Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune
disease that significantly impacts global health and affects ~1% of
the population (1). This disease
is characterized by persistent synovitis, which progressively
destroys joint structures and results in substantial loss of
function. Effective management of synovitis, the primary
pathological manifestation of RA, is crucial for preventing joint
erosion and mitigating disability in patients (2).
Histopathological examination of synovial biopsies
remains the most direct method of assessing the severity of
synovitis. The General Synovitis Score (GSS) is a widely used
literature-supported method for scoring synovial inflammation based
on histopathological changes observed in the synovium with
hematoxylin and eosin (H&E) staining (3). It primarily evaluates three aspects:
Synovial hyperplasia, stromal activation and inflammatory
infiltration. Each aspect is semi-quantitatively scored from 0-3,
with a total score >4 indicating inflammatory arthritis.
Previous studies have confirmed a definite correlation between the
GSS and the activity level of RA (4,5).
Studies of the histopathological features of the
synovium in patients with RA have consistently found that the
synovium exhibits characteristic features, including synovial
hyperplasia, stromal activation, inflammatory infiltration,
neovascularization and ectopic lymphoid neogenesis (6-8).
However, the GSS omits one of the most crucial histopathological
features of the RA synovium: Neovascularization. This omission may
result in the GSS failing to fully capture the inflammatory
characteristics of the RA synovium.
Observations at the Rheumatology and Immunology
Department of the First Affiliated Hospital of Nanchang University
(Nanchang, China) on RA synovial pathology have revealed that most
patients exhibit varying degrees of neovascularization. Although
synovial hyperplasia is widely acknowledged as a hallmark
pathological change in almost all RA cases (8,9),
some patients with RA exhibit thinning of the synovial lining, a
decrease in the number of synoviocytes, loosening of the
arrangement, or even complete synovial disappearance. This
phenomenon is referred to as synoviocyte detachment. Patients with
synoviocyte detachment typically have high levels of inflammatory
markers, indicating increased disease activity (10). In such cases, the GSS often fails
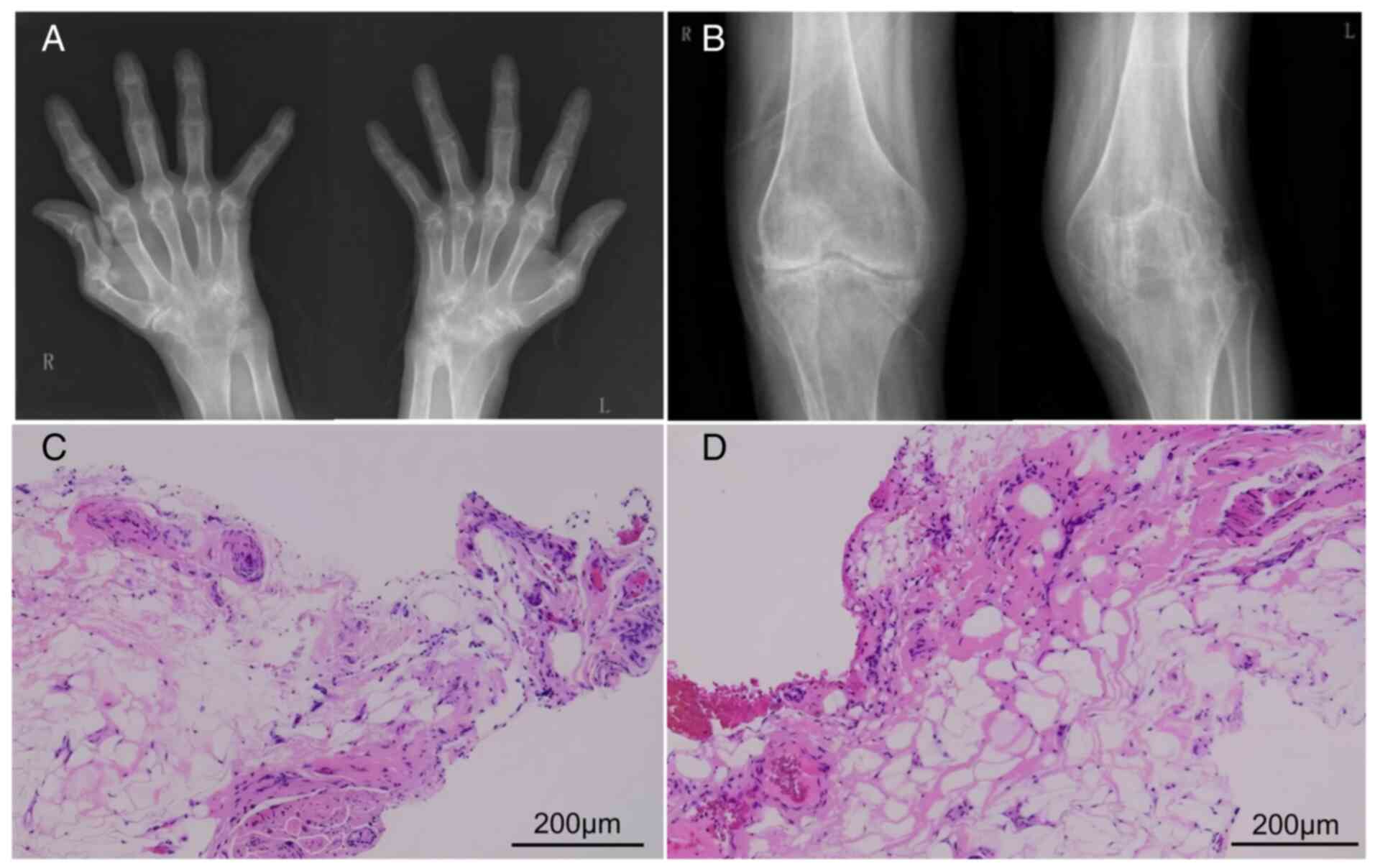
to accurately reflect disease activity. For example, in the present
study, one RA patient with moderate to severe disease activity had
a GSS of only 1 (Fig. 1).
