Introduction
Chronic myeloid leukemia (CML), a clonal
myeloproliferative neoplasm arising from pluripotent hematopoietic
stem cells, is characterized by the Philadelphia chromosome and the
resulting BCR-ABL1 fusion gene (1,2).
This genetic abnormality leads to the production of a
constitutively active tyrosine kinase, which drives the
pathogenesis of CML (3,4). While CML typically follows a
predictable course through chronic, accelerated and blast crisis
phases, its clinical presentation may be heterogeneous. A subset of
patients with CML present with marked thrombocytosis and when the
platelet count reaches or exceeds 1,000x109/l, the
condition is defined as CML with thrombocytosis (CML-T). This
marked thrombocytosis significantly increases the risk of
thromboembolic events, making CML-T a clinically challenging
subtype of CML (5-7).
Distinguishing CML-T from other myeloproliferative neoplasms,
particularly essential thrombocythemia (ET), which is also
characterized by elevated platelet counts (≥450x109/l),
is paramount for accurate diagnosis and treatment (8). Although early clinical presentations
can be similar, the underlying genetic abnormalities and
therapeutic approaches differ. Management of ET primarily focuses
on symptom control with agents like aspirin, hydroxyurea,
anagrelide, or interferon-α (IFN-α), but these treatments are
generally not curative (9). By
contrast, CML-T, like other forms of CML, relies on tyrosine kinase
inhibitors (TKIs), such as imatinib, to target the BCR-ABL1 fusion
protein and control disease progression (10). Combining imatinib with IFN-α has
shown synergistic potential in CML, potentially leading to improved
outcomes (11,12). However, there is no established
standard of care for CML-T, especially in cases with extreme
thrombocytosis, and the optimal treatment strategy remains to be
defined and warrants further study. The present report described a
patient with CML-T presenting with an exceptionally high platelet
count of 3,798x109/l and the unusual finding of normal
spleen size, posing a significant diagnostic challenge. The
patient's successful treatment with imatinib and interferon-α,
resulting in complete hematological and cytogenetic remission,
highlights the potential of this combination therapy in managing
this rare and complex clinical entity. The present case underscored
the need for further research into the efficacy and safety of
combination therapy in CML-T, particularly in cases with extreme
thrombocytosis.
Case presentation
Patient information
A 66-year-old female patient was admitted to
Shandong Second Medical University (Weifang, China) in August 2023
with recurrent chest tightness and pain. The patient's medical
history was notable for hypertension, diabetes mellitus and
coronary artery disease for 20 years. The family history was
noncontributory for thrombocytosis or other hematologic
malignancies. The present case report was approved by the Medical
Ethics Committee of the Affiliated Hospital of Shandong Second
Medical University (approval no. wyfy-2024-qt-051; date of
approval: September 18, 2024; Weifang, China).
Diagnosis
On presentation, the patient's platelet count was
markedly elevated at 3,798x109/l (normal
range:150-450x109/l). Peripheral blood smear analysis
revealed 5% blasts. Bone marrow aspiration and biopsy were
performed as part of the diagnostic workup. Peripheral blood and
bone marrow aspirate smears were collected before treatment and
stained with Wright-Giemsa stain for 1 min at room temperature,
followed by staining with a buffer solution for ~15 min at room
temperature. The slides were then examined microscopically at
1,000x magnification. The bone marrow biopsy sample was fixed in 4%
neutral buffered formalin at room temperature for at least 6 h,
underwent gradient ethanol dehydration, xylene clearing and
paraffin embedding following standard protocols, was sectioned at 3
µm thickness and stained with hematoxylin and eosin at room
temperature for 3 min each. Microscopic evaluation was performed at
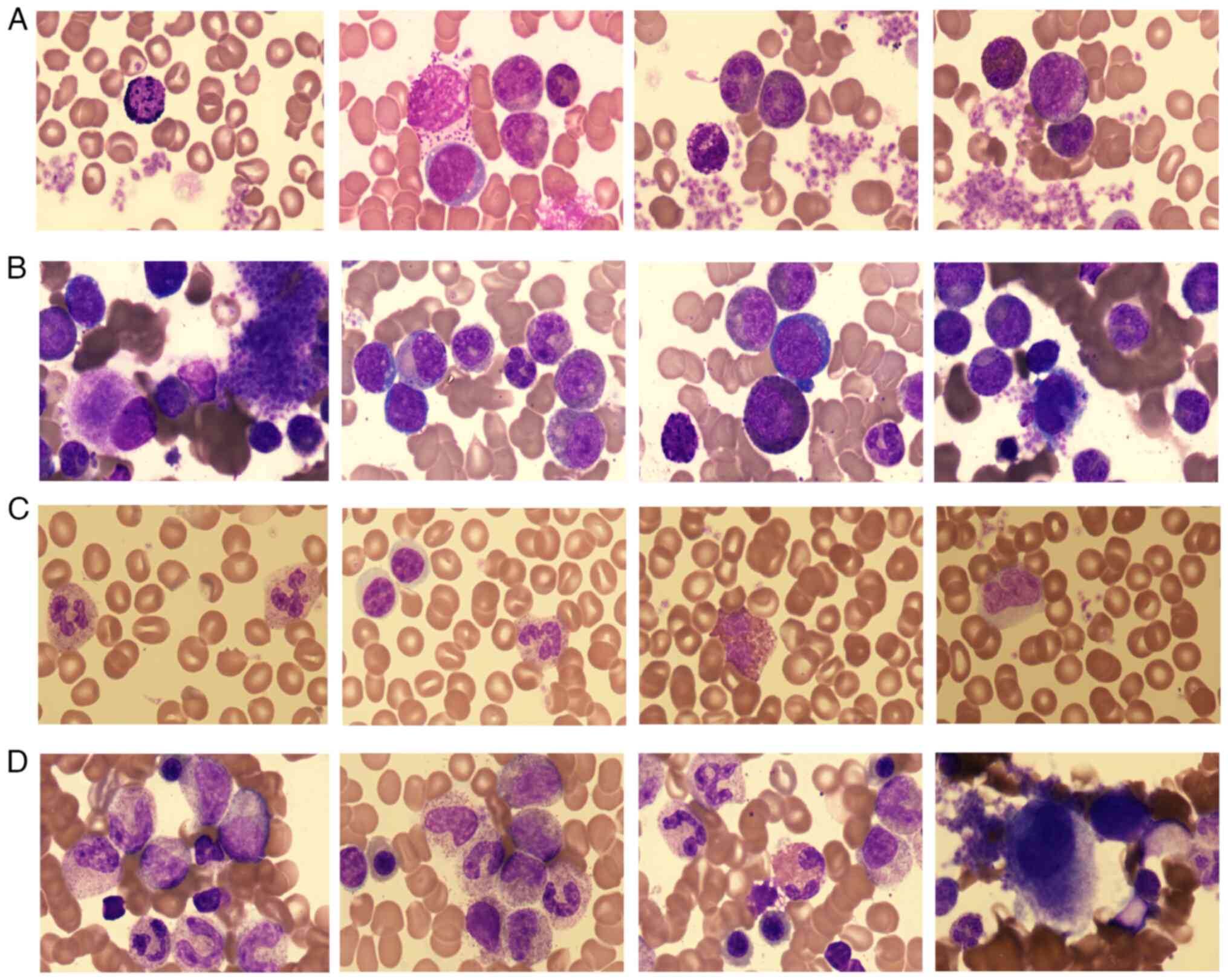
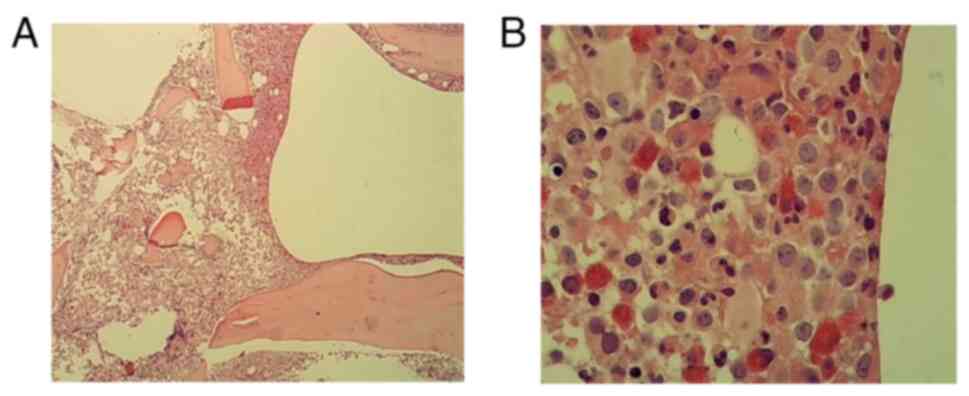
x40 and x400 magnification. Results showed hypercellularity with
myeloid predominance, marked megakaryocytic hyperplasia and
prominent platelet aggregation (Fig.
