Introduction
Breast cancer (BC) is the most common type of cancer
in women worldwide (1). The
American Cancer Society estimated that 300,590 new cases of
invasive BC in women would be diagnosed in 2023 worldwide (2). Breast tumors are heterogeneous and
are pathologically classified by immunohistochemical staining. The
key proteins for classification of breast tumors are estrogen
receptor (ER) α, progesterone receptor (PR) and human epidermal
growth factor receptor 2 (HER2). Additionally, factors such as
tumor stage, nodal status, tumor grade, molecular subtype and the
proliferation marker MIB-1 have prognostic relevance. Therapy is
planned for the corresponding patient with BC depending on all
these characteristics. Molecular subtyping has been implemented in
clinical practice as an important tool for risk-adapted therapy in
patients with BC (3-5).
Treatment options include surgery, radiotherapy, endocrine therapy,
chemotherapy and targeted therapies, which vary depending on
factors such as TNM status and molecular biology (6,7).
Despite improvements in systemic treatment concepts since 1990, BC
was projected to be the second leading cause of
malignancy-associated deaths in women in 2023(2). One of the major challenges is the
increase in treatment resistance. Current research is focused on
exploring the mechanisms by which tumor cells become resistant to
endocrine therapy and chemotherapy (8-10).
The development of predictive biomarkers for treatment response is
crucial for clinicians to detect resistance early and adjust
management accordingly.
Chemotherapy represents a well-established and
occasionally life-saving treatment option, despite its known severe
side effects (6). Overall survival
is markedly increased after the application of chemotherapy in BC
(11,12). Combined epirubicin and paclitaxel
are the cornerstones of early BC therapy (8,11,13,14).
Epirubicin is an anthracycline, and the mode of action of
anthracyclines is diverse, although most importantly, they
intercalate into DNA molecules, thus inhibiting topoisomerase II (a
key enzyme in cell division) and generating radicals that lead to
DNA degradation (15). Paclitaxel
is a taxol-based chemotherapeutic and, as such, acts as a
microtubule-stabilizing agent, impacts depolymerization and
triggers apoptosis (11,16). Gemcitabine is a nucleosid analog
that plays an important role in the treatment of metastatic BC
(14). After being phosphorylated,
gemcitabine exhibits cytotoxic activity by inhibiting the enzyme
ribonucleotide reductase, which itself catalyzes sufficient and
correct DNA synthesis (14,17).
Another treatment option for BC is endocrine
therapy. The selective ER degrader fulvestrant is a therapeutic
agent with antiestrogenic properties. Compared with the most common
antihormonal therapeutics such as tamoxifen, fulvestrant does not
exhibit estrogen agonistic effects, including increased risk of
endometrial carcinoma or thromboembolic disease (18,19).
Therefore, it has become of great interest in BC therapy.
As well as chemotherapy and antihormonal therapy,
bone-targeted therapeutics are of outstanding importance for some
patients, especially those receiving antihormonal therapies. Not
only in advanced but also in early BC therapy, bisphosphonates and
denosumab have shown to crucially affect bone health, quality of
life, overall and disease-free survival (20), and skeletal-related events such as
bone and joint pain, fractures and malignant hypercalcemia can be
prevented by these agents (21,22).
Among all bisphosphonates, zoledronic acid has been shown to be the
most efficient. Its mode of action is to inhibit the proliferation
of osteoclasts, thus leading to their cell death (20).
Long non-coding RNAs (lncRNAs) are non-coding
(nc)RNA sequences of >200 nucleotides in length. The number of
lncRNA genes in the human genome is ~3-fold that of protein-coding
genes (23). Accumulating evidence
suggests that lncRNAs are involved in a wide range of cellular
processes affecting protein, DNA, RNA expression and interactions
(24). A previous study showed
evidence for regulatory roles of lncRNA in facilitating
carcinogenesis, invasion-metastasis and chemoresistance in multiple
cancer types (25). Several
lncRNAs have been implicated in BC. For example, HOX transcript
antisense RNA (HOTAIR) is known to be correlated with tamoxifen
resistance (26). The lncRNA
cytoskeleton regulator RNA (CYTOR) is highly expressed in
triple-negative BC (27). However,
the expression of lncRNAs and their role as possible biomarkers for
therapy resistance or cancer screening remain to be elucidated.
Cancerogenesis is a very complex process in which a
number of factors can play a role. Mutations and copy number
variations have a high relevance. However, a number of studies have
also shown that ncRNAs play an important role in the field of
oncology (25-27).
The group of ncRNA contain a wide variety of regulatory RNAs,
included are for example piwiinteracting RNAs (piRNAs), microRNAs
(miRNAs), small cajal body-specific RNAs (scaRNAs), small nucleolar
RNAs (snoRNAs) and lncRNAs, to name but a few (28). For lncRNAs, previous studies have
shown evidence of their regulatory role in facilitating
carcinogenesis, invasion, metastasis and chemoresistance in
different type of cancers, as reviewed by Majidinia and Yousefi
(25). In addition, a number of
studies have already demonstrated the role of lncRNAs in breast
cancer (26,27,29-31).
Ki-67 is an established prognostic biomarker in BC
(32), and it has been shown that
Ki67 dynamics can indicate therapy response (33). Cyclin D1 is a cell cycle regulator
reported to be overactive in cancer tissue (34). Notably, it has been shown to be
involved in lncRNA-mediated tamoxifen resistance in BC (35).
The present study analyzed the potential effects
triggered by different BC treatments on the transcriptional
expression of 12 pre-selected lncRNAs and the proliferation markers
Cyclin D1 and Ki-67 in six different cell lines. Intracellular
analysis of these lncRNAs and their correlation with Ki-67 and
Cyclin D1 is expected to indicate a potential biomarker function,
since, hypothetically, altered lncRNA expression after treatment
that correlates with a simultaneous alteration of Ki-67 and cyclin
D1 may highlight the predictive potential of lncRNAs An association
with BC has already been described for some of the aforementioned
pre-selected lncRNAs, while others have so far only been described
in other tumor types (Table
I).
 | Table IReported function of pre-selected
lncRNAs investigated. |
Table I
Reported function of pre-selected
lncRNAs investigated.
| A, Tumor-promoting
properties in BC |
|---|
| Pre-selected
lncRNA | Reported
function | Affected
genes/proteins/pathways | (Refs.) |
|---|
|
CYTOR/linc00152 | Promotes cell
proliferation, tumorigenesis, invasion and metastasis | EZH2 | (34,44) |
| HOTAIR | Promotes cell
proliferation, invasion and metastasis. Prognostic marker for
metastasis Positively correlated with tamoxifen resistance | EZH2; estrogen
receptor protein | (33,45) |
| MALAT-1/NEAT2 | Promotes cell
proliferation, invasion and migration |
Phosphatidylinositid-3-Kinase-AKT
pathway | (65) |
| CCAT2 | Promotes cell
proliferation, tumorigenesis and inhibits apoptosis Positively
correlated with tamoxifen resistance | ERK/MAPK signaling
pathway TGF-β signaling pathway | (63,64) |
| BCAR4 | Promotes cell
proliferation, metastasis and tumor aggressiveness Endocrine
resistance | ERBB2/ERBB3
signaling pathway | (61) |
| TERC | Promotes cell
proliferation | | (66) |
| WSPAR/lncTCF7 | No data for BC, but
Wnt signaling dysfunction mediates progression of triple-negative
BC Promotes cell proliferation, invasion and metastasis in
colorectal cancer | Wnt/β-catenin
pathway | (71,72) |
| B,
Tumor-suppressing properties in BC |
| Pre-selected
lncRNA | Reported
function | Affected
genes/proteins/pathways | (Refs.) |
| BC4 | Downregulated in
BC | Undefined | (67,73) |
| FTX | Tumor
suppressor | Wnt/β-catenin
pathway | (73) |
| JPX | Downregulated in
BC | XIST (X
inactivate-specific transcript) → AKT Phosphorylation | (62,68) |
| linc00312 | Downregulated in BC
Suppression of proliferation, colony forming ability, migration and
invasiveness of BC cell lines | Suppression of
Cyclin B1 Increase of cadherin 1 (CDH1) Decrease of vimentin | (74,75) |
| NKILA (NF-ĸB
interacting long non-coding RNA) | - Tumor
suppressor | NF-ĸB-pathway | (59,60) |
Materials and methods
Cell culture conditions and
treatments
Established BC cell lines with a varying range of
hormone receptor states (Table
II) were incubated in a humidified atmosphere at 37˚C and 5%
CO2. MCF-7 cells were cultured in RPMI-1640 medium
(Thermo Fisher Scientific, Inc.), while i) BT-474, ii) T-47D,
SK-BR-3, BT-20 and MDA-MB-231 were cultured in DMEM-F12 (Thermo
Fisher Scientific, Inc.) in the presence of i) insulin (2.5 µl/ml)
alone or, ii) insulin (2.5 µl/ml) and estrogen (1 µ/ml)
supplemented with 1% HEPES buffer (MilliporeSigma), 1% 100 U/ml
penicillin/streptomycin (MilliporeSigma) and 5% FBS (Gibco; Thermo
Fisher Scientific, Inc. Table
II).
 | Table IIMolecular classification of breast
cancer cell lines investigated. |
Table II
Molecular classification of breast
cancer cell lines investigated.
