Introduction
Spinal cord injury (SCI) is a severe central nervous
system injury typically caused by external trauma, such as car
accidents, falls or sports activities (1). This type of injury can lead to
partial or complete destruction of the spinal cord, resulting in a
series of complex pathophysiological changes, including primary and
secondary injuries. Primary injury is usually due to the direct
mechanical force acting on the spinal cord, such as vertebral
fractures, dislocations or ligament tears, leading to compression,
contusion or complete transection of the spinal cord. Secondary
injury refers to the series of biological reactions that occur
following primary injury, including neuroinflammation, disruption
of the blood-brain barrier, oxidative stress, apoptosis and
demyelination (2). These secondary
responses further exacerbate neuronal damage, leading to extensive
neurological dysfunction and markedly increase the difficulty of
SCI patient rehabilitation. Current treatment strategies for SCI
mainly focus on surgical intervention and pharmacological therapy
in the acute phase, aiming to mitigate secondary injury and promote
nerve regeneration (3). However,
these conventional treatments have limited efficacy in restoring
neurological function and are unable to reverse the long-term
disabilities caused by the injury. Therefore, developing more
effective therapies to promote nerve regeneration and functional
recovery has become a focal point of SCI research.
In recent years, with the rapid development of stem
cell technology, the application of stem cell therapy in SCI
treatment has gained widespread attention. Stem cells, particularly
mesenchymal stem cells (MSCs), have emerged as a potential
treatment option for SCI due to their multilineage differentiation
potential, low immunogenicity and immunoregulatory abilities
(4). Among the various sources of
MSCs, human umbilical cord MSCs (hUCMSCs) have become a research
focus due to their easy accessibility, minimal ethical concerns and
excellent differentiation and proliferation potential. hUCMSCs can
differentiate into multiple types of neural cells, such as neurons
and astrocytes and an also modulate the microenvironment of the
injury site by secreting bioactive molecules, thereby suppressing
inflammatory responses and promoting nerve regeneration (5,6).
Specifically, the mechanisms through which hUCMSCs contribute to
SCI repair include the following: First, hUCMSCs can secrete
various cytokines and growth factors, such as brain-derived
neurotrophic factor (BDNF) and nerve growth factor, to directly
promote neuronal survival and regeneration (7,8).
Second, hUCMSCs can modulate the inflammatory response at the
injury site, inhibiting the activation of inflammatory cells and
reducing further damage to neurons from secondary injury. In
addition, hUCMSCs can secrete exosomes and microRNAs (miRNAs), such
as miR-124-3p, which regulate neuronal differentiation and axon
growth, playing a significant role in neural repair (9,10).
miR-124-3p is an miRNA highly expressed in the
nervous system, considered to play a crucial role in neuronal
differentiation and functional maintenance (11). Studies have shown that miR-124-3p
is markedly upregulated following nerve injury, promoting neuronal
regeneration and axonal repair by targeting multiple signaling
pathways (12,13). For example, PPG alleviates
ischemia-induced neuronal injury and microglial inflammation by
regulating the miR-124-3p/tumor necrosis factor receptor-associated
factor 6/NF-κB pathway (13).
Furthermore, miR-124-3p has been found to regulate
neuroinflammatory responses by modulating macrophage polarization,
thereby reducing the release of inflammatory factors and mitigating
secondary inflammatory responses following neural injury (14). In the context of SCI repair, the
role of miR-124-3p is attracting attention and is regarded as a
potential biomarker. Changes in its expression level can reflect
the progress of neural repair and provide a new method for
monitoring clinical treatment efficacy. Studies have indicated that
miR-124-3p upregulation is closely associated with neurological
recovery, highlighting its promising application prospects in SCI
treatment (15). Despite the
emerging understanding of the mechanisms through which hUCMSCs and
miR-124-3p contribute to SCI repair, challenges remain. For
instance, the heterogeneity of hUCMSCs may lead to inconsistent
therapeutic outcomes. In addition, although the role of miR-124-3p
in SCI repair has been partially validated, its complex mechanism
of action requires further elucidation.
In conclusion, the combined application of hUCMSCs
and miR-124-3p offers new hope for SCI treatment. The in-depth
exploration of this research direction will not only help improve
SCI treatment outcomes but also lay the theoretical foundation for
future clinical applications. As research progresses, hUCMSCs and
miR-124-3p are expected to become key tools in SCI treatment,
providing patients with improved rehabilitation opportunities and
improved quality of life.
Materials and methods
Ethical statement
All animal experiments were approved by the Animal
Ethics Committee of the Xinjiang Medical University (Urumqi, China;
approval no. IACUC-20230321-07). All procedures involving animals
complied with the principles of laboratory animal management and
protection (16), with measures
taken to minimize the number of animals used and their
suffering.
The study adhered to the Animal Research: Reporting
of In Vivo Experiments (ARRIVE) guidelines to ensure
transparency and reproducibility. Humane endpoints were established
and animals were sacrificed if they exhibited severe pain,
sustained weight loss exceeding 15%, or significant loss of
mobility. The total duration of the experiment was 35 days, from
the establishment of the model to the final data collection. A
total of 36 healthy 8-week-old female specific pathogen-free-grade
Sprague-Dawley rats weighing 220-250 g were purchased from the
Animal Experiment Center of Xinjiang Medical University (Urumqi,
China), all of which successfully completed the study without any
mortality. Animal health and behavior were monitored twice daily
(morning and afternoon), focusing on body weight, mobility, dietary
intake, hydration and wound recovery. To minimize suffering and
distress, all surgical procedures were performed under anesthesia
with 1% sodium pentobarbital (40 mg/kg). Animals were housed in
individualized cages maintained under controlled conditions,
including a temperature of 22±2˚C, a relative humidity of 50-60%
and a 12-h light/dark cycle. They were provided with free access to
soft food and adequate hydration. Mortality was confirmed through
the absence of spontaneous respiration, fixed and dilated pupils,
cessation of heartbeat and lack of reflex activity.
hUCMSCs
The hUCMSCs used in the present study were purchased
from Xinjiang Western Saiou Biotechnology Co., Ltd. (cat. no.
WC-2023128). The hUCMSCs used in the experiments were at passages
3-5 and underwent standardized cultivation and quality control by
the company to ensure their multilineage differentiation potential
and the expression of typical mesenchymal stem cell surface
markers.
Characterization of hUCMSCs
The characterization of hUCMSCs included the
following processes.
Surface marker detection
The surface markers of hUCMSCs were analyzed by flow
cytometry. Cells were washed with PBS, digested with trypsin and
collected by centrifugation (300 x g; 5 min; 4˚C). A total of
1x106 cells were suspended in 100 µl PBS and
corresponding FITC- or PE-conjugated antibodies were added,
including anti-CD73, anti-CD90 and anti-CD105 for positive markers
and anti-CD34, anti-CD45 and anti-HLA-DR as negative controls (BD
Biosciences). Following mixing, the cells were incubated in the
dark for 30 min at 4˚C, washed twice with PBS and analyzed using a
flow cytometer (FACSCalibur; BD Biosciences). The results were
processed using FlowJo software (version 10.10; FlowJo LLC).
