Introduction
Islet transplantation is a promising treatment for
patients with type 1 diabetes mellitus who frequently have severe
hyperglycemia and glycemic instability (1,2).
Islet transplantation using the Edmonton protocol can successfully
restore long-term endogenous insulin production and glycemic
stability in patients with type 1 diabetes mellitus (3). The administration of
immunosuppressive therapy that causes less damage to pancreatic
islet cells is a critical consideration. Before the Edmonton
protocol, immunosuppressants, such as tacrolimus, cyclosporine,
azathioprine, glucocorticoids, and antilymphocyte globulin were
used similar to other organ transplantation. Because
glucocorticoids were found to enhance insulin resistance, while
tacrolimus and cyclosporine were observed to suppress insulin
secretion and cause kidney damage, the Edmonton protocol introduced
a new immunosuppressive therapy using sirolimus and
anti-interleukin (IL)-2 receptor antibodies (daclizumab) with
low-dose tacrolimus. However, the results of the Edmonton protocol
at that time were unsatisfactory with a 5-year insulin withdrawal
rate of 7.5% (4). The
Collaborative Islet Transplant Registry (CITR) protocol with
anti-thymocyte globulin and anti-tumor necrosis factor (TNF) α
antibody (etanercept) instead of anti-IL-2 receptor antibodies,
further improves islet transplant outcomes (1,5).
Reportedly, the 4-year graft survival rate was approximately 90%
for transplant cases between 2007 and 2010, and the 3-year insulin
withdrawal rate was 44%.
In 1991, Izumori (6) discovered a new enzyme for
synthesizing rare sugars and established a method to mass produce
rare sugars. According to the International Rare Sugar Association,
monosaccharides and their derivatives are rare in nature. D-allose,
the C3 epimer of D-glucose, is a rare sugar produced from
D-fructose that has various antioxidant, anti-inflammatory, and
immunosuppressive effects (7,8).
Previous reports (8,9) indicate that D-allose is
immunosuppressive without toxic side effects, unlike FK506
(tacrolimus), as D-allose induced a dose-dependent suppression of
segmented neutrophils. In liver transplantation, prolonged liver
allograft survival in rats can be achieved using a combination of
FK506 and D-allose than either drug individually (8). D-allose has been shown to have a
protective effect owing to its anti-inflammatory and
immunosuppressive effects, and antioxidant activity against
ischemia-reperfusion injury in several organs such as the liver
(9), brain (10,11),
and skin (12,13).
Based on accumulated evidence of the antioxidant
effects of D-allose in transplanted cells, we previously focused on
improving insulin secretion after transplantation of pancreatic
islets. We demonstrated that D-allose treatment of isolated islet
cultures prior to transplantation restored islet function and
increased transplant success rates (14). D-allose has been suggested to
improve the function of damaged islets through its antioxidant
activity.
In the present study, we further examined whether
intravenous D-allose administration could improve insulin secretion
when pancreatic islets were transplanted into type 1 diabetes model
mice. This would suggest that D-allose is useful both as an
immunosuppressant in human pancreatic islet transplantation and as
a protective agent against cell damage through its
anti-inflammatory effects. Furthermore, D-allose is safe enough to
be administered intravenously, therefore it may be possible to
implement it quickly in clinical settings.
Materials and methods
Animals
Male BALB/c mice aged 8-12 weeks purchased from CLEA
Japan, Inc. (Tokyo, Japan) and Japan SLC, Inc. (Shizuoka, Japan)
were used for all the experiments. All mice were bred and handled
according to the Experimental Animal Research Guide. This study was
approved by the Animal Care and Use Committee of Kagawa University.
Since only male mice were used in our previous study (14), we conducted experiments using male
mice as a continuation of the previous study in this study. A total
of 60 mice were used as a mouse model of type 1 diabetes by STZ
induction, and 138 healthy mice were used for islet extraction for
islet transplantation. The handling of euthanasia of mice used in
this study was performed according to the AVMA guidelines for the
euthanasia of animals (2013 edition) (15). Animals will be closely monitored
after treatment and will be euthanized if they have difficulty
feeding or ingesting water, show rapid and unrecoverable weight
loss (>25% in 7 days), or show signs of weakness such as
abnormal posture, breathing problems, bleeding or anemia, or if the
experiment is stopped and the animal is euthanized. Healthy mice
whose pancreas was removed to extract the islets were sacrificed by
severing the vena cava. The mice with one kidney removed, from
which the islets were transplanted, were euthanized with carbon
dioxide within a week because they became hyperglycemic and
debilitated. Euthanasia by carbon dioxide was carried out in a
designated area of the animal experimental facility. The carbon
dioxide replacement was performed at a rate of 30%/min at our
facility. In detail, mice were placed in cages and 100%
CO2 gas was flowed from a compressed carbon dioxide gas
cylinder at a flow rate of 5 l/min for 5 min. During this time, the
mice stopped breathing and their eyeballs became discolored. After
5 min, the flow of CO2 was stopped, the container was
sealed, and more than 5 min were allowed to pass. Death was then
confirmed by palpation to check the heartbeat.
Creation of diabetic mice
Streptozotocin (STZ) 200 mg/kg body weight (BW) was
administered via the tail vein to prepare type 1 diabetes model
mice. A diabetic mouse model was defined as one in which the casual
blood glucose level was >400 mg/dl for >2 consecutive days.
Blood was collected by making a small incision in the skin at the
tip of the mouse's tail. Less than 1 µl of blood is needed to
measure blood glucose, so a little more than this was
collected.
Islet isolation and culture
method
Under inhalation anesthesia with isoflurane
(introduction: O2 0.5L, isoflurane 4-5%, maintenance: O2 0.5L,
isoflurane 2-3%) or sevoflurane (introduction: O2 0.5L, sevoflurane
5%, maintenance: O2 0.5L, isoflurane 2.5-4%), depending on the
anesthesia machine, cold Hanks' Balanced Salt Solution (HBSS;
Sigma, St. Louis, MO, USA) containing dissolved collagenase
(collagenase type V; Wako Pure Chemical Industries, Ltd., Osaka,
Japan) at a concentration of 2 mg/ml (0.2%) was injected through
the common bile duct under microscope, to dilate the pancreas,
which was then removed. The pancreas was digested by warm bathing
at 37˚C for 25 min. Subsequently, concentration gradient
centrifugation was performed using Histopaque 1077 (Sigma) and
HBSS, and the solution layer containing the islets was recovered.
Then, the islets were cultured with RPMI1640 medium (Sigma)
supplemented with 5.6 mmol/l glucose and 5% fetal bovine serum at
37˚C and 5% CO2 for 2 h.