1), as well as a markedly cellular marrow with an increased
myeloid-to-erythroid ratio and a significant increase in
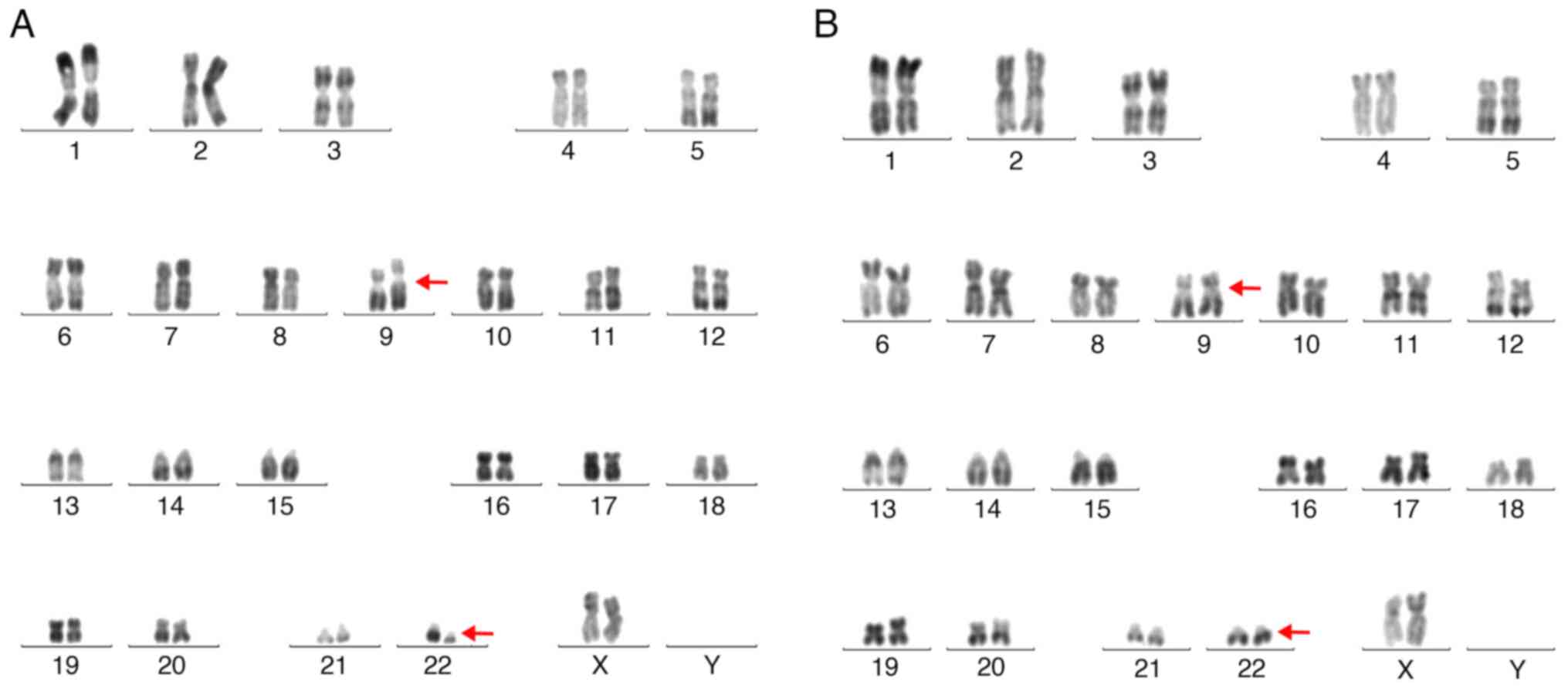
predominantly small megakaryocytes on biopsy (Fig. 2). The cytogenetic analysis
identified the Philadelphia chromosome t (9;22) (q34;q11.2;
Fig. 3). Reverse
transcription-quantitative PCR (RT-qPCR) was performed to detect
the BCR-ABL1 p210 transcript, with an expression level of 70.78% on
the International Scale (IS) (Fig.
4). RNA was extracted from 1x106 cells using the
Lab-Aid 896 Blood Total RNA Extraction Kit (Xiamen Zeesan Biotech
Co., Ltd.). RNA purity and concentration were assessed using a
Thermo Scientific NanoDrop 2000 Spectrophotometer (Thermo Fisher
Scientific, Inc.). cDNA synthesis was performed, and qPCR was
carried out using TaqMan Gene Expression Master Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.) in a 20 µl reaction
volume. The forward primer sequences for BCR-ABL1 p210 were
5'-TCCGCTGACCATCAACAAGGA-3' and 5'-TCCGCTGACCATCAATAAGGA-3', and
the reverse primer sequence was 5'-CACTCAGACCCTGAGGCTCAA-3'. ABL1
served as the reference gene with the following primer sequences:
Forward 5'-TGGAGATAACACTCTAAGCATAACTAAAGGT-3' and reverse
5'-GATGTAGTTGCTTGGGACCCA-3'. PCR cycling conditions were: 50˚C for
20 min, 95˚C for 10 min, followed by 40 cycles of 95˚C for 15 sec
and 60˚C for 60 sec. Quantification was performed using the
standard curve method. Experiments were performed with three
biological replicates, each in triplicate (technical replicates).
To exclude ET, targeted sequencing was performed to screen for
mutations within CALR (exon 9), JAK2 (exons 12,14, and 16), MPL
(exon 10), and CSF3R (exons 14 and 17), which represent the most
frequent mutational hotspots in myeloproliferative neoplasms, and
no mutations were detected in this analysis.