Osteogenic differentiation. Passage 3 hUCMSCs
were seeded in a 6-well plate and when the cells reached ~70%
confluence, they were cultured in osteogenic induction medium
(containing 100 nM dexamethasone, 10 mM β-glycerophosphate and 50
µg/ml ascorbic acid). After 21 days, the cells were fixed with 4%
paraformaldehyde for 15 min at room temperature, stained with
Alizarin Red for 30 min at room temperature and observed under a
light microscope (Olympus Corporation) at x400 magnification. Three
random fields were examined to assess osteogenic nodules, with
fields selected to avoid overlaps.
Chondrogenic differentiation. Cells were
suspended in chondrogenic induction medium containing 10 ng/ml
TGF-β3 and cultured at 37˚C in a 5% CO2 incubator for 3
weeks. The cartilage matrix was observed using Alcian Blue
staining, performed for 30 min at room temperature.
Adipogenic differentiation. Cells were seeded
in a 6-well plate and cultured in adipogenic induction medium
(containing 1 µM dexamethasone, 10 µg/ml insulin, 0.5 mM
isobutylmethylxanthine and 200 µM indomethacin) at 37˚C in a 5%
CO2 incubator for 14 days. After fixation with 4%
paraformaldehyde for 15 min at room temperature, the cells were
stained with Oil Red O for 30 min at room temperature and lipid
droplets were observed under a light microscope (Olympus
Corporation) at x400 magnification. Three fields were randomly
selected to avoid overlapping regions.
Rat spinal cord injury model
SD rats were selected due to their anatomical and
physiological similarity to humans, making them a widely accepted
model for SCI studies. Female rats were used to ensure consistent
hormonal levels and reduced variability in experimental outcomes
compared to males. Additionally, their physiological structure
facilitates easier assistance with urination after SCI model
establishment, which is critical for postoperative care. An
improved Allen method (17) was
used to establish the SCI model and the entire procedure was
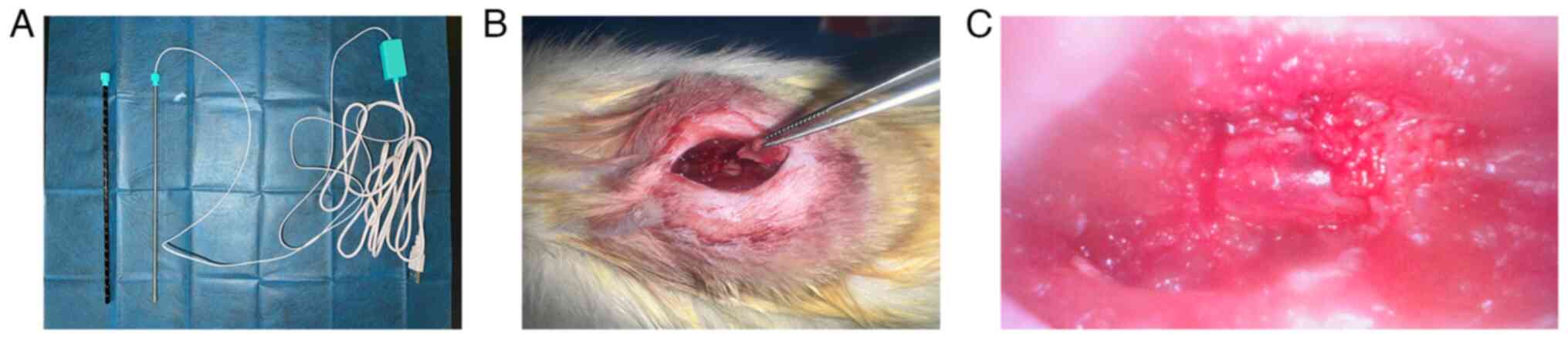
performed under neuroendoscopy (Fig.
1). First, the rats were anesthetized with 1% sodium
pentobarbital (40 mg/kg) via intraperitoneal injection and placed
in a prone position with T9-T11 as the surgical range and T10 as
the center. The surgical area was shaved and disinfected with
iodine. A 2.5 cm longitudinal skin incision was made and
subcutaneous fat and fascia were bluntly dissected. The surgical
knife was used to sharply dissect along the spinous processes,
retracting the muscles on both sides and the supraspinous and
interspinous ligaments of T9-T11 were cut to fully expose the
spinous processes and lamina of T9-T10. A mosquito hemostat was
used to gently lift the T10 spinous process and an ophthalmic
scissor was used to carefully cut open the lamina on both sides of
the T10, lifting the posterior wall of the vertebral canal, with
partial removal of the lateral walls to fully expose the T10 spinal
segment. A small elliptical iron plate (~5 mm² in area and 1 mm in
thickness) was then placed over the T10 segment and a 20 g
Kirschner wire was dropped freely from a height of 3.5 cm using a
modified 1 ml syringe sleeve to strike the T10 spinal segment.
Following the strike, significant congestion and edema were
observed at the corresponding site, with transient tail flicks and
sustained hindlimb spasms and tremors in the rats. Finally, the
surgical area was irrigated with sterile saline and the incision
was closed layer by layer.
The experimental animals were randomly divided into
three groups: Sham-operated, model and hUCMSC groups, with 12 rats
in each group. In the sham-operated group, the spinal cord was
exposed and the incision was immediately closed. In the model
group, the SCI model was established after exposing the spinal
cord, followed by an intrathecal injection of 20 µl PBS. In the
hUCMSC group, the SCI model was similarly established, followed by
an intrathecal injection of 20 µl hUCMSCs under neuroendoscopy.
Evaluation of hindlimb motor function
in rats
On days 1, 3, 7, 14 and 21 post-SCI, hindlimb motor
function in rats was assessed using the Basso, Beattie and
Bresnahan (BBB) score and the Rivlin inclined plate tests (18,19).
The BBB score comprehensively evaluates hindlimb function in terms
of early joint movement, mid-stage gait and coordinated movement
and fine paw movement during locomotion. The scoring range was
0-21, with 0 indicating complete paralysis and 21 indicating normal
hindlimb function. This method was used to evaluate the recovery of
hindlimb function. The Rivlin inclined plate test involved placing
rats on a flat inclined plate made of a 1-cm thick wooden board
with a 0.5-cm thick rubber pad. The inclined plate was gradually
tilted from 0˚ at 5˚ increments and the maximum angle at which the
rat could remain on the plate without slipping for 5 sec was
recorded using a protractor. All functional assessments were
independently completed by two blinded researchers, with each rat
being evaluated three times and the average value was taken as the
final result to ensure data accuracy and reliability.
Sample collection
Hindlimb motor function was evaluated on days 1, 3,
7, 14 and 21 and three rats were randomly selected at each time
point. Following intraperitoneal overdose anesthesia with 1%
pentobarbital sodium (80 mg/kg), the rats were sacrificed by
cervical dislocation and the chest was opened to expose the
ascending aorta and heart. Each rat was perfused with 0.9% saline
to remove circulating blood and the spinal cord tissue from the
injury site (0.5-1 cm) was collected on ice for pathological
examination and reverse transcription-quantitative (RT-q) PCR.
Samples for pathological examination were fixed in 4%
paraformaldehyde at 4˚C for 24 h, dehydrated in an ethanol gradient
(70, 80, 90, 95 and 100% ethanol, for 1 h each), cleared in xylene
(10 min twice) and embedded in paraffin at 60˚C for 2 h. The
tissues were sectioned into 5-µm thick spinal cord rings for
hematoxylin and eosin (H&E) staining, Nissl staining and
immunofluorescence. Samples for RT-qPCR were preserved in RNA
stabilization solution for subsequent miRNA extraction and
analysis. Samples for western blotting were stored at -80˚C.
