH in
underdeveloped countries. However, the generalizability of the
results is limited by the single-center study design and small
sample size, and further studies with larger clinical samples are
required to address these limitations.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Joint project of
Chongqing Health Commission and Science and Technology Bureau
(grant no. 2024MSXM163) and Science and Technology of Nanchuan
District, Chongqing (grant no. Cx 202209).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
AC conceived the study, the design of the
methodology and writing, reviewing and editing the manuscript. JS
was responsible for the conception of the study, the formal
analysis of the data, reviewing and editing the manuscript. JP and
TL performed the software analyses and data curation. LC and QW
performed CT images. AC, JS, LC, WQ and TL confirm the authenticity
of all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Ethics approval for the present study was obtained
from the Ethics Committee of Nanchuan Hospital of Chongqing Medical
University (approval no. YXYJ-2022-013; Chongqing, China), and
written informed consent was obtained from all patients before the
study began.
Patient consent for publication
Written informed consent for publication was
obtained from all participants involved in the present study. The
consent process adhered to ethics guidelines and institutional
protocols to ensure that patients were fully informed about the
nature, scope and potential implications of the publication of
their clinical data. All patient information was anonymized to
protect privacy and confidentiality, in accordance with the
principles of The Declaration of Helsinki.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ihara M and Yamamoto Y: Emerging evidence
for pathogenesis of sporadic cerebral small vessel disease. Stroke.
47:554–560. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Woo D, Comeau ME, Venema SU, Anderson CD,
Flaherty M, Testai F, Kittner S, Frankel M, James ML, Sung G, et
al: Risk factors associated with mortality and neurologic
disability after intracerebral hemorrhage in a racially and
ethnically diverse cohort. JAMA Netw Open.
5(e221103)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhang S, Wang Z, Zheng A, Yuan R, Shu Y,
Zhang S, Lei P, Wu B and Liu M: Blood pressure and outcomes in
patients with different etiologies of intracerebral hemorrhage: A
multicenter cohort study. J Am Heart Assoc.
9(e016766)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tu WJ and Wang LD: Special Writing Group
of China Stroke Surveillance Report. China stroke surveillance
report 2021. Mil Med Res. 10(33)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kaur P and Sharma S: Recent advances in
pathophysiology of traumatic brain injury. Curr Neuropharmacol.
16:1224–1238. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
McGurgan IJ, Ziai WC, Werring DJ, Al-Shahi
Salman R and Parry-Jones AR: Acute intracerebral haemorrhage:
Diagnosis and management. Pract Neurol. 21:128–136. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rabinstein AA, Atkinson JL and Wijdicks
EFM: Emergency craniotomy in patients worsening due to expanded
cerebral hematoma: To what purpose? Neurology. 58:1367–1372.
2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Akhigbe T and Zolnourian A: Role of
surgery in the management of patients with supratentorial
spontaneous intracerebral hematoma: Critical appraisal of evidence.
J Clin Neurosci. 39:35–38. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hanley DF, Thompson RE, Muschelli J,
Rosenblum M, McBee N, Lane K, Bistran-Hall AJ, Mayo SW, Keyl P,
Gandhi D, et al: Safety and efficacy of minimally invasive surgery
plus alteplase in intracerebral haemorrhage evacuation (MISTIE): A
randomized, controlled, open-label, phase 2 trial. Lancet Neurol.
15:1228–1237. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kobata H and Ikeda N: Recent updates in
neurosurgical interventions for spontaneous intracerebral
hemorrhage: Minimally invasive surgery to improve surgical
performance. Front Neurol. 12(703189)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Scaggiante J, Zhang X, Mocco J and Kellner
CP: Minimally invasive surgery for intracerebral hemorrhage: An
updated meta-analysis of randomized controlled trials. Stroke.
49:2612–2620. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mansour A, Loggini A, El Ammar F,
Alvarado-Dyer R, Polster S, Stadnik A, Das P, Warnke PC, Yamini B,
Lazaridis C, et al: Post-trial enhanced deployment and technical
performance with the MISTIE procedure per lessons learned. J Stroke
Cerebrovasc Dis. 30(105996)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hou D, Lu Y, Wu D, Tang Y and Dong Q:
Minimally invasive surgery in patients with intracerebral
hemorrhage: A meta-analysis of randomized controlled trials. Front
Neurol. 12(789757)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Roth A, Buttrick SS, Cajigas I, Jagid JR
and Ivan ME: Accuracy of frame-based and frameless systems for deep
brain stimulation: A meta-analysis. J Clin Neurosci. 57:1–5.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Thanvi BR, Sprigg N and Munshi SK:
Advances in spontaneous intracerebral haemorrhage. Int J Clin
Pract. 66:556–564. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Alerhand S and Lay C: Spontaneous
intracerebral hemorrhage. Emerg Med Clin North Am. 35:825–845.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tosi U and Souweidane MM: The future of
neuroendoscopy: Looking ahead through a lens. World Neurosurg.