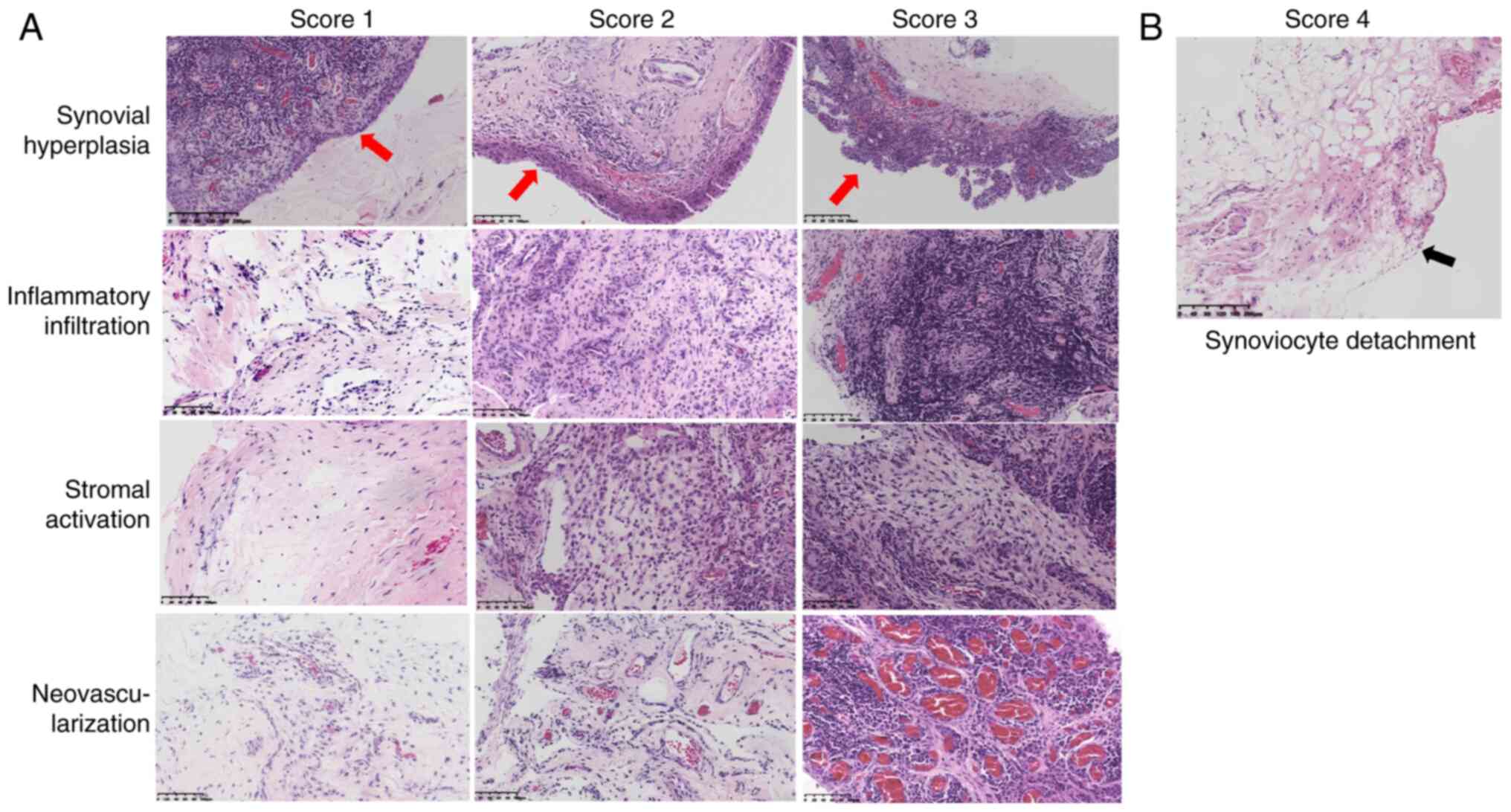
To address this problem, the present study added
neovascularization and synoviocyte detachment to the GSS items,
resulting in an enhanced scoring system: The modified GSS (mGSS).
This system semi-quantitatively assesses five key aspects of RA
synovitis: Synovial hyperplasia, synoviocyte detachment, stromal
activation, inflammatory infiltration and neovascularization
(Fig. 2).
The present cross-sectional study was conducted to
demonstrate the efficacy and reliability of the mGSS in assessing
RA synovitis. The present study investigated the association of
synovial neovascularization and synoviocyte detachment with disease
activity in patients with RA and compared the correlations of the
GSS and the mGSS with clinical disease activity.
Patients and methods
Patients
Between March 2023 and December 2023, 60 patients
diagnosed with RA who underwent synovial biopsy at the Rheumatology
and Immunology Department of The First Affiliated Hospital of
Nanchang University (Nanchang, China) were enrolled. All patients
met the 2010 American College of Rheumatology/European Alliance of
Associations for Rheumatism diagnostic criteria for RA (11). The present study was approved by
the Ethical Review Board of The First Affiliated Hospital of
Nanchang University [ethics approval number IIT (2023); clinical
ethics review no. 011]. Written informed consent was obtained from
all patients before they underwent synovial biopsy. Clinical data,
including sex, age, disease duration, medication history,
anti-citrullinated protein antibody (ACPA), rheumatoid factor (RF),
erythrocyte sedimentation rate (ESR), C-reactive protein (CRP),
Tender 28-Joint Count (TJC-28), Swollen 28-Joint Count (SJC-28) and
patient global assessment (PGA) were collected (12). The disease activity score in 28
joints CRP (DAS28-CRP)=0.56 x TJC-28 + 0.28 x SJC-28+ 0.36 x ln
(CRP + 1) + 0.014 x PGA + 0.96.
