Introduction
For most patients with stage III non-small cell lung
cancer (NSCLC), adding surgical treatment after radiochemotherapy
may not improve prognosis (1,2).
However, recent years have seen encouraging results from studies
incorporating immune checkpoint inhibitors (ICIs) into induction
therapy for resectable lung cancer (3-6),
leading to considerations of introducing this treatment modality to
patients at later stages. Several driver gene mutations [such as
epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase
and proto-oncogene tyrosine-protein kinase] have been proven to be
associated with poor outcomes of treatment with ICIs (7-9).
This may be due to driver genes affecting the expression of
programmed death-ligand 1 (PD-L1) in tumor and immune cells, tumor
mutational burden (TMB) and the tumor infiltration of immune cells
(7). However, studies on the
efficacy of ICIs in patients with rearranged during transfection
(RET) fusion are relatively few. RET fusions are generally thought
to respond poorly to immunotherapy, for reasons similar to other
driver genes (10-12).
The present study reports the case of a patient with stage IIIC
lung adenocarcinoma harboring RET fusions and high expression of
PD-L1. The patient underwent single-port thoracoscopic lobectomy
and systematic lymph node dissection after treatment with
tislelizumab plus chemotherapy, and the results suggested that the
patient achieved major pathological remission. This case highlights
the potential therapeutic value of ICIs in patients with RET
fusion.
Case presentation
A 61-year-old female patient was admitted to the
Yueyang Central Hospital (Yueyang, China) due to blood in sputum
for 1 month. In January, 2023, contrast-enhanced computed
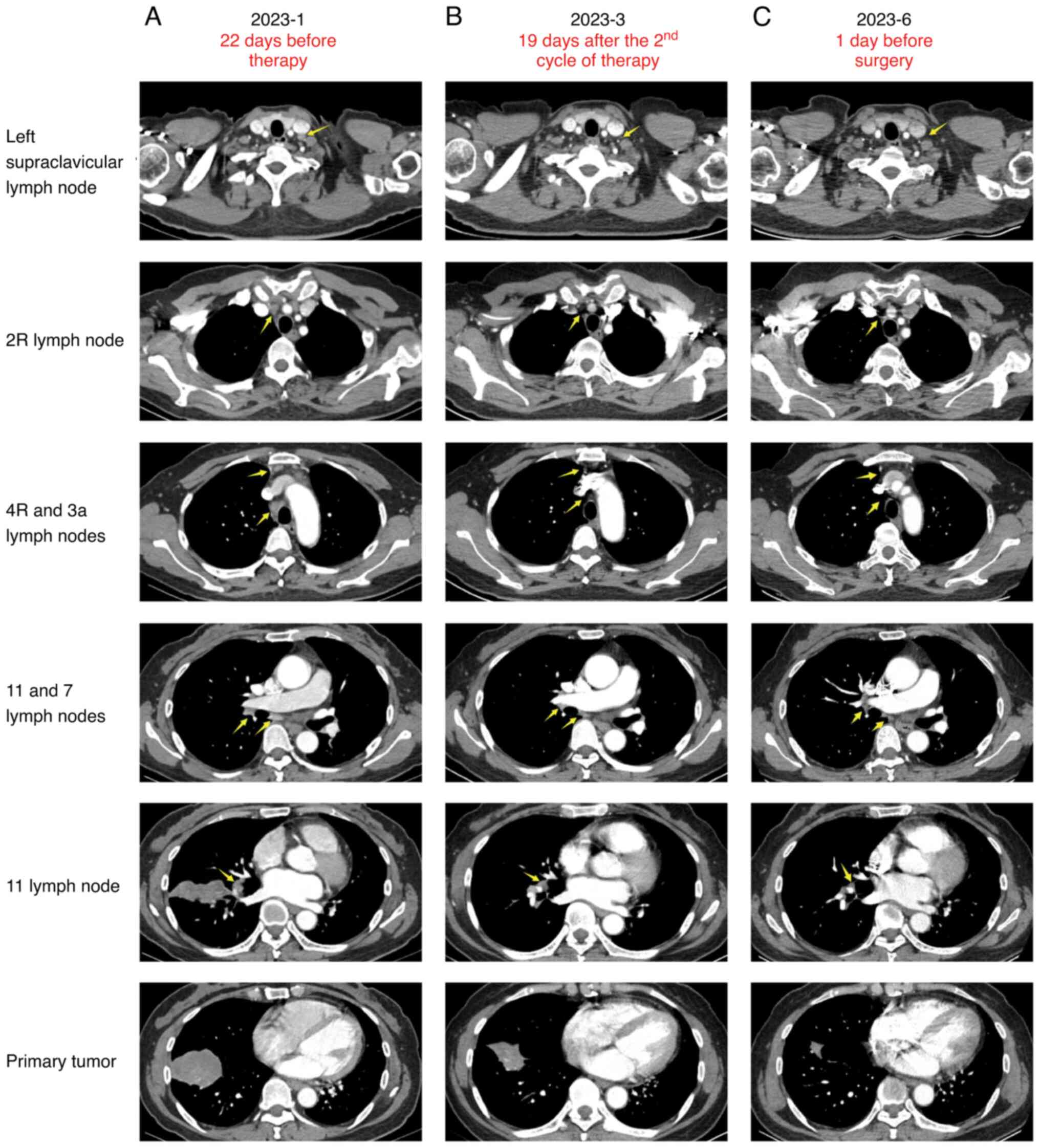
tomography (CT) revealed a 60x41x39 mm mass in the right lower
lung, accompanied by localized atelectasis and multiple enlarged
lymph nodes in the interlobar and mediastinal regions (Fig. 1A). A positron emission tomography
(PET)/CT scan performed 12 days later showed abnormal
18F-fluorodeoxyglucose accumulation in the right lower
lung tumor, with a maximum standardized uptake value (SUVmax) of
19.0, multiple lymph nodes with increased glucose metabolism in the
mediastinum (zones 2R, 4R, 3a and 7), left supraclavicular region
and between the right lung lobes, suggesting metastasis (Fig. 2A). The metabolism of the thoracic