H&E and Nissl staining
The embedded rat spinal cord tissue was sectioned
and fixed in 4% paraformaldehyde at 4˚C for 24 h. Paraffin-embedded
sections were deparaffinized with environmentally friendly
deparaffinization solution, dehydrated in an ethanol gradient and
washed with distilled water. H&E staining was performed at room
temperature for 5 min in hematoxylin and 2 min in eosin, followed
by dehydration and clearing. Following drying, histological and
morphological changes were observed under a light microscope (Leica
Microsystems GmbH). For Nissl staining, suitable spinal cord
sections were selected, washed with PBS, placed on slides, stained
with Nissl staining solution for 20 min at room temperature,
dehydrated, cleared and mounted for observation under a light
microscope (Leica Microsystems GmbH) at x200 magnification. Three
fields were randomly selected to avoid overlapping regions.
Bromodeoxyuridine (BrdU) labeling
Each group of rats was intraperitoneally injected
with BrdU saline solution (10 mg/100 g) twice daily (at 8-h
intervals) on days 1, 2, 5, 6, 12, 13, 19 and 20 before
sacrifice.
Immunofluorescence staining
Spinal cord tissues fixed in 4% paraformaldehyde at
4˚C for 24 h were embedded in paraffin. The tissue was dehydrated
in an ethanol gradient (70, 80, 90, 95 and 100%, for 1 h each),
cleared in xylene (10 min twice) and embedded in paraffin at 60˚C
for 2 h. Paraffin blocks were sectioned into 10-µm thick slices.
Sections were treated with 0.5% Triton X-100 for 20 min, followed
by 0.6% H2O2 for 15 min to remove endogenous
peroxidase activity. A drop of 10% normal goat serum (Gibco; Thermo
Fisher Scientific, Inc.) was applied and the sections were
incubated at 37˚C for 30 min to block nonspecific binding. Primary
antibodies were then added: Anti-BrdU antibody (mouse origin;
dilution, 1:200; Sigma-Aldrich; Merck KGaA; cat. no. B8434) and
anti-neuron-specific enolase (NSE) antibody (mouse origin;
dilution, 1:50; Abcam; cat. no. ab180943) and incubated overnight
at 4˚C. The next day, sections were washed three times with PBS for
5 min each and secondary antibodies were added: Anti-mouse
Cy3-conjugated secondary antibody (dilution, 1:500; Abcam; cat. no.
ab97035) for BrdU-positive cell detection and anti-mouse
FITC-conjugated secondary antibody (dilution, 1:1,000; Abcam; cat.
no. ab6785) for NSE-positive cell detection, followed by incubation
at 37˚C for 30 min. DAPI staining (dilution, 1:1,000; 10 min at
room temperature; Abcam; cat. no. ab228549) was used to label cell
nuclei. Following washing with PBS, the sections were observed and
imaged under a fluorescence microscope (Olympus Corporation) to
analyze the number of BrdU and NSE double-positive cells.
RT-qPCR
Total RNA was extracted from spinal cord tissue
using TRIzol® reagent (Thermo Fisher Scientific, Inc.),
according to the manufacturer's instructions and RNA concentration
and purity (A260/A280) were measured using a NanoDrop 2000
spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific,
Inc.). RNA (1 µg) was reverse transcribed into cDNA using the
PrimeScript RT Reagent Kit (Takara Bio, Inc.). The RT reaction
conditions were 37˚C for 15 min, 85˚C for 5 sec and holding at 4˚C.
RT-qPCR was performed using SYBR Green PCR Master Mix (Takara Bio,
Inc.) on a 7500 Fast Real-Time PCR System (Thermo Fisher
Scientific, Inc.). Each reaction mixture contained a total volume
of 20 µl, including 10 µl SYBR Green mix, 0.4 µM forward primer,
0.4 µM reverse primer, 2 µl cDNA template and nuclease-free water
up to 20 µl. Primer sequences are shown in Table I. All RT-qPCR experiments were
repeated three times and the relative expression level of
miR-124-3p was calculated using the 2-ΔΔCq method
(20), with U6 as the internal
control.
 | Table ISequence of each gene and internal
reference primer. |
Table I
Sequence of each gene and internal
reference primer.
| Name of primer | Primer Sequences
(5'-3') |
|---|
| U6 forward |
CTCGCTTCGGCAGCACA |
| U6 reverse |
AACGCTTCACGAATTTGCGT |
|
rno-miR-124-3p-RT |
CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGGGCATTCA |
| rno-miR-124-3p
forward |
ACACTCCAGCTGGGTAAGGCACGCGGTG |
| Universal
reverse |
TGGTGTCGTGGAGTCG |
Western blotting
To analyze BDNF protein expression, total protein
was extracted from the rat spinal cord injury site using RIPA lysis
buffer (Beijing Solarbio Science & Technology Co., Ltd.) and
concentrations were measured with a BCA assay (Thermo Fisher
Scientific, Inc.; cat. no. 23225). Equal amounts (30 µg) of protein
were separated on 10% SDS-PAGE gels and transferred onto PVDF
membranes (MilliporeSigma; cat. no. IPVH00010). Membranes were
blocked with 5% non-fat milk in TBST (0.1% Tween-20) at room
temperature for 1 h, then incubated overnight at 4˚C with anti-BDNF
primary antibody (1:1,000, Abcam; cat. no. ab108319). After three
washes, the membranes were incubated with an HRP-conjugated
secondary antibody (1:10,000, Abcam; cat. no. ab6721) for 1 h at
room temperature. Protein bands were detected with ECL (Thermo
Fisher Scientific, 34580) and quantified using ImageJ software
(version 1.53; National Institutes of Health), normalized to GAPDH
(1:10,000, Abcam; cat. no. ab181602) expression.
Statistical analysis
All data analyses were performed using SPSS 21.0
(IBM Corp.) and GraphPad 7.0 software (Dotmatics). Measurement data
were expressed as the mean ± standard deviation. Differences
between two unpaired groups with normal distribution and
homogeneous variance were analyzed using an unpaired t-test, while
differences among multiple groups were analyzed using one-way
ANOVA. Post hoc multiple comparisons were performed using Tukey's
HSD test when significant differences were observed. P<0.05 was
considered to indicate a statistically significant difference.