Synovial biopsy and tissue
processing
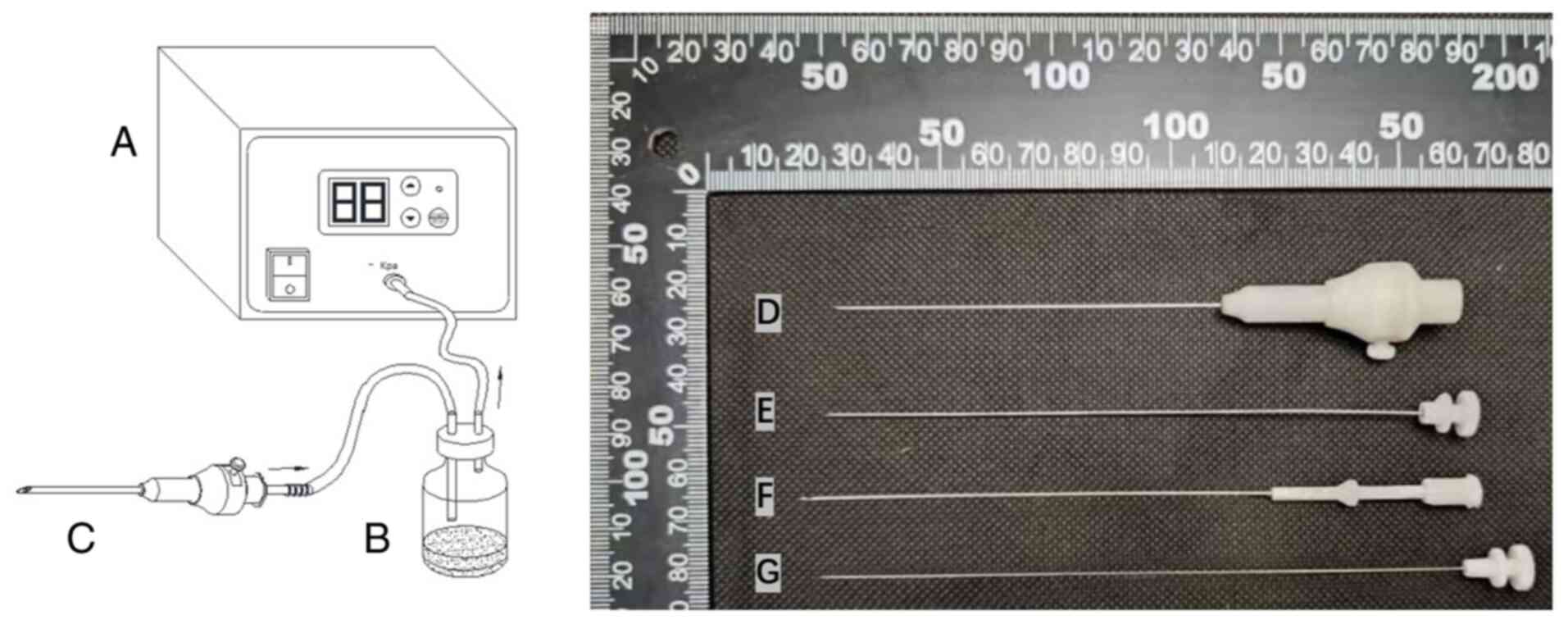
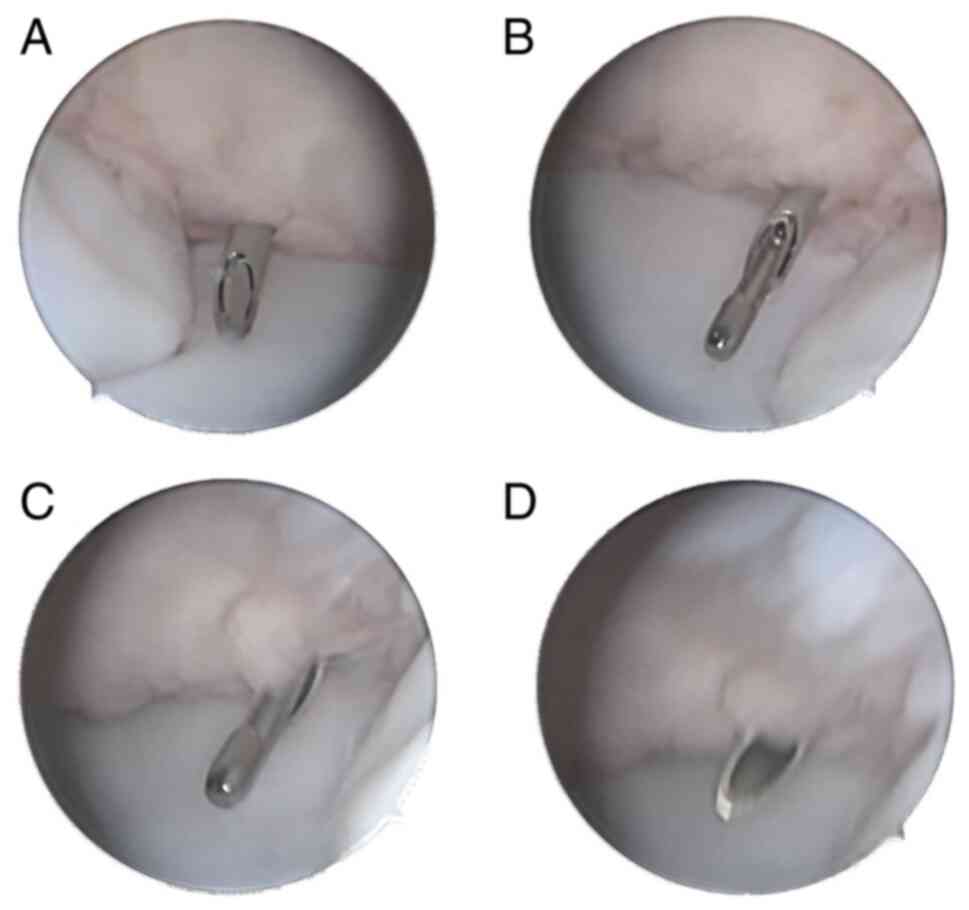
A novel synovial biopsy device was used in the
present study (Fig. 3). A
negative-pressure device was attached to the synovial biopsy needle
to assist in capturing synovial tissue. The needle biopsy process
is illustrated in Fig. 4. The
biopsy sites were distributed as follows: 45 knee joints (75.0%),
nine wrist joints (15.0%), two elbow joints (3.3%) and four ankle
joints (6.7%). A total of six fragmented synovial pieces, each
measuring ~3x6 mm, were obtained from the joint of each patient.