vertebrae was mildly elevated, and no tumors or bone destruction
were observed on the CT scan. It was then diagnosed as degenerative
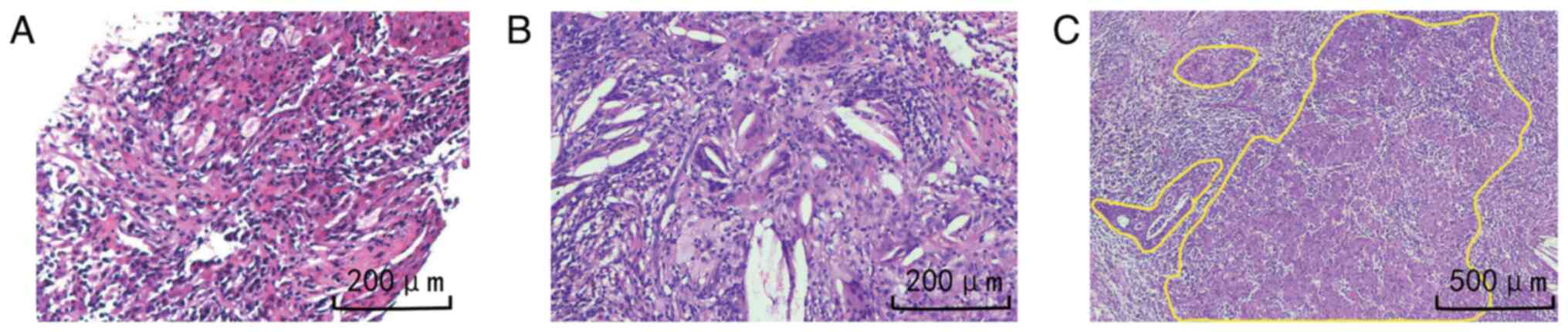
changes of the spine. The pathology report from the percutaneous
lung biopsy indicated poorly differentiated adenocarcinoma
(Fig. 3A). Gene mutation analysis
showed a 10.57% abundance of RET-ABCC2 (R11:A7) gene fusion, 5.36%
EPB41L3-RET (E5'UTR:R13) gene fusion and 4.93% PIK3CA gene mutation
(exon 10 c.1633G>A p.Glu545Lys), while no other mutations were
detected. The genetic testing was performed by Burning Rock Biotech
using their clinically validated LungCore® 9-gene Panel
(targeting EGFR, MET, ERBB2, KRAS, BRAF, PIK3CA, ALK, ROS1 and
RET). Library preparation and hybridization capture were conducted
with Burning Rock's proprietary LungCore® NGS Kit,
followed by sequencing on an Illumina Miseq DX platform. All
experimental procedures strictly adhered to Burning Rock's ISO
15189-certified standard operating protocols, which encompass DNA
extraction, hybridization capture, sequencing and bioinformatics
analysis (13). Experimental
details were described in Burning Rock's clinical test report
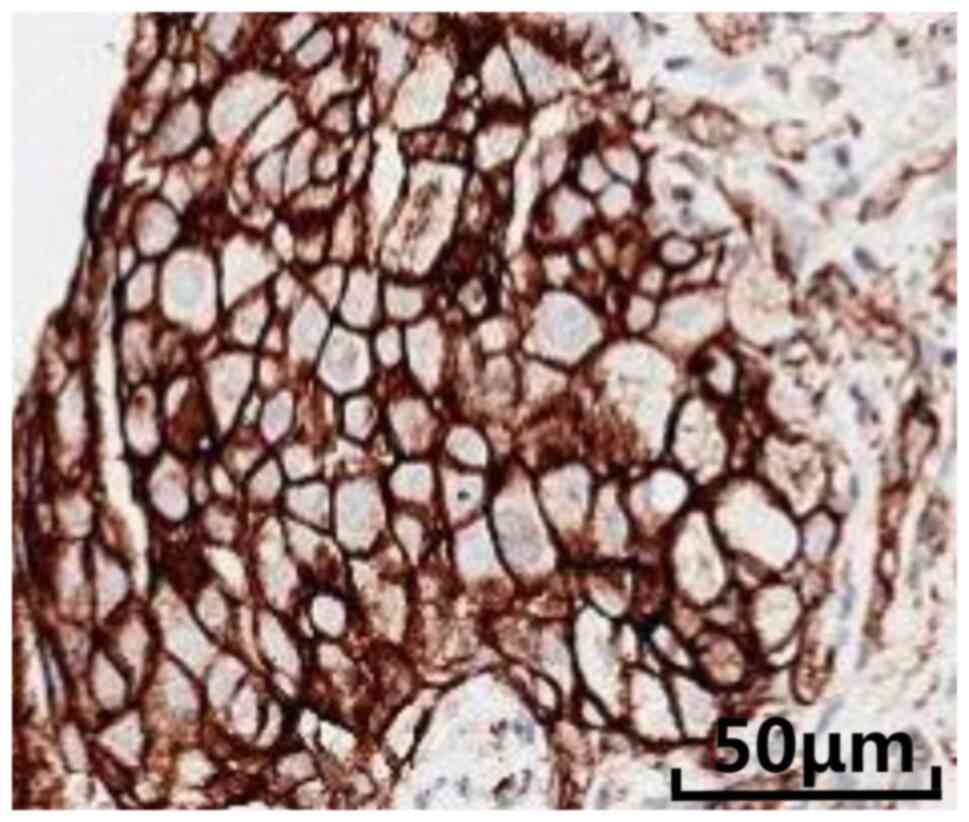
(available upon request). Assessment of PD-L1 expression showed a
tumor cell proportion score of 100% (Fig. 4).
Concurrent chemoradiotherapy followed by
immunotherapy with durvalumab for 1 year is the current standard of
care for patients with unresectable stage III NSCLC (14,15).
However, the patient had a strong desire for surgery and refused
pralsetinib or selpercatinib due to financial reasons. Therefore,
from February 2023 to April 2023, four cycles of chemotherapy with
780 mg of pemetrexed and 110 mg of cisplatin, plus 200 mg of
tislelizumab immunotherapy were administered.
After the second cycle of induction therapy, a CT
with contrast scan showed that the primary tumor had shrunk to
35x22x20 mm. The lymph nodes with increased glucose metabolism in
the left supraclavicular, 2R, 4R and 3a stations significantly
reduced in size. The subcarinal and interlobar lymph nodes had also
shrunk (Fig. 1B). After four
cycles of induction therapy, a PET/CT scan was performed. The
results showed complete metabolic response, with a primary tumor
SUVmax of 3.2, indicating that tumor activity was suppressed
(Fig. 2B). Therefore, after
consulting with specialists (a thoracic surgeon, a respiratory
physician, a medical oncologist, a radiation oncologist, a