Echocardiography demonstrated left ventricular
hypertrophy and a reduced left ejection fraction, with an LVEF of
55% (normal range, 50-70%) and an electrocardiogram showed ST-T
segment changes and T-wave inversion. All cardiac enzymes were
within normal limits except for an elevated NT-proBNP level of
2,914.34 pg/ml (normal <125 pg/ml). Chest and abdominal computed
tomography scans showed no evidence of pulmonary embolism or
hepatosplenomegaly. Liver and kidney function tests and lipid
profile were within normal limits. Based on these findings, the
patient was diagnosed with extreme CML-T complicated by acute
myocardial infarction.
Treatment and outcomes
At the initiation of treatment, the patient's
platelet count was markedly elevated at 3,798x109/l,
along with a white blood cell count of 38.75x109/l and a
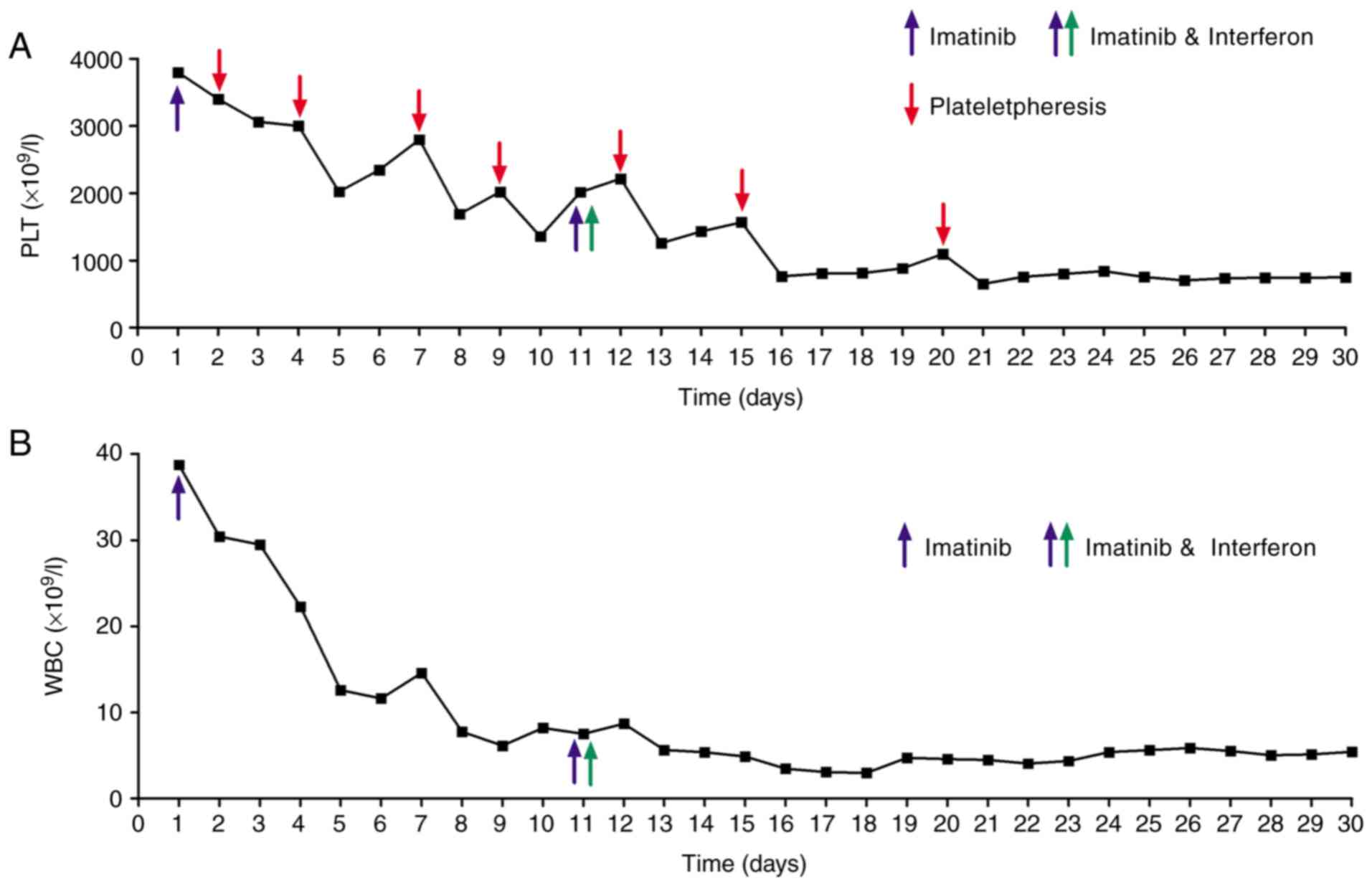
hemoglobin level of 117 g/l. Imatinib was initiated at 400 mg once
daily. Imatinib therapy promptly normalized the leukocyte count;
however, the reduction in platelet count was less pronounced. To
mitigate the risk of thrombosis due to extreme thrombocytosis, the
patient received seven sessions of therapeutic plateletpheresis.
Despite these interventions, the platelet count remained at
1,356x109/l on day 10. After 10 days of imatinib
monotherapy, the patient experienced episodes of chest tightness,
shortness of breath and angina. Electrocardiography findings were
consistent with acute subendocardial myocardial infarction. At the
time of these cardiac events, the patient's platelet count was
still markedly elevated at 1,356x109/l, suggesting a
potential correlation between the extreme thrombocytosis and the
myocardial infarction. Following treatment with aspirin,
ticagrelor, isosorbide mononitrate and rosuvastatin, the patient's
symptoms subsequently improved. Subsequent BCR-ABL1 kinase domain
mutation analysis revealed no DNA or amino acid mutations,
excluding imatinib resistance. Given the inadequate response of
thrombocytosis to imatinib monotherapy, IFN-α was initiated on day
11 at a dose of 30 µg once daily via subcutaneous injection and
this combination therapy led to a more rapid reduction in platelet
count. After 20 days, the patient's clinical symptoms improved and
discharge to home treatment followed. At discharge, the white blood
cell count was 5.44 x109/l, hemoglobin was 106 g/l and
platelet count was 752x109/l. which, although markedly
reduced, remained above the threshold for complete remission.
Notably, the patient experienced episodes of chest tightness,
shortness of breath and angina during treatment.
Electrocardiography findings were consistent with acute
subendocardial myocardial infarction. These symptoms resolved
following anticoagulant and antiplatelet therapy. The patient also
experienced mild adverse events, including hypocalcemia, liver
injury, fever and dizziness, all of which were managed with
supportive care. Following discharge, the patient continued
treatment with oral imatinib 400 mg once daily. The IFN-α regimen
was adjusted to 30 µg twice weekly via subcutaneous injection.