Results
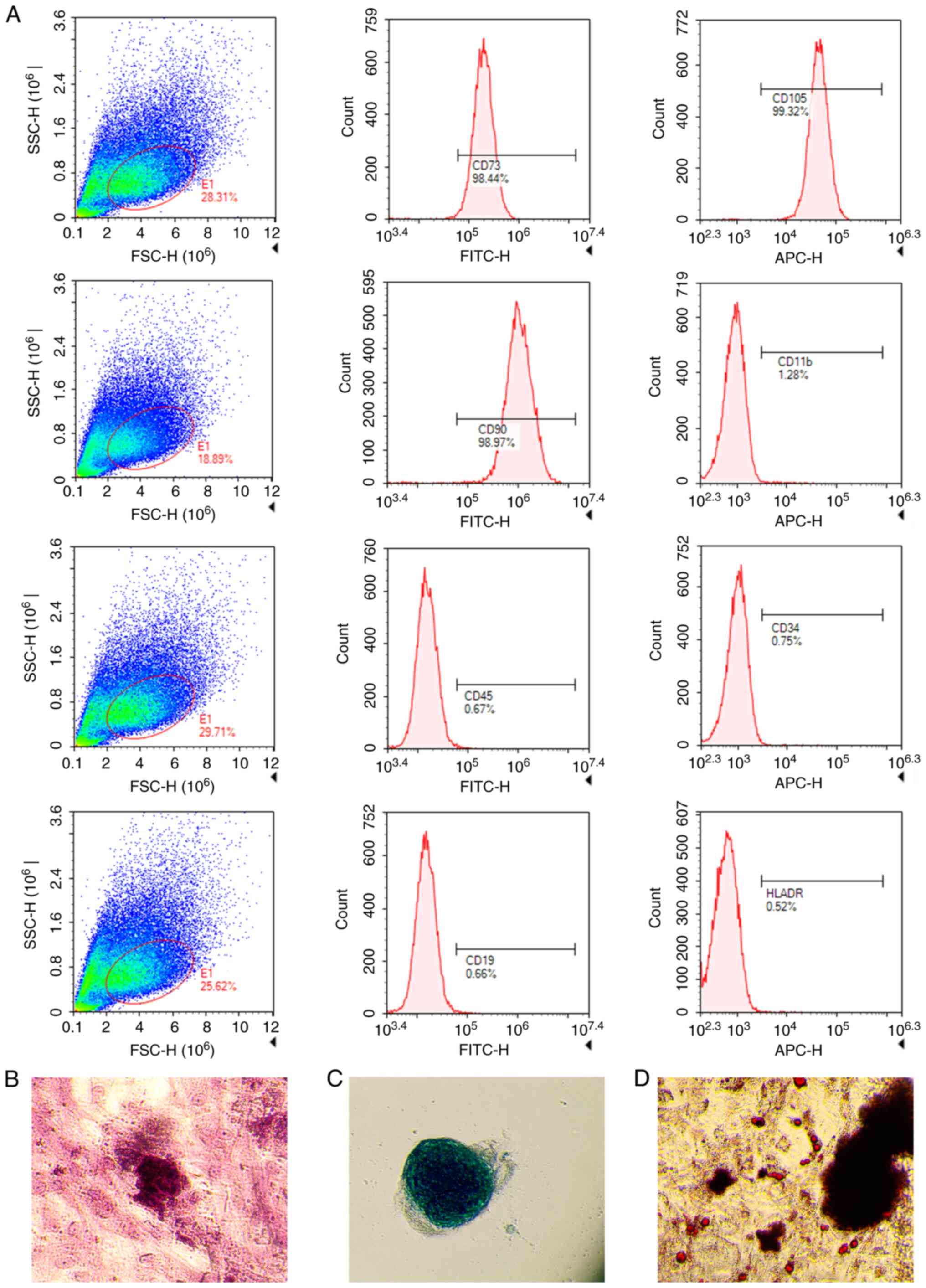
Identification of hUCMSCs
At passage 3, the isolated cells were analyzed for
surface marker expression using flow cytometry. The results showed
that the positive rates for CD73, CD105 and CD90 were 98.44, 99.32
and 98.97%, respectively, while those for CD11b, CD45, CD34, CD19
and human leukocyte antigen (HLA)-DR were 1.28, 0.67, 0.75, 0.66
and 0.52%, respectively. These results confirmed that most of the
isolated cells were hUCMSCs (Fig.
2A). Subsequently, osteogenic, adipogenic and chondrogenic
differentiation experiments were performed on these hUCMSCs. The
results showed that these cells exhibited a good differentiation
ability under different conditions, successfully differentiating
into osteocytes (Fig. 2B),
chondrocytes (Fig. 2C) and
adipocytes (Fig. 2D), further
confirming that we successfully isolated hUCMSCs with multilineage
differentiation potential.
Evaluation of hindlimb motor function
in rats
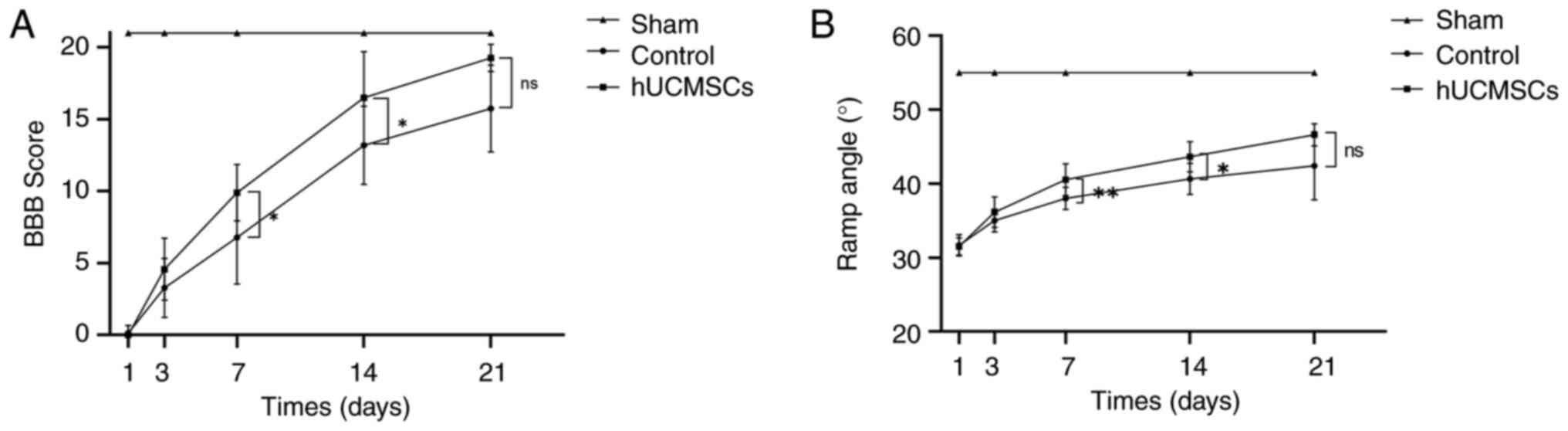
The BBB scores of all rats were 21 before surgery,
indicating normal hindlimb motor function. On days 1, 3 and 21
post-SCI, there were no significant differences in BBB scores
between the control and the hUCMSC groups (P>0.05). However, on
days 7 and 14 post-surgery, the BBB scores in the hUCMSC group were
significantly higher compared with those in the control group
(P<0.05; Fig. 3A). This
indicated that intrathecal transplantation of umbilical cord
mesenchymal stem cells had a significant promoting effect on
hindlimb motor function recovery at these time points. Similarly,
the results of the Rivlin inclined plate test showed that all
groups of rats had an angle of 55˚ before surgery, indicating
normal hindlimb motor function. On days 1, 3 and 21 post-SCI, there
were no significant differences between the control and hUCMSC
groups in the Rivlin inclined plate test (P>0.05). However, on
days 7 and 14 post-surgery, the results in the hUCMSC group were
significantly improved compared with those in the control group,
with statistically significant differences (P<0.05; Fig. 3B).