pathologist and a palliative care physician) in a multiple
disciplinary team, surgery was scheduled. One day before the
surgery, the patient had another CT with contrast scan. The results
showed that the primary tumor focus had shrunk to 21x16x12mm. The
lymph nodes with increased glucose metabolism in the left
supraclavicular and 3a groups were no longer visible on the CT scan
(Fig. 1C). On the 48th day after
the end of induction therapy, the patient underwent right lower
lobectomy and systematic lymph node dissection under general
anesthesia with single-port thoracoscopy. The surgery successfully
disconnected the right lower pulmonary artery, vein and bronchus,
and performed en bloc resection of the mediastinal lymph
nodes at stations 2, 4, 7 and 9 outside the membrane. The
pathological examination revealed a tumor focus of 20x20x10 mm (the
length and width of the maximum cross-sectional area and the
corresponding vertical height on the gross specimen). The
hematoxylin and eosin (H&E) staining was performed according to
standard histopathological protocols. In brief, formalin-fixed,
paraffin-embedded tissue sections (4-µm thickness) were
deparaffinized in xylene, rehydrated through a graded ethanol
series (100, 95, 70%) and rinsed in distilled water. Sections were
stained with Harris' hematoxylin for 5 min, followed by
differentiation in 1% acid ethanol (1% HCl in 70% ethanol) and
bluing in 0.2% ammonia water. Counterstaining was performed with
eosin Y for 1 min. Finally, sections were dehydrated through graded
ethanols, cleared in xylene and mounted with a neutral resin-based
mounting medium. After total excision of the tumor focus, a
therapeutic response assessment was performed. Under the
microscope, the examination primarily showed proliferative and
hyalinized fibrous tissue with considerable infiltration of
inflammatory cells, cholesterol crystals and multinucleated giant
cells (Fig. 3B). Only one slide
(out of four tumor bed slides, each ~15x15 mm) showed a 2-mm
diameter cancer tissue with some tumor cell degeneration (Fig. 3C). The remaining tumor cells were
~0.5%, indicating that pathological remission of major pathological
response (MPR) had been achieved. No tumor residue was found in any
of the lymph nodes sent for inspection, suggesting the patient had
achieved pathological staging ypT1aN0M0. The patient was discharged
on the sixth day after surgery.
