Introduction
Osteosarcoma is one of the most common malignant
neoplasms of bone with rapid progression and poor prognosis. It is
prevalent in children and adolescents. Common symptoms include pain
at affected extremities, joint pain and a palpable mass upon
examination. The current standard of care for osteosarcoma commonly
involves surgery to remove the tumor and chemotherapy for
metastatic disease. Combination of surgery with neoadjuvant and
postoperative chemotherapy has revealed a 5 year survival rate of
68% in patients with primary osteosarcoma (1). Despite the success of combined
treatments in primary osteosarcoma, the patients with pulmonary
metastasis have poor prognosis with a 5 year survival of 33%
(2,3). The development of treatments for
osteosarcoma has been limited due to failures in optimizing
existing treatments and unsuccessful discovery of new effective
agents for last decades. Novel therapeutic strategies are required
to fulfill the urgent clinical needs. Treatments harnessing immune
system have gained attention for osteosarcoma owing to cellular
signature and immune-features of osteosarcoma tumor (4). It is of interest to explore a
therapeutic modality which combines anticancer agent and cellular
immunotherapy.
Natural killer (NK) cells are a subgroup of large
granular lymphocytes, which account for 5-15% of peripheral
lymphocytes (5). NK cells play
essential roles in immune responses against malignant
transformation and viral clearance. The cells recognize target
cells through a complex set of activating and inhibitory receptors
expressed on NK cell surface (6).
Outcomes of NK cell cytolytic response to target cells depend on
the balance between activation and inhibition signaling (7). NK group 2, member D (NKG2D) protein
is one of NK activating receptors, which is expressed on NK cells,
NK T cells and CD8+ T. NKG2D recognize NKG2D ligands
(NKG2DL) expressed on cancer cells, which include the MHC class I
polypeptide-related proteins A (MICA), MICB and unique long 16
binding proteins 1 to 6 (ULBP1-6). Expression of NKG2DL is
upregulated by stress situations such as viral infection or
cancerous transformation (8,9).
NKG2DL is expressed on a variety of cancer cells, suggesting NKG2DL
to be putative targets for treatment (10). It is of interest to explore the use
of potent tumoricidal agents which can increase the expression of
NKG2DLs in a combination with NK cell infusion against
osteosarcoma.
Sesamin is a predominant lignan in sesame seeds,
which has been consumed in Asian countries for hundreds of years.
It has been revealed to exert several pharmaceutical activities
including anti-inflammation and anticancer activities (11). Sesamin has been demonstrated to
inhibit cell proliferation through cell cycle arresting and
apoptosis induction in various cancer cell types (12-15).
Moreover, sesamin enhances anticancer effects of several
chemotherapeutic agents on several malignancies (16,17).
However, there is limited information about effects of sesamin on
osteosarcoma and its use in combination with other therapeutic
modalities.
It was hypothesized that NKG2DLs on osteosarcoma
cells are inducible, leading to improved efficacy of NK cell-based
immunotherapy for osteosarcoma. Modulations of NKG2DL expression
mediated by sesamin in osteosarcoma were evaluated. The
cytotoxicity of NK cells mediated by upregulated NKG2DL expression
was examined.
Materials and methods
Cell culture
Human osteosarcoma cell line MG63 (CRL-1427) and
human NK cell line NK-92 (CRL-2407) were purchased from the
American Type Culture Collection. MG63 cells were cultured and
maintained in Dulbecco's modified Eagle's medium (DMEM; cat. no.
D6429; MilliporeSigma) supplemented with 10% fetal bovine serum
(FBS; cat. no. F8192; MilliporeSigma) and 100 µg/ml
penicillin/streptomycin (cat. no. P4333; MilliporeSigma) within a
humidified atmosphere containing 5% CO2 at 37˚C. Sesamin
(cat. no. SMB00705; MilliporeSigma) was prepared in dimethyl
sulfoxide at a concentration of 50 mM, stored as aliquots at -20˚C,
and diluted with a cell culture medium for use. For treatments with
sesamin, cells were treated with sesamin with serial concentrations
in serum-free DMEM for 24 or 48 h. After treatments, resulting
cells were washed with phosphate-buffered saline (PBS; pH 7.2) for
following analyses. NK-92 cells were cultured and maintained in
complete RPMI-1640 media (cat. no. R8758; MilliporeSigma) (10% FBS,
1% HEPES, 1% L-glutamine, 1% penicillin-streptomycin) and 300 U/ml
recombinant human IL-2 (Proleukin; IOVANCE Biotherapeutics). NK-92
cells were activated by culturing in complete medium containing 500
IU/ml IL-2 and 50 ng/ml IL-15 (R&D Systems, Inc.) for 24 h.
Activated NK-92 cells were harvested for following experiments.
Cell viability assay
Cell viability was evaluated using MTT assay. Cells
(x105) in culture media treated with sesamin were
incubated with
(3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) (0.5
mg/ml) at 37˚C for 4 h. MTT solution was aspirated and the formazan
crystals were dissolved in 200 µl DMSO. Number of viable cells was
directly proportional to the production of formazan determined by
measuring the absorbance at 570 nm.
Cell cycle analyses
After treatment with sesamin, 5x105 of
MG-63 cells were digested by trypsin, washed with PBS, and fixed
with 70% alcohol on ice for 30 min. Cells were subsequently washed
with cold PBS, suspended in 200 µl staining solution (PBS with 1%
BSA, 10 mg/ml propidium iodide, 0.25 mg/ml RNase A) and incubated
at 37˚C in the dark for 30 min. Resulting cells were analyzed using
population of each phase of cell cycle, determined using BD FACS
Canto II with the FlowJo v.10 software package (BD
Biosciences).