178:311–316. 2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Eftekhar B: A smartphone app to assist
scalp localization of superficial supratentorial lesions-technical
note. World Neurosurg. 85:359–363. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sack J, Steinberg JA, Rennert RC, Hatefi
D, Pannell JS, Levy M and Khalessi AA: Initial experience using a
high-definition 3-dimensional exoscope system for
microneurosurgery. Oper Neurosurg (Hagerstown). 14:395–401.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sternbach GL: The Glasgow coma scale. J
Emerg Med. 19:67–71. 2000.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tang D, Soto JM and Zhang L: A novel
laser-based stereotactic localization device for intracranial mass
resection. Brain Hemorrhages. 2:106–110. 2021.
|
|
22
|
Kothari RU, Brott T, Broderick JP, Barsan
WG, Sauerbeck LR, Zuccarello M and Khoury J: The ABC of measuring
cerebral haemorrhage volume. Stroke. 27:1304–1305. 1996.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Haggag H and Hodgson C: Clinimetrics:
Modified Rankin scale (mRS). J Physiother. 68(281)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Keep RF, Hua Y and Xi G: Intracerebral
haemorrhage: Mechanisms of injury and therapeutic targets. Lancet
Neurol. 11:720–731. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Muschelli J, Sweeney EM, Ullman NL, Vespa
P, Hanley DF and Crainiceanu CM: PItcHPERFeCT: Primary intracranial
hemorrhage probability estimation using random forests on CT.
Neuroimage Clin. 14:379–390. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Patel S, Maria-Rios J, Parikh A and Okorie
ON: Diagnosis and management of elevated intracranial pressure in
the emergency department. Int J Emerg Med. 16(72)2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Canac N, Jalaleddini K, Thorpe SG,
Thibeault CM and Hamilton RB: Review: Pathophysiology of
intracranial hypertension and noninvasive intracranial pressure
monitoring. Fluids Barriers CNS. 17(40)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liang KS, Ding J, Yin CB, Peng LJ, Liu ZC,
Guo X, Liang SY, Zhang Y and Zhou SN: Clinical study on minimally
invasive liquefaction and drainage of intracerebral hematoma in the
treatment of hypertensive putamen hemorrhage. Technol Health Care.
25:1061–1071. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Cao Y, Yu S, Zhang Q, Yu T, Liu Y, Sun Z,
Zhao M, Wang W and Zhao JZ: Chinese Stroke Association Stroke
Council Guideline. Chinese stroke association guidelines for
clinical management of cerebrovascular disorders: Executive summary
and 2019 update of clinical management of intracerebral
haemorrhage. Stroke Vasc Neurol. 5:396–402. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Akhigbe T, Okafor U, Sattar T, Rawluk D
and Fahey T: Stereotactic-guided evacuation of spontaneous
supratentorial intracerebral hemorrhage: Systematic review and
meta-analysis. World Neurosurg. 84:451–460. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Choo YS, Chung J, Joo JY, Kim YB and Hong
CK: Borderline basal ganglia hemorrhage volume: Patient selection
for good clinical outcome after stereotactic catheter drainage. J
Neurosurg. 125:1242–1248. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wu R, Zhang J, Wang Z, Wang Z, Zhang X and
Yun Q: Clinical effects of neuroendoscopic hematoma evacuation for
hypertensive intracerebral hemorrhage. Am J Transl Res.
14:1084–1091. 2022.PubMed/NCBI
|
|
33
|
Xu L, Lu X, Zhang C and Wang W: Clinical
efficacy of neuroendoscopy combined with intracranial pressure
monitoring for the treatment of hypertensive intracerebral
hemorrhage. World Neurosurg. 187:e210–e219. 2024.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang Z, Qu J and Zhao H: Comparison of the
clinical effects of stereotactic aspiration and craniotomies in the
treatment of hypertensive intracerebral hemorrhages. Int J Clin Exp
Med. 12:5357–5364. 2019.
|
|
35
|
Hu JL, Zhang C and Li JM: Curative effect
of minimally invasive puncture and drainage assisted with alteplase
on treatment of acute intracerebral hemorrhage. J Acute Dis.
6:28–32. 2017.
|
|
36
|
Ojo OA and Onyia CU: Proposal of
modification in management strategy for intracranial hemorrhage in
low- and middle-income countries. Clin Neurol Neurosurg. 181:21–23.
2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Tang D, Liu J, Xiao C, Xie D, Fu X, Cui S,
He B, Li M and Zhang L: A novel frameless laser stereotaxis system
for neurosurgical interventions. World Neurosurg. 174:175–182.