Islet transplantation and examination
of blood glucose level
Transplantation of 15 islet equivalents (IEQ)/g (per
BW of recipient mice) of pancreatic islets using islets extracted
from two healthy mice was performed under the capsule of one of
kidneys in each diabetic model mouse. The method of transplantation
under the renal capsule followed our previous study (14). Of the 19 diabetic model mice
transplanted with pancreatic islets, D-allose, which was supplied
by the Kagawa University Rare Sugar Research Center (Kagawa,
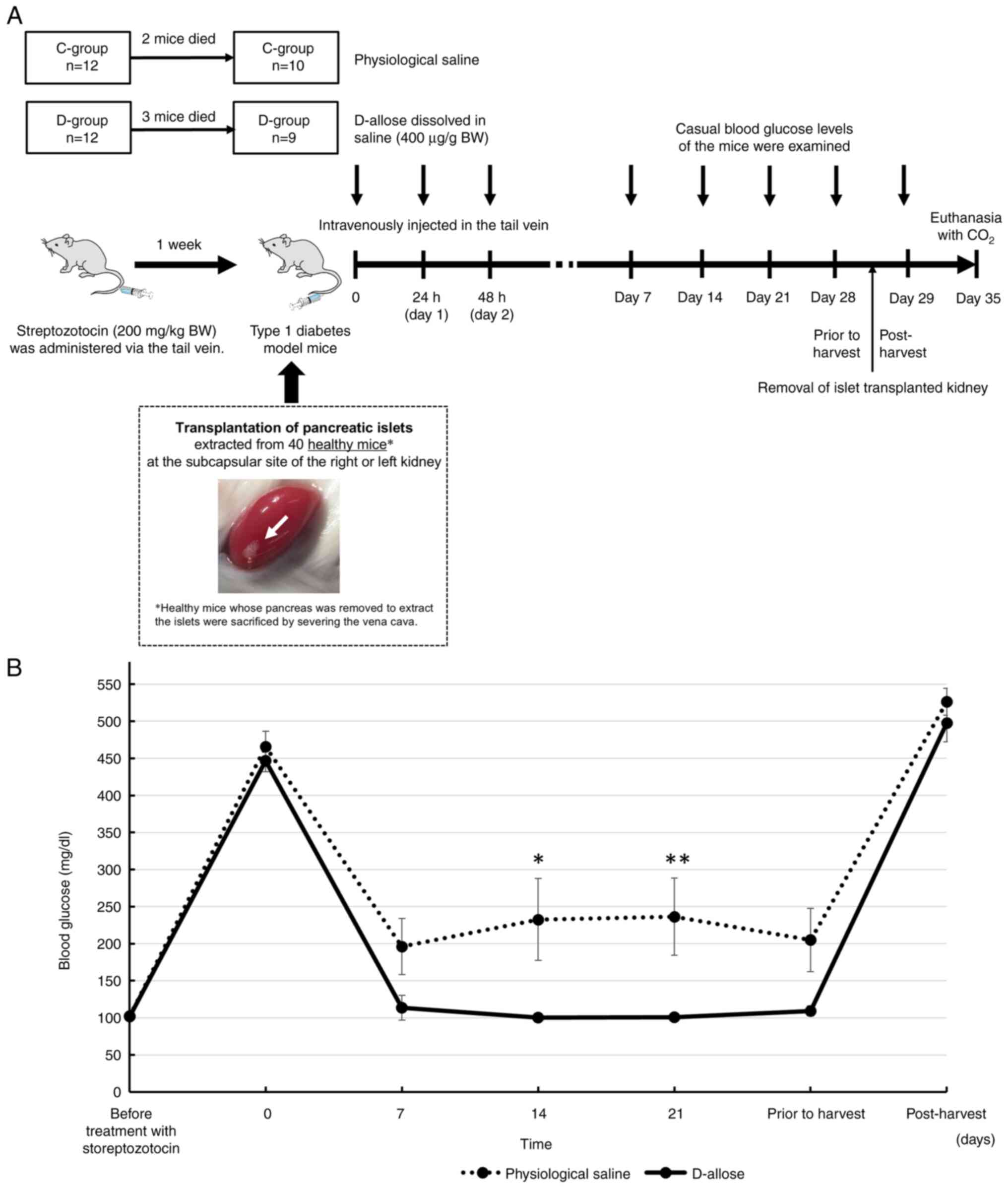
Japan), was administered to 9 mice (D-group), while physiological
saline was administered to 10 mice as the control group (C-group)
(Fig. 1A). D-allose (400 µg/g BW)
and physiological saline were intravenously injected in the tail
vein three times (0, 24, and 48 h after transplantation). Dose
concentrations of D-allose were determined according to previous
papers reported from our institution (16,17).
All mice were permitted ad libitum access to food
and water during this study. Casual blood glucose levels of the
mice were examined at several time points (before treatment with
streptozotocin, transplantation of pancreatic islets after
treatment with streptozotocin (0 day), 7, 14, 21 days, and the day
before and after extraction of transplanted islets).
IPGGT
Five mice each from the D-group and C-group, were
prepared in the same manner as above and fasted for 12 h overnight
on the seventh day after transplantation (Fig. 2A). Then, IPGGT was performed. Blood
glucose was monitored at various time points (0, 15, 30, 60, 90,
and 120 min) after intraperitoneal injection of glucose solution (2
g glucose/kg BW) by cutting the tail and gently massaging the blood
onto a glucose test strip.