| First author/s,
year | Cell line | Receptor
status | (Refs.) |
|---|
| Brooks, 1973 | MCF7 | ER+, PR+, HER2neu
- | (76) |
| Judge, 1983 | T-47D | ER+, PR+, HER2neu
- | (77) |
| Lasfargues,
1978 | BT-474 | ER+, PR+, HER2neu
+ | (78) |
| Trempe, 1976 | SK-BR-3 | ER-, PR-, HER2neu
+ | (58) |
| Lasfargues,
1958 | BT-20 | ER-, PR-, HER2neu
- | (57) |
| Brinkley, 1980 | MDA-MB-231 | ER-, PR-, HER2neu
- | (79) |
Cells were plated in 6-well culture plates and
incubated to 70-80% confluence. Pharmaceutical compounds dissolved
in DMSO (MilliporeSigma) were applied to in vitro models in
parallel monotherapy. Control cells received equivalent DMSO
volumes. In vitro compound concentrations geared to expected
serum levels in patients in the clinical setting are listed in
Table III. Following
application, cells were incubated for 18 h at 37˚C and 5%
CO2.
 | Table IIIConcentration of the medications
used. |
Table III
Concentration of the medications
used.
| Medication | Concentration | Supplier |
|---|
| Epirubicin | 5 µg/ml | Onkovis |
| Fulvestrant | 16 ng/ml | CC-Pharma GmbH |
| Gemcitabine | 20 µg/ml | Abmol
Bioscience |
| Paclitaxel | 5 nM | LC
Laboratories |
| Zoledronic
acid | 5 ng/ml | Novartis
International AG |
Treatment response was assessed visually. Therefore,
cell cultures were carefully analyzed, and the cells were
quantified before and after compound treatment. In case of 50%
reduction of cells in a cell culture flask, treatment response was
assessed positive. Additionally, Ki67 reduction was used to
determine a positive treatment effect. All experiments were
performed in triplicate (Table
III).
Total RNA isolation
Total RNA of cultured cells was obtained after cell
lysis with QIAzol Lysis Reagent® (Qiagen GmbH) using the
EURx Total RNA Purification Kit (Roboklon, GmbH) according to the
manufacturer's protocol. Assessment of RNA quantity was performed
by ultraviolet-spectrometry (NanoDrop ND1000; NanoDrop
Technologies; Thermo Fisher Scientific, Inc.). RNA samples were
stored at -80˚C until further processing.
Reverse transcription-quantitative
(RT-q)PCR
The RT reaction mixture consisted of 5 µl Maxima
RT-buffer (Thermo Scientific, Inc.), 1 µl 5 µM random hexamer (IDT
DNA, Leuven, Belgium), 1 µl 5 mM dNTPs (Roboklon GmbH), 0.25 µl
Maxima reverse transcriptase (Thermo Scientific, Inc.), 0.25 µl
SUPERase in RNase inhibitor (Thermo Fischer Scientific, Inc.) and 2
µg RNA in a volume of 25 µl. The reaction was carried out at 65˚C
for 1 min, followed by 25˚C for 10 min, 50˚C for 30 min and 85˚C
for 5 min and in a Nexus Thermal Cycler (Eppendorf SE). Processed
cDNA was stored at -20˚C until further analysis.
The relative expression levels of specific mRNAs and
lncRNAs were assessed by qPCR by using a SYBR Green assay in
duplicate. cDNA (1 µl) was mixed with 9 µl Master Mix containing
6.45 µl nuclease-free water, 1 µl 10X qPCR buffer, 0.5 µl 5 mM
dNTPs (Jena Bioscience), 0.5 µl specific qPCR primer (Biomers), 0.5
µl SYBR Green 1 µM (Jena Bioscience) and 0.25 U Hot Start
Taq-Polymerase (Jena Bioscience). Primers consisted of a specific
primer pair for each mRNA, lncRNA type and for the endogenous
control genes (Tables IV and
V). A negative control (10 µl
mastermix; no cDNA) and a minus-RT control (no RNA for Reverse
Transcription; 1 µl unspecific cDNA; 9 µl mastermix) were added in
order to evaluate if specific or unspecific products were
amplified. qPCR was performed on a LightCycler96 (Roche Diagnostics
GmbH) at 95˚C for 120 sec, followed by 40 cycles at 95˚C for 10
sec, 60˚C for 20 sec and 72˚C for 10 sec.
 | Table IVSpecific lncRNA primer sequences for
reverse transcription-quantitative PCR. |
Table IV
Specific lncRNA primer sequences for
reverse transcription-quantitative PCR.
| Primer for
lncRNA | | Sequence |
|---|
| BC4 | Sense |
5'-CCTTCCTTCGCACCACTAAA-3' |
| | Antisense |
5'-CGAGGAGGCATGGGTAAATATG-3' |
| BCAR 4 | Sense |
5'-CGAGGCTAAGAGTAGGAGTGATA-3' |
| | Antisense |
5'-GCGAGGTGCTAGCGATTATT-3' |
| CCAT2 | Sense |
5'-TCTCAACTGCCCAGGTAATATG-3' |
| | Antisense |
5'-GTTGGGACTTGCTGGTAGAA-3' |
| CYTOR | Sense |
5'-GATGGCTTGAACATTTGGTCTTC-3' |
| | Antisense |
5'-TCCTGTTTCATCTCCCAGTTATTC-3' |
| FTX | Sense |
5'-CCAGTTTGCCTCCCTCTTT-3' |
| | Antisense |
5'-CAGCACCTCATTCAACCTAGT-3' |
| HOTAIR | Sense |
5'-GTGTAGACCCAGCCCAATTTA-3' |
| | Antisense |
5'-GGCTGGACCTTTGCTTCTAT-3' |
| JPX | Sense |
5'-GAGTCCACCACCACCATAATC-3' |
| | Antisense |
5'-GCATGTCTTCCAGCACCATA-3' |
| linc312 | Sense |
5'-GACGCTGTTGAAGGAAGAAATG-3' |
| | Antisense |
5'-CCAAAGGAATCAGACCAGGAG-3' |
| MALAT-1 | Sense |
5'-GATTTGAGCGGAAGAACGAATG-3' |
| | Antisense |
5'-TGCCATGTGCCTGGAATTA-3' |
| NKILA | Sense |
5'-GAATTGCTTTGGAAGGAGCATAG-3' |
| | Antisense |
5'-CTGAACTGGGTGTCCTGTATTT-3' |
| TERC | Sense |
5'-CGAGGTTCAGGCCTTTCAG-3' |
| | Antisense |
5'-CATGTGTGAGCCGAGTCC-3' |
| WSPAR | Sense |
5'-GTCCTTGGACCTGAGCTAAC-3' |
| | Antisense |
5'-GGCTGGCATATAACCAACAATG-3' |
 | Table VSpecific mRNA primer sequences for
reverse transcription-quantitative PCR. |
Table V
Specific mRNA primer sequences for
reverse transcription-quantitative PCR.
| Primer for
mRNA | | Sequences |
|---|
| ALAS 1 | Sense |
5'-AGCGCAACGTCAAACTCAT-3' |
| | Antisense |
5'-TTTTAGCAGCATCTGCAACC-3' |
| Cyclin D1 | Sense |
5'-GGGTTGTGCTACAGATGATAGAG-3' |
| | Antisense |
5'-AGACGCCTCCTTTGTGTTAAT-3' |
| Ki-67 | Sense |
5'-GACCTCCAAACTGGCTCCTAATC-3' |
| | Antisense |
5'-GCTGCCAGATAGAGTCAGAAAG-3' |
| TOP2α | Sense |
5'-GACGCTTCGTTATGGGAAGATA-3' |
| | Antisense |
5'-GGGCCAGTTGTGATGGATAA-3' |
All results from RT-qPCR were normalized against the
geometric mean of Alas1 and TOP2α using the DD-CTq
method (36) Each statistically
significant lncRNA alteration was compared to Ki67-alterations.
Only in case of simultaneous expression alteration, altered lncRNAs
expression was considered relevant in terms of biomarker properties
(Tables IV and V).
Statistical analysis
The influence of treatment on lncRNA expression
levels in different BC cell lines was investigated using a linear
regression t-tests with factors treatment (epirubicin, fulvestrant,
gemcitabine, paclitaxel or zoledronic acid) compared to control
conditions and cell line as factorial predictors, including an
interaction term as coefficients. For calculation the Statistic
program R was used [R Core Team (Release year 2017; http://www.R-project.org/)]. This scenario inherently
suggested complex associations where both the type of cell line and
the treatment could affect the expression levels. A linear model
with interaction terms allows for the examination of these effects
in a nuanced manner, including how the effect of treatment may vary
across different cell lines, where simpler tests cannot
accommodate. The use of regression analysis with interaction terms
provides a comprehensive, efficient, and statistically rigorous
method for investigating complex relationships in biological data.
It is well-suited to address the specific research questions posed
in the study, offering insights into the nuanced effects of
treatments across different BC cell lines that simpler statistical
tests could not provide (37).
lncRNA expression levels were graphically visualized as boxplots,
showing the medians, quartiles and interquartile range (IQR) in the
cell lines BT-474, MCF-7, T-47D, SK-BR-3, BT-20 and MDA-MB-231
under control conditions. The estimated coefficients represented
the average effects on the lncRNA expression levels under treatment
were visualized in a heatmap that represented the fold change.
Results
In the present study, the potential regulatory
effects of epirubicin, gemcitabine, paclitaxel, fulvestrant and
zoledronic acid on the expression levels of pre-selected lncRNAs in
six BC cell lines were analyzed (Table IV). The characteristics of the six
investigated cell lines (MCF-7, T-47D, BT-474, SK-BR-3, BT-20 and
MDA-MB-231) are summarized in Table
II. Regression analysis was based on the target-control value
of BT-20 and was selected as intercept (Fig. 1, Fig.
2, Fig. 3 and Fig. 4) The parameter estimates from the
regression model with their 95% confidence intervals are presented
in Table SI).