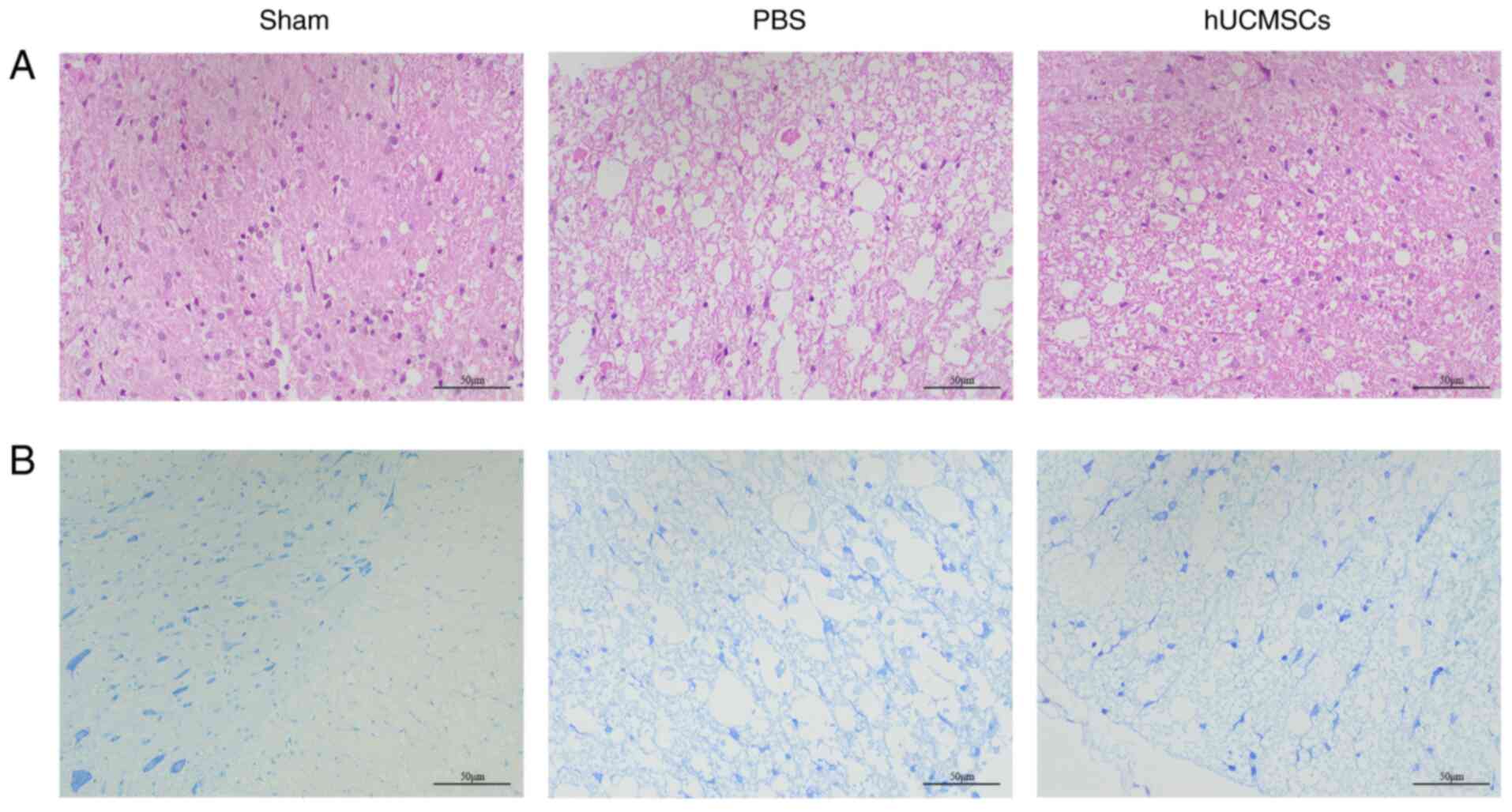
H&E and Nissl staining
H&E and Nissl staining were performed on day 7
post-SCI to observe the histological changes during the significant
improvement of hindlimb motor function. The H&E staining
results showed no obvious scar tissue or cavitation structures in
the spinal cord tissue of the sham-operated group, with numerous
intact neurons observed. By contrast, the control group showed
substantial scar tissue and vacuolar necrotic regions. In the
hUCMSC group, the number of neurons was markedly higher than in the
control group and the vacuolar necrotic regions were notably
reduced, indicating a favorable therapeutic effect (Fig. 4A). Nissl staining results further
supported these observations. The sham-operated group displayed
numerous normally distributed neurons, while the control group
showed severe neuronal damage and widespread cavitation. In
comparison, the hUCMSC group showed neuronal distribution
intermediate between the sham-operated and control groups,
suggesting that hUCMSCs promoted neuronal repair to a certain
extent (Fig. 4B).
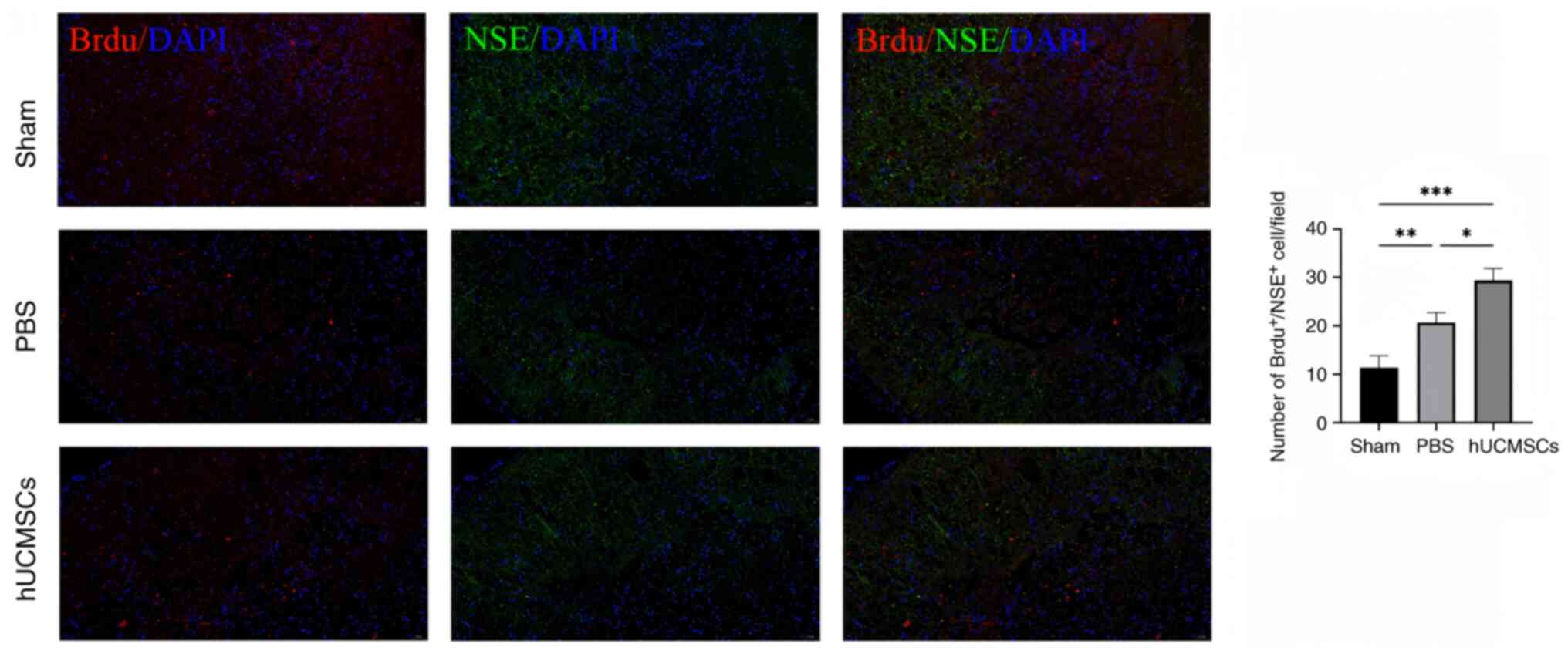
BrdU+/NSE+ expression in spinal cord
tissue from rats
Representative images were selected for display on
day 7 post-SCI (Fig. 5).