These samples were fixed in 10% formalin with 0.01 mol/l phosphate
buffer at room temperature (20-25˚C) for 24 h. Fixed samples were
dehydrated through a graded ethanol series, cleared in xylene and
embedded in molten paraffin wax at 60˚C. The paraffin-embedded
tissues were sectioned at a thickness of 4 µm. H&E staining of
sections was performed and the samples were examined under light
microscopy (objective lens 10x). The sections were stained with
hematoxylin (1%) at room temperature (~25˚C) for 8 min,
differentiated for 5 sec, blued for 30 sec and counterstained with
eosin (1%) for 2 min at room temperature. The slides were
independently reviewed by two experienced histopathologists and
disagreements were referred for further evaluation by a senior
specialist.
Histopathology assessment
Histopathological examination assessed synovial
lining hyperplasia, stromal activation, inflammatory infiltration
and neovascularization, with each aspect scored semi-quantitatively
from 0-3. Synoviocyte detachment was scored as 4. The GSS includes
synovial lining hyperplasia, stromal activation and inflammatory
infiltration, with a total score ranging from 0-9. The mGSS
incorporates synovial hyperplasia (0-3) or synoviocyte detachment
(4), stromal activation (0-3),
neovascularization (0-3) and inflammatory infiltration (0-3),
resulting in a total score ranging from 0-13 (Fig. 2). Synovial hyperplasia, stromal
activation and inflammatory infiltration were scored as per the GSS
(13).
As for neovascularization, both Tak et al
(14) and de Bois et al
(15) assessed the severity of
neovascularization by evaluating vascular density, using the number
of blood vessels counted under high-power fields and defining
thresholds at 3, 9, 16 and 22. Inspired by this methodology, the
present study adopted a similar approach and found it effective.
Specifically, neovascularization was scored based on the number of
blood vessels in synovial tissue, using the following criteria: 0:
0-3; 1: 4-9; 2: 10-15; 3: ≥16 vessels per field under a 20x
objective lens using light microscopy. For this scoring system,
capillaries, venules and arterioles were equally weighted to ensure
a comprehensive evaluation.
Statistical analysis
Statistical analyses were performed using SPSS 26.0
(IBM Corp.). The normality of the data distribution was assessed
using the Kolmogorov-Smirnov test. Normally distributed continuous
data are presented as the mean (standard deviation). Unpaired
two-tailed Student's t-test was used to analyze the differences
between two normally distributed groups. Categorical data such as
sex are presented as the number of subjects (percentage). For
comparisons between two groups, the Mann-Whitney U test was applied
for non-normally distributed data. For comparisons involving more
than two groups, one-way ANOVA was applied for normally distributed
continuous data, followed by Bonferroni's post hoc test. For
non-normally distributed data, the Kruskal-Wallis test was used,
with Dunn's post hoc test for multiple comparisons. Parametric data
underwent correlation analyses using Spearman's correlation
coefficient. P<0.05 was considered to indicate a statistically
significant difference.
Results
Demographic and disease
characteristics of all patients
The baseline characteristics of the 60 patients
enrolled in the present study are summarized in Table I. Among the patients, 53 (88.3%)
were female, with a mean age of 53.9 years and a mean disease
duration of 97.6 months. Serological tests indicated that 38
(63.3%) patients were ACPA-positive and 45 (75.0%) were
RF-positive. The mean DAS28-CRP score was 4.71±1.02.
 | Table IBaseline characteristics of all
patients. |
Table I
Baseline characteristics of all
patients.
| Characteristic | All patients
(n=60) |
|---|
| Age, years | 53.9±11.3 |
| Sex, female, n
(%) | 53 (88.3%) |
| Disease duration,
months | 97.6±84.9 |
| ACPA positive, n
(%) | 38 (63.3) |
| RF positive, n
(%) | 45 (75.0) |
| ESR, mm/h | 45.57±29.51 |
| CRP, mg/l | 34.96±35.13 |
| TJC-28 | 5.33±3.74 |
| SJC-28 | 4.93±3.73 |
| PGA | 66.22±11.54 |
| DAS28-CRP | 4.71±1.02 |
Correlation between synovial
neovascularization and clinical activity in patients with RA
The synovial histopathology of the 56 patients with
RA (93.3%) had varying degrees of neovascularization. Among these,
23 had mild neovascularization (score, 1), 16 had moderate
neovascularization (score, 2) and 17 had severe neovascularization
(score, 3) (Fig. 5A). Patients
with severe neovascularization had significantly higher DAS28-CRP,
ESR and CRP levels than those with mild or moderate
neovascularization (P<0.05) (Fig.
5B, E and F). However, no significant statistical
difference was observed in ACPA levels across the mild, moderate
and severe neovascularization groups (Fig. 5C). The mean RF level in patients
with severe neovascularization was higher than that in patients
with moderate neovascularization (P<0.05; Fig. 5D). The severity of
neovascularization was significantly correlated with DAS28-CRP, ESR
and CRP levels (all P<0.001) with correlation coefficients of
0.49, 0.44 and 0.51, respectively (Table II).