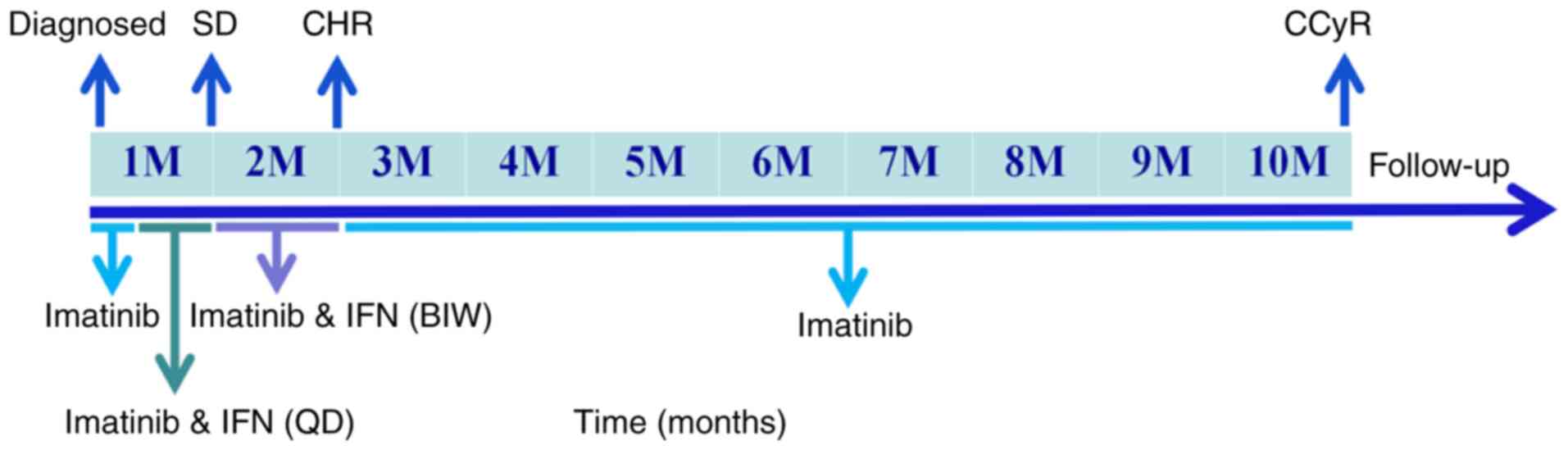
After one month of this adjusted combination therapy, a follow-up
complete blood count revealed further hematologic improvement, with
a white blood cell count of 5.21x109/l, hemoglobin of
112 g/l and platelet count of 311x109/l (Fig. 5). Complete hematological response
(CHR) was confirmed by peripheral blood and bone marrow
examination, with findings demonstrating normal white blood cell,
platelet, and absolute neutrophil counts, absence of blasts and
immature myeloid cells in peripheral blood, normocellular bone
marrow with normal maturation, and <5% blasts. IFN-α was then
discontinued and the patient continued on imatinib monotherapy 400
mg once daily. After 10 months, cytogenetic analysis showed no
detectable Philadelphia chromosome, indicating complete cytogenetic
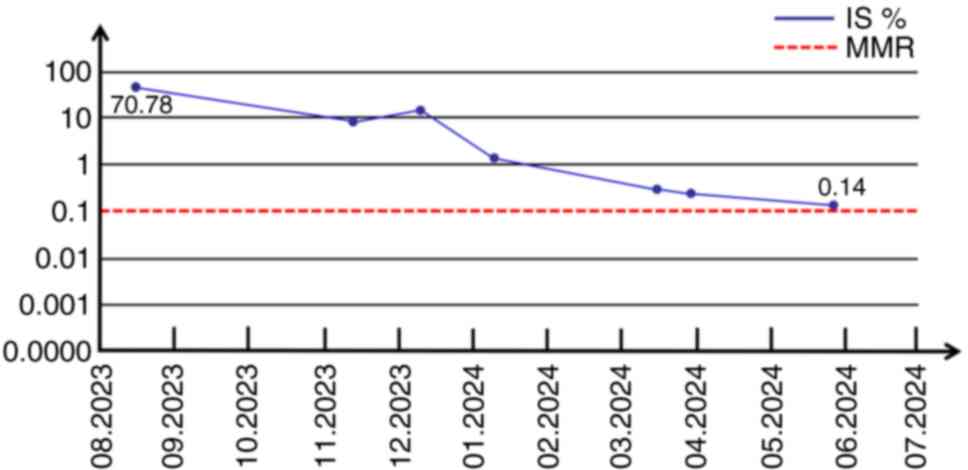
remission (CCyR). Since diagnosis in August 2023, the patient has
received seven BCR-ABL1 fusion gene transcript level assessments,
demonstrating a progressive reduction in transcript levels. The
most recent evaluation in June 2024 revealed a BCR-ABL1 transcript
level of 0.14% IS, approaching major molecular remission (MMR),
defined as a BCR-ABL1 transcript level less than 0.1%. It is worth
noting that imaging studies consistently showed a normal spleen
size throughout the treatment course.
Follow-up and outcome
As of the most recent follow-up in September 2024,
the patient remains on imatinib therapy and is stable, with no
evidence of disease relapse. The patient's blood counts have
remained within the normal range (Fig.
6).
Discussion
CML-T is a rare subtype of CML, defined by platelet
counts that typically reach or exceed 1,000x109/l.
Although no standardized treatment for CML-T is currently
available, previous studies have demonstrated a favorable response
to imatinib therapy (10,13). Given the distinct therapeutic
approaches required, differentiating CML-T from ET is crucial for
treatment decision-making. The diagnosis of ET is primarily
established by elevated platelet counts, increased bone marrow
megakaryocytes and the presence of CALR, JAK2, MPL, or CSF3R
mutations (14,15). By contrast, the presence of the
Philadelphia chromosome or BCR-ABL rearrangement with isolated
thrombocytosis should be diagnosed as CML, not ET, according to the
World Health Organization diagnostic criteria (16). The patient presented with a
platelet count of 3,798x109/l, significantly higher than
previously reported levels. This highlighted the rarity of CML-T
and the challenges associated with its diagnosis, suggesting a
potential unique mechanism underlying thrombocytosis in this
patient. Notably, despite the extreme thrombocytosis, the patient's
spleen size remained normal, adding to the diagnostic complexity.
Although splenomegaly is a typical feature in most patients with
CML, this patient consistently lacked splenomegaly throughout the
disease course. Previous literature has documented cases of CML
with concomitant myelofibrosis or thrombocytosis without
splenomegaly (17,18). Additionally, research suggests that
~40% of patients with CML are asymptomatic in the early stages of
the disease, with diagnosis often relying solely on laboratory
abnormalities. Furthermore, the clinical presentation of CML can
vary across different geographical regions (19,20).
These findings highlight that factors such as the stage of CML and
individual patient variability can influence the presence or
absence of splenomegaly. The diagnosis and assessment of CML
necessitate a comprehensive evaluation incorporating a
multi-faceted approach rather than relying solely on spleen
size.
