Introduction
Moyamoya disease (MMD), first described by Suzuki
and Takaku (1) in 1969, is a
chronic cerebrovascular disease presenting with an abnormal vessel
net resembling a ‘puff of smoke’ at the skull base on angiography.
MMD presents with progressive occlusion of the terminal portion of
the internal carotid artery and circle of Willis, which is
recognized as a major cause of ischemic and hemorrhagic strokes
(2). The etiology and pathogenesis
of MMD remain to be fully elucidated; however, numerous studies
have demonstrated that genetic factors, immune function and
hemodynamic factors may be responsible for disease progression
(3-5).
An autopsy case report demonstrated that marked narrowing of
cerebral arteries and fibrocellular intimal thickening, which was
composed of smooth muscle cells, existed in a patient with MMD
(6). The proteomic profiling of
MMD has revealed that abnormal endothelial cell proliferation may
be induced by the upregulation of focal adhesion-related proteins
(7). However, there are still no
efficient and sensitive biomarkers for MMD diagnosis or treatment
and there is an urgent need to elucidate molecular mechanisms
underlying MMD initiation and progression to identify therapeutic
and diagnostic targets.
Disulfidptosis is a newly identified form of cell
death caused by the vulnerability of the actin cytoskeleton to
disulfide stress induced by the collapse of aberrant disulfide
bonds in actin cytoskeleton proteins and F-actin (8). The amino acid transporter protein
solute carrier family 7 member 11 helps transport cystine to cancer
cells, which promotes tumor growth. Cystine, a toxic disulfide,
accumulates in tumor cells and triggers disulfidptosis when cells
lack glucose, and NADPH production decreases, which can convert
cystine into non-toxic molecules (8,9).
RNF213 was identified as a susceptibility gene for MMD.
RNF213 knockdown in vascular smooth muscle cells led to
alteration in cytoskeletal organization and contractility (10). The protein profiling of MMD also
revealed upregulated cytoskeletal proteins (7). Furthermore, exosomal micro (mi)RNA
profiling revealed significant differences in the expression of
miRNAs involved in vascular cytoskeleton reconstruction in MMD
(11). The dysregulation of the
cytoskeleton in MMD may be involved in abnormal disulfidptosis,
which is caused by the vulnerability of the actin cytoskeleton, and
aberrant disulfidptosis may be involved in abnormal cell
proliferation during the pathogenesis of MMD.
The present study aimed to identify possible
pathogenic mechanisms of MMD, and comprehensive bioinformatics
analyses were used to explore disulfidptosis-related genes (DRGs)
in MMD using the Gene Expression Omnibus (GEO) database. The
expression profiles of DRGs in MMD were analyzed, and consistency
clustering analysis was performed to construct subclusters of MMD.
Functional enrichment analysis was also performed for different
molecular subtypes of MMD. Furthermore, hub genes with intersecting
gene sets of differential DRGs were identified among different
molecular subtypes and between patients with MMD and controls.
Immune infiltration was further explored and its correlation with
hub genes was assessed. Analyses of signaling pathways and
transcription regulation were used to explore the functions and
regulatory mechanisms of hub genes in MMD. The whole study provides
comprehensive analyses of disulfidptosis in MMD and new insights
into the molecular pathological mechanisms of MMD.
Materials and methods
Data processing
This study was approved by the Ethics Committee of
Beijing Tiantan Hospital (Beijing, China; approval no.
KY-2023-2024-02). To identify relevant gene expression datasets, a
comprehensive search was performed using the keyword ‘moyamoya
disease’ in the GEO database (https://www.ncbi.nlm.nih.gov/geo/), selecting studies
with Homo sapiens as the species. Datasets with a sample
size greater than six were included to ensure sufficient
statistical power and four microarray datasets (GSE189993,
GSE157628, GSE141024 and GSE141022) were obtained (12,13).
For each dataset, gene expression files were downloaded and the
same platform, GPL16699, was used for data analysis. These datasets
were composed of gene expression profiles from middle cerebral
artery (MCA) samples. Specifically, the matrix data file of
GSE189993 included the gene expression data of 32 participants (21
patients with MMD and 11 healthy controls), and GSE157628 contained
the gene expression data of 20 participants (11 patients with MMD
and 9 healthy controls). The matrix data file GSE141024 was
obtained from eight participants (four patients with MMD and four
healthy controls), and GSE141022 included the expression profiles
from eight participants (four patients with MMD and four healthy
controls). Detailed sample information for each dataset, including
the number of patients with MMD and control subjects, is presented
in Table SI. The R package SVA
was used to correct the microarrays and display the status of the
batches before and after correction using principal component
analysis.