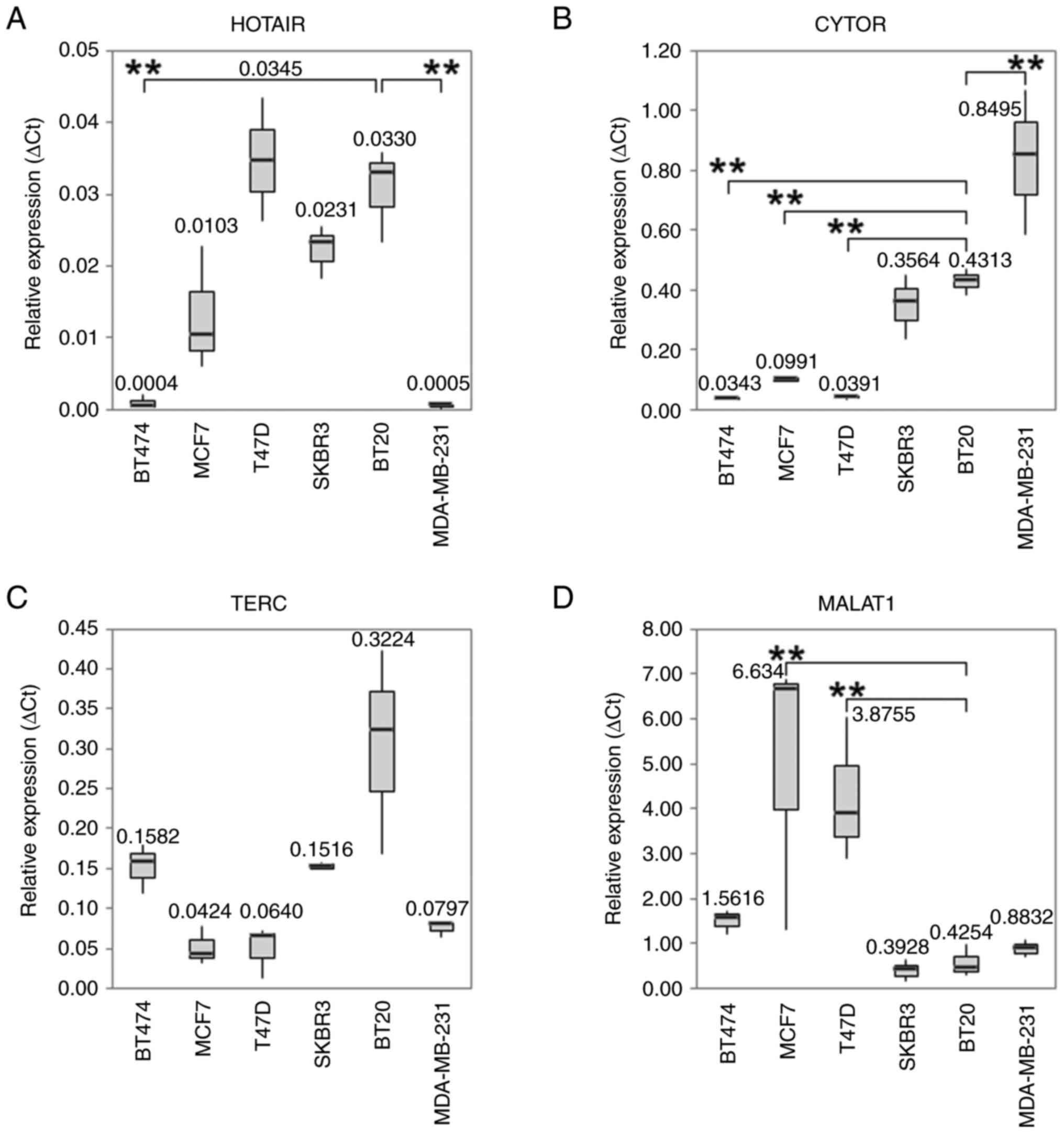 | Figure 1Boxplots with median values of mRNA
expression levels. The median values of mRNA expression levels of
(A) HOTAIR, (B) CYTOR, (C) TERC and (D) MALAT-1 under control
conditions in different BC cell lines. The significance level
refers to the calculation of the linear regression model. The
untreated BT20 cells served as an intercept. (A) The expression
level of HOTAIR was lower in BT-474 and MDA-MB-231 (P<0.001).
The IQR for PCR is from left to right: 0.0008, 0.0083, 0.0086,
0.0036, 0.0063 and 0.0003. (B) Under standard control conditions
CYTOR was overexpressed in MDA-MB-231 cells (P<0.001). In the
cell lines MCF7, T-47D and BT-474 CYTOR was downregulated
(P<0.001). The IQR for PCR is from left to right: 0.0068,
0.0070, 0.0050, 0.1069, 0.0429 and 0.2405. (C) TERC shows no
significant differences in the expression of the different cell
lines. The IQR for PCR is from left to right: 0.0307, 0.0230,
0.0299, 0.0046, 0.1266 and 0.0089. (D) MALAT-1 expression levels
under standard culture conditions revealed elevated expression in
the ER-positive cell lines MCF-7 (P<0.001) and T-47D (P=0.001).
The IQR for PCR is from left to right: 0.2615, 2.7902, 1.5664,
0.2396, 0.3329 and 0.1853. All Box plots demonstrate median (thick
black line), lower and upper quantile range (box lines), and
minimum and maximum values. Based on triplicate experiments,
reverse transcription-quantitative PCR. **P<0.001 vs.
the intercept. IQR, interquartile range; HOTAIR, HOX transcript
antisense RNA; CYTOR, cytoskeleton regulator RNA; TERC, telomerase
RNA component; MALAT-1. metastasis associated lung adenocarcinoma
transcript 1. |
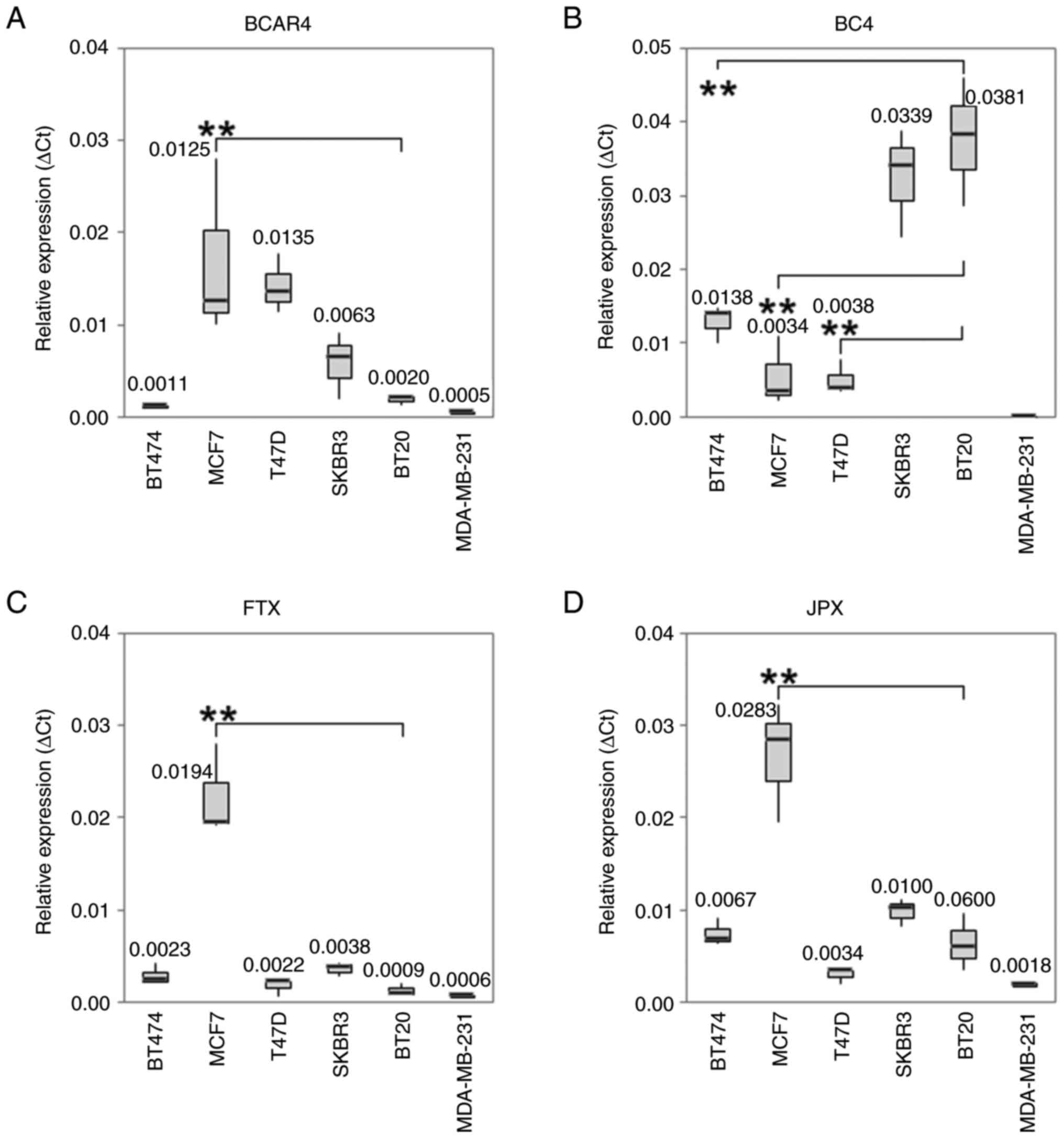 | Figure 2Boxplots with median values of mRNA
expression levels. The median values of mRNA expression levels of
(A) BCAR4, (B) BC4, (C) FTX and (D) JPX under control conditions in
different BC cell lines. The significance level refers to the
calculation of the linear regression model. The untreated BT20
cells served as an intercept. (A) In MCF-7 cells a statistically
significant higher BCAR4 expression level was observed. The IQR for
PCR is from left to right: 0.0002, 0.0089, 0.0031, 0.0035, 0.0005
and 0.0001. (B) The RNA expression level of BC 4 was significant
reduced in MCF7, T-47D and BT-474 cells (P<0.001). The IQR for
PCR is from left to right: 0.0024, 0.0043, 0.0021, 0.0072, 0.0086
and 0. (C) MCF-7 cells expressed a significant higher RNA level of
FTX. The IQR for PCR is from left to right: 0.0010, 0.0044, 0.0008,
0.0008, 0.0006 and 0.0001. (D) The expression level of JPX was
statistically significant higher in MCF-7 cells. The IQR for PCR is
from left to right: 0.0014, 0.0063, 0.0007, 0.0014, 0.0030 and
0.0002. All Box plots demonstrate median (thick black line), lower
and upper quantile range (box lines), and minimum and maximum
values. Based on triplicate experiments, reverse
transcription-quantitative PCR. **P<0.001 vs. the
intercept. IQR, interquartile range; BCAR4, breast cancer
antiestrogen resistance 4. |
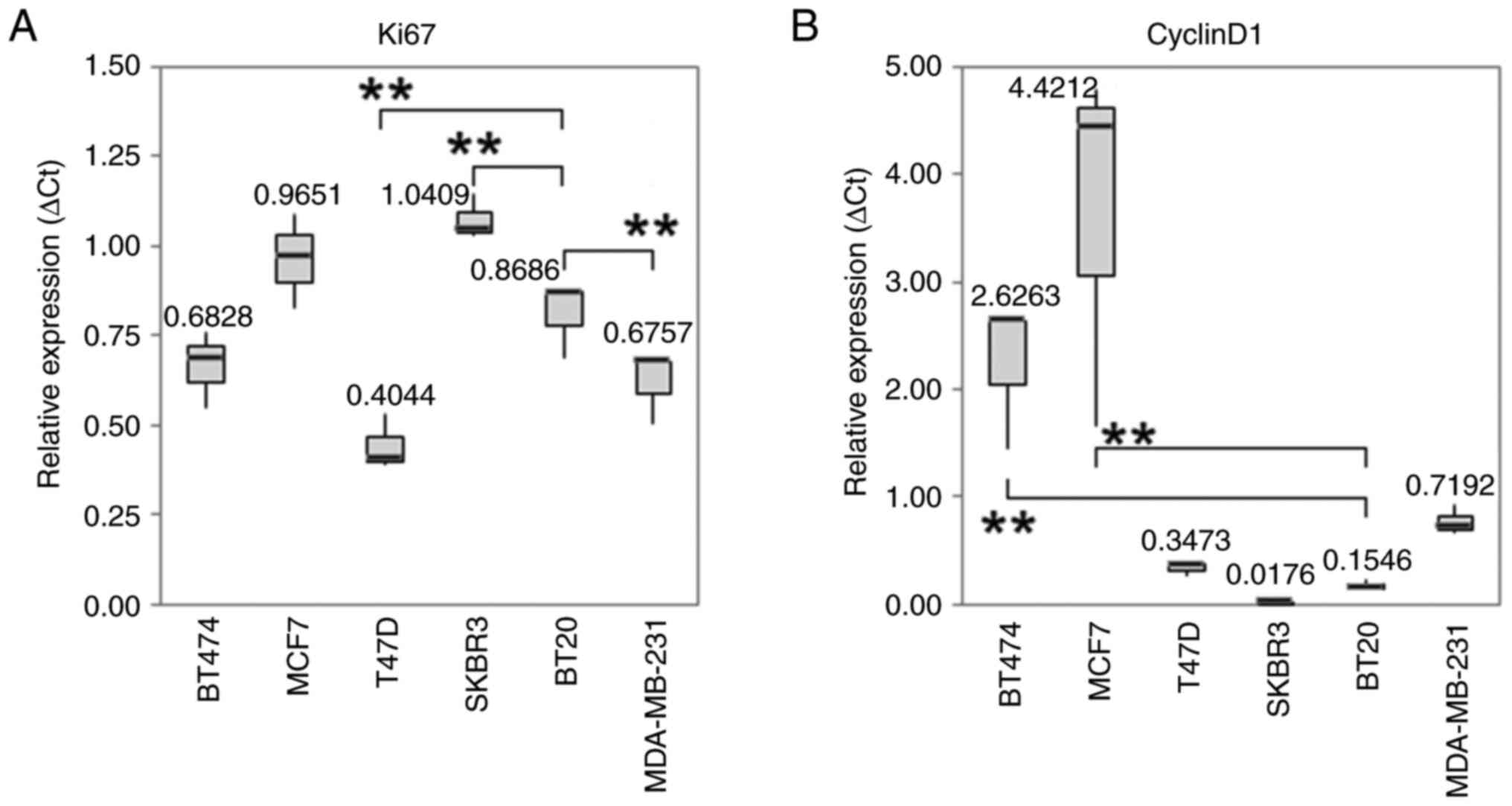 | Figure 3Boxplots with median values of mRNA
expression levels. The median values of mRNA expression levels of
(A) Ki67 and (B) cyclin D1 under control conditions in different BC
cell. The significance level refers to the calculation of the
linear regression model. The untreated BT20 cells served as an
intercept. (A) The RNA expression level of the proliferation marker
Ki-67 was overexpressed in SK-BR-3 (P=0.001). In T-47D cells
(P<0.001) and MDA-MB-231 cells (P=0.01) were a downregulation of
Ki-67 detectable. The IQR for PCR is from left to right: 0.1051,
0.1304, 0.0700, 0.0589, 0.0943 and 0.0860. (B) Under standard
culture conditions cyclin D1 was statistically significant higher
expressed in MCF-7 and BT-474 cells. The IQR for PCR is from left