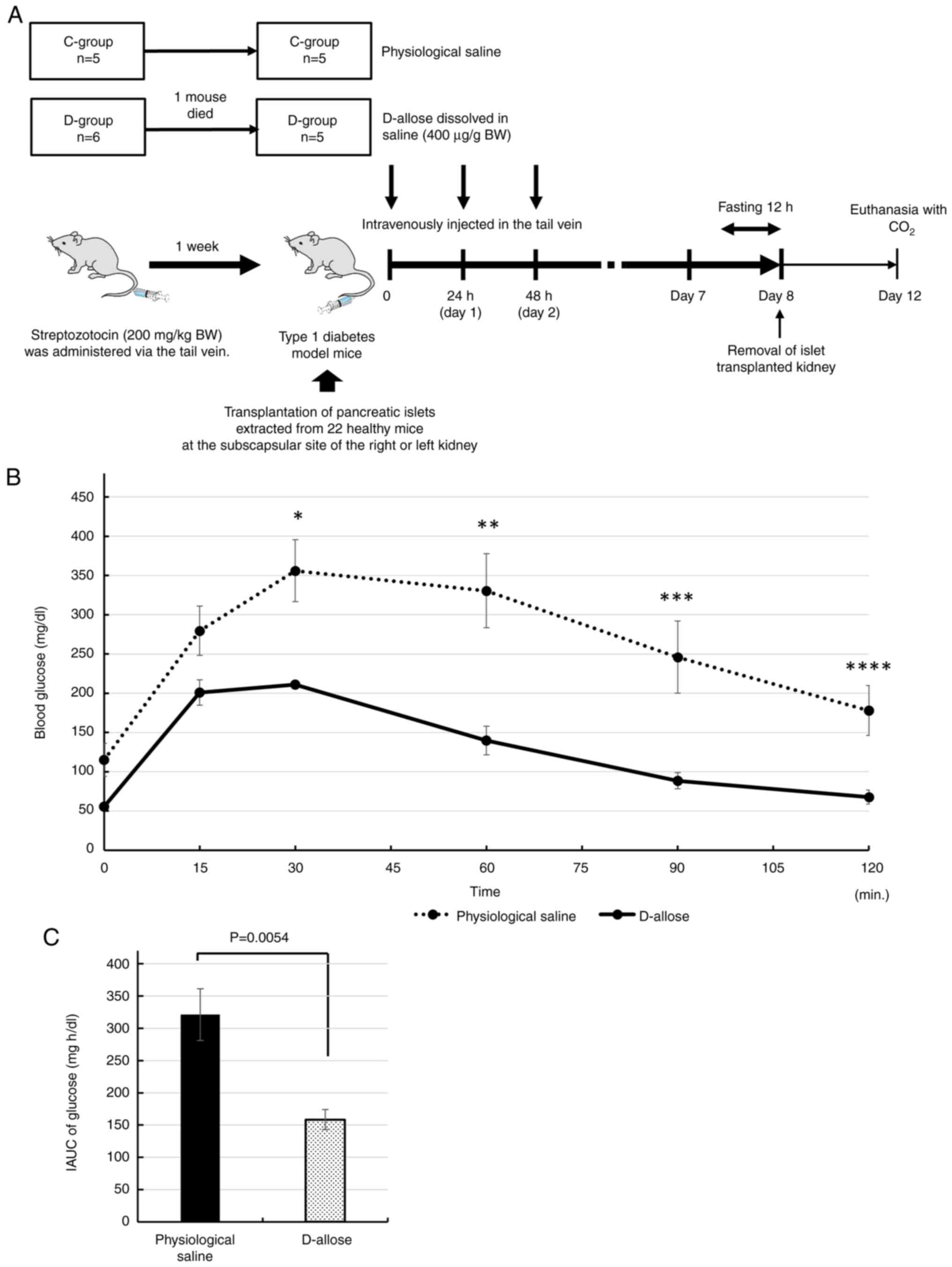 | Figure 2(A) Flow chart of the experiment for
the intraperitoneal glucose tolerance test. (B) Blood glucose
levels were monitored at various time points (0, 15, 30, 60, 90 and
120 min) after intraperitoneal injection of glucose in the D- (n=5)
and C-groups (n=5). There were significant differences in the blood
glucose levels between the D- and C-groups at 30, 60, 90 and 120
min (*P=0.0066, **P=0.0054,
***P=0.01 and ****P=0.01, respectively). (C)
IAUC of blood glucose concentration. The IAUC of glucose was
significantly decreased in the D-group compared with the C-group
(P=0.0054). BW, body weight; C-group, mice that received
physiological saline as a control; D-group, mice that received an
intravenous injection of D-allose; IAUC, incremental area under the
curve. |
The incremental area under the curve (IAUC) of blood
glucose concentration was calculated using the method described by
Wolever and Jenkins (18).
Histological examination of the
effects of D-allose administration into recipients
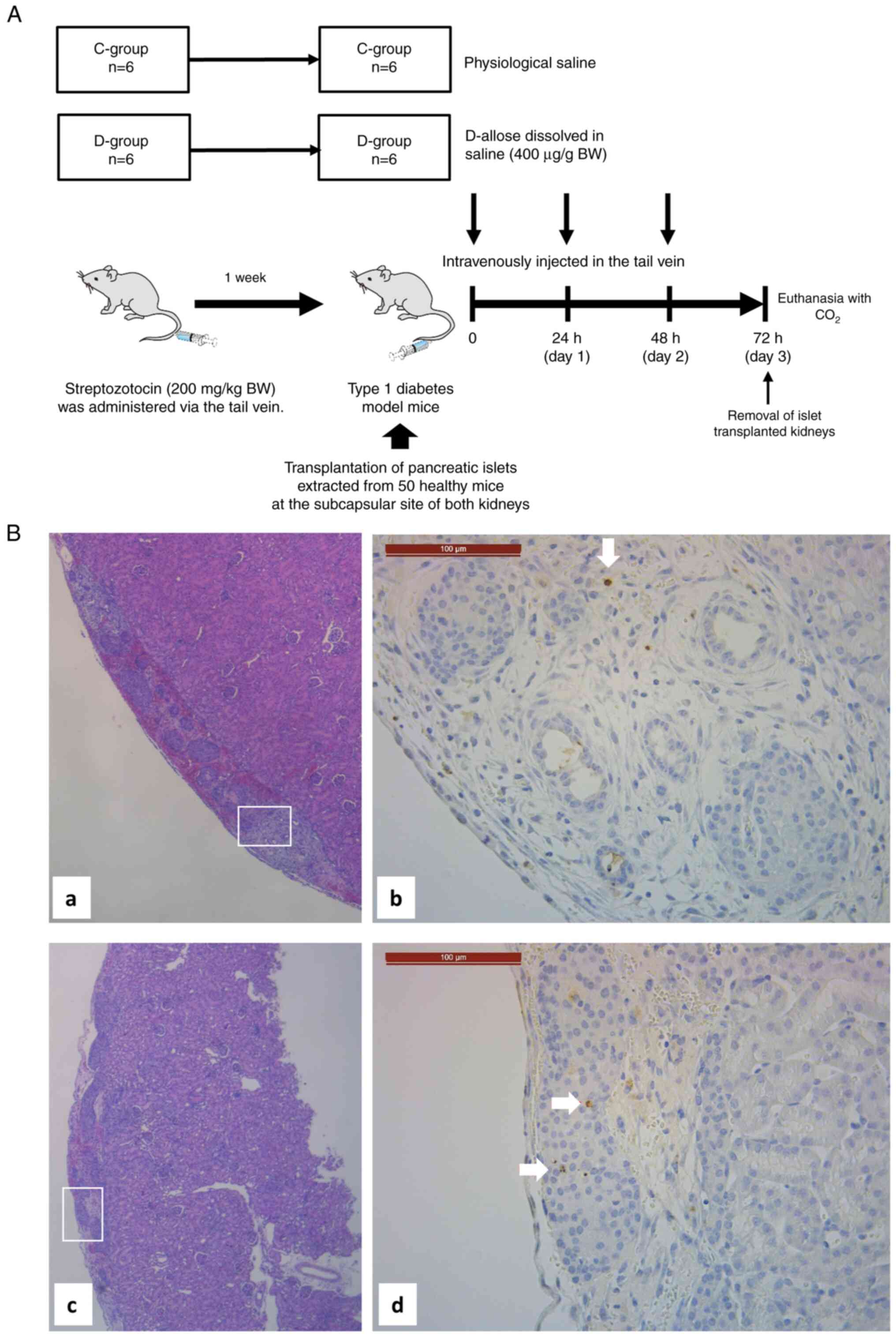
To evaluate apoptotic islet cells, 30 IEQ/g (per BW
of recipient mouse) of pancreatic islets was transplanted under the
bilateral renal capsule of non-diabetic BALB/c mice.