Consistency clustering analysis of
DRGs
The DRG gene set was obtained from a study by Liu
et al (8). Based on the
expression profiles of the DRGs for each sample, the data were
clustered into discrete molecular clusters, and 90% of the total
samples were clustered using 300 iterations (8). A consensus cumulative distribution
function (CDF) curve was constructed and a consensus matrix heatmap
analysis was performed to choose the best cluster number κ (κ=3) to
distinguish different molecular subtypes of MMD (C1, C2 and C3) for
further analyses.
Differential expression analysis
Differential expression analysis was performed using
the R package ‘limma’ with R (version 4.2.2) to analyze the
differences in gene expression between subjects with MMD and
controls with the Mann-Whitney U-test. Differences in the
expression profiles were also identified among the molecular
subtypes of MMD with the Kruskal-Wallis H-test. Differentially
expressed genes were selected using the conditions of P<0.05,
|log2fold change (FC)|>1. Heatmaps were drawn to show
the gene expression differences using the R package ‘ggplot2’ with
R (version 4.2.2). Differential DRGs were obtained and further
analyzed using Spearman correlation analysis in patients with MMD
and controls.
Functional enrichment analysis
Functional enrichment analysis was used to explore
the molecular functions and mechanisms of the differentially
expressed DRGs in MMD. Gene ontology (GO) enrichment analysis and
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment
analysis were performed using the R package ‘clusterProfiler’ with
R (version 4.2.2). P- and q-values of less than 0.05 were
considered to indicate statistical significance.
Identification of hub genes in
MMD
Based on the results of the differential expression
analysis that selected genes with a P-value <0.05 and
|log2FC|>1, the intersecting differentially expressed genes were
selected from four comparison groups, which included C1 vs. C2, C1
vs. C3, C2 vs. C3, and MMD vs. controls, which included 29
differentially expressed genes. Using the intersecting gene sets,
they were ranked according to the average functional similarity
correlation using Friend analysis and the top five genes with the
highest correlation were selected as candidate hub genes.
Furthermore, intergroup differential expression analysis was
performed using the Kruskal-Wallis H-test with candidate hub genes
among C1, C2, C3 and the controls, which were used to select
significantly differentially expressed genes among these groups as
hub genes for further study.
Immune infiltration analysis
Immune cell profiling was performed using the
CIBERSORT algorithm, a robust method for evaluating immune cell
composition in tissue samples. The CIBERSORT tool is based on a
reference set of 547 gene markers representing 22 distinct human
immune cell types (14). A
differential analysis of immune cell expression was conducted to
investigate the immune characteristics across different molecular
MMD subtypes with the Kruskal-Wallis H-test. This analysis revealed
significant differences in immune cell infiltration between
patients with MMD and healthy controls with the Mann-Whitney
U-test. Additionally, Spearman correlation analysis was performed
to explore the potential associations between the expression of hub
genes and the relative proportions of specific immune cell
types.
Gene set enrichment analyses (GSEA) of
hub genes
The differences in the signaling pathways of the hub
genes between the high- and low-expression groups were further
analyzed using GSEA. The annotated gene set of version 7.0 was
obtained from the MsigDB database (https://www.gsea-msigdb.org/gsea/msigdb).
Significantly enriched gene sets (with an adjusted P<0.05) were
sorted based on consistency scores.
Gene set variation analysis
(GSVA)
GSVA, a nonparametric and unsupervised method, is
used for evaluating the enrichment of transcriptome gene sets. Gene
sets were downloaded from the molecular signature database and the
GSVA algorithm was used to comprehensively score each gene set and
evaluate the differential biological functions of the hub genes in
different samples.
Transcription regulation analysis
The prediction of transcription factors was
performed with hub genes using the R package ‘RcisTarget’ with R
(version 4.2.2). All calculations were based on motifs, and the
normalized enrichment scores of motifs were scored using motifs
from the database and annotation files based on motif similarity
and gene sequences. Visualization of a comprehensive
transcriptional regulatory network of hub genes for disulfideptosis
in MMD was constructed using Cytoscape (version 3.2.1; http://www.cytoscape.org/).