Starting from 21 days after the surgery, the patient
received 200 mg of intravenous tislelizumab treatment every 21
days. Before and after the treatment, the oncologist conducted a
medical history inquiry and physical examination of the patient.
The patient underwent a CT scan of the chest and upper abdomen with
contrast every 3 months after the surgery. Tumor markers in the
blood were also reviewed every 3 months, including carcinoembryonic
antigen, carbohydrate antigen (CA)-125, CA-50, CA-199,
neuron-specific enolase, squamous cell carcinoma antigen and
cytokeratin 19 fragment. As of the submission of the article, no
tumor recurrence was found and the markers were normal.
Discussion
While most studies on neoadjuvant ICI therapy focus
on patients with resectable NSCLC, the use of it as induction
therapy in advanced-stage NSCLC is controversial. Deng et al
(16) reported 51 cases of
unresectable stage III B NSCLC that underwent immunotherapy
combined with chemotherapy, 31 of which then underwent minimally
invasive surgical resection. During the follow-up period, the
disease-free survival/progression-free survival (PFS) of these 31
surgical patients was significantly longer than that of the
remaining 20 patients who did not undergo surgery. Zheng et
al (17) also analyzed 59
patients with initial unresectable stage IIIB NSCLC who received
induction pembrolizumab combined with chemotherapy and obtained
similar results. The RATIONALE-315 study (18) showed that tislelizumab plus
platinum-doublet chemotherapy could achieve a 56.2% MPR rate and a
40.7% pathological complete response (pCR) rate for patients with
resectable stage II-IIIA NSCLC. It is esteemed that this treatment
modality could also improve the survival of patients with advanced
NSCLC.
Concurrent chemoradiotherapy has been the
cornerstone of treatment of unresectable, locally advanced NSCLC
and the PACIFIC study (NCT02125461) (19) established the foundation for
consolidative immunotherapy after concurrent chemoradiotherapy to
become the standard treatment for unresectable locally advanced
NSCLC, as it reported a median overall survival (OS) of 47.5 months
and a 5-year OS of 42.9% for the immunomaintenance therapy regimen
after concurrent chemoradiotherapy. However, in the real world,
~55% of stage III lung cancer patients do not meet the inclusion
criteria of the study (20). Of
note, the 5-year OS rate for stage IIIC lung cancer is only ~13%
(21). Historically, the mainstay
of treatment for unresectable locally advanced lung cancer has
evolved from radiotherapy alone, to induction chemotherapy followed
by radiotherapy, to concurrent chemoradiotherapy, and finally to
concurrent chemoradiotherapy followed by immunomaintenance therapy
(22). However, the approach of
local treatment after systemic induction therapy has not been
widely studied. Theoretically, the treatment effect of chemotherapy
plus immunotherapy followed by radiotherapy should not differ
significantly from that of concurrent chemoradiotherapy followed by
immunomaintenance therapy. However, if the tumor shrinks
significantly enough, patients may have the opportunity for radical
surgical resection. According to the Chinese Society of Clinical
Oncology guidelines (15), for
unresectable stage III lung cancer, systemic induction therapy may
be chosen after multiple disciplinary team discussion. If radical
resection is deemed feasible, surgery can then be performed. This
approach is classified as a category 2 recommendation. Due to the
patient's strong resistance to radiotherapy and the patient's
strong desire for the opportunity to undergo radical surgical
resection, the team decided to give the patient the chance.