Western blotting
After treatments, cells were lysed using lysis
buffer (50 mM Tris-HCl, pH 7.5, 1% Nonidet P-40, 1 mM
phenylmethylsulfonyl fluoride and 1 mM NaF). Cell lysates were
centrifuged at 4˚C, 15,000 x g for 20 min. Supernatants were
collected and assessed for protein concentration using the Bradford
assay (cat. no. B6916; MilliporeSigma). A total of 20 µg of total
protein was subjected to a 12.5% SDS-polyacrylamide gel
electrophoresis followed by transferring onto a nitrocellulose
membrane (MilliporeSigma). The resulting membranes were blocked
with 5% w/v skimmed milk in PBS for 30 min at room temperature and
then incubated for 2 h at room temperature with primary antibodies
at a ratio of 1:1,000, which were against p21 (cat. no. MA5-31479),
cyclin B1 (cat. no. MA1-155), cyclin-dependent kinase 1 (CdK1; cat.
no. MA5-15631) and β-actin (cat. no. MA5-15739; all from
Invitrogen; Thermo Fisher Scientific, Inc.), respectively. Blots
were subsequently incubated with 1:2,000 dilution of horseradish
peroxidase-conjugated secondary antibodies (cat. no. 31430;
Invitrogen; Thermo Fisher Scientific, Inc.) for 1 h at room
temperature and antigen-antibody complexes were displayed using ECL
chemiluminescence (Millipore Sigma). The protein bands were
quantified with the ImageJ software (version 1.54; National
Institutes of Health).
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA of cells after treatment was isolated
using TRIzol® (MilliporeSigma) according to the
manufacturer's protocol. Reverse transcription was performed using
SuperScript IV VILO Master Mix (cat. no. 11756050; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Quantification of mRNA levels of the genes of interest was
performed using the ABI PRISM 7700 sequence detection system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) with
designated primer pairs. FastStart Universal SYBR Green Master
(Roche Applied Science) was used for qPCR. The thermocycling
conditions for qPCR included initial denaturation at 95˚C for 5
min, 40 cycles of amplification (at 95˚C for 30 sec, 58˚C for 30
sec and 72˚C for 30 sec) and at 72˚C for 5 min. The threshold cycle
numbers were calculated using the 2-ΔΔCq relative value
method (18) and normalized to
GAPDH. Pairs of primers used for genes of interest are listed in
Table I. Each qPCR reaction was
repeated at least three times.
 | Table IPrimers used for reverse
transcription-quantitative PCR analysis. |
Table I
Primers used for reverse
transcription-quantitative PCR analysis.
| Gene name | Variant | Primer sequence
(5'-3') |
|---|
| p21 | NM_001374509.1 | F:
GAAGACCATGTGGACCTG |
| | | R:
GCGTTTGGAGTGGTAGA |
| Cyclin B1 | NM_031966.4 | F:
CTTATACTAAGCACCAAATC |
| | | R:
GTTGGCTAAATCTTGAACT |
| CdK1 | NM_001786.5 | F:
GACTAGAAAGTGAAGAGGAAG |
| | | R:
TACTGACCAGGAGGGATAGAA |
| MICA | NM_001177519. | F:
GCCATGAACGTCAGGAATTT |
| | | R:
GACGCCAGCTCAGTGTGATA |
| MICB | NM_005931 | F:
TCTTCGTTACAACCTCATGGTG |
| | | R:
TCCCAGGTCTTAGCTCCCAG |
| ULBP1 | NM_025218.4 | F:
CTTGACATTCAAGTGGAGAAT |
| | | R:
GTCCATTGAAGAGGAACTGCC |
| GAPDH | NM_002046.7 | F:
AATGGAAATCCCATCACCATCT |
| | | R:
CAGCATCGCCCCACTTG |
NK cell elimination activities
A total of 5x103 MG-63 cells were seeded
into a well of the 96-well culture plates with complete DMEM media
and incubated for 24 h, followed by treatments with or without
sesamin for another 48 h. Resulting MG-63 cells were co-cultured
with activated NK-92 cells at a range of effector: target (E:T)
ratios (1:5, 1:3, 1:1, 3:1, 5:1 and 10:1) for 4 h at 37˚C with 5%
CO2. After 4 h of co-culture, NK-92 cells were removed
and MTT assay was carried out to assess viable cells by measuring
the absorbance at 570 nm.
ELISA
Concentrations of IFN-γ secreted by activated NK-92
cells in cultures with MG-63 cells were measured using ELISA
according to manufacturer's protocol. The levels of IFN-γ in the
culture medium was detected using IFN-γ ELISA Kit (R&D systems,
Inc.).
Flow cytometry
A total of 1x106 MG-63 cells were
cultured with different concentrations of sesamin. The expression
of the NKG2DLs was analyzed by immunofluorescence staining using
anti-MICA (1:50; cat. no. MA5-36026; Invitrogen; Thermo Fisher
Scientific, Inc.), anti-MICB (1:50; cat. no. MA5-29422; Invitrogen;
Thermo Fisher Scientific, Inc.), anti-ULBP-1 (1:50; cat. no.
MA5-38655; Invitrogen; Thermo Fisher Scientific, Inc.) and
phycoerythrin. In all experiments, cells were stained at room
temperature for 15 min with propidium iodide (1 µg/µl) to assess
cell viability. Data acquisition and flow cytometric analysis were
carried out on a BD FACSCanto II using the FlowJo v10 software
package (FlowJo LLC).
Statistical analysis
Data are presented as the mean ± SD of the three
independent experiments with conditions set up in three or six
replicates. Comparisons were made by unpaired Student's t-test
between two groups. One-way and two-way ANOVA were performed to
assess the statistical significance of differences in measured
variables between multiple groups with Tukey's post hoc test.