Significant thrombocytosis, a hallmark of CML-T, is
associated with increased blood viscosity, promoting thrombosis and
elevating the risk of thromboembolic events (21). The patient experienced recurrent
angina during treatment, potentially attributable to thrombosis
secondary to extreme thrombocytosis. The mechanisms underlying the
profound thrombocytosis observed in CML-T remain incompletely
understood. The patient's extreme thrombocytosis and high BCR-ABL1
p210 fusion gene expression level (IS: 70.78%) suggested a
potential role for BCR-ABL1 overexpression, and previous research
has demonstrated a correlation between the BCR-ABL1 fusion gene and
elevated platelet counts in CML (22). However, the precise mechanisms by
which BCR-ABL1 directly influences megakaryocyte differentiation
and platelet production remain unclear and warrant further
investigation. BCR-ABL1 overexpression is hypothesized to disrupt
normal megakaryocyte development, leading to excessive platelet
production. Imatinib, a targeted BCR-ABL1 tyrosine kinase
inhibitor, restores megakaryocyte function and reduces platelet
counts in CML (23,24). BCR-ABL1p210, the most prevalent
variant in CML, is associated with thrombocytosis, disease
progression and adverse prognosis, as well as response to IFN-α and
imatinib therapy (25-32).
Other BCR-ABL1 variants, such as p185, p190 and p230, have also
been implicated in lymphoid progenitor cell transformation and
thrombocytosis in CML. Furthermore, these variants also contribute
to favorable outcomes, including complete remission and improved
long-term survival, in patients with Philadelphia
chromosome-positive acute lymphoblastic leukemia (33-40)
(Table I). Furthermore, two major
BCR-ABL1 mRNA transcript types exist, e14a2 and e13a2, with e14a2
associated with higher platelet counts and e13a2 with higher white
blood cell counts (41). BCR-ABL1
transcript typing was not performed in this case and future
research should explore the relationship between BCR-ABL1
transcript type and thrombocytosis in CML-T. Furthermore, the
pathogenesis of thrombocytosis in CML-T may involve additional
molecular mechanisms beyond BCR-ABL1. Mutations in genes such as
MPL, THPO and JAK2, as well as dysregulated expression of
thrombopoietin, IL-6 and other inflammatory mediators, have been
implicated in driving platelet production in various thrombocytosis
contexts, a finding that warrants further investigation (14,42-45).
 | Table IRoles of different BCR-ABL variants
in leukemia. |
Table I
Roles of different BCR-ABL variants
in leukemia.
| First author,
year | Cancer type | BCR-ABL
variants | Clinical
significance | (Refs.) |
|---|
| Ten Bosch et
al, 1998 | CML | BCR-ABLp210 | Promotes
thrombopoiesis through CrkL phosphorylation | (25) |
| Bennour et
al, 2013 | CML | BCR-ABLp210 | Correlated with
platelet counts in patients with CML | (26) |
| Arana-Trejo et
al, 2002 | CML | BCR-ABLp210/p
190/p230 | Associated with
platelet counts, splenomegaly and chromosomal abnormalities | (27) |
| Polampalli et
al, 2008 | CML | BCR-ABLp210 | Associated with
myeloid blast crisis in patients with CML | (28) |
| Al-Achkar et
al, 2016 | CML | BCR-ABLp210 | Associated with
prognosis in patients with CML | (29) |
| Pane et al,
1999 | CML | BCR/ABLp 210 | Affecting the
responsiveness of patients with CML to IFN-α | (30) |
| Zhao et al,
2015 | CML | BCR/ABLp
190/p210 | Participation in
imatinib resistance through ABL kinase domain mutations | (31) |
| Zhang et al,
2022 | CML | BCR-ABLp210 | Associated with
thrombocytosis in patients with CML | (32) |
| Puil et al,
1994 | CML | BCR-ABLp185/p
210 | Participates in the
occurrence of CML through Ras signaling pathway | (33) |
| Liu et al,
1999 | CML | BCR-ABLp185 | Activates
megakaryocytes through JAK2/STAT5 signaling | (34) |
| Verma et al,
2009 | CML | BCR-ABLp190 | Induce rapid
transformation of lymphoid progenitor cells and poor prognosis | (35) |
| Melo et al,
1997 | CML | BCR-ABLp230 | Associated with
thrombocytosis in patients with CML | (36) |
| Balatzenko et
al, 2008 | CML | BCR-ABLp190 | Associated with
extreme thrombocytosis in patients with CML | (37) |
| Adnan-Awad et
al, 2008 | CML |
BCR-ABLp190/210 | Upregulating
interferon receptor expression through Src signaling | (38) |
| Gleissner et
al, 2002 | Ph(+) ALL | BCR-ABLp210 | Associated with
long-term survival of patient | (39) |
| Qiu et al,
2016 | Ph(+) ALL | BCR-ABLp190/p
210 | Associated with CR
and long-term survival of patient | (40) |
Imatinib, the first-generation BCR-ABL1 TKI approved
by the US Food and Drug Administration, significantly improves CML
treatment by competitively binding to the BCR-ABL1 kinase domain,
thereby inhibiting kinase activity, promoting apoptosis in leukemic
cells and ultimately improving the prognosis of patients with CML
(46,47). Previous reports suggest imatinib as
a valuable treatment option for patients with CML presenting with
the rare complication of thrombocytosis (48). Despite its efficacy in reducing
platelet counts in CML, imatinib may increase bleeding risk
potentially due to platelet apoptosis, aggregation inhibition,
platelet derived growth factor receptor (PDGFR) downregulation and
megakaryocyte apoptosis via PI3K/Akt pathway (49-51).
Conversely, increased platelet activation after imatinib treatment
in CML has also been reported (52). Close monitoring of platelet-related
parameters during imatinib therapy is therefore warranted.
Prolonged imatinib therapy may lead to drug resistance, potentially
due to acquired BCR-ABL1 mutations, including point mutations,
insertions and deletions, which compromise TKI therapy efficacy and
potentially lead to treatment failure (53). While second- and third-generation
TKIs show potential in overcoming resistance, they are associated
with inherent cardiovascular risks, such as thrombotic vascular
occlusion and heart failure. Consequently, caution is advised when
prescribing these newer TKIs to patients with pre-existing
cardiovascular conditions (3,54-58).
IFN-α demonstrates myelosuppressive activity,
inhibiting the uncontrolled clonal proliferation of hematopoietic
cells in MPNs, including CML and ET. Before the advent of TKIs,
IFN-α was the standard first-line treatment for CML, particularly
in patients ineligible for allogeneic hematopoietic stem cell
transplantation (59-61).
Additionally, IFN-α significantly reduces platelet counts in ET and
other MPNs with thrombocytosis, probably through megakaryocyte
normalization (62-64).
Furthermore, its immunomodulatory effects enhance natural killer
cell activity, leading to the destruction of CML cells (65). While imatinib has largely
superseded IFN-α in CML treatment, it remains a valuable
therapeutic option for patients intolerant to imatinib (66). Moreover, IFN-α can promote monocyte
differentiation into anti-tumor dendritic cells and activate CD8+ T
cells, thus bolstering anti-tumor immunity in CML (67).
Combined IFN-α and imatinib therapy shows additive
effects in chronic-phase CML, improving hematological responses,
extending event-free survival and enhancing MMR rates (11,12,68).
In the present case, initial imatinib monotherapy effectively
controlled leukocyte counts but failed to adequately address
persistently elevated platelet levels, thereby increasing the risk
of thromboembolic events, as evidenced by recurrent angina.