to right: 0.5895, 1.5604, 0.0607, 0.0077, 0.0430 and 0.1269. All
Box plots demonstrate median (thick black line), lower and upper
quantile range (box lines), and minimum and maximum values. Based
on triplicate experiments, reverse transcription-quantitative PCR.
**P<0.001 vs. the intercept. IQR, interquartile
range. |
 | Figure 4The influence of treatment on lncRNA
expression levels of the different BC cell lines. Expression
profiles of selected lnc-RNAs in BT-474, MCF-7, T-47D, SKBR-3,
BT-20 and MDA-MB-231cells under control conditions and after
different breast cancer treatments. Lnc-RNA-expressions were
determined in triplicates by real-time quantitative PCR and
calculated using ∆Ct method based on reference value (geometric
mean of housekeeping genes Alas1 and TOP2α) to determine expression
of single lncRNAs. After calculating the expression level. The data
were visualized in a heatmap according to the color key. It
represents the fold change of the ∆∆Ct values compared to the same
cell line untreated. lncRNA, long non-coding RNA; CCAT2, colon
cancer associated transcript 2 RNA; CYTOR, cytoskeleton regulator
RNA; HOTAIR, HOX transcript antisense RNA; MALAT-1, metastasis
associated lung adenocarcinoma transcript 1; NKILA, NF-ĸB
interacting long non-coding RNA TERC, telomerase RNA component;
WSPAR, WNT signaling pathway activating non-coding RNA. |
Basal lncRNA and Ki-67/Cyclin D1
expression levels
The expression level of HOTAIR was lower in the
BT-474 and MDA-MB-231 cell lines (P<0.001; Fig. 1A) compared to the intercept. TERC
was highly expressed in BT-20 cells (P<0.001; Fig. 1C). metastasis associated lung
adenocarcinoma transcript 1 (MALAT-1) expression levels under
standard culture conditions revealed elevated expression in the
ER-positive cell lines MCF-7 (P<0.001) and T-47D (P=0.001;
Fig. 1D). Under standard control
conditions, CYTOR was overexpressed in BT-20 and MDA-MB-231 cells
(P<0.001), and downregulated in MCF-7, T-47D and BT-474 cells
(Fig. 1B). The expression level of
lncRNA BC4 was increased in BT-20 cells (P<0.001), and reduced
in MCF-7, T-47D and BT-474 cells (P<0.001; Fig. 2B). Higher BCAR4 and FTX RNA
expression levels were observed in MCF-7 cells (P<0.001;
Fig. 2A and C). The expression level of JPX was higher
in BT-20 and MCF-7 cells (P<0.001; Fig. 2D). The mRNA expression level of the
proliferation marker Ki-67 was elevated in BT-20 (P<0.001) and
SK-BR-3 (P=0.001) cells. Downregulation of Ki-67 was detectable in
T-47D (P<0.001) and MDA-MB-231 (P=0.01) cells (Fig. 3A). Under standard culture
conditions, Cyclin D1 was overexpressed in MCF-7 and BT-474 cells
(P<0.001; Fig. 3B).
lncRNA and Ki-67/Cyclin D1 expression
levels following treatment with epirubicin, gemcitabine,
paclitaxel, fulvestrant and zoledronic acid
Treatment with epirubicin triggered an increase in
HOTAIR expression in BT-20, SK-BR-3 and T-47D cells (P<0.001)
and an increase in CYTOR expression in BT-20, MDA-MB-231 and BT-474
cells (P<0.001). In SK-BR-3 cells, the CYTOR-increasing
drug-dependent effect did not reach statistical significance but
demonstrated a pronounced trend. The expression levels of lncRNA
BC4 increased in BT-20 and SK-BR-3 cells (P<0.001), and the
expression of NF-ĸB interacting long non-coding RNA (NKILA)
increased in MCF-7 cells (P<0.001). A slight upregulation of
BCAR4 was observed in T-47D cells (P=0.05). For JPX, downregulation
was observed in BT-474, SK-BR-3, BT-20 and MCF-7 cells. However,
the decrease was only significant in BT-474 cells. In response to
epirubicin, the expression of Ki-67 decreased in MCF-7 and BT-474
cells (P<0.001; data not shown). The same trend was observed in
MDA-MB-231 cells, albeit it was not statistically significant
(P=0.05). In BT-474 cells, epirubicin-dependent downregulation was
observed for Cyclin D1 (P=0.01; Fig.