However, the application of immunotherapy in
patients exhibiting driver gene positivity remains another subject
of debate. The AEGEAN trial (23)
showed promising therapeutic effects of neoadjuvant durvalumab with
chemotherapy. However, a subgroup analysis of 51 EGFR
mutation-positive patients, who were included before the protocol
revision, did not provide substantial evidence of clinical benefit
with durvalumab as compared to placebo (24). The KEYNOTE-671 trial (25), which included 33 patients with EGFR
mutations, indicated an improvement in event-free survival in the
pembrolizumab group. However, the limited sample size of the study
necessitates further investigation. A phase 2 clinical trial
(CTONG2104) reported by Zhang et al (26) involved 18 patients with EGFR
mutation who underwent neoadjuvant sintilimab with chemotherapy and
subsequent surgery. The MPR rate was 44%, while the pCR rate was
zero. Despite the less-than-optimal pCR rate, the MPR results
appeared favorable when compared with previous results from
preoperative targeted treatment [in the NEOS study (27), in which the patients were treated
with osimertinib 80 mg orally once per day for six weeks, followed
by surgical resection, the MPR rate was 10.7% (3/28) and the pCR
rate was 3.6% (1/28)].
RET fusion accounts for only 1-2% of lung
adenocarcinoma cases (28-30).
Although selective RET inhibitors, such as selpercatinib and
pralsetinib, significantly improved the prognosis for patients with
advanced lung cancer with RET fusions (31-33),
the potential benefit of ICIs in individuals with RET fusions
remains elusive, largely because these patients are usually
excluded from clinical trials or specifically analyzed.
Small-sample retrospective studies suggested that patients with RET
fusions may not benefit from ICIs (10-12).
One possible cause is that RET fusions are closely related to low
PD-L1 expression and low TMB (10). Previous studies have indicated that
ICIs may be more effective for patients with high PD-L1 levels and
high TMB (34,35). In the study by Lu et al
(36), out of 20 patients who
underwent PD-L1 testing, only 5 (25%) had PD-L1 expression with a
tumor cell proportion score >50%. In the study by Offin et
al (37), only 5 out of 26
patients (19%) had PD-L1 expression >50%. In the study by Yan
et al (10), only 22 out of
129 patients (17.8%) had PD-L1 expression >50%. Among the 28
patients who underwent ICI treatment, only 7 (25%) had PD-L1
expression >50%, and 15 (53%) had PD-L1 expression ≥1%. Despite
the small number of patients, those with PD-L1 expression ≥1%
showed a trend towards prolonged PFS compared to those with PD-L1
expression <1%. In this present case, the expression of PD-L1
was very high. This may be a significant reason for the
effectiveness of ICI. The patient was not tested for the TMB for
financial reasons. Since a high TMB is more likely to occur in
smoking patients (38,39), it may be suspected that this factor
has little effect in this case. In addition, mutations in driver
genes may alter the tumor immune microenvironment, thereby
affecting the efficacy of immunotherapy (8). However, contrasting findings have
emerged from a recent study suggesting that RET mutations may
actually offer a positive predictive response to ICI therapy
(40), proposing that RET fusion
and RET point mutations may trigger distinct molecular pathways in
cancer. RET fusion lung cancers may also be heterogeneous in terms
of concomitant genetic alterations. Lu et al (36) reported 2 cases of KIF5B-RET fusion
with high levels of PD-L1 expression, which showed good clinical
benefits from ICIs. The two patients received pembrolizumab and
durvalumab, respectively. In the present case with very high PD-L1
expression, tislelizumab combined with chemotherapy achieved
remarkable results. To the best of our knowledge, the present study
is the first report of the successful treatment of a patient with
advanced lung adenocarcinoma and RET fusion using tislelizumab with
chemotherapy, who was initially not eligible for surgery, then
became eligible and was subjected to surgery.
Currently, there is a lack of research focusing on
the impact of PIK3CA mutation on the effectiveness of immunotherapy
for lung cancer. PIK3CA mutation is significantly associated with
poor survival (41) and may result
in resistance to EGFR-tyrosine kinase inhibitors in patients with
NSCLC (42). PIK3CA mutations may
also promote tumor lymph node metastasis (43).