Transplantation was performed in six mice in the D-group and six
mice in the C-group (Fig. 3A).
Mice in the D-group were intravenously injected 400 µg/g BW of
D-allose dissolved in physiological saline, through the tail vein,
24 and 48 h after transplantation. Mice in the C-group were
intravenously injected with physiological saline. On the 3rd
postoperative day, the islet-transplanted kidney was removed. The
excised right kidney was fixed in formalin, and the left kidney was
immediately stored at -80˚C.
The kidney tissue samples of each group were fixed
in 4% paraformaldehyde for 48 h. The sample was embedded in
paraffin wax and cut into 4 µm slices. After defatting, the
sections underwent hematoxylin and eosin staining, followed by
dehydration and sealing. The stained sections were observed under a
microscope (Olympus, Japan).
Apoptotic cells were examined by terminal
deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling
(TUNEL) staining, using an In Situ Apoptosis Detection Kit (MK-500;
Takara Bio Inc., Tokyo, Japan) according to the manufacturer's
instructions. Briefly, cells were fixed in 4% paraformaldehyde and
permeabilized with 0.1% Triton X-100. Endogenous peroxidases were
inactivated by incubating the cells in 3% hydrogen peroxide for 5
min. After TUNEL staining with TdT enzyme for 90 min at 37˚C in the
dark, sections were treated with streptavidin-HRP, and apoptotic
cells were visualized using DAB staining, resulting in brown
staining. Hematoxylin was used for counterstaining. TUNEL-positive
cells were examined under a microscope. Three fields were randomly
selected for each sample, and the number of apoptotic cells was
calculated.
Reverse transcription-quantitative PCR
(RT-qPCR)
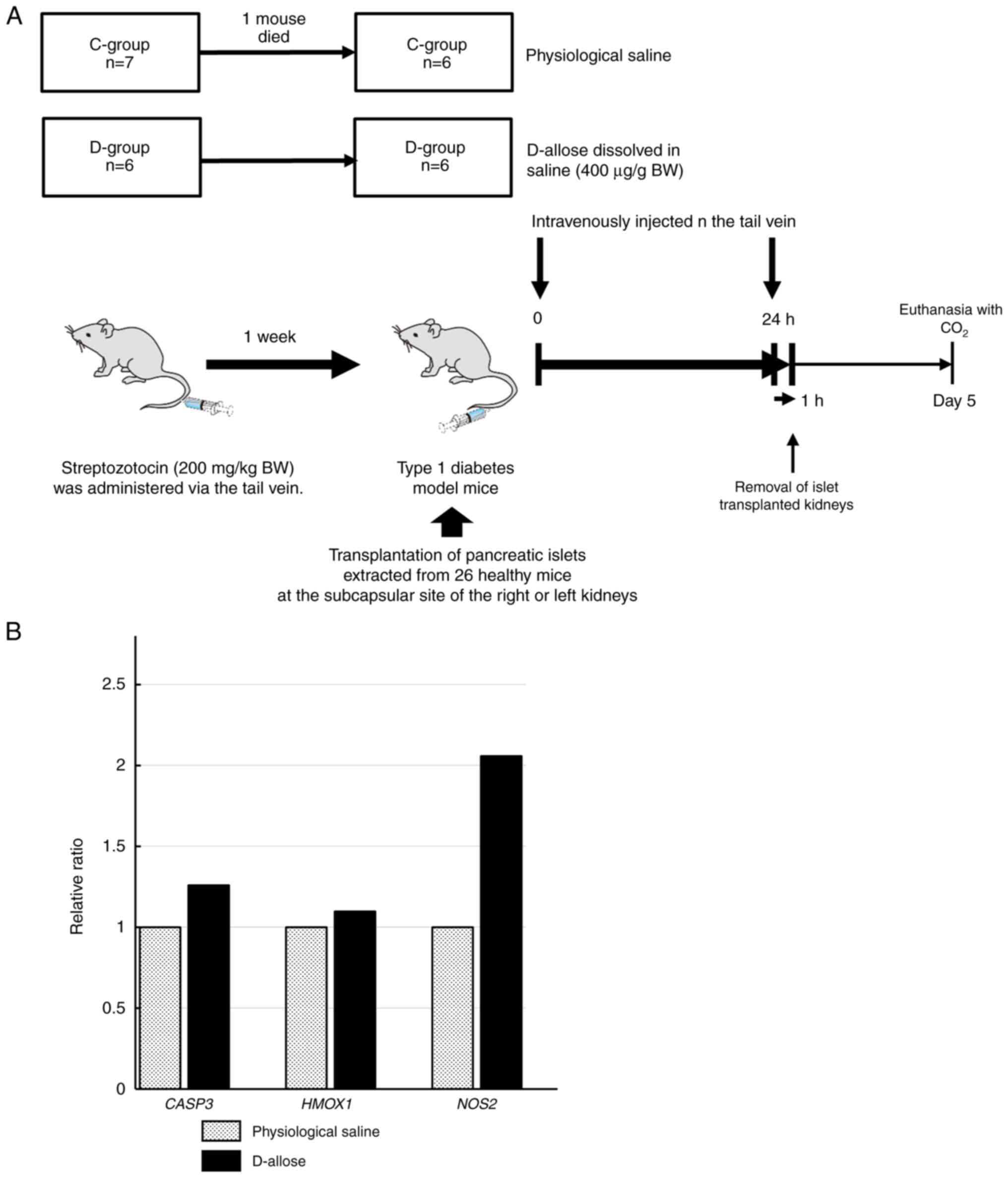
The gene expression of caspase 3 (CASP3),
heme oxygenase 1 (HMOX1), and nitric oxide synthase 2
(NOS2) in engrafted cells with or without D-allose
administration, was examined using RT-qPCR. Six diabetic model mice
in both the D- and C-groups received islet transplants (Fig. 4A).