MMD-related gene correlation
analysis
MMD-related genes were obtained from the GeneCards
database (https://www.genecards.org/) and
differential expression analysis was performed using the R package
‘limma’ with R (version 4.2.2) to compare the expression level of
MMD-related genes between disease and control groups with the
Mann-Whitney U-test. The relationship between MMD-related genes and
hub genes was visualized using a bubble chart and Pearson
correlation analysis.
Endothelial migration and
proliferation-related gene correlation analysis
Endothelial migration- and proliferation-related
genes were obtained from the GeneCards database. Pearson
correlation analysis was used to explore the relationship between
endothelial migration- and proliferation-related genes and hub
genes.
Statistical analysis
All analyses were performed using R (version 4.2.2),
and P<0.05 was considered to indicate statistical significance.
Comparisons between two groups were performed using the
Mann-Whitney U-test and comparisons between three or more groups
were performed using the Kruskal-Wallis H-test. All of the
correlation analyses used in the study were Pearson correlation
analyses.
Participants and sample
preparation
A total of three Chinese and Han individuals were
enrolled who underwent digital subtraction angiography to check for
MMD at the Department of Neurosurgery, Beijing Tiantan Hospital,
Capital Medical University (Beijing, China) from July 2021 to
December 2022(15). Detailed
consultations and physical examinations of patients with MMD were
performed to ensure that they did not have any underlying diseases,
such as hypertension, diabetes, hyperlipidemia or hyperthyroidism,
or any surgical history, which could have affected the results of
this study. Table SII shows the
clinical and demographic characteristics of the patients. 3 MMD
patients (1 male and 2 females) with an age range of 39 to 51 years
were included. And 3 healthy controls (1 male and 2 females) with
an age range of 30 to 45 years were included. In addition, three
healthy controls (age range, 18-45 years) were recruited with
clinical notice in Beijing Tiantan Hospital from July 2021 to
December 2022. Written informed consent was obtained from all
participants in accordance with the Declaration of Helsinki and the
study protocol was approved by the Ethics Committee of Beijing
Tiantan Hospital (Beijing, China; approval no. KY 2020-045-02),
which informed the patients that the blood samples would be used in
studies of MMD pathogenesis in the future. Blood samples (2 ml)
were collected from all patients with MMD and centrifuged at 1,500
x g for 10 min at room temperature, followed by storage at -80˚C
for analysis.
Enzyme-linked immunosorbent assay
(ELISA)
The serum of six participants was obtained after
centrifugation and a standard solution was prepared according to
the manufacturer's instructions. The Human WDR27 ELISA Kit (cat.
no. E16199h; Elabscience; https://www.elabscience.cn/), Human NEIL2 ELISA Kit
(cat. no. abx534990), Human MSMO1 ELISA Kit (cat. no. abx385151;
both from Abbexa) and Human OSBPL11 ELISA Kit (cat. no. MBS166713;
MyBioSource, Inc.) were obtained to perform the assays. Standard,
blank and sample wells were used in the ELISA kits. To the standard
wells, 100 µl of diluted standard solution was added, while 100 µl
of standard diluent buffer was added to the blank wells, and 100 µl
of the sample was added to the remaining wells, which were
incubated at 37˚C for 1 h. After adding detection reagents A and B
to each well by suction and washing, each well was sealed and
incubated at 37˚C in the dark for 20 min. Subsequently, 50 µl stop
solution was added to each well and the optical density value at
450 nm was measured. Data were analyzed and visualized using
GraphPad Prism 9 (version 9.4.0; Dotmatics) and Adobe Illustrator
(version 26.3.1; Adobe Systems, Inc.) was used to organize and
combine figures. All data are expressed as the mean ± standard
deviation. Statistical differences between groups were tested using
one-way ANOVA with Tukey's post-hoc test. P<0.05 was considered
to indicate statistical significance.
Results
Differential expression analysis of
DRGs in MMD
The GSE189993, GSE157628, GSE141024 and GSE141022
datasets were downloaded from the GEO database and expression
profile data were obtained from 68 participants (40 patients with
MMD and 28 controls). The results showed that the batch effect
among microarrays was reduced after correction (Fig. 1A). The expression levels of DRGs in
samples from MMD and controls were analyzed and the results
demonstrated that LRPPRC (FC=-10.4 and P=0.01), NCKAP1 (FC=0.49 and
P=7.33x10-5) and NDUFS1(FC=0.86 and P=0.04) genes were
differentially expressed in MMD compared with the controls
(Fig. 1B). A heatmap was drawn to
show the expression of DRGs in each sample (Fig. 1C). In addition, a correlation
analysis of these genes between the control and MMD samples was
conducted, as shown in Fig.