Immunofluorescence results showed that the number of
BrdU+/NSE+ double-positive cells in the
sham-operated group was significantly lower than that in the other
two groups, while the number of BrdU+/NSE+
double-positive cells in the hUCMSC group was significantly higher
than that in the control group (P<0.05). These results suggested
that more proliferating cells differentiated into neurons following
an intrathecal injection of hUCMSCs, or that hUCMSCs may enhance
neurogenesis and repair by promoting neuronal proliferation.
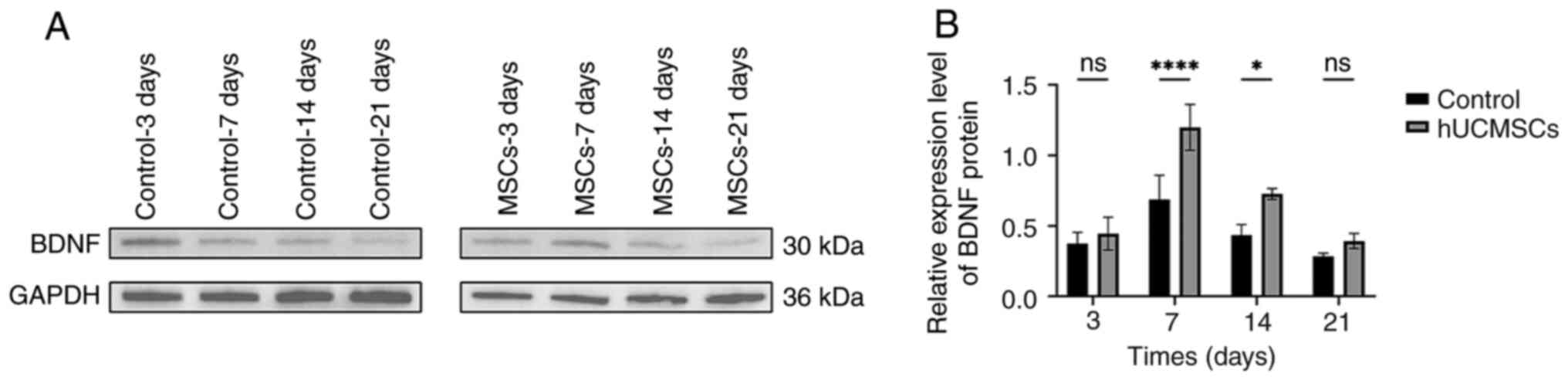
BDNF protein expression in rat spinal
cord tissue following hUCMSCs transplantation
Western blotting was used to analyze the expression
of neurorepair-related proteins in the control and hUCMSC groups in
the SCI rat model, specifically investigating the changes in BDNF
protein expression at different time points following the
intrathecal transplantation of hUCMSCs. The results showed that
BDNF expression in the spinal cord tissue of the hUCMSC group was
significantly increased compared with the control group on days 7
and 14 (P<0.05; Fig. 6).
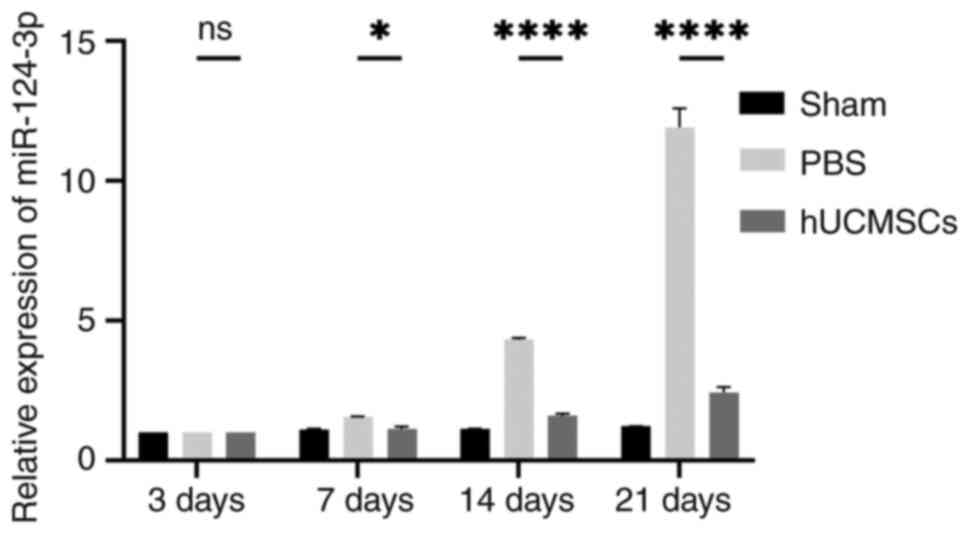
Comparison of miR-124 expression in
spinal cord tissue among the three groups
In the present study, RT-qPCR was used to analyze
the expression levels of miR-124-3p in spinal cord tissue in each
experimental group, with U6 as an internal reference gene for
normalization. The results showed that miR-124-3p expression in the
hUCMSC group was significantly decreased on days 7, 14 and 21
post-SCI, as compared with the control group (P<0.05) and over
time, the rate of increase in miR-124-3p expression in the hUCMSC
group was markedly lower than that in the control group compared to
day 3. The sham-operated group showed no significant changes in
miR-124-3p expression, with stable expression levels (Fig. 7). These data indicated that
miR-124-3p expression is closely associated with the repair process
following spinal cord injury, particularly in neural repair
following hUCMSCs transplantation, where miR-124-3p showed a
significant reduction in upregulation.
Discussion
Spinal cord injury (SCI) is a severe disease of the
CNS that often results in permanent loss of neurological function
(1). However, the current
pharmacological and non-pharmacological treatment options have
limited efficacy in promoting spinal cord repair. Therefore,
developing more effective therapeutic strategies is a major focus
of scientific research. In recent years, cell transplantation
therapies, particularly stem cell-based treatments, have been
regarded as promising approaches for treating SCI. hUCMSCs are
ideal candidate cells for treating SCI due to their
multidirectional differentiation potential, immunoregulatory
capabilities and low immunogenicity (21). Studies have shown that hUCMSCs can
target damaged tissues, differentiate into functional cells, or
secrete immunomodulatory factors, thereby exerting significant
therapeutic effects in vivo (22). During the repair of SCI, specific
biomarkers can serve as effective indicators for monitoring the
repair process (23). Among them,
miR-124-3p has attracted considerable attention due to its
regulatory role in the nervous system, particularly in
neuroregeneration. The aim of the present study was to investigate
the changes in miR-124-3p expression following intrathecal
transplantation of hUCMSCs in SCI repair and evaluate its potential
as a monitoring marker for SCI repair.
The main findings of the present study indicated
that intrathecal transplantation of hUCMSCs hag a significant
therapeutic effect on SCI repair, especially in promoting neuronal
proliferation and neuroregeneration. Specifically,
immunofluorescence of rat spinal cord tissue post-transplantation
revealed that the number of BrdU+/NSE+
double-positive cells in the hUCMSC group was markedly higher than
that in the control group, suggesting that more proliferating cells
were differentiating into neurons. This implied that hUCMSCs may
play a role in neural repair by promoting neuronal regeneration.