4).
Following treatment with gemcitabine, the expression
level of CYTOR significantly increased in BT-20 and BT-474 cells.
The increase of CYTOR expression in SK-BR-3 cells followed a
statistical trend. The RNA expression level of BC4 significantly
increased in BT-20 and in SK-BR-3 cells, although without
statistical significance. In MCF-7 cells, the expression of NKILA
increased (P=0.05), whereas the expression of JPX decreased
(P=0.05). For Ki-67, gemcitabine-dependent downregulation was
observed in the cell lines SK-BR-3 (P<0.001), BT-474 (P=0.001),
BT-20 (P=0.01) and MDA-MB-231 (P=0.01). The mRNA expression level
of Cyclin-D1 decreased in BT-474 cells (Fig. 4).
Paclitaxel led to an increased expression level of
lncRNA FTX in MCF-7 cells (P=0.001). The other pre-selected lncRNAs
showed no alterations of their expression levels after treatment
with paclitaxel (Fig. 4).
Following treatment with fulvestrant, the expression of JPX
decreased in MCF-7 cells (P=0.01), while other alterations did not
reach statistical significance (Fig.
4). No zoledronic acid-driven alterations in the expression
levels of the pre-selected lncRNAs were observed in any of the cell
lines investigated (Fig. 4).
Discussion
The present study analyzed the potential effects
triggered by different BC treatments on the transcriptional
expression of pre-selected lncRNAs and the proliferation markers
Cyclin D1 and Ki-67 in six different BC cell lines. Following
treatment with epirubicin and gemcitabine, the levels of the
proliferation marker Ki-67 decreased in all six cell lines,
indicating a decreased proliferation after chemotherapy and thus
treatment response, while Cyclin D1 levels only decreased in the
cell line BT-474. Therefore, in the present study, Ki-67 was
considered to serve as a predictive marker for the six cell lines
analyzed following treatment with epirubicin and gemcitabine, while
Cyclin D1 could only be used for interpretation in the BT-474 cell
line.
LncRNA CYTOR has emerged as an important player in
cancer progression across various malignancies, underscoring its
potential both as a biomarker and a therapeutic target. Recent
research agrees with the present observations that CYTOR expression
is dysregulated in multiple cancer types, which is further altered
following treatment with chemotherapy agents such as epirubicin and
gemcitabine (38). Specifically,
the current study noted an upregulation of CYTOR in selected cell
lines under chemotherapy, coinciding with a concurrent
downregulation of Ki67, indicating a potential therapeutic
response. This observation is supported by a body of evidence
suggesting the involvement of CYTOR in cancer cell proliferation,
migration and invasion. For instance, a previous study demonstrated
the role of CYTOR in modulating key signaling pathways implicated
in cancer progression, such as the PI3K/Akt signaling pathway, thus
highlighting its contribution to enhanced cancer cell survival and
proliferation (39). Furthermore,
dysregulation of CYTOR has been associated with chemoresistance in
certain cancer types, similar to the present findings. Elevated
CYTOR expression has been linked to resistance against
gemcitabine-based chemotherapy in pancreatic cancer (40) and to tamoxifen therapy resistance
in BC (41).
Additionally, studies have begun to unravel the
molecular mechanisms underlying the function of CYTOR, elucidating
its important role in cancer biology. For example, previous
research has highlighted its involvement in regulating apoptosis
and cell cycle progression, thereby contributing to cancer therapy
resistance (38-42).
Such evidence suggests that targeting CYTOR could offer a new
avenue for combating treatment resistance and improving patient
outcomes. With the role of CYTOR in cancer biology becoming
increasingly clear, it can be concluded that CYTOR may serve not
only as a marker of disease progression but also as a potential
target for therapeutic intervention, especially in the context of
chemoresistance (42,43).
The alignment of the present study with these
findings underscores the importance of further investigating CYTOR
in cancer research, particularly its response under chemotherapy,
to develop more effective treatments. In the current study, CYTOR
was consistently expressed in every cell line and underwent a
marked treatment-induced regulation. Under control conditions,
CYTOR expression was high in the triple-negative BC cell lines
BT-20 and MDA-MB-231 (P<0.001). A lower expression level was
observed in the hormone-receptor positive cell lines MCF-7 and
T-47D (P<0.001). The increased expression levels of CYTOR in
triple-negative BC cell lines support the hypothesis that CYTOR is
associated with higher aggressiveness and worse overall survival in
different tumor types, as demonstrated by Liang et al
(44).
Following treatment with epirubicin and gemcitabine,
the levels of the proliferation marker Ki-67 decreased in all cell
lines, although to a different extent. The RNA expression of CYTOR
increased following therapy with epirubicin and gemcitabine. After
epirubicin treatment, this increase was most pronounced in the
triple-negative BC cell lines BT-20 and MDA-MB-231 (P<0.001).
The same effect was observed in BT-474 cells. Under the influence
of gemcitabine, the expression level of CYTOR increased in BT-20
and BT-474 cells. Wu et al (27) described a proliferation-stimulation
effect of CYTOR in vitro and in vivo. It appears that
cell lines with a high proliferation activity and responsiveness to
chemotherapy (epirubicin and gemcitabine) cause reactive CYTOR
overexpression. This confirms the results reported by Van
Grembergen et al (45), who
showed that CYTOR was essential for the proliferation in MDA-MB-231
BC cells. Knockdown of CYTOR leads to cell accumulation at the end
of the S phase and in the G2/M phase. Since epirubicin
and gemcitabine have their maximum toxic effect in the
S/G2 phase, the increased expression of CYTOR observed
during treatment can be interpreted as an adaptation of the cancer
cells to try to maintain proliferation. Based on the present
results, it may be hypothesized that CYTOR will be overexpressed if
the treatment is effective. In the current study, treatment with
paclitaxel, fulvestrant or zoledronic showed no significant
alteration in the expression levels of Ki-67 or CYTOR.
The lncRNA HOTAIR is observed to be upregulated in
various cancer types (46). In the
present study, HOTAIR was consistently expressed in the selected
cell lines. Following treatment with epirubicin, the RNA expression
level of HOTAIR increased. This is in agreement with previous
findings, such as those by Teschendorff et al (47), who found a correlation between
overexpression of HOTAIR and carboplatin resistance in ovarian
cancer. The epirubicin-dependent overexpression of HOTAIR may also
be a sign of resistance. A statistically significant increase in
HOTAIR expression was observed in BT-20, SK-BR-3 and T-47D cells.
None of the cell lines analyzed in the present study showed a
simultaneous significant decrease in the proliferation marker
Ki-67. This contrasts with cell lines lacking HOTAIR
overexpression, which displayed an epirubicin-dependent decline in
Ki-67 levels. According to the current findings and recent
research, HOTAIR is overexpressed under therapies such as
paclitaxel, cisplatin, paclitaxel and doxorubicin, temozolomide and
sunitinib (48-52).
Of note, the present and other studies have shown that HOTAIR
knockout can lead to increased chemotherapy efficacy, suggesting
that HOTAIR may indicate resistance (48,53).
Additionally, the current study contributed to the understanding of
the role of HOTAIR in treatment response, particularly in the
context of epirubicin therapy. Furthermore, there is a relevant
study on NSCLC and EGFR-TKI therapy that shows conflicting results
(54), where HOTAIR reduction was
demonstrated post-therapy, contrasting with the present findings.
Wang et al (55) were able
to show, in a pancreatic cancer cell line, that treatment with
gemcitabine led to overexpression of HOTAIR, which promoted cell
proliferation and migration and reduced the apoptosis rate. After
suppression of HOTAIR with small interfering RNA, the sensitivity
to gemcitabine increased. This process could be reversed by
lentivirus-induced expression of HOTAIR, so that chemotherapy
resistance can be assumed by induction of HOTAIR after gemcitabine
administration (55). Thus, HOTAIR
may be a target for new therapy against chemotherapy resistance. In
the present study on BC cell lines, overexpression of HOTAIR was
only significantly detectable after epirubicin treatment, while the
alterations observed after gemcitabine treatment were not
statistically significant. In addition, a study has shown
overexpression of HOTAIR in association with endocrine resistance,
more precisely tamoxifen resistance (26). The fulvestrant-dependent alteration
of the expression level of HOTAIR was examined in the present
study. However, no significant alteration in HOTAIR was measurable
after fulvestrant treatment. The results of the current study are
congruent with those of Milevskiy et al (56), who hypothesized that the
association between estrogen and HOTAIR may play a role in
endocrine resistance. The authors used well-documented
tamoxifen-resistant, fulvestrant-resistant and estrogen-independent
sublines of MCF7 cells (56).
HOTAIR expression increased in both low-estrogen MCF7X and
tamoxifen-resistant TAMR cells, which was consistent with a
repressive role of the estrogen/ER pathway. After fulvestrant
treatment, HOTAIR expression did not increase significant.