1D.
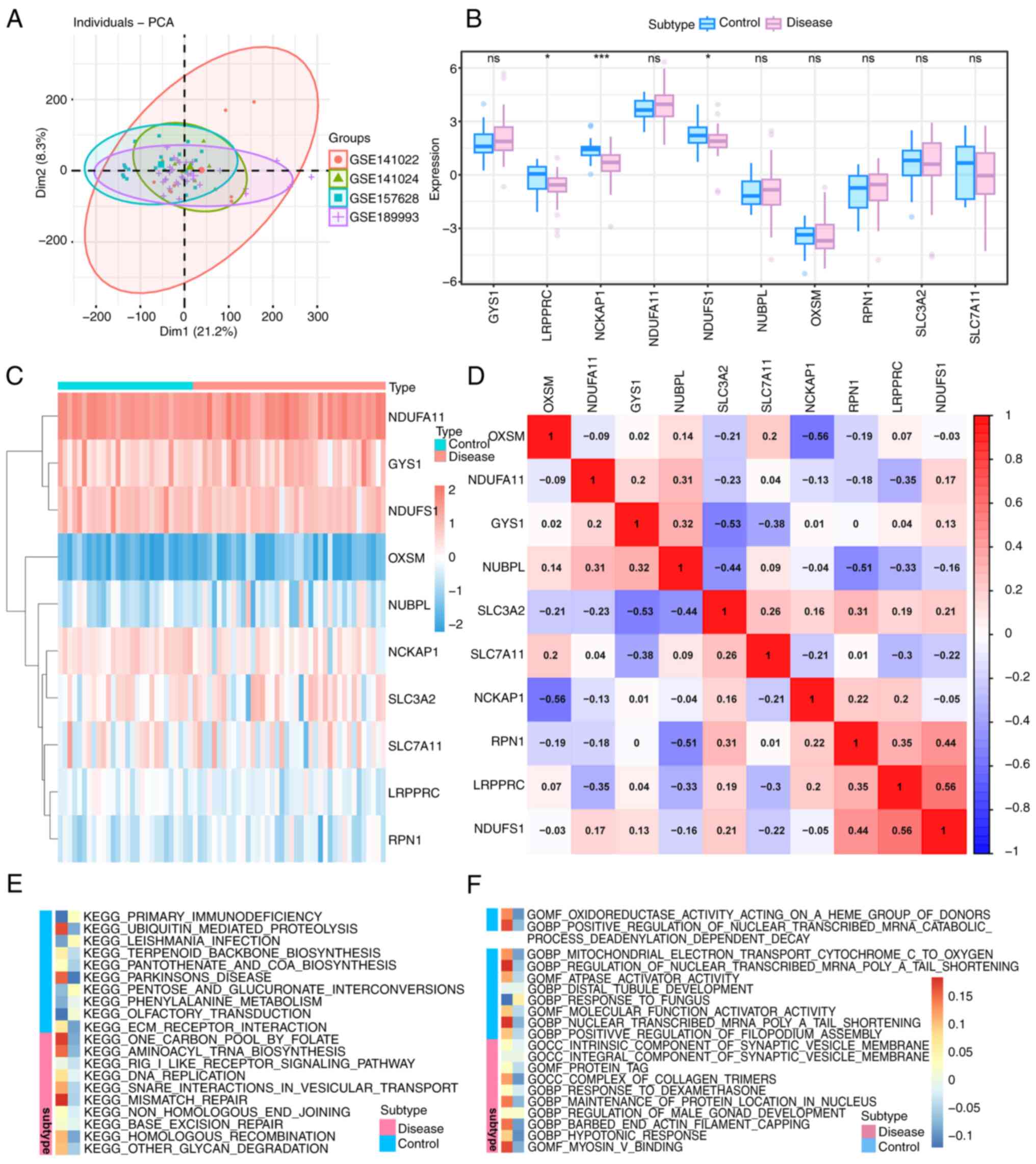 | Figure 1Differential analysis and functional
enrichment analysis with gene datasets. (A) PCA plots after
correlation. The different colored circles represent different
datasets. (B) Box plot of DRGs between MMD group and control group.
Blue represents the control group and red represents the MMD group.
*P<0.05; ***P<0.001; ns, no
significance. (C) Heatmap of DRG expression in MMD and controls.