The present study presented a case of metastatic RET
fusion NSCLC treated with induction tislelizumab with chemotherapy,
leading to an MPR. However, the potential benefits of this
treatment modality in broader patient groups remain unclear due to
the relatively brief mid-term follow-up in the present case. To
evaluate the safety and effectiveness of this novel treatment
strategy, further exploration through extensive clinical trials is
necessary. Considering the low incidence rate of RET fusions in
patients with lung cancer, more retrospective data are needed to
demonstrate the value of immunotherapy in these patients. For
patients with initially unresectable locally advanced lung cancer,
it is esteemed that prospective randomized controlled trials will
determine whether surgery is more effective than radiotherapy when
systemic therapy can downstage the disease.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by Yueyang Central Hospital
(grant no. YYSZXYN2023005).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
DHT and TC conceived the study. RQ extracted and
organized the original data. DHT wrote the main part of the
original manuscript, and FW and QZ wrote a literature review of the
progress in the discussion section. QL analyzed and interpreted the
patient's imaging results, and LH interpreted the patient's
pathology results. DHT and TC confirm the authenticity of all the
raw data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The research protocol was approved by the Ethics
Committee of Yueyang Central Hospital (Yueyang, China; approval no.
2024-054), and the patient provided written consent for the plan.
This study was also conducted in accordance with the Declaration of
Helsinki.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of the present case report and the
accompanying associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Albain KS, Swann RS, Rusch VW, Turrisi AT
III, Shepherd FA, Smith C, Chen Y, Livingston RB, Feins RH, Gandara
DR, et al: Radiotherapy plus chemotherapy with or without surgical
resection for stage III non-small-cell lung cancer: A phase III
randomised controlled trial. Lancet. 374:379–386. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Eberhardt WE, Pöttgen C, Gauler TC,
Friedel G, Veit S, Heinrich V, Welter S, Budach W, Spengler W,
Kimmich M, et al: Phase III study of surgery versus definitive
concurrent chemoradiotherapy boost in patients with resectable
stage IIIA(N2) and selected IIIB non-small-cell lung cancer after
induction chemotherapy and concurrent chemoradiotherapy (ESPATUE).
J Clin Oncol. 33:4194–4201. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Provencio M, Nadal E, Insa A,
García-Campelo MR, Casal-Rubio J, Dómine M, Majem M,
Rodríguez-Abreu D, Martínez-Martí A, De Castro Carpeño J, et al:
Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell
lung cancer (NADIM): An open-label, multicentre, single-arm, phase
2 trial. Lancet Oncol. 21:1413–1422. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Provencio M, Serna-Blasco R, Nadal E, Insa
A, García-Campelo MR, Casal Rubio J, Dómine M, Majem M,
Rodríguez-Abreu D, Martínez-Martí A, et al: Overall survival and
biomarker analysis of neoadjuvant nivolumab plus chemotherapy in
operable stage IIIA non-small-cell lung cancer (NADIM phase II
trial). J Clin Oncol. 40:2924–2933. 2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Forde PM, Spicer J, Lu S, Provencio M,
Mitsudomi T, Awad MM, Felip E, Broderick SR, Brahmer JR, Swanson
SJ, et al: Neoadjuvant nivolumab plus chemotherapy in resectable
lung cancer. N Engl J Med. 386:1973–1985. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Provencio M, Nadal E, González-Larriba JL,
Martínez-Martí A, Bernabé R, Bosch-Barrera J, Casal-Rubio J, Calvo
V, Insa A, Ponce S, et al: Perioperative nivolumab and chemotherapy
in stage III non-small-cell lung cancer. N Engl J Med. 389:504–513.
2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dantoing E, Piton N, Salaün M, Thiberville
L and Guisier F: Anti-PD1/PD-L1 immunotherapy for non-small cell
lung cancer with actionable oncogenic driver mutations. Int J Mol
Sci. 22(6288)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Seegobin K, Majeed U, Wiest N, Manochakian
R, Lou Y and Zhao Y: Immunotherapy in non-small cell lung cancer
with actionable mutations other than EGFR. Front Oncol.
11(750657)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Grant MJ, Herbst RS and Goldberg SB:
Selecting the optimal immunotherapy regimen in driver-negative
metastatic NSCLC. Nat Rev Clin Oncol. 18:625–644. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yan N, Zhang H, Shen S, Guo S and Li X:
Response to immune checkpoint inhibitor combination therapy in
metastatic RET-mutated lung cancer from real-world retrospective
data. BMC Cancer. 24(178)2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bhandari NR, Hess LM, Han Y, Zhu YE and
Sireci AN: Efficacy of immune checkpoint inhibitor therapy in
patients with RET fusion-positive non-small-cell lung cancer.