Therefore, IFN-α was added to the treatment regimen to further
manage platelet counts and mitigate thrombotic risk. Although this
combined therapy may offer benefits for patients with CML,
particularly those with extreme thrombocytosis, including enhanced
platelet reduction and the possibility of complete remission, as
observed in this patient, it is crucial to acknowledge the
increased risk of adverse events such as myelosuppression,
hepatotoxicity and flu-like symptoms (69-72).
This patient experienced neutropenia and liver injury during
combined therapy, which were successfully managed with symptomatic
treatment. Therefore, implementing IFN-α alongside imatinib
necessitates carefully considering the risk-benefit profile and
close clinical monitoring.
In conclusion, the present case report highlighted
the successful treatment of a patient with CML-T and severe
thrombocytosis using combined IFN-α and imatinib therapy,
emphasizing the challenges in diagnosing and managing this rare
condition. While imatinib monotherapy initially failed to control
platelet counts adequately, the addition of IFN-α led to complete
hematologic and cytogenetic remission, suggesting this combination
may be a promising strategy for similar cases. However, as this is
a single-center report with a limited sample size, larger
prospective studies, ideally multicenter, randomized controlled
trials, are crucial to validate these findings and establish
optimal treatment regimens. Further research should investigate the
long-term efficacy and safety of the combination therapy, refine
patient selection criteria and explore alternative therapeutic
approaches like novel TKIs. Additionally, elucidating the molecular
mechanisms driving severe thrombocytosis in CML-T, including the
role of BCR-ABL1 mutations, remains essential for developing
targeted therapeutic interventions.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MXJ was the primary investigator, leading the study
design, data analysis and manuscript drafting. DLD conducted the
literature review, revised the manuscript, prepared the figures and
confirmed the authenticity of the raw data. ZZL contributed to data
collection and experimental procedures. HYW independently verified
the authenticity of all data cited in the manuscript, reviewed the
manuscript and confirmed the treatment course. LC reviewed and
edited the manuscript for final submission. DLD and HYW confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present case report was approved by the Medical
Ethics Committee of the Affiliated Hospital of Shandong Second
Medical University (grant no. wyfy-2024-qt-051; date of approval:
September 18, 2024; Weifang, China).
Patient consent for publication
Written informed consent was obtained from the
patient's family for the publication of this report and the
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jabbour E and Kantarjian H: Chronic
myeloid leukemia: 2020 update on diagnosis, therapy and monitoring.
Am J Hematol. 95:691–709. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rinaldi I and Winston K: Chronic myeloid
leukemia, from pathophysiology to treatment-free remission: A
narrative literature review. J Blood Med. 14:261–277.
2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Osman AEG and Deininger MW: Chronic
myeloid leukemia: Modern therapies, current challenges and future
directions. Blood Rev. 49(100825)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Arber DA, Orazi A, Hasserjian RP, Borowitz
MJ, Calvo KR, Kvasnicka HM, Wang SA, Bagg A, Barbui T, Branford S,
et al: International consensus classification of myeloid neoplasms
and acute leukemias: Integrating morphologic, clinical and genomic
data. Blood. 140:1200–1228. 2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Thakral B, Saluja K, Malhotra P, Sharma
RR, Marwaha N and Varma S: Therapeutic plateletpheresis in a case
of symptomatic thrombocytosis in chronic myeloid leukemia. Ther
Apher Dial. 8:497–499. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ebrahem R, Ahmed B, Kadhem S and Truong Q:
Chronic myeloid leukemia: A case of extreme thrombocytosis causing
syncope and myocardial infarction. Cureus. 8(e476)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Turakhia SK, Murugesan G, Cotta CV and
Theil KS: Thrombocytosis and STAT5 activation in chronic
myelogenous leukaemia are not associated with JAK2 V617F or
calreticulin mutations. J Clin Pathol. 69:713–719. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chiatamone Ranieri S, Arleo MA, Trasarti
S, Bizzoni L, Carmosino I, De Luca ML, Mohamed S, Mariggiò E,
Scalzulli E, Rosati S, et al: Clinical and prognostic features of
essential thrombocythemia: Comparison of 2001 WHO Versus 2008/2016
WHO criteria in a large single-center cohort. Clin Lymphoma Myeloma
Leuk. 21:e328–e333. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Haznedaroglu IC: The therapeutic goals of
essential thrombocythemia under the clouds of over-treatment and
under-treatment. Expert Opin Pharmacother. 14:1431–1436.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Verma SP, Subbiah A, Jacob SE and Basu D:
Chronic myeloid leukaemia with extreme thrombocytosis. BMJ Case
Rep. 2015(bcr2014204564)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Talpaz M: Interferon-alfa-based treatment
of chronic myeloid leukemia and implications of signal transduction
inhibition. Semin Hematol. 38 (Suppl 8):S22–S27. 2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Palandri F, Castagnetti F, Iacobucci I,
Martinelli G, Amabile M, Gugliotta G, Poerio A, Testoni N, Breccia
M, Bocchia M, et al: The response to imatinib and interferon-alpha
is more rapid than the response to imatinib alone: A retrospective
analysis of 495 Philadelphia-positive chronic myeloid leukemia
patients in early chronic phase. Haematologica. 95:1415–1419.
2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu Z, Fan H, Li Y and Liu C: Analysis of
clinical characteristics and efficacy of chronic myeloid leukemia
onset with extreme thrombocytosis in the era of tyrosine kinase
inhibitors. Onco Targets Ther. 10:3515–3520. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tefferi A and Barbui T: Polycythemia vera
and essential thrombocythemia: 2021 update on diagnosis,
risk-stratification and management. Am J Hematol. 95:1599–1613.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jang MA and Choi CW: Recent insights
regarding the molecular basis of myeloproliferative neoplasms.
Korean J Intern Med. 35:1–11. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Byun YJ, Park BB, Lee ES, Choi KS and Lee
DS: A case of chronic myeloid leukemia with features of essential
thrombocythemia in peripheral blood and bone marrow. Blood Res.
49:127–129. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hiruma K, Saitoh H, Someya K and Kashimura
M: Hematologic abnormalities in a patient with chronic myelogenous
leukemia with advanced myelofibrosis were improved by G-CSF. Rinsho
Ketsueki. 35:135–141. 1994.PubMed/NCBI(In Japanese).
|
|
18
|
Shah NP: Front-line treatment options for
chronic-phase chronic myeloid leukemia. J Clin Oncol. 36:220–224.
2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Granatowicz A, Piatek CI, Moschiano E,
El-Hemaidi I, Armitage JD and Akhtari M: An overview and update of
chronic myeloid leukemia for primary care physicians. Korean J Fam
Med. 36:197–202. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rajabto W and Angkasa YK: Asymptomatic
chronic-phase chronic myeloid leukemia BCR-ABL. (+) without
splenomegaly: A case report. Niger J Clin Pract. 25:373–375.
2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Galvez C and Stein BL: Thrombocytosis and
thrombosis: Is there really a correlation? Curr Hematol Malig Rep.
15:261–267. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zheng Y, Wen J and Li J: Pediatric chronic
myeloid leukemia presenting with extreme thrombocytosis and acute
upper gastrointestinal hemorrhage: A case report. J Pediatr Hematol
Oncol. 43:e1049–e1051. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Turroni S, Tolomeo M, Mamone G, Picariello
G, Giacomini E, Brigidi P, Roberti M, Grimaudo S, Pipitone RM, Di
Cristina A and Recanatini M: A natural-like synthetic small
molecule impairs bcr-abl signaling cascades and induces
megakaryocyte differentiation in erythroleukemia cells. PLoS One.