Different colors represent expression levels in each sample. Red
indicates upregulation and blue indicates downregulation. (D)
Heatmap of correlation analysis among various DRGs in patients with
MMD. Red indicates a positive correlation and blue indicates a
negative correlation. (E) KEGG enrichment analysis of DRGs in MMD.
(F) GO enrichment analysis of DRGs in MMD. The two different
columns indicate the different groups, the red one is the disease
group and the blue one is the control group. PCA, principal
component analysis; GO, gene ontology; BP, Biological Process; CC,
Cellular Component; MF, Molecular Function; KEGG, Kyoto
Encyclopedia of Genes and Genomes; DRG, disulfide-ptosis-related
gene; MMD, moyamoya disease. |
Functional enrichment analysis of
differentially expressed genes
GO and KEGG enrichment analyses were performed and
the results are shown as a heatmap in Fig. 1E and F. GO enrichment analysis revealed that
the barbed-end actin filament capping process (P=0.005) and myosin
V binding (P=0.007) were significantly enriched in differentially
expressed genes in MMD (Fig. 1F).
KEGG enrichment analysis revealed that DNA replication (P=0.03) and
aminoacyl tRNA biosynthesis (P=0.02) pathways were significantly
enriched (Fig. 1E).
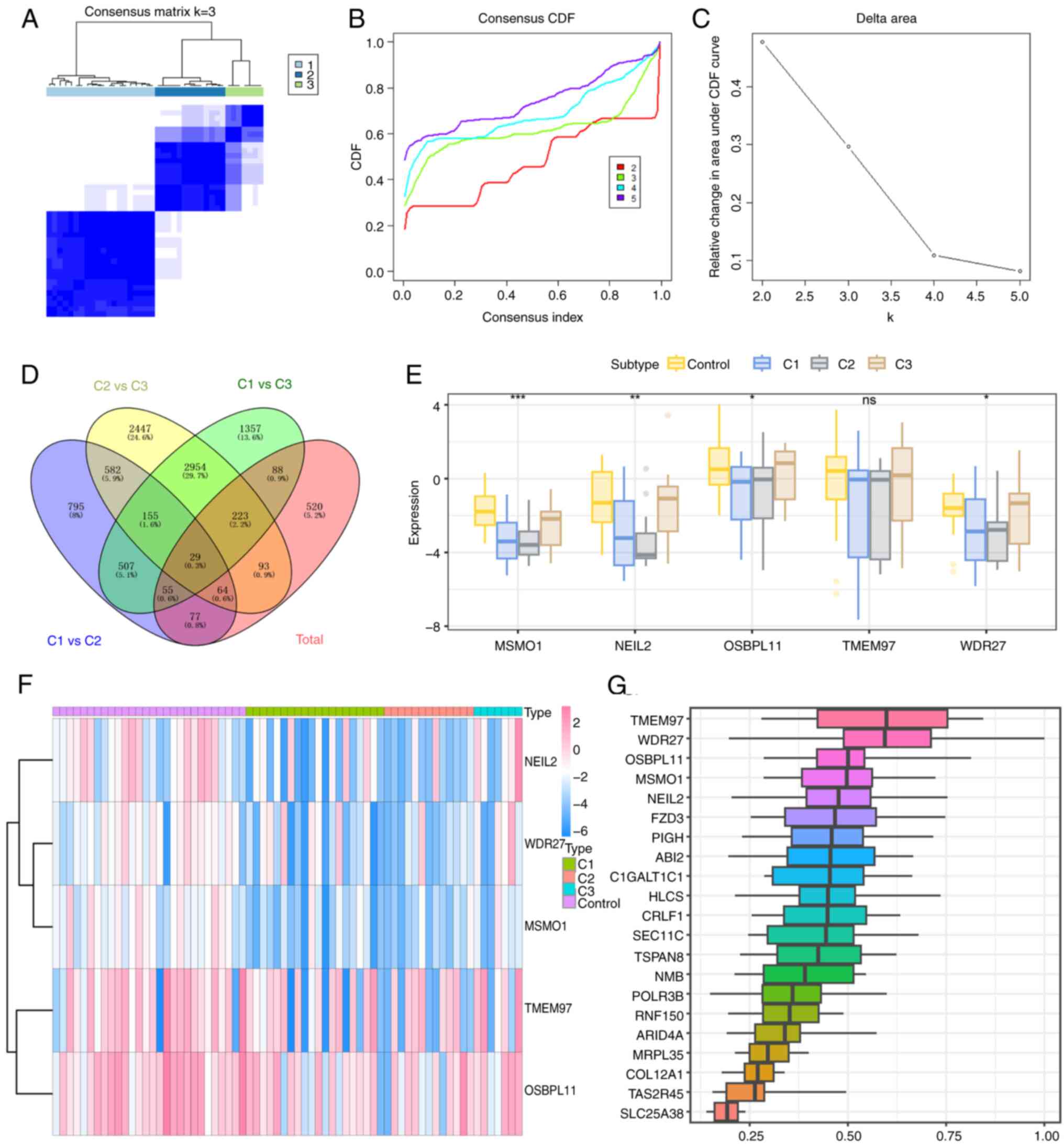
Molecular subtypes classification
based on DRGs in MMD
Consistency clustering was performed and a molecular
classification of MMD based on DRG expression levels was
constructed. Although the CDF curve showed that κ=2 was the best
choice of molecular subtypes classification, the results showed
that the boundaries of the subtypes in the samples were clearer
when κ=3 compared with κ=2 in Fig.