Immunotherapy. 13:893–904. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Akers KG, Oskar S, Zhao B, Frederickson AM
and Arunachalam A: Clinical outcomes of PD-1/PD-L1 inhibitors among
patients with advanced or metastatic non-small cell lung cancer
with BRAF, ERBB2/HER2, MET, or RET alterations: A systematic
literature review. J Immunother. 47:128–138. 2024.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Burning Rock Dx. LungCore®
9-gene Panel Overview. Available online: https://www.brbiotech.com/service/c4 (Accessed on
2025-1-31).
|
|
14
|
Ettinger DS, Wood DE and Aisner DL: NCCN
guidelines version 2.2024 non-small cell lung cancer. National
Comprehensive Cancer Network, Plymouth Meeting, PA, 2024.
|
|
15
|
Chinese Society of Clinical Oncology
Guidelines Working Committee: Chinese Society of Clinical Oncology
(CSCO) guidelines for the diagnosis and treatment of non-small cell
lung cancer, 2024 edition. People's Medical Publishing House,
Beijing, p14, 2024.
|
|
16
|
Deng H, Liu J, Cai X, Chen J, Rocco G,
Petersen RH, Brunelli A, Ng CSH, D'Amico TA, Liang W and He J:
Radical minimally invasive surgery after immuno-chemotherapy in
initially-unresectable stage IIIB non-small cell lung cancer. Ann
Surg. 275:e600–e602. 2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zheng J, Li Y, Jin C, Ruan K, Sun K, Chen
H, Wang M, Zhang S and Zhou J and Zhou J: Efficacy and surgical
safety of sequential surgical resection after pembrolizumab plus
chemotherapy for initial unresectable stage IIIB non-small cell
lung cancer. Lung Cancer. 184(107326)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yue D, Wang W, Liu H, Chen Q, Chen C,
Zhang J, Bai F and Wang C: LBA58 Pathological response to
neoadjuvant tislelizumab (TIS) plus platinum-doublet (PtDb)
chemotherapy (CT) in resectable stage II-IIIA NSCLC patients (pts)
in the phase III (Ph3) RATIONALE-315 trial. Ann Oncol. 34 (Suppl
2)(S1299)2023.
|
|
19
|
Spigel DR, Faivre-Finn C, Gray JE, Vicente
D, Planchard D, Paz-Ares L, Vansteenkiste JF, Garassino MC, Hui R,
Quantin X, et al: Five-year survival outcomes from the PACIFIC
trial: Durvalumab after chemoradiotherapy in stage III
non-small-cell lung cancer. J Clin Oncol. 40:1301–1311.
2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jung HA, Noh JM, Sun JM, Lee SH, Ahn JS,
Ahn MJ, Pyo H, Ahn YC and Park K: Real world data of durvalumab
consolidation after chemoradiotherapy in stage III non-small-cell
lung cancer. Lung Cancer. 146:23–29. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mahul BA, Stephen BE, Frederick LG, Byrd
DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess
KR, Sullivan DC, et al: AJCC cancer staging manual, 8th edition.
Springer, New York, NY, p450, 2010.
|
|
22
|
Puri S, Saltos A, Perez B, Le X and Gray
JE: Locally advanced, unresectable non-small cell lung cancer. Curr
Oncol Rep. 22(31)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Heymach JV, Harpole D, Mitsudomi T, Taube
JM, Galffy G, Hochmair M, Winder T, Zukov R, Garbaos G, Gao S, et
al: Perioperative durvalumab for resectable non-small-cell lung
cancer. N Engl J Med. 389:1672–1684. 2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
He J, Gao S, Reck M, Harpole D, Mitsudomi
T, Taube JM, Lee KY, Horio Y, Runglodvatana Y, Aperghis M, et al:
OA12.06 Neoadjuvant durvalumab + chemotherapy followed by adjuvant
durvalumab in resectable EGFR-mutated NSCLC (AEGEAN). J Thorac
Oncol. 18 (11 Suppl):S72–S73. 2023.
|
|
25
|
Wakelee H, Liberman M, Kato T, Tsuboi M,
Lee SH, Gao S, Chen KN, Dooms C, Majem M, Eigendorff E, et al:
Perioperative pembrolizumab for early-stage non-small-cell lung
cancer. N Engl J Med. 389:491–503. 2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang C, Sun YX, Yi DC, Jiang BY, Yan LX,
Liu ZD, Peng LS, Zhang WJ, Sun H, Chen ZY, et al: Neoadjuvant
sintilimab plus platinum-based chemotherapy in EGFR-mutant NSCLC:
An updated analysis of a phase 2 prospective trial
(NEOTIDE/CTONG2104). Cell Rep Med. 5(101615)2024.
|
|
27
|
Lv C, Fang W, Wu N, Jiao W, Xu S, Ma H,
Wang J, Wang R, Ji C, Li S, et al: Osimertinib as neoadjuvant
therapy in patients with EGFR-mutant resectable stage II-IIIB lung
adenocarcinoma (NEOS): A multicenter, single-arm, open-label phase
2b trial. Lung Cancer. 178:151–156. 2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ferrara R, Auger N, Auclin E and Besse B:
Clinical and translational implications of RET rearrangements in
non-small cell lung cancer. J Thorac Oncol. 13:27–45.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Adashek JJ, Desai AP, Andreev-Drakhlin AY,
Roszik J, Cote GJ and Subbiah V: Hallmarks of RET and co-occuring
genomic alterations in RET-aberrant cancers. Mol Cancer Ther.