In contrast to the established body of research, the
findings of the present study on the expression of 10 lncRNAs NF-ĸB
interacting long non-coding RNA (NKILA), breast cancer antiestrogen
resistance 4 (BCAR4), colon cancer associated transcript 2 RNA
(CCAT2), metastasis associated lung adenocarcinoma transcript 1
(MALAT1), telomerase RNA component (TERC), WNT signaling pathway
activating non-coding RNA (WSPAR), BC4, FTX, JPX and long
intergenic non-coding RNA (lincRNA) 00312 do not present a
consistent pattern of regulation. This deviation underscores a
complex interaction that may be influenced by the specific
conditions of the present experimental setup, including cell line
selection, drug dosage, and treatment duration, which are crucial
factors as outlined in the referenced publications. For instance,
Lasfargues and Ozzello (57) as
well as Trempe (58) have
emphasized the heterogeneity of breast cancer cell lines, which
might explain the sporadic regulation patterns observed in the
present study, contrasting with the uniform responses reported in
previous studies (4,5).
The effect of different chemotherapeutic agents on
lncRNA expression, particularly epirubicin and gemcitabine, also
reveals a disparity when compared to literature. Khasraw et
al (13) and Seidman (14) discuss the general mechanisms and
effects of these drugs in breast cancer treatment but do not delve
into their specific impacts on lncRNA regulation. The present
findings that NKILA, BCAR4 and others exhibit varied expression
under these treatments suggested a more complex interaction than
previously understood, further diverging from studies that did not
observe such effects (10,17).
Focusing on the body of literature on the single
lncRNAs, especially NKILA, BCAR 4 and JPX should be discussed in
more detail. Previous studies have shown NKILA to be involved in
suppressing breast cancer metastasis through inhibition of NF-κB
signaling (59,60). The present observation of NKILA
upregulation in MCF-7 cells under epirubicin and gemcitabine
treatment could suggest a stress response mechanism. However, the
lack of a consistent response across cell lines and treatments
indicates a potential context-dependent regulation.
Highlighted in the work of Godinho et al
(61), BCAR4 is known to drive
endocrine resistance in breast cancer. The present findings of
BCAR4 upregulation in MCF-7 untreated cells and T-47D cells under
epirubicin treatment could reflect its role in resistance
mechanisms, yet the absence of this trend across other treatments
and cell lines underscores the variability in its expression and
potential function.
Involved in X chromosome inactivation and
regulation, JPX shows downregulation under epirubicin treatment in
BT-474 and gemcitabine in MCF-7(62). This suggests a potential
involvement in stress response or treatment resistance mechanisms,
albeit in a cell line-specific manner.
In addition, CCAT2, associated with breast cancer
growth and metastasis, showed no significant changes, suggesting
that its effect may lie in metastatic processes rather than direct
treatment response (63,64). MALAT1, linked to poor prognosis and
tumor growth, was more highly expressed in ER-positive cell lines,
hinting at a fundamental role in tumorigenesis (65). TERC, crucial for telomere
maintenance, exhibited cell line-specific upregulation in BT-20,
indicating a complex interplay with treatment conditions (66). Lesser-studied lncRNAs such as WSPAR
and BC4 showed minimal or specific regulation, underscoring the
need for further research to clarify their functions in breast
cancer biology and treatment responses.
WSPAR has been described in association with
colorectal and hepatocellular cancer. WSPAR overexpression
correlates with cell proliferation, invasion and metastatic growth.
However, no function of WSPAR has been reported in BC thus far. In
the present study, only the hormone receptor-positive cell lines
MCF-7 and T-47D showed WSPAR expression. However, the expression
levels showed no significant drug-induced alterations.
Ding et al (67) described the downregulation of BC4
in BC, which was confirmed in the present study, since each BC cell
line showed low BC4 expression. After treatment with epirubicin and
gemcitabine, the expression level of BC4 increased in BT-20 and
SK-BR-3 cells, while Ki-67 expression levels decreased in both cell
lines at the same time after treatment with gemcitabine only.
Epirubicin did not induce a significant alteration in Ki-67
expression in BT-20 or SK-BR-3 cells. Due to the divergent
expression-alteration of Ki-67, it is difficult to interpret the
meaning of the increase in BC4 expression during therapy. Thus,
further studies are needed to elucidate the meaning of BC4
overexpression for therapy efficiency monitoring.
The lncRNAs TERC, MALAT-1 and linc312 were
consistently expressed in each cell line, but showed no significant
treatment-dependent alterations. In addition, the expression of
CCAT2 showed no significant therapy modification. Notably, CCAT2
was consistently expressed in every cell line with the exception of
BT-474.
BCAR4, a lncRNA associated with endocrine resistance
(61), showed limited
therapy-dependent alterations in the present study. The expression
level of BCAR4 decreased (P=0.01) after epirubicin treatment only
in T-47D cells, without any alterations in Ki-67 or Cyclin D1
expression.
Wu et al (59) reported a negative correlation
between the expression level of NKILA and tumor/metastasis
proliferation. Liu et al (60) have shown NKILA to be involved in
suppressing breast cancer metastasis through inhibition of NF-κB
signaling. In the present study, the effect of epirubicin in NKILA
expression was observed only in the MCF-7 cell line.
The expression level of the lncRNA JPX was higher in
MCF-7 and BT-20 cells and lower in MDA-MB-231 cells. Following
epirubicin treatment, a decrease in JPX expression was detectable
in BT-20, BT-474, MCF-7 and SKBR-3 cells. However, a reduction in
the expression level of Ki-67 was observed at the same time only in
BT-474 and MCF-7 cells. Fulvestrant also led to a decrease in JPX
expression level in MCF-7. The interpretation of the role of
downregulation of JPX is therefore challenging. Huang et al
(68) reported that suppression of
JPX via reduced expression of the tumor suppressor Xist led to
reduced inactivation of AKT phosphorylation and thus to increased
cell viability. Overexpression of XIST markedly inhibited BC cells
proliferation, migration and invasion via sponging to microRNA-155
in BC (69).
The present study has certain limitations. The
current study investigated whether there were any connections
between selected BC drugs and pre-selected RNAs; future studies
should focus on the identification of the signaling pathways
involved.
Although paclitaxel is an established, potent drug
in the treatment of BC, overall, it rarely appeared to have effects
on the different cell lines analyzed in the present study. The
concentration of the drugs, including paclitaxel, was based on the
maximum serum levels during in vivo therapy under clinical
conditions. The duration of drug application was based on the
half-value period of the drug and findings from our preliminary
experiments. Li et al (70)
observed a clear proliferation-inhibiting effect of paclitaxel with
prolonged exposure (48 instead of 18 h) and double concentration
(10 instead of 5 nM). However, with an incubation time of 48 h at
10 nM, the drug dose of such study is within a range that is not
reached clinically in BC therapy. The reaction of cells in
vitro cannot, however, represent the exact behavior of an
organism with metabolism and redistribution. The conditions may
suggest that paclitaxel, in the present study, was applied in an
excessively low concentration for finding effects in proliferation
and lncRNA expression. Considering the interpretation of these
results, it is not certain whether lncRNAs hold predictive value
for paclitaxel in reality. Potential effects could have been missed
in the current study due to the aforementioned lower dosage and
exposure of the drug. Therefore, further explorations with adjusted
experimental conditions should be pursued in the future. As
potential avenues for future research, a step-wise procedure could
be envisioned. Firstly, in vitro experiments should be
performed with increased dosages and longer exposure of paclitaxel.
Secondly, in vivo models could be introduced to mimic the
clinical metabolic conditions in a more realistic manner. Lastly,
in vitro and in vivo results could be validated in
clinical trials by analyzing the breast tissue or liquid samples of
patients under paclitaxel treatment. Another limitation is that the
expression of the lncRNAs and the proliferation markers are
regulated by a number of factors. Further studies must confirm
whether the discovered correlations can really be causally linked.
An additional limitation was that the response to the treatment was
assessed visually. At a 50% reduction of cells in a cell culture
flask, the response to treatment was assessed as positive. A
viability assay would be useful for further projects.
In summary, the strength and innovative findings of
this study relate to the significant correlation of lncRNA
alterations to established disease biomarkers such as Ki67. CYTOR
and HOTAIR showed significant expression alterations under
different chemotherapeutic treatments (especially under
anthracyclines) simultaneously to Ki67 downregulation and thus
suggesting biomarker properties for treatment response. In clinical
practice, CYTOR and HOTAIR could potentially be used to determine
treatment effects under anthracyclines specifically in tissue and
liquid biopsies. Based on the findings of the present study, the
upregulation of HOTAIR and CYTOR potentially indicate treatment
response whereas their downregulation or expression level
maintenance might indicate treatment failure. As the proper therapy
response is crucial for overall and progression free survival,
these findings could be game-changing for the management of
chemotherapy patients. Thus, further studies must be undertaken to
analyze HOTAIR and CYTOR with regards to therapy response. In the
present study, the other 10 lncRNAs did not show significant
expression alterations under the aforementioned treatments. This
could mean that they are not suitable for an early detection of
therapy response under the given therapeutics. Furthermore, it
might also mean that the treatment doses in the present study were
not adequate.
In conclusion, the present study analyzed in
vitro the potential effects of various drugs on the
transcriptional expression of pre-selected lncRNAs to indicate
therapy resistance and response in BC. The results revealed that
lncRNA CYTOR may be an appropriate biomarker for the response to
treatment with both epirubicin and gemcitabine, while NKILA may be
a marker for treatment response. Furthermore, HOTAIR overexpression
may suggest therapy resistance to epirubicin, while BC4
transcriptional overexpression may indicate therapy response to
gemcitabine and epirubicin. Overall, treatment-dependent
alterations were not observed in every cell line investigated and
the extent of the alteration differed, which may reflect the
heterogeneity of BC disease even in vitro. However, the
therapy-dependent alterations shown in the current study suggest
that lncRNAs may hold potential to serve as biomarkers for
treatment response or resistance. In addition, lncRNAs may be
explored as a prognostic and diagnostic molecule in patients with
metastatic BC. However, the expression of lncRNAs and their role as
potential biomarkers remain to be fully clarified. Further research
should focus on the elucidation of the pathways involved in lncRNAs
regulatory mechanisms and the analysis of lncRNAs for monitoring of
BC treatment in vivo.