Correlations between the protein expression levels with NK
cell-mediated cytotoxicity were examined using Pearson's
correlation analysis. All statistical analyses were conducted using
SigmaPlot 10 (Systat Software, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
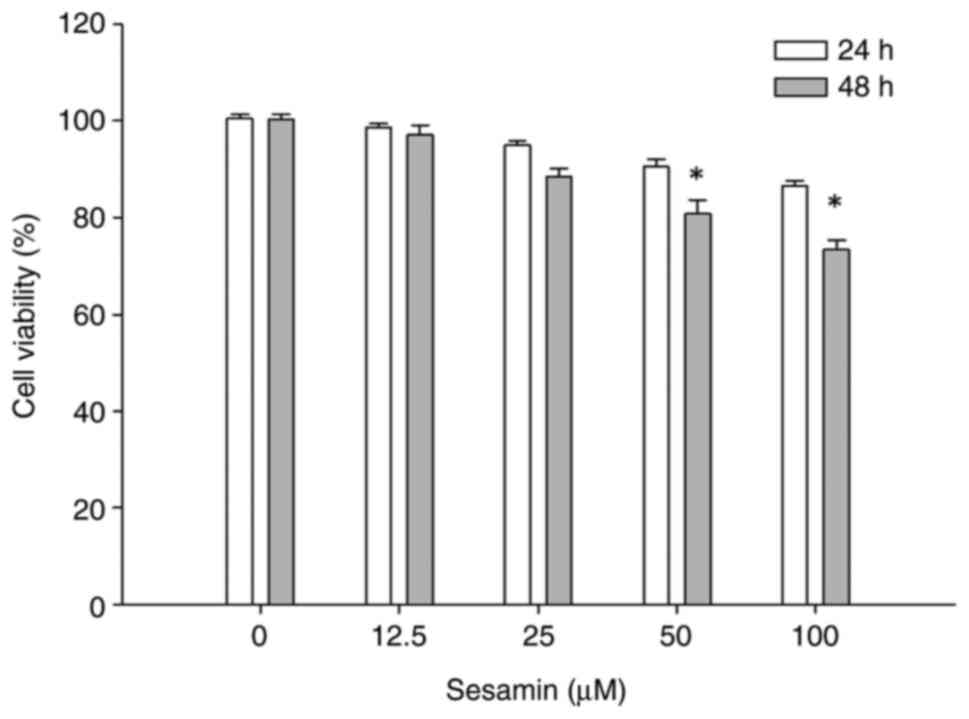
Sesamin reduces cell viability of MG63
osteosarcoma cells
The effect of sesamin on growth of osteosarcoma
cells was determined using MTT assay. MG-63 osteosarcoma cells were
treated at various concentrations of sesamin (0,12.5,25,50 and 100
µM) for 24 h or 48 h and measured for cell viability. No
significant inhibitory effects were observed at 24 h. The cells
responded time-dependently to sesamin treatment within the
concentrations tested. The viability of MG-63 cells was decreased
to 80.7±2.9 and 73.3±1.9% of controls in response to the incubation
with 50 and 100 µM of sesamin for 48 h, respectively (P<0.05)
(Fig. 1). The doses of sesamin
significantly inhibiting viability of MG-63 cells were chosen for
subsequent experiments.
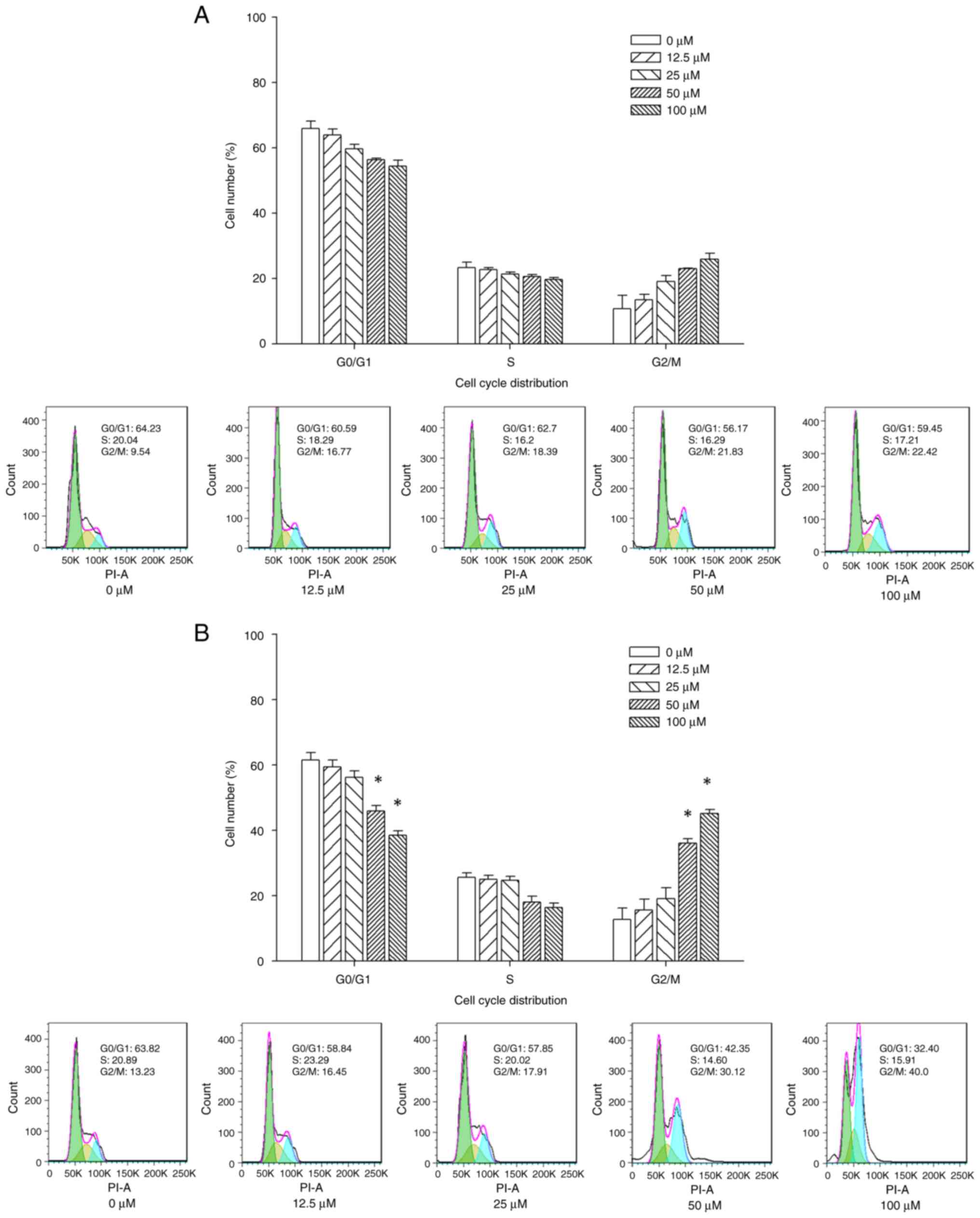
Sesamin induces cell cycle arrest at
G2/M phase in MG-63 osteosarcoma cells
The mechanism underlying sesamin-induced
proliferation inhibition was investigated. Changes in cell cycle
distribution in MG-63 cells treated with sesamin at designated
concentrations were determined using flow cytometry. MG-63 cells
exposed to sesamin at different concentrations for 24 h exhibited
G2/M cell cycle arrest (Fig. 2A). Treatment of MG-63 cells with 0,
12.5, 25, 50 and 100 µM sesamin for 48 h resulted in an increase in
proportion of G2/M phase cells from 12.8±3.5, 15.6±3.4,
19.2±3.2, 36.1±1.4 and 45.2±1.2%, respectively (Fig. 2B). The results revealed that
sesamin induced G2/M cell cycle arrest in a dose- and
time-dependent manner.