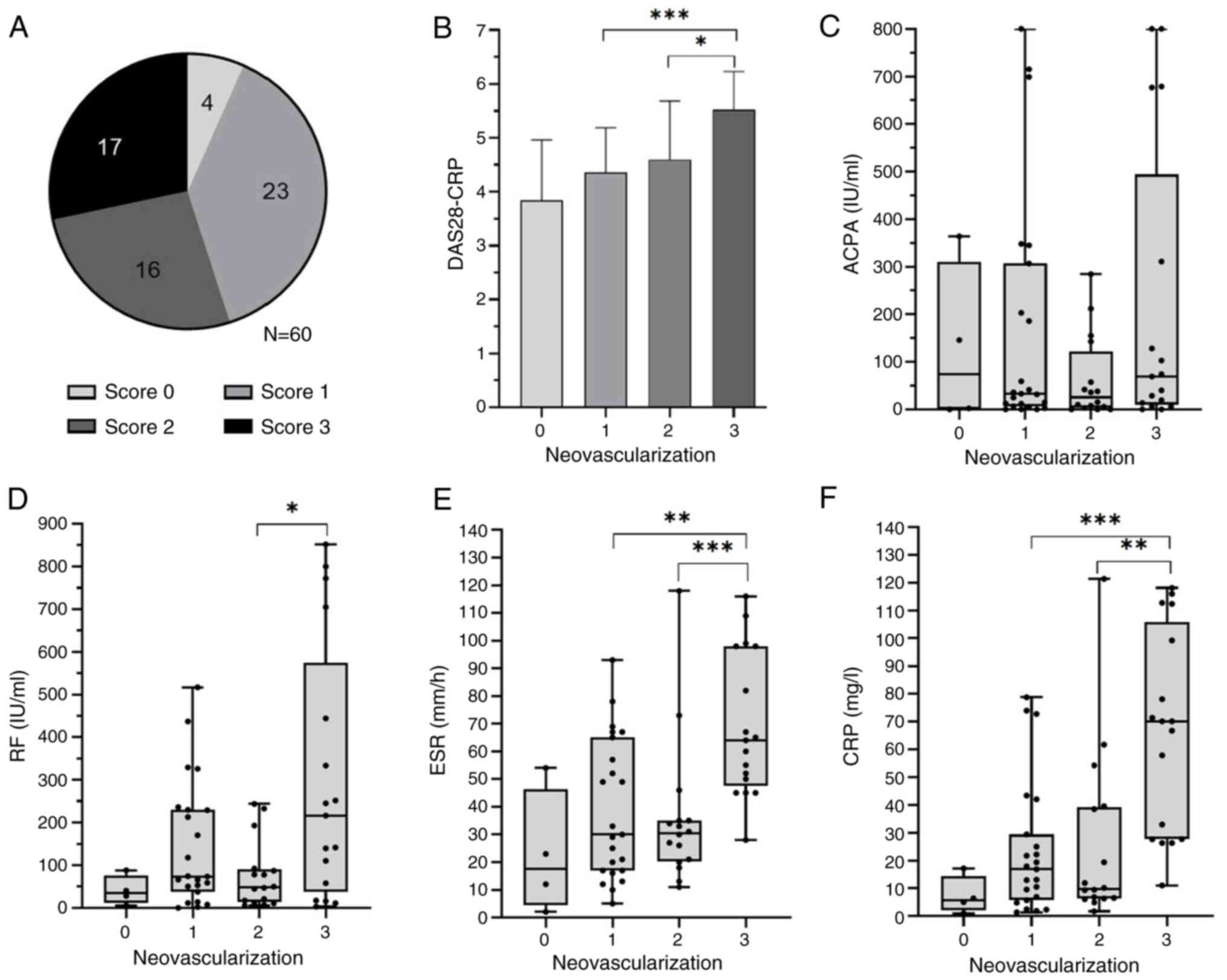 | Figure 5Comparison of clinical and serological
parameters with neovascularization scores in patients with
rheumatoid arthritis. (A) Severity of neovascularization was scored
form 0-3. A score of 0 indicates no neovascularization (four
patients), a score of 1 indicates mild neovascularization (23
patients), a score of 2 indicates moderate neovascularization (16
patients) and a score of 3 indicates severe neovascularization (17
patients). Patients with severe neovascularization had
significantly higher (B) DAS28-CRP) levels compared to those with
mild and moderate neovascularization. (C) ACPA levels showed no
statistically significant differences among the mild, moderate, and
severe groups. (D) RF level in patients with severe
neovascularization was higher than in those with moderate
neovascularization. Patients with severe neovascularization had
significantly higher (E) ESR and (F) CRP levels compared to those
with mild and moderate neovascularization. *P<0.05,
**P<0.01, ***P<0.001. DAS28-CRP,
disease activity score in 28 joints-C-reactive protein; ESR,
erythrocyte sedimentation rate; CRP, C-reactive protein; RF,
rheumatoid factor. |
 | Table IICorrelation between synovial
neovascularization and disease activity indicators in patients with
rheumatoid arthritis. |
Table II
Correlation between synovial
neovascularization and disease activity indicators in patients with
rheumatoid arthritis.
|
Neovascularization | ACPA, IU/ml | RF, IU/ml | ESR, mm/h | CRP, mg/l | DAS28-CRP |
|---|
| None (Score 0) | 74.15
(0.58-309.50) | 34.45
(11.63-76.15) | 17.50
(4.50-46.25) | 5.73
(2.03-14.47) | 3.90±1.10 |
| Mild (Score 1) | 35.90
(12.0-306.83) | 73.55
(37.50-230.16) | 30.00
(17.00-65.00) | 16.85
(5.73-29.46) | 4.40±0.78 |
| Moderate (Score
2) | 7.78
(0-117.78) | 48.57
(13.78-91.06) | 30.50
(20.25-35.00) | 9.80
(6.36-39.28) | 4.60±1.09 |
| Severe (Score
3) | 40.10
(0-555.50) | 216.47
(37.42-574.71) | 64.00
(47.50-98.00) | 70.10
(27.69-105.77) | 5.53±0.71 |
| Correlation
coefficient, ρ | 0.08 | 0.22 | 0.44 | 0.51 | 0.49 |
| P-value | 0.570 | 0.085 | <0.001 | <0.001 | <0.001 |
Comparison of disease activity in
patients with synoviocyte proliferation and synoviocyte
detachment
Among the patients, 36 (60%) had synoviocyte
proliferation, with DAS28-CRP scores ranging from 1.88-5.88. A
total of nine patients (16%) had synoviocyte detachment,
corresponding to DAS28-CRP scores ranging from 3.75-5.89. The group
with synoviocyte detachment had higher levels of ESR, CRP and
DAS28-CRP compared with patients with synovial hyperplasia
(P<0.05; Table III).