In the D-group, 400 µg/g BW of D-allose dissolved in
saline was intravenously administered via the tail vein immediately
after transplantation and 24 h after transplantation. In the
C-group, saline alone was administered intravenously. After 1 h,
the transplanted islets were extracted alone with the surrounding
renal parenchyma under anesthesia. The extracted tissue was
immediately stored at liquid nitrogen temperature, and subsequently
at -80˚C.
Bead-type cell disruption was performed on the
excised tissue, and RNA was extracted using the spin column method.
For RNA, contamination with impurities was confirmed by absorbance
measurement (NanoDrop 2000, Thermo Fisher Scientific), and RNA
concentrations of >100 ng/µl, A260/280 ratio >1.8, and
A260/230 ratio >1.5 were accepted. cDNA was synthesized from the
extracted RNA by reverse transcription using the PrimeScript RT
Master Mix (Takara Bio). The amount of RNA was adjusted such that
the total RNA was 500 ng/2 µl of the absorbance PrimeScript RT
Master Mix. qPCR was performed to examine the mRNA expression of
CASP3, HMOX1, NOS2, and actin beta
(ACTB) using TaqMan probe Mm01195085_m1, Mm00516005_m1,
Mm00440502_m1, and Mm02619580_g1 respectively (Thermo Fisher
Scientific) on the Applied Biosystems real-time PCR system ViiA ™
7. The qPCR conditions were applied according to the manufacturer's
instructions. The mRNA expression level of each gene was normalized
to that of ACTB, and the expression levels were analyzed
using the 2-ΔΔCq method (19).
Statistical analysis
All values are expressed as means ± standard error
(SE). Comparisons of glucose values at each timepoint, IAUC, the
number of engrafted cells and mRNA expression levels between the
two groups were statistically analyzed using an unpaired t-test.
Statistical significance was set at p<0.05.
Results
Improvement of casual blood glucose
level in D-allose-administered mice
The average ± SE of casual blood glucose level in
the D-group (n=9) at 0, 7, 14, 21, and 28 (prior to harvest) days
was 446.67±14.7, 113.56±16.71, 100.44±5.58, 100.89±4.29, and
109.44±6.7 mg/dl, respectively, while that in the C-group (n=10) at
0, 7, 14, 21, and 28 days was 465.7±20.78, 196.1±37.83,
232.7±55.32, 236.4±52.07, and 205±42.98 mg/dl, respectively
(Fig. 1B). There was a significant
difference in casual blood glucose levels between the D- and
C-groups at 14 and 21 days (P=0.038 and 0.025, respectively).
IPGGT
After intraperitoneal injection of glucose solution,
the average ± SE of the blood glucose level in the D-group (n=5) at
0, 15, 30, 60, 90, and 120 min was 55.8±2.96, 200.8±16.17,
211±5.08, 140±18.21, 88.6±10.39, and 67.8±8.97 mg/dl, respectively,
while that in the C-group (n=5) at 0, 15, 30, 60, 90, and 120 min
was 115.2±21.11, 279.6±31.24, 356±39.47, 330.6±47.06, 246±45.8, and
178±31.82 mg/dl, respectively (Fig.
2B). There was a significant difference between blood glucose
levels in the D- and C-groups at 30, 60, 90, and 120 min (P=0.0066,
0.0054, 0.01 and 0.01, respectively).
Based on the alteration of glucose in IPGGT, IAUC of
glucose in the C- and D-groups was 321.2±40.22 and 158.53±15.46
mg-h/dl, respectively (Fig. 2C).
The IAUC of glucose was significantly lower in the D-group than
that in the C-group (P=0.0054).
HE and TUNEL staining to evaluate
apoptosis
There was no difference in the morphology of the
transplanted islets under pathological staining between the control
and D-allose groups (Fig. 3B).
The representative TUNEL staining images were shown
in Fig. 3. The mean number of
engrafted cells in the C- and D-groups was 469.8±123.2 and
479.67±84.14, respectively (Table
I). No statistically significant difference was observed in the
number of engrafted cells between the groups (P=0.95). Among them,
few apoptotic cells were observed in both the groups (Table I).
 | Table INumber of engrafted cells and
apoptotic cells in the C- and D-groups. |
Table I
Number of engrafted cells and
apoptotic cells in the C- and D-groups.
| | C-group | D-group |
|---|
| | Case | | Case | |
|---|
| Cells | 1 | 2 | 3 | 4 | 5 | 6 | Mean | 1 | 2 | 3 | 4 | 5 | 6 | Mean |
|---|
| Engrafted
cells | 264 | 332 | 1,016 | 560 | 473 | 174 | 470 | 847 | 404 | 529 | 503 | 332 | 263 | 480 |
| Apoptotic
cells | 0 | 1 | 2 | 2 | 1 | 0 | 1 | 3 | 0 | 0 | 1 | 2 | 0 | 1 |
CASP3, HMOX1, and NOS2 mRNA expression
in the engrafted cells
The gene expression associated with apoptosis
(CASP3) and cellular damage (HMOX1 and NOS2)
was examined to determine how D-allose maintains insulin secretion
in engrafted cells. The expression level of each gene is shown as a
relative ratio to that of the D-group, with the gene expression
level in the C-group set to 1. NOS2 mRNA expression (ratio:
2.06) in the engrafted cells of the D-group tended to be higher
than that of the C-group (P=0.07) (Fig. 4B). CASP3 (ratio: 1.26) and
HMOX1 (ratio: 1.1) mRNA expression was not significantly
different in the engrafted cells between the C- and D-groups.
Discussion
In the present study, we found that intravenous
D-allose administration at the time of islet transplantation
effectively improved type 1 diabetes mellitus in mice. D-allose is
manufactured from D-allulose, which is already in the food supply
as a low-calorie sweetener. Although D-allose has not been
confirmed to exist in nature until now, it has recently been found
in umbilical cord blood (20).
Among rare sugars, D-allulose (also known as D-psicose) has a
hypoglycemic effect and is expected to be useful in the prevention
of type 2 diabetes (21,22). D-Allulose has been reported to
dose-dependently suppress blood glucose levels after glucose
loading in the OGTT of diabetic volunteer subjects in clinical
trials (21). In addition, animal
studies have shown that D-allulose was a glucagon-like peptide-1
(GLP-1) releasing substance that limits feeding and hyperglycemia
through the vagus nerve (22). On
the other hand, D-allose is expected to be useful in the treatment
of type 1 diabetes because of its usefulness in maintaining cells
following islet transplantation, as in this study, because of its
antioxidant and anti-inflammatory effects as previously reported
(7,8).