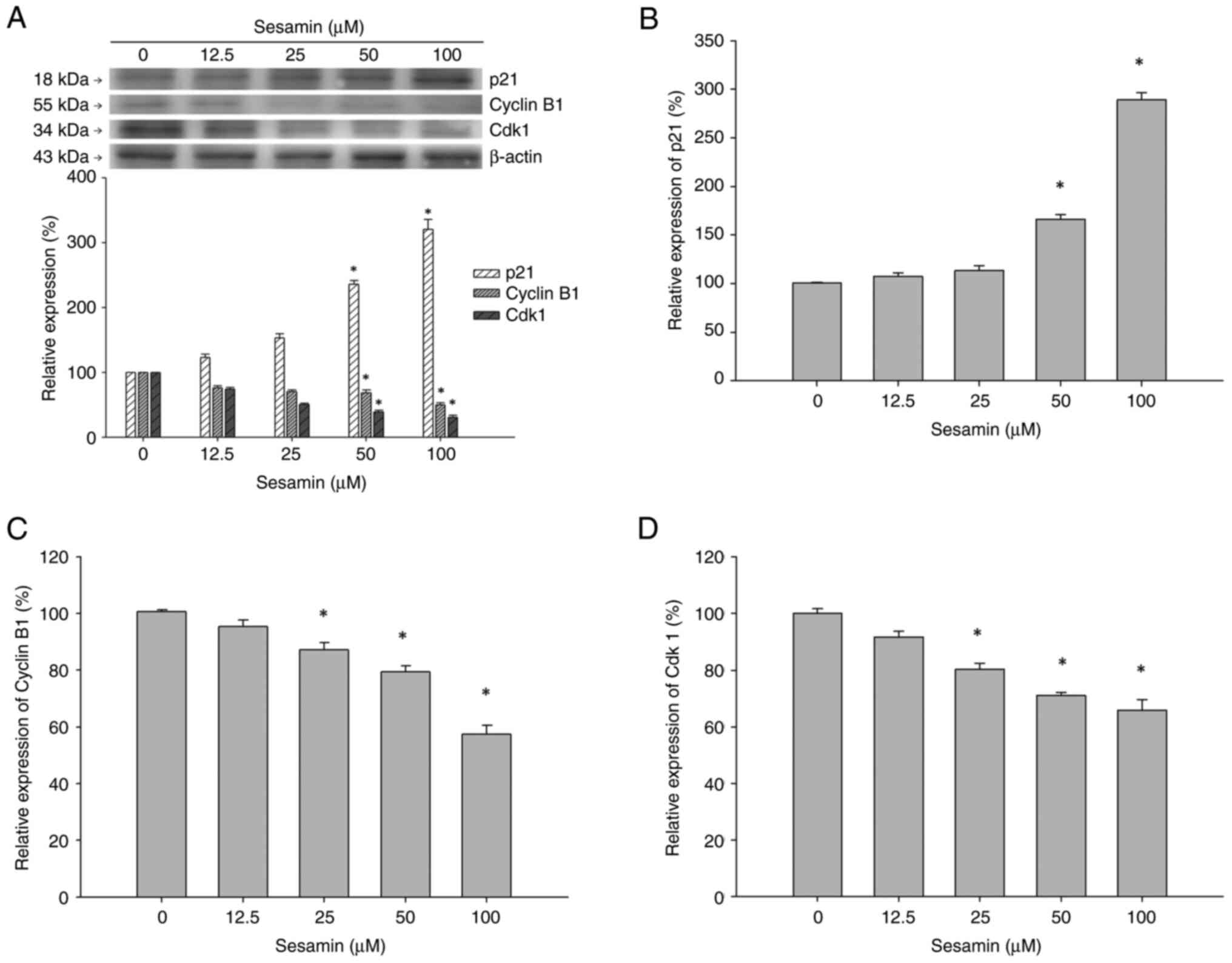
To determine the molecular events involved in
sesamin-induced cell cycle arrest, changes in expression of
proteins involved in G2/M phase including p21, Cdk1 and
cyclin B1 in sesamin-treated MG-63 cells were assessed. The protein
levels of p21, cyclinB1 and Cdk1 were detected using western
blotting in MG-63 cells treated with 0, 12.5, 25, 50 and 100 µM
sesamin for 48 h (Fig. 3A).
Sesamin induced an increase in expression of protein p21, which is
an inhibitor of Cdk/cyclin complex in MG-6 cells. The results
revealed that sesamin inhibited the expression of cyclin B1 and
Cdk1 at 48 h, which are involved in the G2/M transition.
The mRNA levels of p21, Cyclin B1 and Cdk 1 gene in MG-63 cells
treated with sesamin for 48 h were validated using RT-qPCR. The
mRNA expression of p21 was significantly upregulated in MG-63 cells
treated with 50 and 100 µM sesamin (Fig. 3B). The mRNA levels of cyclin B1 and
Cdk1 were significantly reduced in comparison with that of 0 µM
MG-63 cells (Fig. 3C and D). The changes in p21, Cyclin B1 and Cdk
1 mRNA expression were parallel to that of the results of western
blotting.