 | Table IIIComparison of clinical
characteristics between patients with rheumatoid arthritis and with
synoviocyte detachment and those with synoviocyte
proliferation. |
Table III
Comparison of clinical
characteristics between patients with rheumatoid arthritis and with
synoviocyte detachment and those with synoviocyte
proliferation.
| Characteristic | Synoviocyte
detachment (n=9) | Synoviocyte
proliferation (n=36) | P-value |
|---|
| Age, years | 60.44±7.99 | 51.86±10.79 | 0.031 |
| Female, n (%) | 8 (88.9) | 31 (86.1) | 0.823 |
| Disease duration,
months | 112.67±90.39 | 89.94±81.14 | 0.466 |
| Moderate-severe
bone erosion, n (%) | 8 (77.8) | 20 (61.1) | 0.047 |
| ACPA positive, n
(%) | 7 (77.8) | 21 (58.3) | 0.267 |
| RF positive, n
(%) | 8 (88.9) | 25 (69.4) | 0.206 |
| ESR, mm/h | 73.44±31.93 | 40.72±25.04 | 0.002 |
| CRP, mg/l | 62.36±40.95 | 30.36±29.64 | 0.010 |
| TJC-28 | 7.33±5.05 | 5.69±3.60 | 0.267 |
| SJC-28 | 7.33±4.97 | 5.22±3.43 | 0.142 |
| PGA | 71.11±13.41 | 65.00±10.89 | 0.158 |
| DAS28-CRP | 5.51±0.95 | 4.77±1.02 | 0.046 |
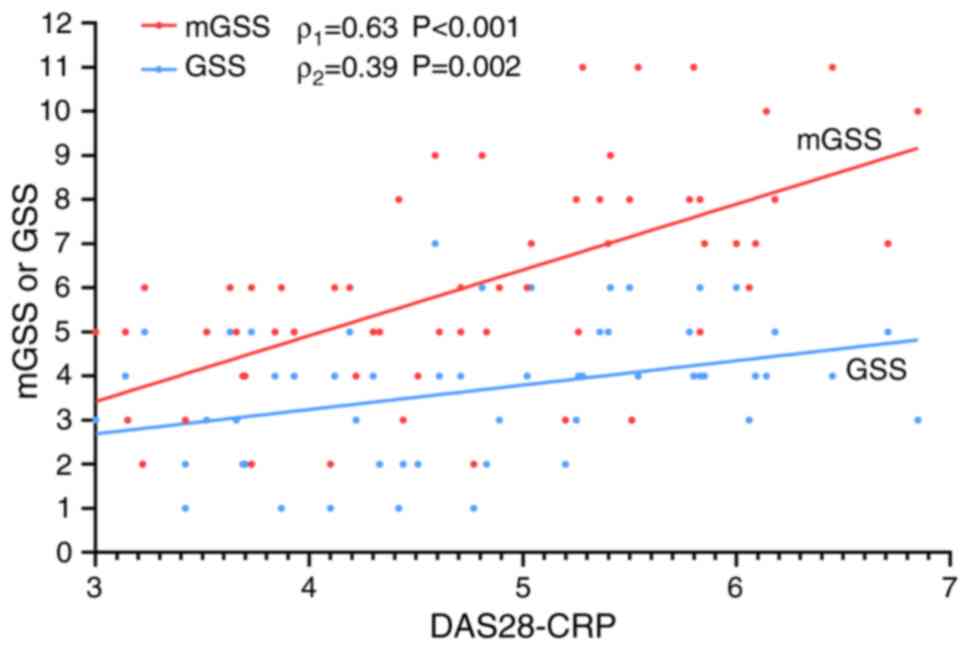
Comparison of the correlations of
DAS28-CRP between GSS and mGSS
Spearman's correlation analysis revealed that the
GSS and the mGSS were significantly correlated with the DAS28-CRP
level (Fig. 6). However, the mGSS
had a stronger correlation (ρ=0.63; P<0.001) than the GSS
(ρ=0.39; P=0.002).
Discussion
Neovascularization is considered to be an early and
key event in the formation and maintenance of synovial inflammation
in RA (16). Persistent
inflammation increases oxygen demand, leading to local hypoxia,
which induces the expression of pro-angiogenic factors, such as
vascular endothelial growth factor (VEGF). This stimulates the
formation of new blood vessels, provides oxygen and nutrients to
proliferating synovial cells and facilitates inflammatory cell
infiltration, thereby exacerbating chronic synovial inflammation
(17,18). The increased endothelial surface
area also creates a substantial capacity for producing cytokines,
adhesion molecules and other inflammatory stimuli. Additionally,
the proliferation of new blood vessels in the synovium facilitates
tissue invasion, supporting the active infiltration of the synovium
into the cartilage, leading to erosion and destruction of the
cartilage (19,20).
In a previous study, the serum VEGF level was
revealed to be higher in patients with RA than in healthy
individuals. Furthermore, the serum VEGF level was correlated with
ESR, CRP, RF, the number of tender and swollen joints, Modified
Health Assessment Questionnaire and PGA of disease activity in
patients with RA (21). Another
study revealed that the serum VEGF level at presentation in
patients with early RA was significantly correlated with the
development of radiographic damage after 1 year, and improvement in
the clinical symptoms of RA was associated with a reduced serum
VEGF level (22). These findings
highlight the pivotal role of neovascularization in fueling the
progression and severity of RA. However, by inhibiting
neovascularization, particularly through blocking the VEGF
signaling pathway, synovial vascular density can be reduced,
inflammatory cell infiltration decreased and symptoms alleviated
(7,23).
In the present study, >90% of the patients with
RA exhibited varying degrees of neovascularization. The synovial
neovascularization score was significantly correlated with disease
activity (DAS28-CRP) and the inflammatory markers, ESR and CRP.
This indicates the widespread presence of synovial vascular lesions
in RA histopathology and a strong association between increased
neovascularization and heightened disease activity in patients with
RA. Therefore, these results suggested that neovascularization may
be a crucial parameter for assessing disease activity in patients
with RA.