The expression of the neurorepair-related protein BDNF was markedly
higher in the hUCMSC group than in the control group, indicating
that hUCMSCs may enhance neuronal survival and regeneration by
promoting BDNF expression. Furthermore, RT-qPCR results showed that
miR-124-3p expression was markedly decreased in the hUCMSCs
transplantation group compared with the control group at the same
time points, which was closely associated with the spinal cord
repair process. This finding was consistent with that of other
studies (14,24), further supporting the key
regulatory role of miR-124-3p in nervous system repair. The
significant changes in miR-124-3p expression not only reflect the
potential mechanism of hUCMSCs in injury repair but also suggested
that miR-124-3p could be an effective molecular marker for
monitoring SCI repair. In motor function assessments, the BBB
scores and Rivlin inclined plate test results of rats in the hUCMSC
group were markedly improved compared with those in the control
group, particularly on days 7 and 14 post-injury, demonstrating the
positive role of hUCMSCs in promoting functional recovery after
SCI.
Existing research shows that hUCMSCs have certain
therapeutic effects on SCI, such as reducing neuronal apoptosis,
promoting motor function recovery and reducing demyelination
(25,26). However, certain studies have raised
concerns regarding the stability of these effects and the
mechanisms involved. For example, systematic reviews and network
meta-analyses have indicated that, although hUCMSCs have certain
therapeutic effects on SCI, their efficacy is not always markedly
improved compared with other treatment strategies when compared to
other types of stem cells (27).
By contrast, the present study employed a multi-layered
experimental design to further verify the significant effects of
hUCMSCs in neuronal regeneration and functional recovery,
particularly in using miR-124-3p as a biomarker for evaluating the
repair process, highlighting the unique advantages of hUCMSCs. In
the field of miRNA research, miR-124-3p has attracted attention for
its regulatory role in the nervous system. A previous study
demonstrated that miR-124-3p can exert neuroprotective effects
during SCI repair by regulating the interaction between glial cells
and neurons and inhibiting the activation of neurotoxic microglia
and astrocytes (24). The present
study further confirmed, through experimental data, the close
association between the upregulation of miR-124-3p and neural
repair following hUCMSCs treatment, strengthening the theoretical
basis for miR-124-3p as an effective monitoring marker in SCI
repair.
Despite the significant therapeutic effects of
intrathecal transplantation of hUCMSCs in SCI repair demonstrated
in the present study, particularly the potential of miR-124-3p as a
biomarker for monitoring the repair process, several limitations
remain. Although animal models are indispensable in SCI research,
they cannot fully represent the pathophysiological processes in
humans. hUCMSCs are derived from different individuals and their
cellular characteristics and functions may exhibit heterogeneity
(28). This heterogeneity may lead
to inconsistencies in treatment outcomes, affecting the
reproducibility of research findings. Factors such as the source of
hUCMSCs, culture conditions and passage number in different
experiments may result in varying therapeutic performances.
Although the present study confirmed the important role of
miR-124-3p in SCI repair, its mechanism of action may be more
complex than revealed in the present study. miR-124-3p not only
participates in neuroregeneration but may also play a role in
inflammation regulation, apoptosis and other processes. However,
the specific functions of miR-124-3p in different cell types and
stages of repair remain unclear and require further research. The
present study mainly focused on the short-term effects of hUCMSCs
transplantation and the changes in miR-124-3p expression. However,
the long-term efficacy and safety of hUCMSCs, particularly the
potential risks of immune responses or tumor formation, lack
systematic long-term follow-up data (28). This is especially important when
translating hUCMSCs to clinical applications. While hUCMSCs and
miR-124-3p have shown significant therapeutic potential in animal
models, translating these findings to human clinical applications
remains challenging. Issues such as optimizing cell
transplantation, monitoring post-transplantation and effectively
regulating miR-124-3p expression in patients need to be addressed
further (29). In conclusion,
while the present study provides new insights into the treatment of
SCI, caution should be exercised when translating these findings
into clinical applications. Future research should validate these
results in larger-scale experiments and explore ways to overcome
these limitations to achieve broader and more effective clinical
applications.
In summary, the present study demonstrated that
intrathecal transplantation of hUCMSCs markedly promoted the
recovery of hindlimb motor function following SCI in rats.
Behavioral assessments, including BBB scoring and Rivlin inclined
plate tests, showed a significant improvement in motor function in
the hUCMSCs-treated group compared with the control group,
particularly on post-surgical days 7 and 14. Histological analysis
using H&E and Nissl staining further confirmed the beneficial
effects of hUCMSCs, showing enhanced neuronal preservation and
reduced necrotic areas. Immunofluorescence also showed an increase
in BrdU+/NSE+ cells in the hUCMSC group,
indicating that hUCMSCs promoted neurogenesis by enhancing cell
proliferation and differentiation. The expression of the
neurotrophic factor BDNF was markedly increased following hUCMSCs
transplantation, further demonstrating their role in enhancing
neurorepair mechanisms. In addition, the downregulation of
miR-124-3p in the hUCMSC group was associated with improved motor
function recovery, suggesting that miR-124-3p could be a potential
biomarker for neural repair.
Overall, the present findings indicated that hUCMSCs
have significant neuroprotective and regenerative effects on SCI,
primarily by promoting neuronal survival, proliferation and
neurotrophic support. These results suggested that hUCMSCs have
great clinical application potential in SCI treatment and
monitoring miR-124-3p in response to stem cell transplantation
presents a novel and promising approach. One limitation of the
present study is the relatively small sample size of 36 rats, which
may affect the statistical power and generalizability of the
results. While this sample size is consistent with previous SCI
studies and was chosen due to resource and ethical considerations,
future studies with larger cohorts are planned to further validate
the therapeutic efficacy of hUCMSCs and the potential of miR-124-3p
as a biomarker for spinal cord injury recovery.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Youth Project of
Natural Science Foundation of Xinjiang Uygur Autonomous Region
Science and Technology Department (China) (grant no.
2021D01C339).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
HQ and YW designed the study. YZ, MiA, NM and WL
performed the experiments and collected the data. WL and YW drafted
the manuscript. MuA, YL and XM analyzed the experimental data. MiA
and NM provided necessary help during the experiments. YW and HQ
provided guidance and funding support for the study. HQ and YZ
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Ethics Committee of Xinjiang Medical University (Urumqi, China;
approval no. IACUC-20230321-07). All procedures used in the animal
experiments adhered to the standards of the principles of
management and protection of experimental animals and the utmost
care was taken to minimize the number of animals used and their
suffering.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hu X, Xu W, Ren Y, Wang Z, He X, Huang R,
Ma B, Zhao J, Zhu R and Cheng L: Spinal cord injury: Molecular
mechanisms and therapeutic interventions. Signal Transduct Target
Ther. 8(245)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sterner RC and Sterner RM: Immune response
following traumatic spinal cord injury: Pathophysiology and
therapies. Front Immunol. 13(1084101)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Eli I, Lerner DP and Ghogawala Z: Acute
traumatic spinal cord Injury. Neurol Clin. 39:471–488.