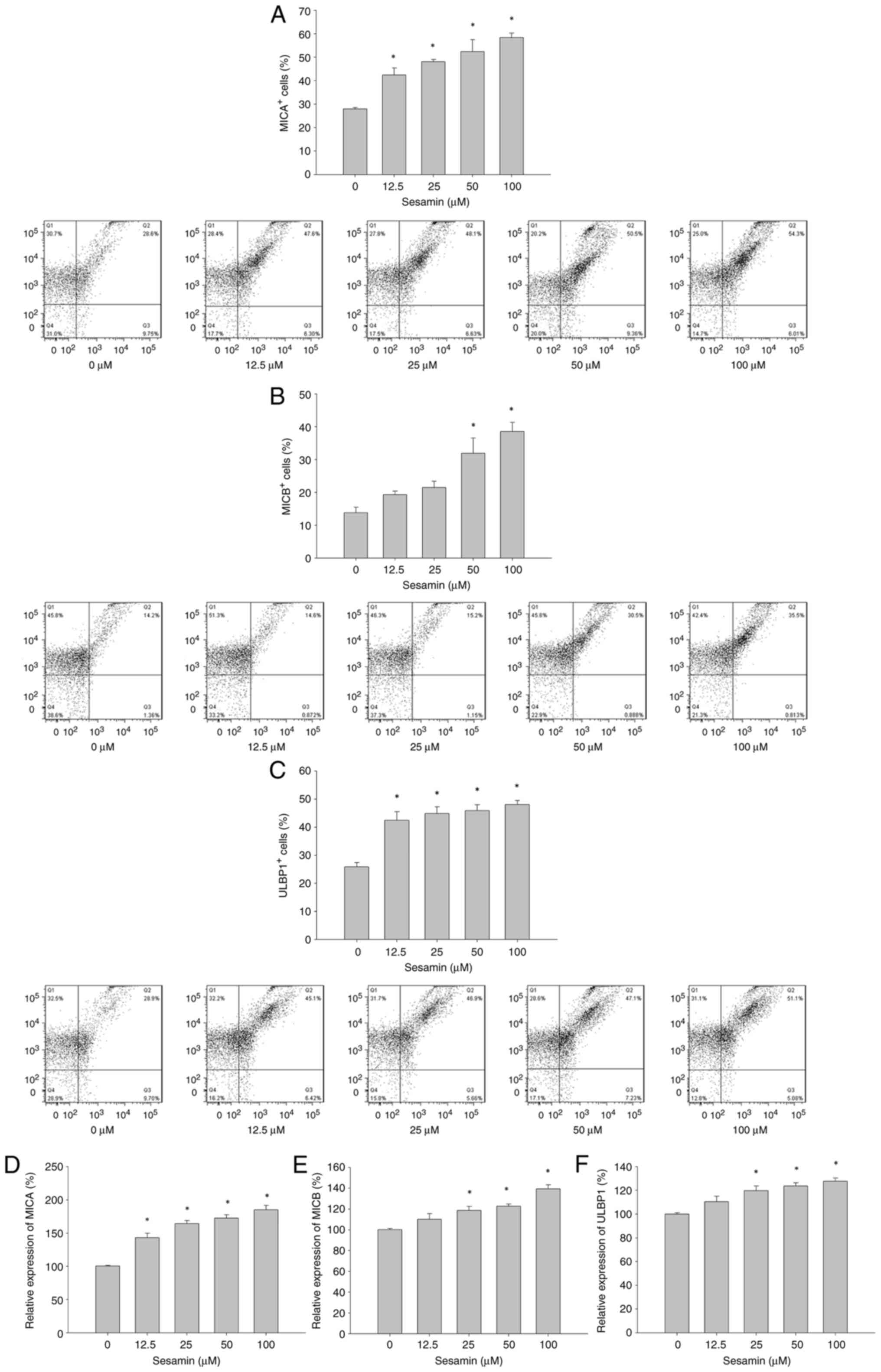
Sesamin increases NKG2D ligand
expression in MG-63 osteosarcoma cells
As increased p21 expression has been revealed in
association with expression of NKG2DLs, it was investigated whether
sesamin has influences on the expression of the ligands for NKG2D
in MG-63 cells. Expression of NKG2DLs in MG-63 cells treated with
or without sesamin were examined by flow cytometry and RT-qPCR.
MG-63 cells constitutively express MICA, MICB and ULBP1 at
different levels (Fig. 4A-C).
Treatment with sesamin for 48 h resulted in increased percentage of
MICA positive MG-63 cells in a dose-dependent manner. The
percentage of MICB positive MG-63 cells was significantly increased
at sesamin concentrations of 50 and 100 µM. Sesamin treatment
increased the percentage of ULBP1 positive MG-63 cells in a
dose-independent manner. Afterwards, the changes in mRNA levels of
MICA, MICB and ULBP1 in MG-63 cells treated sesamin were
investigated. The results of RT-qPCR revealed that mRNA expression
of MICA, MICB and ULBP1 was significantly upregulated in MG-63
cells treated with sesamin for 48 h (Fig. 4D-F).
Sesamin enhances susceptibility of
MG-63 osteosarcoma cells to NK cell-mediated cytotoxicity
Since sesamin upregulated the expression of NKG2DLs
in MG-63 cells, it was next investigated whether sesamin primes
MG-63-cells for NK cell-mediated antitumor activity by increasing
NKG2DL expression. MG-63 cells were treated with sesamin at various
concentration for 48 h and were subsequently subjected to
co-culture with NK-92 cells. NK-92 cells inhibited the viability of
untreated MG-63 cells up to 50% at an E:T ratio of 10:1 (Fig. 5). Treatment with sesamin enhanced
MG-63 cell susceptibility to NK-92 cell elimination, resulting in a
significant decrease in viability of MG-63 cells in a
dose-dependent manner. Data obtained from cytotoxicity assay using
E:T ratios of 1:1, 3:1 or 5:1 were used to calculate
IC50 values, resulting in IC50 of 75.1, 51.1
or 16.8 µM, respectively.
The possible correlation between NKG2DL expression
and NK cell-mediated cytotoxicity in MG-63 osteosarcoma cells was
investigated. The correlation coefficient between MICA and NK
cell-mediated cytotoxicity was significant at E:T ratios of 1:3
(P=0.04) and 1:1 (P=0.04) (Table
II). The correlation between MICB levels in MG-63 cells and NK
cell mediated elimination was significant at all E:T ratios. A
significant correlation was detected between percentage of NKG2DL
positive cells and NK-mediated cytotoxicity. There was no
correlation between ULBP1 and NK-cell mediated killing at all E:T
ratios tested.
 | Table IICorrelation between natural killer
cell-mediated cytotoxicity and sesamin-induced NKG2DL
expression. |
Table II
Correlation between natural killer
cell-mediated cytotoxicity and sesamin-induced NKG2DL
expression.