Another notable feature of RA synovium is synovial
hyperplasia. The normal synovial lining consists of 2-3 layers of
synoviocytes. However, in RA, chronic inflammation and aberrant
cellular signaling drive the excessive proliferation of
synoviocytes, leading to a synovial lining that can expand to 6-10
layers (8,24). This hyperplasia promotes the
development of invasive pannus tissue and exacerbates joint damage
(25). Consistent with previous
studies, the current study found that most patients with RA (60%)
had synovial hyperplasia. However, it was observed that some
patients with RA did not have synovial hyperplasia and synoviocyte
proliferation; by contrast, 16% of the patients had thinning of the
synovial lining with synoviocyte detachment.
Physiopathological observations suggest that chronic
mild inflammation typically leads to cell proliferation, whereas
severe acute inflammation can result in cell necrosis and
detachment (26,27). Substantial evidence indicates that
chronic persistent inflammation within the synovial membrane leads
to synoviocyte proliferation in RA (28). The mechanisms underlying
synoviocyte proliferation are multifaceted and include the effects
of inflammatory mediators, oxidative-stress-induced DNA damage and
the infiltration of bone marrow-derived cells (29,30).
However, long-term chronic inflammation also results
in the release of various inflammatory mediators, including TNF-α,
IL-1 and IL-6, which can induce apoptosis of synoviocytes (31,32).
There are two types of synoviocytes: Macrophage-like synoviocytes
and fibroblast-like synoviocytes (FLS), with FLS being the major
component of the synovial lining layer. In the early stages of RA,
FLS have enhanced proliferative and antiapoptotic capabilities,
leading to synovial hyperplasia. However, in later stages,
prolonged inflammatory stimulation and cellular metabolic stress
may cause these cells to gradually lose their proliferative
ability, resulting in synovial thinning (20,33).
Thus, synoviocyte necrosis and detachment can occur in two
scenarios: During severe inflammation or as a late-stage
manifestation. The present study showed that patients with
synoviocyte detachment had higher disease activity but did not have
longer disease duration than patients with synoviocyte
proliferation. Therefore, it was hypothesized that synoviocyte
detachment is more closely associated with severe inflammation in
patients with RA. Thus, it is proposed that in synovitis scoring,
the presence of synoviocyte detachment should be assigned a score
of 4, higher than the score of 3 for severe synovial
hyperplasia.
In response to these findings, the present study
proposed the mGSS, which includes assessments of neovascularization
and synoviocyte detachment alongside traditional parameters. The
correlation between the mGSS and DAS28-CRP showed a trend towards
superiority over the GSS (correlation coefficient, 0.62 vs. 0.37).
This suggested that the enhanced scoring system aligns more closely
with the clinical realities of RA, offering a more comprehensive
assessment tool that can improve disease management strategies.
The present study had some limitations. First, it
was a single-center study with a relatively small sample size,
which may affect the generalizability of the findings. Second, the
local sampling from different sites during minimally invasive
synovial biopsy, the selection of synovial histopathology sections
and manual readings are susceptible to subjective bias, which may
also lead to result bias. Future studies with larger sample sizes
are required to ensure the objectivity and accuracy of the
results.
In conclusion, although the GSS provides a
fundamental framework for evaluating synovial inflammation, it does
not fully encompass the intricate histopathology of RA. The mGSS,
with its inclusion of neovascularization and synoviocyte
detachment, offers a more detailed and clinically relevant
evaluation of synovial histopathology. The present study not only
highlighted the association of these histopathological features
with RA activity, but also set the stage for future research to
validate and refine the mGSS, potentially establishing a new
standard for assessing and managing RA.
Acknowledgements
The authors would like to acknowledge Dr Xie Tian
(Nanchang Jialin Medical Pathology Laboratory, Nanchang, China) for
preparing the logistics of the project.
Funding
Funding: The present study was supported by the Foundation of
Jiangxi Provincial Health Commission Science and Technology Project
(grant no. SKJP220212536).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
DW and YD analyzed the data and drafted the initial
manuscript. YH, JZ and WL collected the data and confirm the
authenticity of all the raw data. YP and ZX independently assessed
the pathological slides of the synovium. RW oversaw the entire
project, designed the experiment and revised the manuscript draft.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical Review
Board of the First Affiliated Hospital of Nanchang University
[approval no. IIT (2023); clinical ethics review no. 011]. Written
informed consent was obtained from all patients/participants,
covering both their participation in the procedure and their
participation in the study. All procedures were conducted in
accordance with The Declaration of Helsinki (as revised in
2013).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Smolen JS, Aletaha D and Mcinnes IB:
Rheumatoid arthritis. Lancet. 388:2023–2038. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Radu AF and Bungau SG: Management of
rheumatoid arthritis: An overview. Cells. 10(2857)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Krenn V, Perino G, Rüther W, Krenn VT,
Huber M, Hügle T, Najm A, Müller S, Boettner F, Pessler F, et al:
15 years of the histopathological synovitis score, further
development and review: A diagnostic score for rheumatology and
orthopaedics. Pathol Res Pract. 213:874–881. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Schmidt T, Najm A, Mussawy H, Burghardt R,
Oehler N, Krenn V, Rüther W and Niemeier A: General synovitis score
and immunologic synovitis score reflect clinical disease activity
in patients with advanced stage rheumatoid arthritis. Sci Rep.
9(8448)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Slansky E, Li J, Häupl T, Morawietz L,
Krenn V and Pessler F: Quantitative determination of the diagnostic
accuracy of the synovitis score and its components. Histopathology.
57:436–443. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Szekanecz Z, Besenyei T, Szentpétery A and
Koch AE: Angiogenesis and vasculogenesis in rheumatoid arthritis.
Curr Opin Rheumatol. 22:299–306. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Leblond A, Allanore Y and Avouac J:
Targeting synovial neoangiogenesis in rheumatoid arthritis.