We first examined casual blood glucose levels in
type 1 diabetes mellitus model mice treated with D-allose or
physiological saline. Mice treated with D-allose showed improved
diabetes mellitus status since the casual blood glucose level was
<150 mg/dl, which was defined as the normal blood glucose level
in BALB/c mice (23), while the
casual blood glucose level in untreated mice remained >200 mg/dl
on average. Although pancreatic islet transplantation decreased
blood glucose levels, the intravenous injection of D-allose was
more effective in decreasing blood glucose levels. Moreover, blood
glucose levels remained low for more than 21 days with three rounds
of intravenous D-allose administration at the time of islet
transplantation, suggesting that the D-allose suppressed the
decline in insulin secretion during the initial engraftment
stage.
Further, we found that D-allose significantly
improved diabetes mellitus, based on the results of IPGGT. Changes
in blood glucose levels in mice treated with D-allose were lower
than those in untreated mice. Consequently, the IAUC of glucose in
D-allose-treated mice was significantly lower than that in
untreated mice. This result suggests that insulin secretion was
rapidly induced by glucose absorption in D-allose-treated mice.
In the present study, pancreatic islet cells were
transplanted under the renal capsule in mice, which differs from
the transplantation method used in humans. However, D-allose did
not cause an increase in the number of grafts but rather prevented
a decline in insulin secretion. Notably, no change in the number of
transplanted cells was observed with or without D-allose, and no
cells underwent significant apoptosis, as determined using TUNEL
staining and CASP3 expression. Therefore, the fact that
blood glucose levels were kept low by adding D-allose suggests that
D-allose maintains the insulin secretion ability of the cells,
which may also contribute to the extension of the insulin
withdrawal period after transplantation into the human body. We
previously demonstrated that D-allose treatment of isolated islet
cultures prior to transplantation restored islet function and
increased transplant success rates (14). Although the steps involved in
D-allose treatment were different, the results were consistent in
that no effect on apoptosis was found, but high insulin secretion
ability was observed. Considering the results of this study and
previous experiments, it is expected that insulin secretion can be
further maintained by transplanting islet cells incubated overnight
in a medium containing D-allose, and intravenous injection of
D-allose after transplantation.
Insulin secretion in transplanted cells may be
affected by inflammatory responses to the engrafted cells and
immunosuppressants against autoimmunity. Since it is known that
D-allose has both anti-inflammatory (10,11)
and immunosuppressive effects (8),
intravenous D-allose administration would be a promising treatment
for pancreatic islet transplantation. In clinical practice,
pancreatic islets are transplanted into the liver via the portal
vein in humans. According to a study using positron-emission
tomography combined with computed tomography (24), approximately half of the pancreatic
islets transplanted into the portal vein disappeared immediately
after transplantation due to inflammatory reactions and thrombosis.
Therefore, suppressing the inflammatory response that occurs during
islet transplantation is thought to be key for improving the
outcomes. There are many reports on the anti-inflammatory effects
of D-allose, including its ability to suppress the expression of
cytokines and chemokines involved in inflammation. A previous study
(10) demonstrated that
intravenous D-allose administration has potent neuroprotective
effects against acute cerebral ischemia/reperfusion in a rat model
of transient middle cerebral artery occlusion. Another report
(11) showed that D-allose
administration repressed the levels of TNF-α, nuclear factor kappa
B (NF-κB), IL-1β, and IL-8 in inflammatory responses in a mice
model of ischemia reperfusion injury. In another model of ischemia
reperfusion injury in skin flap, D-allose decreased monocyte
chemoattractant protein-1 (MCP-1), TNF-α, IL-1β, and IL-6 levels in
the injured skin flap. There appears to be a consensus regarding
the anti-inflammatory effect of D-allose, and this effect is
potentially involved in maintaining insulin secretion rather than
in cell transplantation. Regarding immunosuppressants for
autoimmunity during transplantation, changes in clinical protocols
have allowed for the extension of insulin withdrawal periods.
Treatment with sirolimus and tacrolimus, used in the Edmonton
protocol, abolishes beta-cell regeneration, leading to a decrease
in insulin secretion (25). The
CITR protocol with anti-thymocyte globulin and anti-TNFα antibody
has improved islet transplant outcomes (1,5).
Previous reports (8,9) indicated that D-allose has an
immunosuppressive capability without toxic side effects unlike
FK506 (tacrolimus), as D-allose induced a dose-dependent
suppression of segmented neutrophils. In liver transplantation,
prolonged liver allograft survival in rats was achieved by a
combination treatment with FK506 and D-allose than either drug
individually (8). The results of
the present study suggest that D-allose could be useful in
maintaining insulin secretion even in pancreatic islet transplants
because of its potential anti-inflammatory and immunosuppressant
effects.
We investigated gene expression related to cellular
damage to determine how D-allose preserves insulin secretion in
engrafted cells. The HMOX1 expression related to
heme-mediated oxidative stress was also examined. Heme released
from heme proteins amplifies the production of reactive oxygen
species (ROS), which are extremely harmful to the body. HMOX1 is
the rate-limiting enzyme in heme degradation, and protects the body
from heme-mediated oxidative stress (26). The induction of HMOX1
expression in gastrointestinal tissues and cells, including the
pancreas, plays a critical role in cytoprotection and resolving
inflammation (27). However, our
results showed that HMOX1 mRNA expression was not
significantly different between engrafted cells in the C- and
D-groups, indicating that the engrafted cells were probably not
subject to strong oxidative stress. In contrast, NOS2 mRNA
expression in the engrafted cells of the D-group tended to be
higher than that in the C-group. When exposed to inflammatory
cytokines such as IL-1β, β-cells express NOS2, the inducible
isoform of nitric oxide (NO) synthase (iNOS). Although previous
reports (28,29) have shown the inhibitory effects of
NO on insulin secretion, inhibition of NOS decreased insulin
secretion from isolated human pancreas (30) and plasma insulin level in healthy
humans (31). These conflicting
results are reportedly due to the varying concentrations of NO
(32). According to a previous
study (32), NO at tens of
nanomolar concentrations facilitates glucose-induced
Ca2+ concentration in β-cells and insulin secretion in a
cGMP-dependent manner, whereas NO at sub-micromolar concentrations
inhibits them in a cGMP-independent manner. Considering this
mechanism, although no significant differences were observed in our
results, it is thought that the slight increase in NOS2
expression caused by the addition of D-allose affected the
stimulation of insulin secretion. However, the direct function of
D-allose was not well understood, although inhibition of
inflammatory cytokines such as TNF-α, IL-1β, and IL-6, and
inhibition of apoptosis have been reported. Recently, D-allose has
been shown to protect the brain from ischemia reperfusion-induced
apoptosis and inflammation by suppressing Galectin-3 expression and
transcriptional processes that inhibit TLR4 and activate PI3K/AKT
phosphorylation (33). In the
present study, a hypoglycemic effect was observed in the
D-allose-treated group. These results suggest that insulin
secretion was promoted by D-allose, which has an inhibitory effect
on inflammatory cytokines induced by islet transplantation.