20:1769–1776. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Osta BE and Ramalingam SS: RET fusion:
Joining the ranks of targetable molecular drivers in NSCLC. JTO
Clin Res Rep. 1(100050)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gainor JF, Curigliano G, Kim DW, Lee DH,
Besse B, Baik CS, Doebele RC, Cassier PA, Lopes G, Tan DSW, et al:
Pralsetinib for RET fusion-positive non-small-cell lung cancer
(ARROW): A multi-cohort, open-label, phase 1/2 study. Lancet Oncol.
22:959–969. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Drilon A, Oxnard GR, Tan DSW, Loong HHF,
Johnson M, Gainor J, McCoach CE, Gautschi O, Besse B, Cho BC, et
al: Efficacy of selpercatinib in RET fusion-positive non-small-cell
lung cancer. N Engl J Med. 383:813–824. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhou C, Solomon B, Loong HH, Park K, Pérol
M, Arriola E, Novello S, Han B, Zhou J, Ardizzoni A, et al:
First-line selpercatinib or chemotherapy and pembrolizumab in RET
fusion-positive NSCLC. N Engl J Med. 389:1839–1850. 2023.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833.
2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Marabelle A, Fakih M, Lopez J, Shah M,
Shapira-Frommer R, Nakagawa K, Chung HC, Kindler HL, Lopez-Martin
JA, Miller WH Jr, et al: Association of tumour mutational burden
with outcomes in patients with advanced solid tumours treated with
pembrolizumab: Prospective biomarker analysis of the multicohort,
open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 21:1353–1365.
2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lu C, Dong XR, Zhao J, Zhang XC, Chen HJ,
Zhou Q, Tu HY, Ai XH, Chen XF, An GL, et al: Association of genetic
and immuno-characteristics with clinical outcomes in patients with
RET-rearranged non-small cell lung cancer: A retrospective
multicenter study. J Hematol Oncol. 13(37)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Offin M, Guo R, Wu SL, Sabari J, Land JD,
Ni A, Montecalvo J, Halpenny DF, Buie LW, Pak T, et al:
Immunophenotype and response to immunotherapy of RET-rearranged
lung cancers. JCO Precis Oncol. 3(PO.18.00386)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Nagahashi M, Sato S, Yuza K, Shimada Y,
Ichikawa H, Watanabe S, Takada K, Okamoto T, Okuda S, Lyle S, et
al: Common driver mutations and smoking history affect tumor
mutation burden in lung adenocarcinoma. J Surg Res. 230:181–185.
2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang X, Ricciuti B, Nguyen T, Li X, Rabin
MS, Awad MM, Lin X, Johnson BE and Christiani DC: Association
between smoking history and tumor mutation burden in advanced
non-small cell lung cancer. Cancer Res. 81:2566–2573.
2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Long JY, Li RZ, Wang DX, Liu H, Tian J,
Ding ZN, Yan LJ, Dong ZR, Hong JG, Tian BW, et al: Comprehensive
molecular analysis identifies RET alterations association with
response of ICIs in multi-immunotherapy cohorts. Int
Immunopharmacol. 126(111281)2024.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wang Y, Wang Y, Li J, Li J and Che G:
Clinical significance of PIK3CA gene in non-small-cell lung cancer:
A systematic review and meta-analysis. Biomed Res Int.
2020(3608241)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chen JY, Cheng YN, Han L, Wei F, Yu WW,
Zhang XW, Cao S and Yu JP: Predictive value of K-ras and PIK3CA in
non-small cell lung cancer patients treated with EGFR-TKIs: A
systemic review and meta-analysis. Cancer Biol Med. 12:126–139.
2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Saal LH, Holm K, Maurer M, Memeo L, Su T,
Wang X, Yu JS, Malmström PO, Mansukhani M, Enoksson J, et al:
PIK3CA mutations correlate with hormone receptors, node metastasis,
and ERBB2, and are mutually exclusive with PTEN loss in human
breast carcinoma. Cancer Res. 65:2554–2559. 2005.PubMed/NCBI View Article : Google Scholar
|