2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wang WM, Jiang C and Bai HM: New insights
in minimally invasive surgery for intracerebral hemorrhage. Front
Neurol Neurosci. 37:155–165. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Greenberg SM, Ziai WC, Cordonnier C,
Dowlatshahi D, Francis B, Goldstein JN, Hemphill JC III, Johnson R,
Keigher KM, Mack WJ, et al: 2022 Guideline for the management of
patients with spontaneous intracerebral hemorrhage: A guideline
from the American heart association/American stroke association.
Stroke. 53:e282–e361. 2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Sondag L, Schreuder FHBM, Boogaarts HD,
Rovers MM, Vandertop WP, Dammers R and Klijn CJM: Dutch ICH Surgery
Trial Study Group, part of the CONTRAST consortium†. Neurosurgical
intervention for supratentorial intracerebral hemorrhage. Ann
Neurol. 88:239–250. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Obayashi S: Cognitive and linguistic
dysfunction after thalamic stroke and recovery process: Possible
mechanism. AIMS Neurosci. 9:1–11. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Shoamanesh A, Patrice Lindsay M,
Castellucci LA, Cayley A, Crowther M, de Wit K, English SW, Hoosein
S, Huynh T, Kelly M, et al: Canadian stroke best practice
recommendations: Management of spontaneous intracerebral
hemorrhage, 7th edition update 2020. Int J Stroke. 16:321–341.
2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chen Y, Li C, Wang Q and Li Z: C-arm CT
scanning combined with simple laser device-assisted puncture
therapy for cerebellar hemorrhage. Front Surg.
11(1421517)2024.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Yan L, Luo J, Ling YC, Dongxue W, Yaxiong
L, Conghui L and Wenchao Z: Comparative analysis of stereotactic
soft-channel and hard-channel aspiration in the treatment of
primary brainstem hemorrhage. J Stroke Cerebrovasc Dis.
33(107956)2024.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zheng Z, Wang Q, Sun S and Luo J:
Minimally invasive surgery for intracerebral and intraventricular
hemorrhage. Front Neurol. 13(755501)2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Haldrup M, Miscov R, Mohamad N, Rasmussen
M, Dyrskog S, Simonsen CZ, Grønhøj M, Poulsen FR, Bjarkam CR,
Debrabant B and Korshøj AR: Treatment of intraventricular
hemorrhage with external ventricular drainage and fibrinolysis: A
Comprehensive systematic review and meta-analysis of complications
and outcome. World Neurosurg. 174:183–196.e6. 2023.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Miyamoto S, Ogasawara K, Kuroda S,
Itabashi R, Toyoda K, Itoh Y, Iguchi Y, Shiokawa Y, Takagi Y,
Ohtsuki T, et al: Japan stroke society guideline 2021 for the
treatment of stroke. Int J Stroke. 17:1039–1049. 2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Peng S, Koch MJ and Amin-Hanjani S:
Spontaneous Intracerebral Hemorrhage (Including posterior fossa).
Acute care neurosurgery by case management: Pearls and Pitfalls.
Springer International Publishing, Cham, pp173-188, 2022.
|
|
49
|
Singh SD, Schreuder FHBM, van
Nieuwenhuizen KM, Jolink WM, Senff JR, Goldstein JN, Boogaarts J,
Klijn CJM, Rinkel GJE and Brouwers HB: Secondary hematoma
evacuation and outcome after initial conservative approach for
patients with cerebellar hematoma larger than 3 cm. Neurocrit Care.
35:680–686. 2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Gupta N: Neurosurgery: Posterior fossa
surgery. Essentials of Geriatric Neuroanesthesia, pp83-103,
2019.
|
|
51
|
Steiner T, Al-Shahi Salman R, Beer R,
Christensen H, Cordonnier C, Csiba L, Forsting M, Harnof S, Klijn
CJ, Krieger D, et al: European stroke organisation (ESO) guidelines
for the management of spontaneous intracerebral hemorrhage. Int J
Stroke. 9:840–855. 2014.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Podolsky-Gondim GG, Cardoso R, Zucoloto
Junior EL, Grisi L, Medeiros M, De Souza SN, Santos MV and Colli
BO: Traumatic brain injury in the elderly: Clinical features,
prognostic factors, and outcomes of 133 consecutive surgical
patients. Cureus. 13(e13587)2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Magid-Bernstein J, Girard R, Polster S,
Srinath A, Romanos S, Awad IA and Sansing LH: Cerebral hemorrhage:
Pathophysiology, treatment, and future directions. Circ Res.
130:1204–1229. 2022.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Shao L, Chen S and Ma L: Secondary brain
injury by oxidative stress after cerebral hemorrhage: Recent
advances. Front Cell Neurosci. 16(853589)2022.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Pan J, Chartrain AG, Scaggiante J, et al:
A summary of modern minimally invasive cerebral haemorrhage removal
techniques. Neurosurgery. 18:710–720. 2020.
|