8(e57650)2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Thiele J, Kvasnicka HM, Varus E, Ollig E,
Schmitt-Graeff A, Staib P and Griesshammer M: Megakaryocyte
features and bcr/abl translocation in chronic myeloid leukemia
following imatinib mesylate (STI571) therapy-a fluorescence in-situ
hybridization study. Leuk Lymphoma. 45:1627–1631. 2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
ten Bosch GJ, Kessler JH, Blom J, Joosten
AM, Gambacorti-Passerini C, Melief CJ and Leeksma OC: BCR-ABL
oncoprotein is expressed by platelets from CML patients and
associated with a special pattern of CrkL phosphorylation. Br J
Haematol. 103:1109–1115. 1998.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bennour A, Ouahchi I, Achour B, Zaier M,
Youssef YB, Khelif A, Saad A and Sennan H: Analysis of the
clinico-hematological relevance of the breakpoint location within
M-BCR in chronic myeloid leukemia. Med Oncol.
30(348)2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Arana-Trejo RM, Ruíz Sánchez R,
Ignacio-Ibarra G, Báez de la Fuente E, Garces O, Gómez Morales E,
Castro Granados M, Ovilla Martínez R, Rubio-Borja ME, Solís Anaya
L, et al: BCR/ABL p210, p190 and p230 fusion genes in 250 Mexican
patients with chronic myeloid leukaemia (CML). Clin Lab Haematol.
24:145–150. 2002.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Polampalli S, Choughule A, Negi N, Shinde
S, Baisane C, Amre P, Subramanian PG, Gujral S, Prabhash K and
Parikh P: Analysis and comparison of clinicohematological
parameters and molecular and cytogenetic response of two Bcr/Abl
fusion transcripts. Genet Mol Res. 7:1138–1149. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Al-Achkar W, Moassass F, Youssef N and
Wafa A: Correlation of p210 BCR-ABL transcript variants with
clinical, parameters and disease outcome in 45 chronic myeloid
leukemia patients. J BUON. 21:444–449. 2016.PubMed/NCBI
|
|
30
|
Pane F, Mostarda I, Selleri C, Salzano R,
Raiola AM, Luciano L, Saglio G, Rotoli B and Salvatore F: BCR/ABL
mRNA and the P210(BCR/ABL) protein are downmodulated by
interferon-alpha in chronic myeloid leukemia patients. Blood.
94:2200–2207. 1999.PubMed/NCBI
|
|
31
|
Junmei Z, Fengkuan Y, Yongping S, Baijun
F, Yuzhang L, Lina L and Qinglan Z: Coexistence of P190 and P210
BCR/ABL transcripts in chronic myeloid leukemia blast crisis
resistant to imatinib. Springerplus. 4(170)2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang X, Sun H, Su Y and Yi H: Long-term
molecular remission after treatment with imatinib in a chronic
myeloid leukemia patient with extreme thrombocytosis harboring rare
e14a3 (b3a3) BCR::ABL1 transcript: A case report. Curr Oncol.
29:8171–8179. 2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Puil L, Liu JX, Gish G, Mbamalu G, Bowtell
D, Pelicci PG, Arlinghaus R and Pawson T: Bcr-Abl oncoproteins bind
directly to activators of the Ras signalling pathway. EMBO J.
13:764–773. 1994.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu RY, Fan C, Garcia R, Jove R and
Zuckerman KS: Constitutive activation of the JAK2/STAT5 signal
transduction pathway correlates with growth factor independence of
megakaryocytic leukemic cell lines. Blood. 93:2369–2379.
1999.PubMed/NCBI
|
|
35
|
Verma D, Kantarjian HM, Jones D, Luthra R,
Borthakur G, Verstovsek S, Rios MB and Cortes J: Chronic myeloid
leukemia (CML) with P190 BCR-ABL: Analysis of characteristics,
outcomes and prognostic significance. Blood. 114:2232–2235.
2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Melo JV: BCR-ABL gene variants. Baillieres
Clin Haematol. 10:203–222. 1997.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Balatzenko G, Guenova M, Stoimenov A,
Jotov G and Toshkov S: Philadelphia chromosome-positive chronic
myeloid leukemia with p190(BCR-ABL) rearrangement, overexpression
of the EVI1 gene and extreme thrombocytosis: A case report. Cancer
Genet Cytogenet. 181:75–77. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Adnan-Awad S, Kim D, Hohtari H, Javarappa
KK, Brandstoetter T, Mayer I, Potdar S, Heckman CA, Kytölä S,
Porkka K, et al: Characterization of p190-Bcr-Abl chronic myeloid
leukemia reveals specific signaling pathways and therapeutic
targets. Leukemia. 35:1964–1975. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Gleissner B, Gökbuget N, Bartram CR,
Janssen B, Rieder H, Janssen JW, Fonatsch C, Heyll A, Voliotis D,
Beck J, et al: Leading prognostic relevance of the BCR-ABL
translocation in adult acute B-lineage lymphoblastic leukemia: A
prospective study of the German Multicenter Trial Group and
confirmed polymerase chain reaction analysis. Blood. 99:1536–1543.
2002.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Qiu LL, Lu YJ, Jing Y, Yu L, Liu DH and
Wang LL: Comparison of clinical outcomes between P190 and P210
trans-cripts in adult Ph chromosome positive acute lymphoblastic
leukemia in the new Era of TKI. Zhongguo Shi Yan Xue Ye Xue Za Zhi.
24:369–374. 2016.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
41
|
Vasconcelos AP, Azevedo IF, Melo FCBC,
Neves WB, Azevedo ACAC and Melo RAM: BCR-ABL1 transcript types
showed distinct laboratory characteristics in patients with chronic
myeloid leukemia. Genet Mol Res. 16:2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Guglielmelli P and Calabresi L: The MPL
mutation. Int Rev Cell Mol Biol. 365:163–178. 2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Stockklausner C, Duffert CM, Cario H,
Knöfler R, Streif W and Kulozik AE: THROMKID-Plus Studiengruppe der
Gesellschaft für Thrombose-und Hämostaseforschung (GTH) and of
Gesellschaft für Pädiatrische Onkologie und Hämatologie (GPOH).
Thrombocytosis in children and adolescents-classification,
diagnostic approach and clinical management. Ann Hematol.