2A-C, which indicated that MMD should be divided into three
clusters.
Identification and expression profiles
of hub genes in MMD
To identify hub genes related to MMD and its
molecular subtypes, the R package limma was used to perform
differential expression analyses of four groups with R (version
4.2.2): C1 vs. C3, C2 vs. C3, C1 vs. C3, and MMD vs. control. The
standard for selecting differentially expressed genes was P<0.05
and |logFC|>1. A total of 2,264 differentially expressed genes
were identified for C1 vs. C2, including 1,207 upregulated and
1,057 downregulated genes. A total of 6,547 differentially
expressed genes were screened for C2 vs. C3, including 2,976
upregulated and 3,571 downregulated genes. For C1 vs. C3, 5,368
differentially expressed genes were detected, including 2,228
upregulated and 3,140 downregulated genes. Finally, 1,149
differentially expressed genes were screened for MMD vs. controls,
including 348 upregulated and 801 downregulated genes. Among the
four gene sets, 29 intersecting genes were identified, which are
shown as Venn plots (Fig. 2D).
Furthermore, the 29 intersecting genes were sorted based on the
average functional similarity relationships among proteins and the
results showed that TMEM97, WDR27, OSBPL11,
MSMO1 and NEIL2 were the top five genes (Fig. 2G). Intergroup analysis of these
five genes in the control and three MMD molecular subtypes was also
performed, and the results showed significant differences among the
WDR27 (P=0.03), OSBPL11 (P=0.04), MSMO1