2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang LT, Liu KJ, Sytwu HK, Yen ML and Yen
BL: Advances in mesenchymal stem cell therapy for immune and
inflammatory diseases: Use of cell-free products and human
pluripotent stem cell-derived mesenchymal stem cells. Stem Cells
Transl Med. 10:1288–1303. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li K, Yan G, Huang H, Zheng M, Ma K, Cui
X, Lu D, Zheng L, Zhu B, Cheng J and Zhao J: Anti-inflammatory and
immunomodulatory effects of the extracellular vesicles derived from
human umbilical cord mesenchymal stem cells on osteoarthritis via
M2 macrophages. J Nanobiotechnology. 20(38)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhou X, Liu X, Liu L, Han C, Xie Z, Liu X,
Xu Y, Li F, Bi J and Zheng C: Transplantation of IFN-γ Primed
hUCMSCs significantly improved outcomes of experimental autoimmune
encephalomyelitis in a mouse model. Neurochem Res. 45:1510–1517.
2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wei P, Jia M, Kong X, Lyu W, Feng H, Sun
X, Li J and Yang JJ: Human umbilical cord-derived mesenchymal stem
cells ameliorate perioperative neurocognitive disorder by
inhibiting inflammatory responses and activating BDNF/TrkB/CREB
signaling pathway in aged mice. Stem Cell Res Ther.
14(263)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Huang J, U KP, Yang F, Ji Z, Lin J, Weng
Z, Tsang LL, Merson TD, Ruan YC, Wan C, et al: Human pluripotent
stem cell-derived ectomesenchymal stromal cells promote more robust
functional recovery than umbilical cord-derived mesenchymal stromal
cells after hypoxic-ischaemic brain damage. Theranostics.
12:143–166. 2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wei Z, Hang S, Wiredu Ocansey DK, Zhang Z,
Wang B, Zhang X and Mao F: Human umbilical cord mesenchymal stem
cells derived exosome shuttling mir-129-5p attenuates inflammatory
bowel disease by inhibiting ferroptosis. J Nanobiotechnology.
21(188)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhao H, Li Y, Chen L, Shen C, Xiao Z, Xu
R, Wang J and Luo Y: HucMSCs-Derived miR-206-knockdown exosomes
contribute to neuroprotection in subarachnoid hemorrhage induced
early brain injury by targeting BDNF. Neuroscience. 417:11–23.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mavroudis I, Balmus IM, Ciobica A, Nicoara
MN, Luca AC and Palade DO: The role of microglial exosomes and
miR-124-3p in neuroinflammation and neuronal repair after traumatic
brain injury. Life (Basel). 13(1924)2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yan Q, Sun SY, Yuan S, Wang XQ and Zhang
ZC: Inhibition of microRNA-9-5p and microRNA-128-3p can inhibit
ischemic stroke-related cell death in vitro and in vivo. IUBMB
Life. 72:2382–2390. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Cheng Z, Li X, Ye X, Yu R and Deng Y:
Purpurogallin reverses neuronal apoptosis and enhances ‘M2’
polarization of microglia under ischemia via mediating the
miR-124-3p/TRAF6/NF-κB axis. Neurochem Res. 48:375–392.
2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li R, Zhao K, Ruan Q, Meng C and Yin F:
Bone marrow mesenchymal stem cell-derived exosomal microRNA-124-3p
attenuates neurological damage in spinal cord ischemia-reperfusion
injury by downregulating Ern1 and promoting M2 macrophage
polarization. Arthritis Res Ther. 22(75)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ge X, Guo M, Hu T, Li W, Huang S, Yin Z,
Li Y, Chen F, Zhu L, Kang C, et al: Increased microglial exosomal
miR-124-3p alleviates neurodegeneration and improves cognitive
outcome after rmTBI. Mol Ther. 28:503–522. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press (US), Washington, DC, 2011.
|
|
17
|
Chen JN, Zhang YN, Tian LG, Zhang Y, Li XY
and Ning B: Down-regulating circular RNA Prkcsh suppresses the
inflammatory response after spinal cord injury. Neural Regen Res.
17:144–151. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Basso DM, Beattie MS and Bresnahan JC: A
sensitive and reliable locomotor rating scale for open field
testing in rats. J Neurotrauma. 12:1–21. 1995.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang X, Hong CG, Duan R, Pang ZL, Zhang
MN, Xie H and Liu ZZ: Transplantation of olfactory mucosa
mesenchymal stromal cells repairs spinal cord injury by inducing
microglial polarization. Spinal Cord. 62:429–439. 2024.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Huang Y, Wu Q and Tam PKH:
Immunomodulatory mechanisms of mesenchymal stem cells and their
potential clinical applications. Int J Mol Sci.
23(10023)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhou H, Shen X, Yan C, Xiong W, Ma Z, Tan
Z, Wang J, Li Y, Liu J, Duan A and Liu F: Extracellular vesicles
derived from human umbilical cord mesenchymal stem cells alleviate
osteoarthritis of the knee in mice model by interacting with METTL3
to reduce m6A of NLRP3 in macrophage. Stem Cell Res Ther.
13(322)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Toader C, Dobrin N, Brehar FM, Popa C,
Covache-Busuioc RA, Glavan LA, Costin HP, Bratu BG, Corlatescu AD,
Popa AA and Ciurea AV: From recognition to remedy: The significance
of biomarkers in neurodegenerative disease pathology. Int J Mol
Sci. 24(16119)2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Jiang D, Gong F, Ge X, Lv C, Huang C, Feng
S, Zhou Z, Rong Y, Wang J, Ji C, et al: Neuron-derived
exosomes-transmitted miR-124-3p protect traumatically injured
spinal cord by suppressing the activation of neurotoxic microglia
and astrocytes. J Nanobiotechnology. 18(105)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang S, Jia Y, Cao X, Feng S, Na L, Dong
H, Gao J and Zhang L: HUCMSCs transplantation combined with
ultrashort wave therapy attenuates neuroinflammation in spinal cord
injury through NUR77/NF-κB pathway. Life Sci.
267(118958)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liao Z, Yang X, Wang W, Deng W, Zhang Y,
Song A, Ni B, Zhao H, Zhang S and Li Z: hucMSCs transplantation
promotes locomotor function recovery, reduces apoptosis and
inhibits demyelination after SCI in rats. Neuropeptides.
86(102125)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu S, Zhang H, Wang H, Huang J, Yang Y,
Li G, Yu K and Yang L: A comparative study of different stem cell
transplantation for spinal cord injury: A systematic review and
network meta-analysis. World Neurosurg. 159:e232–e243.
2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Akhlaghpasand M, Tavanaei R, Hosseinpoor
M, Yazdani KO, Soleimani A, Zoshk MY, Soleimani M, Chamanara M,
Ghorbani M, Deylami M, et al: Safety and potential effects of
intrathecal injection of allogeneic human umbilical cord
mesenchymal stem cell-derived exosomes in complete subacute spinal
cord injury: A first-in-human, single-arm, open-label, phase I
clinical trial. Stem Cell Res Ther. 15(264)2024.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Subbarayan R, Murugan Girija D, Raja STK,
Krishnamoorthy A, Srinivasan D, Shrestha R, Srivastava N and Ranga
Rao S: Conditioned medium-enriched umbilical cord mesenchymal stem
cells: A potential therapeutic strategy for spinal cord injury,
unveiling transcriptomic and secretomic insights. Mol Biol Rep.
51(570)2024.PubMed/NCBI View Article : Google Scholar
|