100:1647–1665. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Rottenstreich A and Bussel JB: Treatment
of immune thrombocytopenia during pregnancy with thrombopoietin
receptor agonists. Br J Haematol. 203:872–885. 2023.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Rubenstein AI, Pierson SK, Shyamsundar S,
Bustamante MS, Gonzalez MV, Milller ID, Brandstadter JD, Mumau MD
and Fajgenbaum DC: Immune-mediated thrombocytopenia and
IL-6-mediated thrombocytosis observed inidiopathic multicentric
Castleman disease. Br J Haematol. 204:921–930. 2024.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wolfe HR and Rein LAM: The evolving
landscape of frontline therapy in chronic phase chronic myeloid
leukemia (CML). Curr Hematol Malig Rep. 16:448–454. 2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Flynn JP and Gerriets V: Imatinib. In:
StatPearls. StatPearls Publishing, Treasure Island, FL, 2025.
|
|
48
|
Gao L, Ren MQ, Tian ZG, Peng ZY, Shi G and
Yuan Z: Management of chronic myeloid leukemia presenting with
isolated thrombocytosis and complex Philadelphia chromosome: A case
report. Medicine (Baltimore). 100(e27134)2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Repsold L, Pool R, Karodia M, Tintinger G,
Becker P and Joubert AM: Apoptotic profiling of chronic myeloid
leukaemia patients' platelets ex vivo before and after treatment
with Imatinib. Cell Biochem Funct. 39:562–570. 2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Sener Y, Okay M, Aydin S, Buyukasik Y,
Akbiyik F and Dikmen ZG: TKI-related platelet dysfunction does not
correlate with bleeding in patients with chronic phase-chronic
myeloid leukemia with complete hematological response. Clin Appl
Thromb Hemost. 25(1076029619858409)2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Shu LL, Jiang QL, Meng FY and Yang M:
Molecular mechanism of imatinib-induced thrombocytopenia in
treatment of patients with CML. Zhongguo Shi Yan Xue Ye Xue Za Zhi.
19:1314–1318. 2011.PubMed/NCBI(In Chinese).
|
|
52
|
Repsold L, Pool R, Karodia M, Tintinger G
and Joubert AM: Ex vivo platelet morphology assessment of chronic
myeloid leukemia patients before and after Imatinib treatment.
Microsc Res Tech. 85:2222–2233. 2022.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Akram AM, Iqbal Z, Akhtar T, Khalida AM,
Sabar MF, Qazi MH, Azize Z, Sajid N, Aleem A, Rasoolh M, et al:
Presence of novel compound BCR-ABL mutations in late chronic and
advanced phase imatinib sensitive CML patients indicates their
possible role in CML progression. Cancer Biol Ther. 18:214–221.
2017.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Al-Ali HK, Heinrich MC, Lange T, Krahl R,
Mueller M, Müller C, Niederwieser D, Druker BJ and Deininger MW:
High incidence of BCR-ABL kinase domain mutations and absence of
mutations of the PDGFR and KIT activation loops in CML patients
with secondary resistance to imatinib. Hematol J. 5:55–60.
2004.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Tanaka R and Kimura S: Abl tyrosine kinase
inhibitors for overriding Bcr-Abl/T315I: From the second to third
generation. Expert Rev Anticancer Ther. 8:1387–1398.
2008.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Dhillon S: Olverembatinib: First approval.
Drugs. 82:469–475. 2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Binzaid AA, Baqal OJ, Soheib M, Nahedh MA,
Samarkandi HH and Aljurf M: Cardiovascular toxicity associated with
tyrosine kinase inhibitor therapy in chronic myeloid leukemia. Gulf
J Oncolog. 1:79–84. 2021.PubMed/NCBI
|
|
58
|
Caocci G, Mulas O, Annunziata M, Luciano
L, Abruzzese E, Bonifacio M, Orlandi EM, Albano F, Galimberti S,
Iurlo A, et al: Long-term mortality rate for cardiovascular disease
in 656 chronic myeloid leukaemia patients treated with second- and
third-generation tyrosine kinase inhibitors. Int J Cardiol.
301:163–166. 2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Robak T: Use of interferon in the
treatment of chronic myeloproliferative disorders. Acta Haematol
Pol. 23 (2 Suppl 1):S30–S37. 1992.PubMed/NCBI(In Polish).
|
|
60
|
Talpaz M, Mercer J and Hehlmann R: The
interferon-alpha revival in CML. Ann Hematol. 94 (Suppl
2):S195–S207. 2015.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Kujawski LA and Talpaz M: The role of
interferon-alpha in the treatment of chronic myeloid leukemia.
Cytokine Growth Factor Rev. 18:459–471. 2007.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Seewann HL: Interferon therapy in
essential thrombocythemia. Wien Med Wochenschr. 143:420–424.
1993.PubMed/NCBI(In German).
|
|
63
|
Koike G, Otsuka T, Shibuya T and Niho Y:
Thrombocytosis in chronic myelogenous leukemia (CML) controlled by
interferon alpha (IFN-alpha). Rinsho Ketsueki. 30:400–403.
1989.PubMed/NCBI(In Japanese).
|
|
64
|
Thiele J, Zirbes T, Kvasnicka HM, Niederle
N, Dammasch J, Schmidt M, Windecker R, Leder LD, Diehl V and
Fischer R: Interferon therapy, but not busulfan restores
normal-sized megakaryopoiesis in CML-a comparative histo- and
immunomorphometric study. Anal Cell Pathol. 11:31–42.
1996.PubMed/NCBI
|
|
65
|
Kong J, Qin YZ, Zhao XS, Hou Y, Liu KY,
Huang XJ and Jiang H: Profiles of NK cell subsets are associated
with successful tyrosine kinase inhibitor discontinuation in
chronic myeloid leukemia and changes following interferon
treatment. Ann Hematol. 100:2557–2566. 2021.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Rüdiger H, andreas H and Michele B:
European LeukemiaNet. Chronic myeloid leukaemia. Lancet.
370:342–350. 2007.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Gabriele L, Borghi P, Rozera C, Sestili
Pandreotti M, Guarini A, Montefusco E, Foà R and Belardelli F:
IFN-alpha promotes the rapid differentiation of monocytes from
patients with chronic myeloid leukemia into activated dendritic
cells tuned to undergo full maturation after LPS treatment. Blood.
103:980–987. 2004.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Simonsson B, Gedde-Dahl T, Markevärn B,
Remes K, Stentoft J, Almqvist A, Björeman M, Flogegård M,
Koskenvesa P, Lindblom A, et al: Combination of pegylated IFN-α2b
with imatinib increases molecular response rates in patients with
low- or intermediate-risk chronic myeloid leukemia. Blood.
118:3228–3235. 2011.PubMed/NCBI View Article : Google Scholar
|
|
69
|
McGlave P, Mamus S, Vilen B and Dewald G:
Effect of recombinant gamma interferon on chronic myelogenous
leukemia bone marrow progenitors. Exp Hematol. 15:331–335.
1987.PubMed/NCBI
|
|
70
|
Foon KA, Sherwin SA, Abrams PG, Stevenson
HC, Holmes P, Maluish AE, Oldham RK and Herberman RB: A phase I
trial of recombinant gamma interferon in patients with cancer.
Cancer Immunol Immunother. 20:193–197. 1985.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Dou XL, Wang SS, Fang JL, Yu L, Ren X,
Huang XJ and Jiang Q: Hepatic adverse events associated with
tyrosine kinase inhibitors in patients with chronic myeloid
leukemia. Zhonghua Nei Ke Za Zhi. 57:649–655. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
72
|
O'Brien S, Kantarjian H and Talpaz M:
Practical guidelines for the management of chronic myelogenous
leukemia with interferon alpha. Leuk Lymphoma. 23:247–252.
1996.PubMed/NCBI View Article : Google Scholar
|