Since islet transplantation-induced inflammatory
cytokines suppress insulin secretion (34), D-allose may promote insulin
secretion by suppressing the expression of inflammatory cytokines
through some molecules. In the future, we would like to clarify the
molecules on which D-allose acts directly.
The following limitations exist for this study.
First, as a continuation of the previous study, only male mice were
analyzed in this study. The reason for using males is that males
are more suitable than females in the generation of STZ-induced
type 1 diabetes model mice since testosterone enhances the
pancreatic β-cell toxicity of STZ (35,36).
In the creation of STZ-induced type 1 diabetic mice, 7 of total 60
(11.7%) died of complications due to hyperglycemia within one week.
Although the mortality rate was higher than the 4.38% described in
the previous report (37), it was
possible that the original condition of the mice may have been a
factor. Although males have been used in studies with rare sugars,
it is possible that different sexes and strains may respond
differently. Considering such differences in humans, we would like
to continue our research including females in the future. Secondly,
in the present study, islets were transplanted under the renal
capsule in mice, since the kidney subcapsular site is in close
proximity to abundant renal parenchymal blood flow, and it is less
susceptible to invasion from the surrounding tissues, making it
difficult for the transplanted islets to be washed away or migrate
elsewhere. In addition, we had already established the technique to
transplant islets under the renal capsule as shown in the previous
report (14). However, in humans,
islets are clinically transplanted into the liver via the portal
vein. According to the previous report (38), islet transplantation under the
renal capsule in humans has been reported to have several
drawbacks, including the need for a large number of islets and the
time required for islet engraftment. Recently, clinical trials have
been conducted using induced pluripotent stem cells (iPSCs) and
embryonic stem cells (ESCs) to produce islet sheets for
subcutaneous transplantation into the abdomen, and favorable
results have been reported (39).
It is possible that the effect of D-allose may differ depending on
the difference in transplantation methods and animal species. In
animal experiments, D-allose was administered to healthy mice for a
long period of time, and blood tests showed no adverse effects on
liver or kidney function (40).
Furthermore, it has been reported that long-term oral
administration of D-allose improved the intestinal microflora of
aged mice (41), indicating that
D-allose is safe in animal studies. Although D-allose is considered
to be a promising rare sugar for treatment with islets
transplantation in animal experiments, no clinical trials in humans
have been conducted so far. In the future, it will be necessary to
consider what route and how much of D-allose should be administered
to humans.
Third, in estimating the reason for the glucose
suppression of D-allose in the present results, we did not examine
insulin measurements in the kidney and islet samples. We will take
this into account when we conduct similar experiments in the future
to confirm the effect of D-allose.
In conclusion, we found that intravenous D-allose
administration at the time of islet transplantation effectively
improved hyperglycemia and maintained stable blood glucose levels
in type 1 diabetes mice. Since there was no difference in the
number of engrafted cells or apoptotic cells with or without
intravenous D-allose administration, D-allose was considered to be
effective in maintaining the cellular function of insulin
secretion.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SN, KK, MO, YS and KO conceived and designed the
study. SN, HM and YA performed mouse experiments and collected
data. SN, HS, AK and TK analyzed the collected data. SN, KK, YS and
KO interpreted the results and wrote the manuscript. KK and KO
confirm the authenticity of all the raw data. All authors reviewed
the results. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
All mice were bred and handled according to the
Experimental Animal Research Guide. The present study was approved
by the Animal Care and Use Committee of Kagawa University (approval
nos. 19646 and 19646-1; Kita-gun, Japan).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Barton FB, Rickels MR, Alejandro R, Hering
BJ, Wease S, Naziruddin B, Oberholzer J, Odorico JS, Garfinkel MR,
Lev M, et al: Improvement in outcomes of clinical islet
transplantation: 1999-2010. Diabetes Care. 35:1436–1445.
2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shapiro AM, Lakey JR, Ryan EA, Korbutt GS,
Toth E, Warnock GL, Kneteman NM and Rajotte RV: Islet
transplantation in seven patients with type 1 diabetes mellitus
using a glucocorticoid-free immunosuppressive regimen. N Engl J
Med. 343:230–238. 2000.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shapiro AM, Ricordi C, Hering BJ,
Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD,
Berney T, Brennan DC, et al: International trial of the Edmonton
protocol for islet transplantation. N Engl J Med. 355:1318–1830.
2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ryan EA, Paty BW, Senior PA, Bigam D,
Alfadhli E, Kneteman NM, Lakey JR and Shapiro AM: Five-year
follow-up after clinical islet transplantation. Diabetes.
54:2060–2069. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bellin MD, Barton FB, Heitman A, Harmon
JV, Kandaswamy R, Balamurugan AN, Sutherland DE, Alejandro R and
Hering BJ: Potent induction immunotherapy promotes long-term
insulin independence after islet transplantation in type 1
diabetes. Am J Transplant. 12:1576–1583. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Izumori K: Izumoring: A strategy for
bioproduction of all hexoses. J Biotechnol. 124:717–722.
2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Murata A, Sekiya K, Watanabe Y, Yamaguchi
F, Hatano N, Izumori K and Tokuda M: A novel inhibitory effect of
D-allose on production of reactive oxygen species from neutrophils.
J Biosci Bioeng. 96:89–91. 2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hossain MA, Wakabayashi H, Goda F,
Kobayashi S, Maeba T and Maeta H: Effect of the immunosuppressants
FK506 and D-allose on allogenic orthotopic liver transplantation in
rats. Transplant Proc. 32:2021–2023. 2000.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hossain MA, Izuishi K and Maeta H:
Protective effects of D-allose against ischemia reperfusion injury
of the rat liver. J Hepatobiliary Pancreat Surg. 10:218–225.
2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gao D, Kawai N, Nakamura T, Lu F, Fei Z
and Tamiya T: Anti-inflammatory effect of D-allose in cerebral
ischemia/reperfusion injury in rats. Neurol Med Chir (Tokyo).
53:365–374. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Huang T, Gao D, Hei Y, Zhang X, Chen X and
Fei Z: D-allose protects the blood brain barrier through
PPARγ-mediated anti-inflammatory pathway in the mice model of
ischemia reperfusion injury. Brain Res. 1642:478–486.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Muneuchi G, Hossain A, Yamaguchi F, Ueno
M, Tanaka Y, Suzuki S and Tokuda M: The rare sugar D-allose has a
reducing effect against ischemia-reperfusion injury on the rat
abdominal skin island flap model. J Surg Res. 183:976–981.