Supplementary Material
The parameter estimates from the
regression model with their 95% confidence intervals.
Acknowledgements
The authors are particularly grateful for continuous
organizational support and technical assistance given by Mrs.
Claudia Nöthling (Department of Obstetrics and Gynecology, Medical
Center, University of Freiburg, Freiburg, Germany). The authors
express their appreciation to Dr Gerta Rücker (Faculty of Medicine,
University of Freiburg, Freiburg, Germany) for her support in the
statistical analysis.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JA, BS, KB, MJ, IJB, TE and SM designed the present
study. MJ, DW and BS performed experiments (cell culture, RNA
isolation and RT-qPCR). JA and SM confirm the authenticity of all
the raw data. BS performed statistical analysis. JA, KB, IG, BS,
TE, IJB and SM analyzed and interpreted the data. JA, TE, KB and BS
wrote the manuscript in consultation with and under critical
revision of DW, MJ, IG, SM and IJB. JA and SM supervised the
overall experimental design. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48.
2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Prat A, Parker JS, Karginova O, Fan C,
Livasy C, Herschkowitz JI, He X and Perou CM: Phenotypic and
molecular characterization of the claudin-low intrinsic subtype of
breast cancer. Breast Cancer Res. 12(R68)2010.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Sørlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:10869–10874. 2001.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Harbeck N and Gnant M: Breast cancer.
Lancet. 389:1134–1150. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Teshome M and Hunt KK: Neoadjuvant therapy
in the treatment of breast cancer. Surg Oncol Clin N Am.
23:505–523. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 365:1687–1717.
2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rani A, Stebbing J, Giamas G and Murphy J:
Endocrine resistance in hormone receptor positive breast
cancer-from mechanism to therapy. Front Endocrinol (Lausanne).
10(245)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gilbert LA and Hemann MT: DNA
damage-mediated induction of a chemoresistant niche. Cell.
143:355–366. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kellokumpu-Lehtinen P, Tuunanen T, Asola
R, Elomaa L, Heikkinen M, Kokko R, Järvenpää R, Lehtinen I, Maiche
A, Kaleva-Kerola J, et al: Weekly paclitaxel-an effective treatment
for advanced breast cancer. Anticancer Res. 33:2623–2627.
2013.PubMed/NCBI
|
|
12
|
Bergh J, Jonsson PE, Glimelius B and
Nygren P: SBU-group. Swedish Council of Technology Assessment in
Health Care: A systematic overview of chemotherapy effects in
breast cancer. Acta Oncol. 40:253–281. 2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Khasraw M, Bell R and Dang C: Epirubicin:
Is it like doxorubicin in breast cancer? A clinical review. Breast.
21:142–149. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Seidman AD: Gemcitabine as single-agent
therapy in the management of advanced breast cancer. Oncology
(Williston Park). 15 (2 Suppl 3):S11–S14. 2001.PubMed/NCBI
|
|
15
|
Bonadonna G, Gianni L, Santoro A, Bonfante
V, Bidoli P, Casali P, Demicheli R and Valagussa P: Drugs ten years
later: Epirubicin. Ann Oncol. 4:359–369. 1993.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Weaver BA: How Taxol/paclitaxel kills
cancer cells. Mol Biol Cell. 25:2677–2681. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Huang P, Chubb S, Hertel LW, Grindey GB
and Plunkett W: Action of 2',2'-difluorodeoxycytidine on DNA
synthesis. Cancer Res. 51:6110–6117. 1991.PubMed/NCBI
|
|
18
|
Carlson RW: The history and mechanism of
action of fulvestrant. Clin Breast Cancer. 6 (Suppl 1):S5–S8.
2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lee CI, Goodwin A and Wilcken N:
Fulvestrant for hormone-sensitive metastatic breast cancer.
Cochrane Database Syst Rev. 1(Cd011093)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Biskup E, Cai F and Vetter M: Bone
targeted therapies in advanced breast cancer. Swiss Med Wkly.
147(w14440)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Van Poznak CH: The use of bisphosphonates
in patients with breast cancer. Cancer control. 9:480–489.
2002.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Coleman R: The use of bisphosphonates in
cancer treatment. Ann N Y Acad Sci. 1218:3–14. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Iyer MK, Niknafs YS, Malik R, Singhal U,
Sahu A, Hosono Y, Barrette TR, Prensner JR, Evans JR, Zhao S, et
al: The landscape of long noncoding RNAs in the human
transcriptome. Nat Genet. 47:199–208. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rinn JL and Chang HY: Genome regulation by
long noncoding RNAs. Annu Rev Biochem. 81:145–166. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Majidinia M and Yousefi B: Long non-coding
RNAs in cancer drug resistance development. DNA Repair (Amst).
45:25–33. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Xue X, Yang YA, Zhang A, Fong KW, Kim J,
Song B, Li S, Zhao JC and Yu J: LncRNA HOTAIR enhances ER signaling
and confers tamoxifen resistance in breast cancer. Oncogene.
35:2746–2755. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wu J, Shuang Z, Zhao J, Tang H, Liu P,
Zhang L, Xie X and Xiao X: Linc00152 promotes tumorigenesis by
regulating DNMTs in triple-negative breast cancer. Biomed
Pharmacother. 97:1275–1281. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kapranov P, Cheng J, Dike S, Nix DA,
Duttagupta R, Willingham AT, Stadler PF, Hertel J, Hackermuller J,
Hofacker IL, et al: RNA maps reveal new RNA classes and a possible
function for pervasive transcription. Science. 316:1484–1488.
2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Malih S, Saidijam M and Malih N: A brief
review on long noncoding RNAs: A new paradigm in breast cancer
pathogenesis, diagnosis and therapy. Tumor Biol. 37:1479–1485.
2016.PubMed/NCBI View Article : Google Scholar : [CrossRef]
[PubMed] 4 Wang J, Ye C, Xiong H, Shen Y, Lu Y, Zhou J and Wang L:
Dysregulation of long non-coding RNA in breast cancer: An overview
of mechanism and clinical implication. Oncotarget 8: 5508-5522,
2017.
|
|
30
|
Wang Q, Gao S, Li H, Lv M and Lu C: Long
Non-coding RNAs inTriple Negative Breast Cancer. J Cell Physiol.
232:3226–3233. 2017.PubMed/NCBI View Article : Google Scholar : [CrossRef]
[PubMed] 279. Warburton AJ and Boone DN: Insights from global
analyses of long noncoding RNAs in breast cancer. Curr Pathobiol
Rep 5: 23-34, 2017.
|
|
31
|
Tian T, Wang M, Lin S, Guo Y, Dai Z, Liu
K, Yang P, Dai C, Zhu Y, Zheng Y, et al: The impact of lncRNA
dysregulation on clinicopathology and survival of breast cancer: A
systematic review and meta-analysis. Mol Ther Nucleic Acids.
12:359–369. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hashmi AA, Hashmi KA, Irfan M, Khan SM,
Edhi MM, Ali JP, Hashmi SK, Asif H, Faridi N and Khan A: Ki67 index
in intrinsic breast cancer subtypes and its association with
prognostic parameters. BMC Res Notes. 12(605)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kreipe H, Harbeck N and Christgen M:
Clinical validity and clinical utility of Ki67 in early breast
cancer. Ther Adv Med Oncol. 14(17588359221122725)2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Montalto FI and De Amicis F: Cyclin D1 in
Cancer: A molecular connection for cell cycle control, adhesion and
invasion in tumor and stroma. Cells. 9(2648)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Shi Q, Li Y, Li S, Jin L, Lai H, Wu Y, Cai
Z, Zhu M, Li Q, Li Y, et al: LncRNA DILA1 inhibits Cyclin D1
degradation and contributes to tamoxifen resistance in breast
cancer. Nat Commun. 11(5513)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Thompson, David M and Yan Daniel Zhao:
Choosing statistical models to assess biological interaction as a
departure from additivity of effects. arXiv:2301.03349, 2023.
|
|
38
|
Cao Q, Wang H, Zhu J, Qi C, Huang H and
Chu X: lncRNA CYTOR promotes lung adenocarcinoma gemcitabine
resistance and epithelial-mesenchymal transition by sponging
miR-125a-5p and upregulating ANLN and RRM2. Acta Biochim Biophys
Sin (Shanghai). 56:210–222. 2024.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chen S, Yang M, Wang C, Ouyang Y, Chen X,
Bai J, Hu Y, Song M, Zhang S and Zhang Q: Forkhead box D1 promotes
EMT and chemoresistance by upregulating lncRNA CYTOR in oral
squamous cell carcinoma. Cancer Lett. 503:43–53. 2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Liu Y, Li M, Yu H and Piao H: lncRNA CYTOR
promotes tamoxifen resistance in breast cancer cells via sponging
miR-125a-5p. Int J Mol Med. 45:497–509. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yang J, Ma Q, Zhang M and Zhang W: LncRNA
CYTOR drives L-OHP resistance and facilitates the
epithelial-mesenchymal transition of colon carcinoma cells via
modulating miR-378a-5p/SERPINE1. Cell Cycle. 20:1415–1430.