Autoimmun Rev. 16:594–601. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Henderson B, Revell PA and Edwards JC:
Synovial lining cell hyperplasia in rheumatoid arthritis: Dogma and
fact. Ann Rheum Dis. 47:348–349. 1988.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Orr C, Vieira-Sousa E, Boyle DL, Buch MH,
Buckley CD, Cañete JD, Catrina AI, Choy EHS, Emery P, Fearon U, et
al: Synovial tissue research: A state-of-the-art review. Nat Rev
Rheumatol. 13:463–475. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang B, Li J, Huang Y and Wu R:
Synoviocyte detachment: An overlooked yet crucial histological
aspect in rheumatoid arthritis. BMC Musculoskelet Disord.
25(829)2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Aletaha D and Smolen JS: Diagnosis and
management of rheumatoid arthritis: A review. JAMA. 320:1360–1372.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Van Riel PL and Renskers L: The disease
activity score (DAS) and the disease activity score using 28 joint
counts (DAS28) in the management of rheumatoid arthritis. Clin Exp
Rheumatol. 34 (Suppl 5):S40–S44. 2016.PubMed/NCBI
|
|
13
|
Krenn V, Morawietz L, Burmester GR, Kinne
RW, Mueller-Ladner U, Muller B and Haupl T: Synovitis score:
Discrimination between chronic low-grade and high-grade synovitis.
Histopathology. 49:358–364. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tak PP, Thurkow EW, Daha MR, Kluin PM,
Smeets TJ, Meinders AE and Breedveld FC: Expression of adhesion
molecules in early rheumatoid synovial tissue. Clin Immunol
Immunopathol. 77:236–242. 1995.PubMed/NCBI View Article : Google Scholar
|
|
15
|
De Bois MH, Arndt JW, Tak PP, Kluin PM,
van der Velde EA, Pauwels EK and Breedveld FC: 99Tcm-labelled
polyclonal human immunoglobulin G scintigraphy before and after
intra-articular knee injection of triamcinolone hexacetonide in
patients with rheumatoid arthritis. Nucl Med Commun. 14:883–887.
1993.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Szekanecz Z, Besenyei T, Paragh G and Koch
AE: Angiogenesis in rheumatoid arthritis. Autoimmunity. 42:563–573.
2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Taylor PC and Sivakumar B: Hypoxia and
angiogenesis in rheumatoid arthritis. Curr Opin Rheumatol.
17:293–298. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Konisti S, Kiriakidis S and Paleolog EM:
Hypoxia-a key regulator of angiogenesis and inflammation in
rheumatoid arthritis. Nat Rev Rheumatol. 8:153–162. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pober JS and Sessa WC: Evolving functions
of endothelial cells in inflammation. Nat Rev Immunol. 7:803–815.
2007.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Bottini N and Firestein GS: Duality of
fibroblast-like synoviocytes in RA: Passive responders and
imprinted aggressors. Nat Rev Rheumatol. 9:24–33. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lee SS, Joo YS, Kim WU, Min DJ, Min JK,
Park SH, Cho CS and Kim HY: Vascular endothelial growth factor
levels in the serum and synovial fluid of patients with rheumatoid
arthritis. Clin Exp Rheumatol. 19:321–324. 2001.PubMed/NCBI
|
|
22
|
Ballara S, Taylor PC, Reusch P, Marmé D,
Feldmann M, Maini RN and Paleolog EM: Raised serum vascular
endothelial growth factor levels are associated with destructive
change in inflammatory arthritis. Arthritis Rheum. 44:2055–2064.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhao H, Duan S, Shi Y, Zhang M, Zhang L,
Jin Z, Fu W, Xiao W, Bai T, Zhang X and Wang Y: Naru-3 inhibits
inflammation, synovial hyperplasia and neovascularization in
collagen-induced arthritis in rats. J Ethnopharmacol.
311(116350)2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tsubaki T, Arita N, Kawakami T,
Shiratsuchi T, Yamamoto H, Takubo N, Yamada K, Nakata S, Yamamoto S
and Nose M: Characterization of histopathology and gene-expression
profiles of synovitis in early rheumatoid arthritis using targeted
biopsy specimens. Arthritis Res Ther. 7:R825–R836. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Liu H, Zhu Y, Gao Y, Qi D, Zhao L, Zhao L,
Liu C, Tao T, Zhou C, Sun X, et al: NR1D1 modulates synovial
inflammation and bone destruction in rheumatoid arthritis. Cell
Death Dis. 11(129)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Federico A, Morgillo F, Tuccillo C,
Ciardiello F and Loguercio C: Chronic inflammation and oxidative
stress in human carcinogenesis. Int J Cancer. 121:2381–2386.
2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Varela ML, Mogildea M, Moreno I and Lopes
A: Acute inflammation and metabolism. Inflammation. 41:1115–1127.
2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu F, Feng XX, Zhu SL, Huang HY, Chen YD,
Pan YF, June RR, Zheng SG and Huang JL: Sonic hedgehog signaling
pathway mediates proliferation and migration of fibroblast-like
synoviocytes in rheumatoid arthritis via MAPK/ERK signaling
pathway. Front Immunol. 9(2847)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ma C, Wang J, Hong F and Yang S:
Mitochondrial dysfunction in rheumatoid arthritis. Biomolecules.
12(1216)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Fearon U, Hanlon MM, Floudas A and Veale
DJ: Cellular metabolic adaptations in rheumatoid arthritis and
their therapeutic implications. Nat Rev Rheumatol. 18:398–414.
2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Firestein GS and Mcinnes IB:
Immunopathogenesis of rheumatoid arthritis. Immunity. 46:183–196.
2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Firestein GS, Nguyen K, Aupperle KR, Yeo
M, Boyle DL and Zvaifler NJ: Apoptosis in rheumatoid arthritis: p53
overexpression in rheumatoid arthritis synovium. Am J Pathol.
149:2143–2151. 1996.PubMed/NCBI
|
|
33
|
Taghadosi M, Adib M, Jamshidi A, Mahmoudi
M and Farhadi E: The p53 status in rheumatoid arthritis with focus
on fibroblast-like synoviocytes. Immunol Res. 69:225–238.
2021.PubMed/NCBI View Article : Google Scholar
|