2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ju J, Hou R and Zhang P: D-allose
alleviates ischemia/reperfusion (I/R) injury in skin flap via
MKP-1. Mol Med. 26(21)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kashiwagi H, Asano E, Noguchi C, Sui L,
Hossain A, Akamoto S, Okano K, Tokuda M and Suzuki Y: Beneficial
effect of D-allose for isolated islet culture prior to islet
transplantation. J Hepatobiliary Pancreat Sci. 23:37–42.
2016.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
American Veterinary Medical Association
(AVMA): AVMA guidelines for the euthanasia of animals: 2013
edition. AVMA, Schaumburg, Il, 2013. https://www.in.gov/boah/files/AVMA_Euthanasia_Guidelines.pdf.
|
|
16
|
Ueki M, Taie S, Chujo K, Asaga T, Iwanaga
Y and Maekawa N: Inhibitory effect of d-allose on neutrophil
activation after rat renal ischemia/reperfusion. J Biosci Bioeng.
104:304–308. 2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nakamura T, Tanaka S, Hirooka K, Toyoshima
T, Kawai N, Tamiya T, Shiraga F, Tokuda M, Keep RF, Itano T and
Miyamoto O: Anti-oxidative effects of d-allose, a rare sugar, on
ischemia-reperfusion damage following focal cerebral ischemia in
rat. Neurosci Lett. 487:103–106. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wolever TM and Jenkins DJ: The use of the
glycemic index in predicting the blood glucose response to mixed
meals. Am J Clin Nutr. 43:167–172. 1986.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: . Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hashimoto F, Nishiumi S, Miyake O,
Takeichi H, Chitose M, Ohtsubo H, Ishimori S, Ninchoji T, Hashimura
Y, Kaito H, et al: Metabolomics analysis of umbilical cord blood
clarifies changes in saccharides associated with delivery method.
Early Hum Dev. 89:315–320. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hossain A, Yamaguchi F, Matsuo T,
Tsukamoto I, Toyoda Y, Ogawa M, Nagata Y and Tokuda M: Rare sugar
D-allulose: Potential role and therapeutic monitoring in
maintaining obesity and type 2 diabetes mellitus. Pharmacol Ther.
155:49–59. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Iwasaki Y, Sendo M, Dezaki K, Hira T, Sato
T, Nakata M, Goswami C, Aoki R, Arai T, Kumari P, et al: GLP-1
release and vagal afferent activation mediate the beneficial
metabolic and chronotherapeutic effects of D-allulose. Nat Commun.
9(113)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kunjathoor VV, Wilson DL and LeBoeuf RC:
Increased atherosclerosis in streptozotocin-induced diabetic mice.
J Clin Invest. 97:1767–1773. 1996.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Eich T, Eriksson O and Lundgren T: Nordic
Network for Clinical Islet Transplantation. Visualization of early
engraftment in clinical islet transplantation by positron-emission
tomography. N Engl J Med. 356:2754–2755. 2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Nir T, Melton DA and Dor Y: Recovery from
diabetes in mice by beta cell regeneration. J Clin Invest.
117:2553–2561. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Sassa S: Biological implication of heme
metabolism. J Clin Biochem Nutr. 38:138–155. 2006.
|
|
27
|
Chang M, Xue J, Sharma V and Habtezion A:
Protective role of hemeoxygenase-1 in gastrointestinal diseases.
Cell Mol Life Sci. 72:1161–1173. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Broniowska KA, Oleson BJ and Corbett JA:
β-Cell responses to nitric oxide. Vitam Horm. 95:299–322.
2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Panagiotidis G, Akesson B, Rydell EL and
Lundquist I: Influence of nitric oxide synthase inhibition, nitric
oxide and hydroperoxide on insulin release induced by various
secretagogues. Br J Pharmacol. 114:289–296. 1995.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Atiya A, Cohen G, Ignarro L and Brunicardi
FC: Nitric oxide regulates insulin secretion in the isolated
perfused human pancreas via a cholinergic mechanism. Surgery.
120:322–327. 1996.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Coiro V, Volpi R, Capretti L, Speroni G,
Caffarri G and Chiodera P: Involvement of nitric oxide in arginine,
but not glucose, induced insulin secretion in normal men. Clin
Endocrinol (Oxf). 46:115–119. 1997.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kaneko Y, Ishikawa T, Amano S and Nakayama
K: Dual effect of nitric oxide on cytosolic Ca2+ concentration and
insulin secretion in rat pancreatic beta-cells. Am J Physiol Cell
Physiol. 284:C1215–C1222. 2003.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Luo Y, Cheng J, Fu Y, Zhang M, Gou M, Li
J, Li X, Bai J, Zhou Y, Zhang L and Gao D: D-allose inhibits
TLR4/PI3K/AKT signaling to attenuate neuroinflammation and neuronal
apoptosis by inhibiting Gal-3 following ischemic stroke. Biol
Proced Online. 25(30)2023.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Auer VJ, Janas E, Ninichuk V, Eppler E,
Weiss TS, Kirchner S, Otto AM and Stangl MJ: Extracellular factors
and immunosuppressive drugs influencing insulin secretion of murine
islets. Clin Exp Immunol. 170:238–247. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Rossini AA, Williams RM, Appel MC and Like
AA: Sex differences in the multiple-dose streptozotocin model of
diabetes. Endocrinology. 103:1518–1520. 1978.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Paik SG, Michelis MA, Kim YT and Shin S:
Induction of insulin-dependent diabetes by streptozotocin.
Inhibition by estrogens and potentiation by androgens. Diabetes.
31:724–729. 1982.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Deeds MC, Anderson JM, Armstrong AS,
Gastineau DA, Hiddinga HJ, Jahangir A, Eberhardt NL and Kudva YC:
Single dose streptozotocin-induced diabetes: Considerations for
study design in islet transplantation models. Lab Anim. 45:131–140.
2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Jindal RM, Sidner RA, McDaniel HB, Johnson
MS and Fineberg SE: Intraportal vs kidney subcapsular site for
human pancreatic islet transplantation. Transplant Proc.
30:398–399. 1998.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Fujikura J, Anazawa T, Toyoda T, Ito R,
Kimura Y and Yabe D: Toward a cure for diabetes: iPSC and
ESC-derived islet cell transplantation trials. J Diabetes Investig.
22(14366)2024.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Iga Y, Nakamichi K, Shirai Y and Matsuo T:
Acute and sub-chronic toxicity of D-allose in rats. Biosci
Biotechnol Biochem. 74:1476–1478. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Shintani T, Yanai S, Kanasaki A, Tanaka M,
Iida T, Ozawa G, Kunihiro T and Endo S: Long-term D-allose
administration favorably alters the intestinal environment in aged
male mice. J Appl Glycosci. 69:97–102. 2022.PubMed/NCBI View Article : Google Scholar
|