| Cell viability
(effector: target ratio) | Ligand expression
(% positive cells) | Correlation
coefficient | P-value |
|---|
| 1:5 | | | |
| | MICA | -0.78 | 0.12 |
| | MICB | -0.95 | 0.01 |
| | ULBP1 | -0.59 | 0.29 |
| | NKG2DL | -0.81 | 0.10 |
| 1:3 | | | |
| | MICA | -0.89 | 0.04 |
| | MICB | -0.96 | 0.01 |
| | ULBP1 | -0.72 | 0.17 |
| | NKG2DL | -0.90 | 0.04 |
| 1:1 | | | |
| | MICA | -0.89 | 0.04 |
| | MICB | -0.98 | <0.001 |
| | ULBP1 | -0.73 | 0.16 |
| | NKG2DL | -0.91 | 0.03 |
| 3:1 | | | |
| | MICA | -0.86 | 0.06 |
| | MICB | -0.98 | <0.001 |
| | ULBP1 | -0.68 | 0.21 |
| | NKG2DL | -0.88 | 0.05 |
| 5:1 | | | |
| | MICA | -0.89 | 0.05 |
| | MICB | -0.98 | <0.001 |
| | ULBP1 | -0.73 | 0.16 |
| | NKG2DL | -0.91 | 0.03 |
| 10:1 | | | |
| | MICA | -0.85 | 0.07 |
| | MICB | -0.96 | 0.01 |
| | ULBP1 | -0.67 | 0.21 |
| | NKG2DL | -0.87 | 0.06 |
Discussion
In the present study, sesamin was revealed to have
induced a G2/M phase cell cycle arrest in MG-63
osteosarcoma cells. Sesamin was reported to have triggered
upregulated expression of NKG2DLs and increased susceptibility of
MG-63 cells to NK cell-mediated elimination. NK cell-mediated
cytotoxicity was positively correlated with levels of NKG2DL
expression.
Osteosarcoma is clinically treated with neoadjuvant
chemotherapy followed by surgical resection and additional
chemotherapy/radiotherapy. Considering the side effects of
chemotherapy and functional impairments after surgical resection, a
broad variety of natural compounds have been considered and
examined for their therapeutic uses against osteosarcoma (19). Sesamin has been demonstrated to
exert anti-neoplastic activities against several malignancies
(20). Treatment with sesamin
causes proliferation arrest at the G1 phase in cell cycle
progression in the breast cancer cells (15). Siao et al (13) reported that sesamin inhibited the
proliferation of MCF-7 cells through inducing Sub-G1 phase arrest
and apoptosis. A previous study revealed that sesamin induced G1
cell cycle arrest and inhibited cyclin D1 and CDK2 expression in
lung cancer (21). In the present
study, it was found that sesamin at a high concentration inhibited
proliferation of MG-63 osteosarcoma cells after long exposure time
up to 48 h through arresting cell cycle at G2/M phase.
The data of the present study revealed that sesamin increased p21
expression in MG-63 cells. The findings are similar to a previous
study revealing that sesamin induced G2/M arrest in
HepG2 cells through the p53/p21 signaling pathway (14). Several cellular stresses cause the
activation of p53 and in turn cell cycle arrest through modulating
downstream regulatory molecules such as p21, cyclins and CDKs
(22-24).
p53 protein is activated in response to DNA damage with results of
G1 arrest (25). On the other
hand, stress-induced increase of p21 and reduction of cyclins have
been reported to be p53-independent (22,26,27).
p21 appears to play a role in induction of G2/M arrest
in responses to DNA damages (28).
In the present study, sesamin caused cell cycle arrest in
p53-deficient MG-63 cells, suggesting that p53 may have no role in
sesamin-induced cell cycle arrest and activation of p21 is
p53-independent.
The cell cycle is regulated through a complex
interaction over time between cyclins and Cdks. Cdks are activated
by cyclins through forming cyclin/Cdk complexes. Interruption of
cyclin B1/Cdk1 complex formation leads to G2/M phase
arrest (29). In the present
study, sesamin arrested MG-63 cells at the G2/M phase
through a decreased formation of cyclin B1/Cdk1 complex. The
results of the present study revealed that sesamin dose-dependently
inhibited cyclin B1 and Cdk1 expression at the transcriptional and
protein levels. Cyclin B1/Cdk1 complex is the primary regulator of
transition from the G2 to M phase. It is suggested that sesamin
induces G2/M phase arrest in MG-63 cells by the
downregulation of Cdk1 and cyclin B1.
NKG2D ligands are stress-inducible proteins, which
are missing or present at very low levels on healthy cells. In
addition to upregulated expression of NKG2DLs in infected cells,
they are also widely expressed in several types of cancer and
hematologic malignancies. Early tumorigenesis involves aberrant
cell proliferation levels which are associated with DNA replication
stress (30). The stress activates
DNA damage response proteins including ataxia telangiectasia
mutated (ATM) and/or ATM and Rad3-related (ATR) kinases, which in
turn cause cell cycle arrest. In addition, NKG2DLs have been
reported to be induced by DNA damage responses or oncogenes
(31,32). Nevertheless, mutations in
suppressive mechanisms and stress pathways contribute to tumor
progression. In the present study, the data revealed that
osteosarcoma MG-63 cells constitutively expressed certain levels of
NKG2DLs. The present study gave substantial evidence that treatment
of MG-63 cells with sesamin results in a concomitant upregulation
of the already expressed NKG2DLs including MICA, MICB and ULBP1
both at the protein and mRNA levels. It was revealed that
sesamin-induced NKG2DLs expression was associated with
G2/M cell cycle arrest in MG-63 cells. The findings are
consistent with previous studies reporting that NKG2DL upregulation
was associated with chemical-induced G2/M cell cycle
arrest in cancer cells (33,34).
It is suggested that exposure to chemotherapeutics leads to
upregulation of NKG2DLs through activation of DNA damage responses
(35). The NKG2DL upregulation is
a consequence of ATR/ATM-induced cell cycle arrest (31,33).
The findings of the present study in p53-deficient MG-63 cells is
supported by previous findings revealing that DNA stress induces
G2/M cell cycle arrest in p53-null cells through ATM/ATR
checkpoint signaling (36).
Increasing evidence has highlighted the role of
NKG2D/NKG2DL interaction in cancer immunotherapy. Strategies
harnessing NKG2D/NKG2DL recognition to achieve cancer control have
been developed with great interests (37,38).
Numerous anticancer drugs have been revealed to increase surface
expression NKG2DL, which pharmacologically induced cell stresses
(39) Upregulated NKG2GL
expression has been reported in cancer cells exposed to ionizing
radiation (40,41). However, current anticancer
therapies have downside effects on NK cell-mediated antitumor
immunity such as NKG2DL shedding leading to cancer immune evasion.