2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yue B, Liu C, Sun H, Liu M, Song C, Cui R,
Qiu S and Zhong M: A Positive Feed-Forward Loop between
LncRNA-CYTOR and Wnt/β-Catenin signaling promotes metastasis of
colon cancer. Mol Ther. 26:1287–1298. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yu J, Shen T, Li Y, Hao T, Yang L, Li H,
Piao XM, Zhang Z, Zhu S, Quan C, et al: CYTOR drives prostate
cancer progression via facilitating AR-V7 generation and its
oncogenic signalling. Clin Transl Med. 13(e1230)2023.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Liang J, Wei X, Liu Z, Cao D, Tang Y, Zou
Z, Zhou C and Lu Y: Long noncoding RNA CYTOR in cancer: A TCGA data
review. Clin Chim Acta. 483:227–233. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Van Grembergen O, Bizet M, de Bony EJ,
Calonne E, Putmans P, Brohée S, Olsen C, Guo M, Bontempi G,
Sotiriou C, et al: Portraying breast cancers with long noncoding
RNAs. Sci Adv. 2(e1600220)2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Malhotra A, Jain M, Prakash H, Vasquez KM
and Jain A: The regulatory roles of long non-coding RNAs in the
development of chemoresistance in breast cancer. Oncotarget.
8:110671–110684. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Teschendorff AE, Lee SH, Jones A, Fiegl H,
Kalwa M, Wagner W, Chindera K, Evans I, Dubeau L, Orjalo A, et al:
HOTAIR and its surrogate DNA methylation signature indicate
carboplatin resistance in ovarian cancer. Genome Med.
7(108)2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Jiang J, Wang S, Wang Z, Cai J, Han L, Xie
L, Han Q, Wang W, Zhang Y, He X and Yang C: HOTAIR promotes
paclitaxel resistance by regulating CHEK1 in ovarian cancer. Cancer
Chemother Pharmacol. 86:295–305. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Guo J, Dou D, Zhang T and Wang B: HOTAIR
promotes cisplatin resistance of osteosarcoma cells by regulating
cell proliferation, invasion, and apoptosis via miR-106a-5p/STAT3
Axis. Cell Transplant. 29(963689720948447)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Wang H, Qin R, Guan A, Yao Y, Huang Y, Jia
H, Huang W and Gao J: HOTAIR enhanced paclitaxel and doxorubicin
resistance in gastric cancer cells partly through inhibiting
miR-217 expression. J Cell Biochem. 119:7226–7234. 2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Wang X, Yu X, Xu H, Wei K, Wang S, Wang Y
and Han J: Serum-derived extracellular vesicles facilitate
temozolomide resistance in glioblastoma through a HOTAIR-dependent
mechanism. Cell Death Dis. 13(344)2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Li D, Li C, Chen Y, Teng L, Cao Y, Wang W,
Pan H, Xu Y and Yang D: LncRNA HOTAIR induces sunitinib resistance
in renal cancer by acting as a competing endogenous RNA to regulate
autophagy of renal cells. Cancer Cell Int. 20(338)2022.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Cheng C, Qin Y, Zhi Q, Wang J and Qin C:
Knockdown of long non-coding RNA HOTAIR inhibits cisplatin
resistance of gastric cancer cells through inhibiting the PI3K/Akt
and Wnt/β-catenin signaling pathways by up-regulating miR-34a. Int
J Biol Macromol. 107 (Pt B):2620–2629. 2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Wang Q, Li X, Ren S, Su C, Li C, Li W, Yu
J, Cheng N and Zhou C: HOTAIR induces EGFR-TKIs resistance in
non-small cell lung cancer through epithelial-mesenchymal
transition. Lung Cancer. 147:99–105. 2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Wang L, Dong P, Wang W, Huang M and Tian
B: Gemcitabine treatment causes resistance and malignancy of
pancreatic cancer stem-like cells via induction of lncRNA HOTAIR.
Exp Ther Med. 14:4773–4780. 2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Milevskiy MJ, Al-Ejeh F, Saunus JM,
Northwood KS, Bailey PJ, Betts JA, McCart Reed AE, Nephew KP, Stone
A, Gee JM, et al: Long-range regulators of the lncRNA HOTAIR
enhance its prognostic potential in breast cancer. Hum Mol Genet.
25:3269–3283. 2016.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Lasfargues EY and Ozzello L: Cultivation
of human breast carcinomas. J Natl Cancer Inst. 21:1131–1147.
1958.PubMed/NCBI
|
|
58
|
Trempe GL: Human breast cancer in culture.
Recent Results Cancer Res 33-41, 1976.
|
|
59
|
Wu W, Chen F, Cui X, Yang L, Chen J, Zhao
J, Huang D, Liu J, Yang L, Zeng J, et al: LncRNA NKILA suppresses
TGF-β-induced epithelial-mesenchymal transition by blocking NF-κB
signaling in breast cancer. Int J Cancer. 143:2213–2224.
2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Liu B, Sun L, Liu Q, Gong C, Yao Y, Lv X,
Lin L, Yao H, Su F, Li D, et al: A cytoplasmic NF-κB interacting
long noncoding RNA blocks IκB phosphorylation and suppresses breast
cancer metastasis. Cancer Cell. 27:370–381. 2015.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Godinho M, Meijer D, Setyono-Han B,
Dorssers LC and van Agthoven T: Characterization of BCAR4, a novel
oncogene causing endocrine resistance in human breast cancer cells.
J Cell Physiol. 226:1741–1749. 2011.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Tian D, Sun S and Lee JT: The long
noncoding RNA, Jpx, is a molecular switch for X chromosome
inactivation. Cell. 143:390–403. 2010.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Redis RS, Sieuwerts AM, Look MP, et al:
CCAT2, a novel long non-coding RNA in breast cancer: expression
study and clinical correlations. Oncotarget. 4:1748–1762.
2013.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Wu ZJ, Li Y, Wu YZ, et al: Long non-coding
RNA CCAT2 promotes the breast cancer growth and metastasis by
regulating TGF-β signaling pathway. European review for medical and
pharmacological sciences. 21:706–714. 2017.PubMed/NCBI
|
|
65
|
Xu S, Sui S, Zhang J, et al:
Downregulation of long noncoding RNA MALAT1 induces
epithelial-to-mesenchymal transition via the PI3K-AKT pathway in
breast cancer. Int J Clin Exp Pathol. 8:4881–4891. 2015.PubMed/NCBI
|
|
66
|
Zhou J, Ding D, Wang M and Cong YS:
Telomerase reverse transcriptase in the regulation of gene
expression. BMB reports. 47:8–14. 2014.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Ding X, Zhu L, Ji T, et al: Long
intergenic non-coding RNAs (LincRNAs) identified by RNA-seq in
breast cancer. PloS one. 9(e103270)2014.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Huang YS, Chang CC, Lee SS, Jou YS and
Shih HM: Xist reduction in breast cancer upregulates AKT
phosphorylation via HDAC3-mediated repression of PHLPP1 expression.
Oncotarget. 7:43256–43266. 2016.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Zheng R, Lin S, Guan L, et al: Long
non-coding RNA XIST inhibited breast cancer cell growth, migration,
and invasion via miR-155/CDX1 axis. Biochemical and biophysical
research communications. 498:1002–1008. 2018.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Li Z, Tian T, Hu X, et al: Six1 mediates
resistance to paclitaxel in breast cancer cells. Biochemical and
biophysical research communications. 441:538–543. 2013.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Pohl SG, Brook N, Agostino M, Arfuso Pohl
SG, Brook N, Agostino M, Arfuso F, Kumar AP and Dharmarajan A: Wnt
signaling in triple-negative breast cancer. Oncogenesis.
6(e310)2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Li T, Zhu J, Wang X, Chen G, Sun L, Zuo S,
Zhang J, Chen S, Ma J, Yao Z, et al: Long non-coding RNA lncTCF7
activates the Wnt/β-catenin pathway to promote metastasis and
invasion in colorectal cancer. Oncol Lett. 14:7384–7390.
2017.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Reiche K, Kasack K, Schreiber S, Lüders T,
Due EU, Naume B, Riis M, Kristensen VN, Horn F, Børresen-Dale AL,
et al: Long non-coding RNAs differentially expressed between normal
versus primary breast tumor tissues disclose converse changes to
breast cancer-related protein-coding genes. PLoS One.
9(e106076)2014.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Chen Y, Qiu F, Huang L, Liu W, Li L, Ji C,
Zeng X, Qiao L, Liu M and Gong X: Long non-coding RNA LINC00312
regulates breast cancer progression through the miR-9/CDH1 axis.
Mol Med Rep. 21:1296–1303. 2020.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Wu J, Zhou X, Fan Y, Cheng X, Lu B and
Chen Z: Long non-coding RNA 00312 downregulates cyclin B1 and
inhibits hepatocellular carcinoma cell proliferation in vitro and
in vivo. Biochem Biophys Res Commun. 497:173–180. 2018.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Brooks SC, Locke ER and Soule HD: Estrogen
receptor in a human cell line (MCF-7) from breast carcinoma. J Biol
Chem. 248:6251–6253. 1973.PubMed/NCBI
|
|
77
|
Judge SM and Chatterton RT Jr:
Progesterone-specific stimulation of triglyceride biosynthesis in a
breast cancer cell line (T-47D). Cancer Res. 43:4407–4412.
1983.PubMed/NCBI
|
|
78
|
Lasfargues EY, Coutinho WG and Redfield
ES: Isolation of two human tumor epithelial cell lines from solid
breast carcinomas. J Natl Cancer Inst. 61:967–978. 1978.PubMed/NCBI
|
|
79
|
Brinkley BR, Beall PT, Wible LJ, Mace ML,
Turner DS and Cailleau RM: Variations in cell form and cytoskeleton
in human breast carcinoma cells in vitro. Cancer Res. 40:3118–3129.
1980.PubMed/NCBI
|


