(P=3.79x10-5) and NEIL2 (P=0.003) genes (Fig. 2E and F). Therefore, these four genes were
considered hub genes for further analysis.
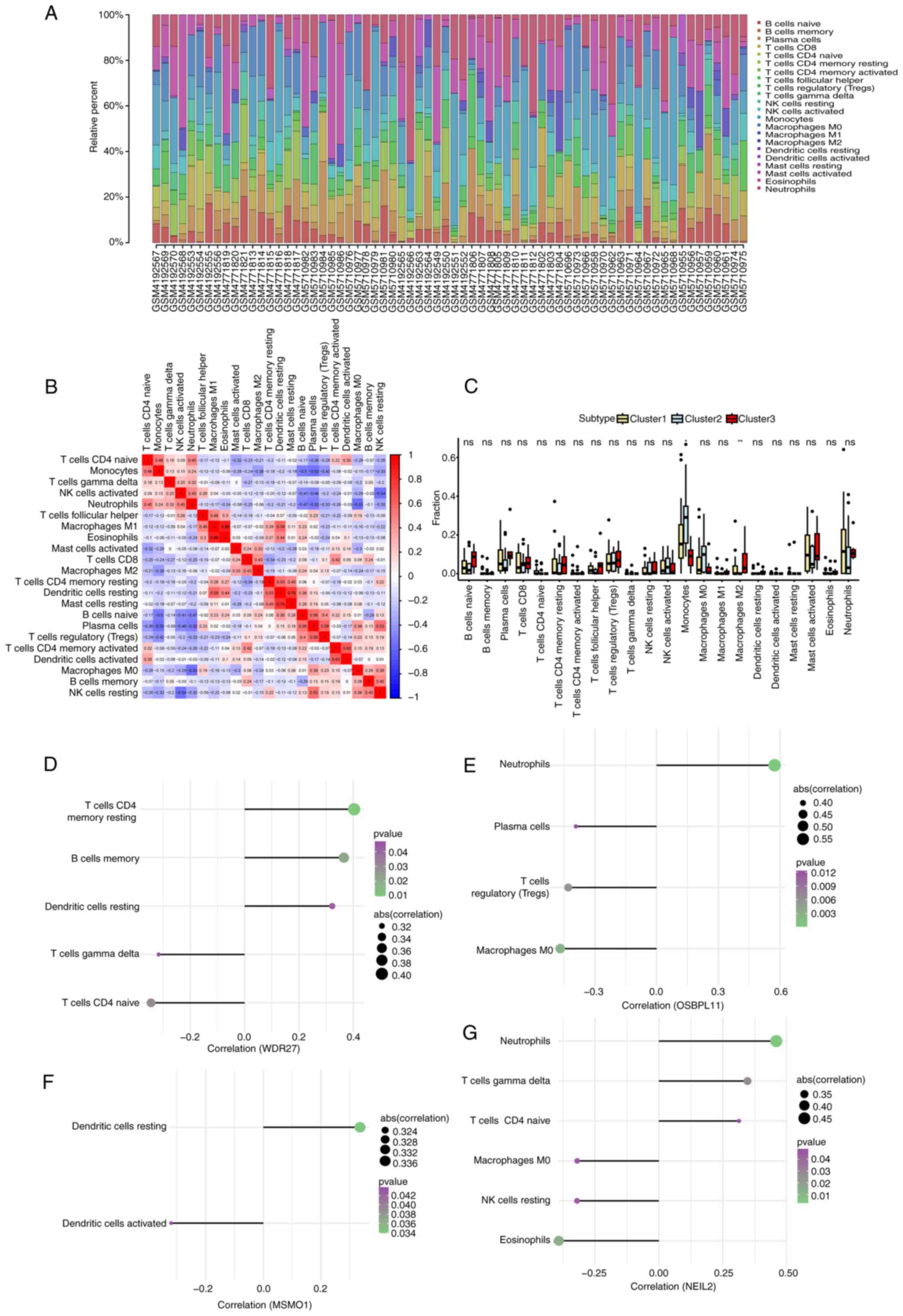
Immune infiltration analysis and
correlation between immune function and hub genes
The distribution of immune cells in each sample is
shown in Fig. 3A. An immune cell
correlation heatmap is also provided (Fig. 3B). Furthermore, the expression of
different immune cells was compared among MMD subtypes. The results
showed significant differences in monocyte (P=0.02) and M2
macrophage (P=0.005) counts among the different molecular subtypes
(Fig. 3C). The correlation between
the hub genes and immune cells in patients with MMD was further
explored. WDR27 was significantly positively correlated with
CD4 memory T cells (r=0.40, P=0.01) and memory B cells (r=0.36,
P=0.02), but negatively correlated with T cells CD4 naive (r=-0.24,
P=0.03) (Fig. 3D). OSBPL11
was significantly positively correlated with neutrophils (r=0.57
and P=0.0001), but negatively correlated with M0 macrophages
(r=-0.47 and P=0.002) and regulatory T cells (Tregs; r=-0.43,
P=0.006; Fig. 3E). MSMO1
was positively correlated with resting dendritic cells (r=0.34,
P=0.03), while it was negatively correlated with activated
dendritic cells (r=-0.32, P=0.04; Fig.
3F). NEIL2 was significantly positively correlated with
neutrophils (r=0.46, P=0.003) and gamma delta T cells (r=0.35,
P=0.03), while it was significantly negatively correlated with
eosinophils (r=-0.39, P=0.01) and resting natural killer cells
(r=-0.32, P=0.04; Fig. 3G).