In the present study, sesamin enhanced susceptibility of
osteosarcoma MG-63 cells to NK cell-mediated cytotoxicity through
inducing KNG2DL expression. The data of the present study revealed
that sesamin increased sensitivity of MG-63 cells to NK cell
killing at a low E:T ratio of 1:3. Among the NKG2DL analyzed, NK
cell-mediated cytotoxicity was significantly correlated with
expression of MICB in presence of sesamin. It is suggested that
sesamin at low concentration may be adequate to achieve favorable
effects of NK-cell immunotherapy against osteosarcoma MG-63 cells.
Sesamin is a natural compound containing several pharmacological
activities including anti-oxidative stress and anti-inflammation.
The anti-inflammatory property of sesamin is suggested to
contribute to addressing tumor microenvironment and ligand
shedding. Oral ingestion of sesamin at doses up to 200 mg/day has
been reported to be safe and tolerable (42). Sesamin is suggested to be a safe
candidate for inducing NKG2DL expression, whereas the other
chemotherapeutic drugs are markedly toxic. Tomimori et al
(43) has reported that the
highest concentration of sesamin in human plasma after ingestion of
50 mg of sesamin was 8 nM (43).
This is substantially lower than the concentrations tested. Further
studies are required to address bioavailability issue and assess
NKG2D/NKG2DL axis in vivo with a consideration of complexity
of tumor cells, tumor microenvironment and NK cells. The
limitations of the present study include in vitro design,
which inherently lacks the challenges of true clinical conditions
such as tumor microenvironment and cell administration. In
addition, one cell line was employed to study the correlation
between the expression of NKG2D ligand and NK cell mediated
elimination. In vivo studies are necessary to validate the
findings of the present study.
In conclusion, within the limitations of the present
study, sesamin induced cell cycle arrest at G2/M phase
in osteosarcoma MG-63 cells and in turn increased NKG2DL expression
in osteosarcoma cells. Sesamin might further enhance the
susceptibility of osteosarcoma cells to NK cell-mediated
cytotoxicity through upregulated expression of NKG2DLs. Therefore,
sesamin represents a potential candidate to improve the efficacy of
NKG2DL-mediated anticancer therapy for osteosarcoma.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SCC, CYK and YJL designed the study. SCC, CYK, HWK,
PTH, CHL and LSW conducted the experiments and analyzed the data.
SCC and CYK confirm the authenticity of all the raw data. SCC, CYK
and YJL prepared and wrote the manuscript. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Li W and Zhang S: Survival of patients
with primary osteosarcoma and lung metastases. J BUON.
23:1500–1504. 2018.PubMed/NCBI
|
|
3
|
Kager L, Zoubek A, Pötschger U, Kastner U,
Flege S, Kempf-Bielack B, Branscheid D, Kotz R, Salzer-Kuntschik M,
Winkelmann W, et al: Primary metastatic osteosarcoma: Presentation
and outcome of patients treated on neoadjuvant cooperative
osteosarcoma study group protocols. J Clin Oncol. 21:2011–2018.
2003.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gomez-Brouchet A, Illac C, Gilhodes J,
Bouvier C, Aubert S, Guinebretiere JM, Marie B, Larousserie F,
Entz-Werlé N, de Pinieux G, et al: CD163-positive tumor-associated
macrophages and CD8-positive cytotoxic lymphocytes are powerful
diagnostic markers for the therapeutic stratification of
osteosarcoma patients: An immunohistochemical analysis of the
biopsies fromthe French OS2006 phase 3 trial. Oncoimmunology.
6(e1331193)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Eller MA and Currier JR: OMIP-007:
Phenotypic analysis of human natural killer cells. Cytometry A.
81:447–449. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lanier LL: NK cell recognition. Annu Rev
Immunol. 23:225–274. 2005.
|
|
7
|
Lanier LL: NKG2D receptor and its ligands
in host defense. Cancer Immunol Res. 3:575–582. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mistry AR and O'Callaghan CA: Regulation
of ligands for the activating receptor NKG2D. Immunology.
121:439–447. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Raulet DH, Gasser S, Gowen BG, Deng W and
Jung H: Regulation of ligands for the NKG2D activating receptor.
Annu Rev Immunol. 31:413–441. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Le Bert N and Gasser S: Advances in NKG2D
ligand recognition and responses by NK cells. Immunol Cell Biol.
92:230–236. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wu MS, Aquino LBB, Barbaza MYU, Hsieh CL,
Castro-Cruz KA, Yang LL and Tsai PW: Anti-Inflammatory and
anticancer properties of bioactive compounds from Sesamum indicum
L.-A review. Molecules. 24(4426)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang X, Qiao J, Zou C, Zhao Y and Huang Y:
Sesamin induces cell cycle arrest and apoptosis through p38/C-Jun
N-terminal kinase mitogen-activated protein kinase pathways in
human colorectal cancer cells. Anticancer Drugs. 32:248–256.
2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Siao AC, Hou CW, Kao YH and Jeng KC:
Effect of sesamin on apoptosis and cell cycle arrest in human
breast cancer mcf-7 cells. Asian Pac J Cancer Prev. 16:3779–3783.
2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Deng P, Wang C, Chen L, Wang C, Du Y, Yan
X, Chen M, Yang G and He G: Sesamin induces cell cycle arrest and
apoptosis through the inhibition of signal transducer and activator
of transcription 3 signalling in human hepatocellular carcinoma
cell line HepG2. Biol Pharm Bull. 36:1540–1548. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yokota T, Matsuzaki Y, Koyama M, Hitomi T,
Kawanaka M, Enoki-Konishi M, Okuyama Y, Takayasu J, Nishino H,
Nishikawa A, et al: Sesamin, a lignan of sesame, down-regulates
cyclin D1 protein expression in human tumor cells. Cancer Sci.
98:1447–1453. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Akl MR, Ayoub NM, Abuasal BS, Kaddoumi A
and Sylvester PW: Sesamin synergistically potentiates the
anticancer effects of γ-tocotrienol in mammary cancer cell lines.
Fitoterapia. 84:347–359. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Akl MR, Ayoub NM and Sylvester PW:
Mechanisms mediating the synergistic anticancer effects of combined
γ-tocotrienol and sesamin treatment. Planta Med. 78:1731–1739.
2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tobeiha M, Rajabi A, Raisi A, Mohajeri M,
Yazdi SM, Davoodvandi A, Aslanbeigi F, Vaziri M, Hamblin MR and
Mirzaei H: Potential of natural products in osteosarcoma treatment:
Focus on molecular mechanisms. Biomed Pharmacother.
144(112257)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Majdalawieh AF, Massri M and Nasrallah GK:
A comprehensive review on the anti-cancer properties and mechanisms
of action of sesamin, a lignan in sesame seeds (Sesamum indicum).
Eur J Pharmacol. 815:512–521. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen Y, Li H, Zhang W, Qi W, Lu C, Huang
H, Yang Z, Liu B and Zhang L: Sesamin suppresses NSCLC cell
proliferation and induces apoptosis via Akt/p53 pathway. Toxicol
Appl Pharmacol. 387(114848)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Karimian A, Ahmadi Y and Yousefi B:
Multiple functions of p21 in cell cycle, apoptosis and
transcriptional regulation after DNA damage. DNA Repair (Amst).
42:63–71. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Pawlik TM and Keyomarsi K: Role of cell
cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol
Biol Phys. 59:928–942. 2004.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Winters ZE: P53 pathways involving G2
checkpoint regulators and the role of their subcellular
localisation. J R Coll Surg Edinb. 47:591–598. 2002.PubMed/NCBI
|
|
25
|
Vogelstein B, Lane D and Levine AJ:
Surfing the p53 network. Nature. 408:307–310. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Yuan L, Zhang Y, Xia J, Liu B, Zhang Q,
Liu J, Luo L, Peng Z, Song Z and Zhu R: Resveratrol induces cell
cycle arrest via a p53-independent pathway in A549 cells. Mol Med
Rep. 11:2459–2464. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shin DY, Sung Kang H, Kim GY, Kim WJ, Yoo
YH and Choi YH: Decitabine, a DNA methyltransferases inhibitor,
induces cell cycle arrest at G2/M phase through p53-independent
pathway in human cancer cells. Biomed Pharmacother. 67:305–311.
2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bunz F, Dutriaux A, Lengauer C, Waldman T,
Zhou S, Brown JP, Sedivy JM, Kinzler KW and Vogelstein B:
Requirement for p53 and p21 to sustain G2 arrest after DNA damage.
Science. 282:1497–1501. 1998.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Porter LA and Donoghue DJ: Cyclin B1 and
CDK1: Nuclear localization and upstream regulators. Prog Cell Cycle
Res. 5:335–347. 2003.PubMed/NCBI
|
|
30
|
Bartkova J, Horejsi Z, Koed K, Krämer A,
Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, et
al: DNA damage response as a candidate anti-cancer barrier in early
human tumorigenesis. Nature. 434:864–870. 2005.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gasser S, Orsulic S, Brown EJ and Raulet
DH: The DNA damage pathway regulates innate immune system ligands
of the NKG2D receptor. Nature. 436:1186–1190. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Boissel N, Rea D, Tieng V, Dulphy N, Brun
M, Cayuela JM, Rousselot P, Tamouza R, Le Bouteiller P, Mahon FX,
et al: BCR/ABL oncogene directly controls MHC class I chain-related
molecule A expression in chronic myelogenous leukemia. J Immunol.
176:5108–5116. 2006.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Soriani A, Zingoni A, Cerboni C, Iannitto
ML, Ricciardi MR, Di Gialleonardo V, Cippitelli M, Fionda C,
Petrucci MT, Guarini A, et al: ATM-ATR-dependent up-regulation of
DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic
agents results in enhanced NK-cell susceptibility and is associated
with a senescent phenotype. Blood. 113:3503–3511. 2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Morelli MB, Amantini C, Santoni M, Soriani
A, Nabissi M, Cardinali C, Santoni A and Santoni G: Axitinib
induces DNA damage response leading to senescence, mitotic
catastrophe, and increased NK cell recognition in human renal
carcinoma cells. Oncotarget. 6:36245–36259. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cerboni C, Fionda C, Soriani A, Zingoni A,
Doria M, Cippitelli M and Santoni A: The DNA damage response: a
common pathway in the regulation of NKG2D and DNAM-1 ligand
expression in normal, infected, and cancer cells. Front Immunol.
4(508)2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Reinhardt HC, Aslanian AS, Lees JA and
Yaffe MB: p53-deficient cells rely on ATM- and ATR-mediated
checkpoint signaling through the p38MAPK/MK2 pathway for survival
after DNA damage. Cancer Cell. 11:175–189. 2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Fuertes MB, Domaica CI and Zwirner NW:
Leveraging NKG2D ligands in immuno-oncology. Front Immunol.
12(713158)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Liu H, Wang S, Xin J, Wang J, Yao C and
Zhang Z: Role of NKG2D and its ligands in cancer immunotherapy. Am
J Cancer Res. 9:2064–2078. 2019.PubMed/NCBI
|
|
39
|
Schmiedel D and Mandelboim O: NKG2D
ligands-critical targets for cancer immune escape and therapy.
Front Immunol. 9(2040)2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Weiss T, Schneider H, Silginer M, Steinle
A, Pruschy M, Polić B, Weller M and Roth P: NKG2D-dependent
antitumor effects of chemotherapy and radiotherapy against
glioblastoma. Clin Cancer Res. 24:882–895. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kim JY, Son YO, Park SW, Bae JH, Chung JS,
Kim HH, Chung BS, Kim SH and Kang CD: Increase of NKG2D ligands and
sensitivity to NK cell-mediated cytotoxicity of tumor cells by heat
shock and ionizing radiation. Exp Mol Med. 38:474–484.
2006.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Sun Y, Ren J, Zhu S, Zhang Z, Guo Z, An J,
Yin B and Ma Y: The effects of sesamin supplementation on obesity,
blood pressure, and lipid profile: A systematic review and
meta-analysis of randomized controlled trials. Front Endocrinol
(Lausanne). 13(842152)2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Tomimori N, Tanaka Y, Kitagawa Y, Fujii W,
Sakakibara Y and Shibata H: Pharmacokinetics and safety of the
sesame lignans, sesamin and episesamin, in healthy subjects.
Biopharm Drug Dispos. 34:462–473. 2013.PubMed/NCBI View Article : Google Scholar
|