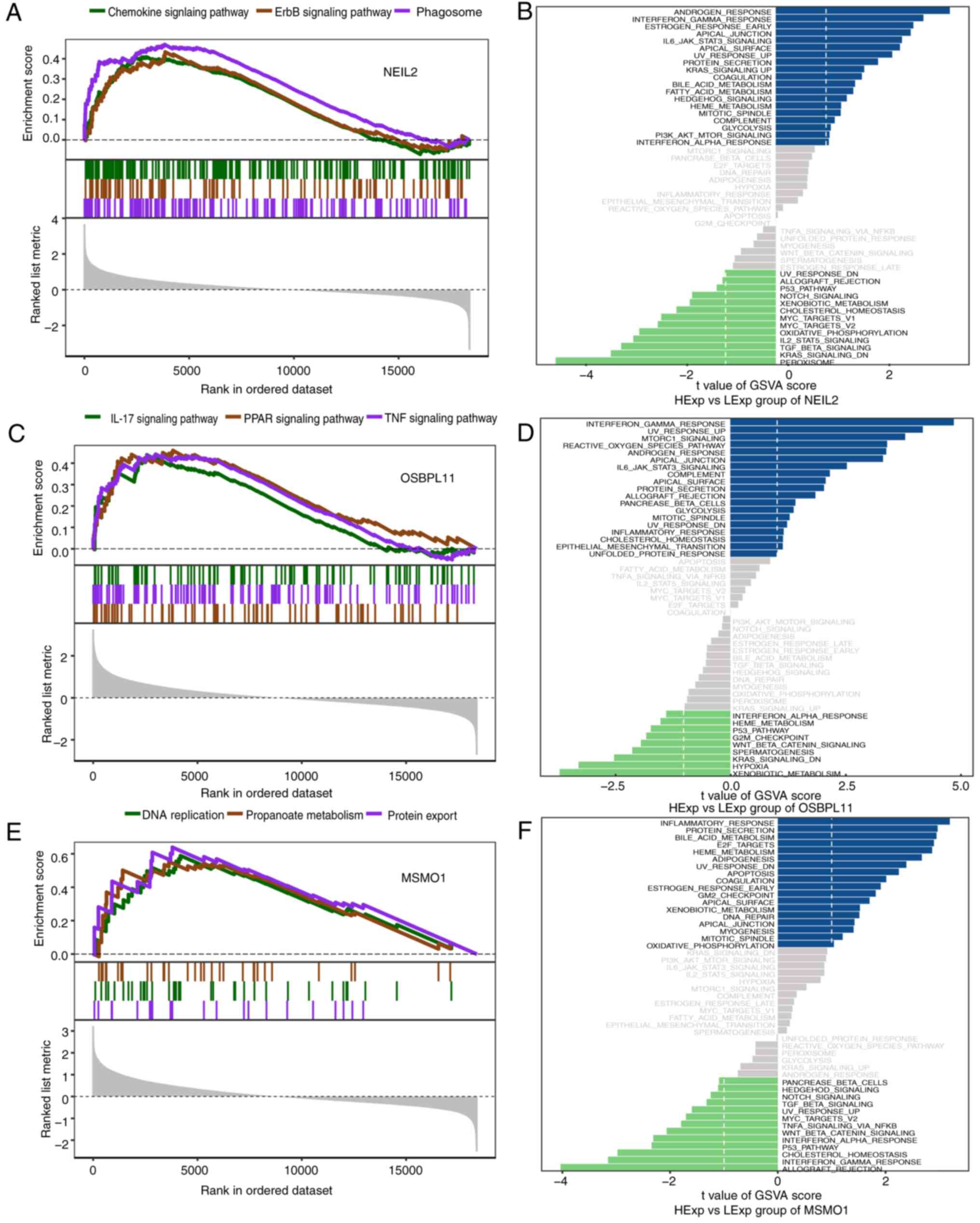
GSEA and GSVA pathway enrichment
analysis
The enriched signaling pathways associated with the
four hub genes and the potential molecular mechanisms by which hub
genes affect the pathogenesis of MMD were investigated. The GSEA
results demonstrated that NEIL2 was enriched in pathways,
including the chemokine signaling pathway (P=0.0002), ErbB
signaling pathway (P=0.006) and phagosome pathway
(P=1.43x10-5; Fig. 4A).
GSVA indicated that high NEIL2 expression was enriched in
the interferon gamma response (GSVA score=2.94) and apical junction
signaling pathways (GSVA score=2.68; Fig. 4B). The pathways enriched by
OSBPL11 included the IL-17 (P=0.002), PPAR (P=0.001) and TNF
signaling pathways (P=0.0003; Fig.
4C). GSVA analysis revealed that high expression of
OSBPL11 was enriched in signaling pathways such as
interferon gamma response (GSVA score=4.78), apical junction (GSVA
score=3.26) and reactive oxygen species pathways (GSVA score=3.35;
Fig. 4D). The pathways enriched by
MSMO1 include DNA replication (P=0.005), propanoate
metabolism (P=0.004) and protein export pathways (P=0.002; Fig. 4E) and high MSMO1 expression
was enriched in signaling pathways including inflammatory response
(GSVA score=3.19), protein secretion (GSVA score=2.96) and HEME
metabolism (GSVA score=2.86; Fig.
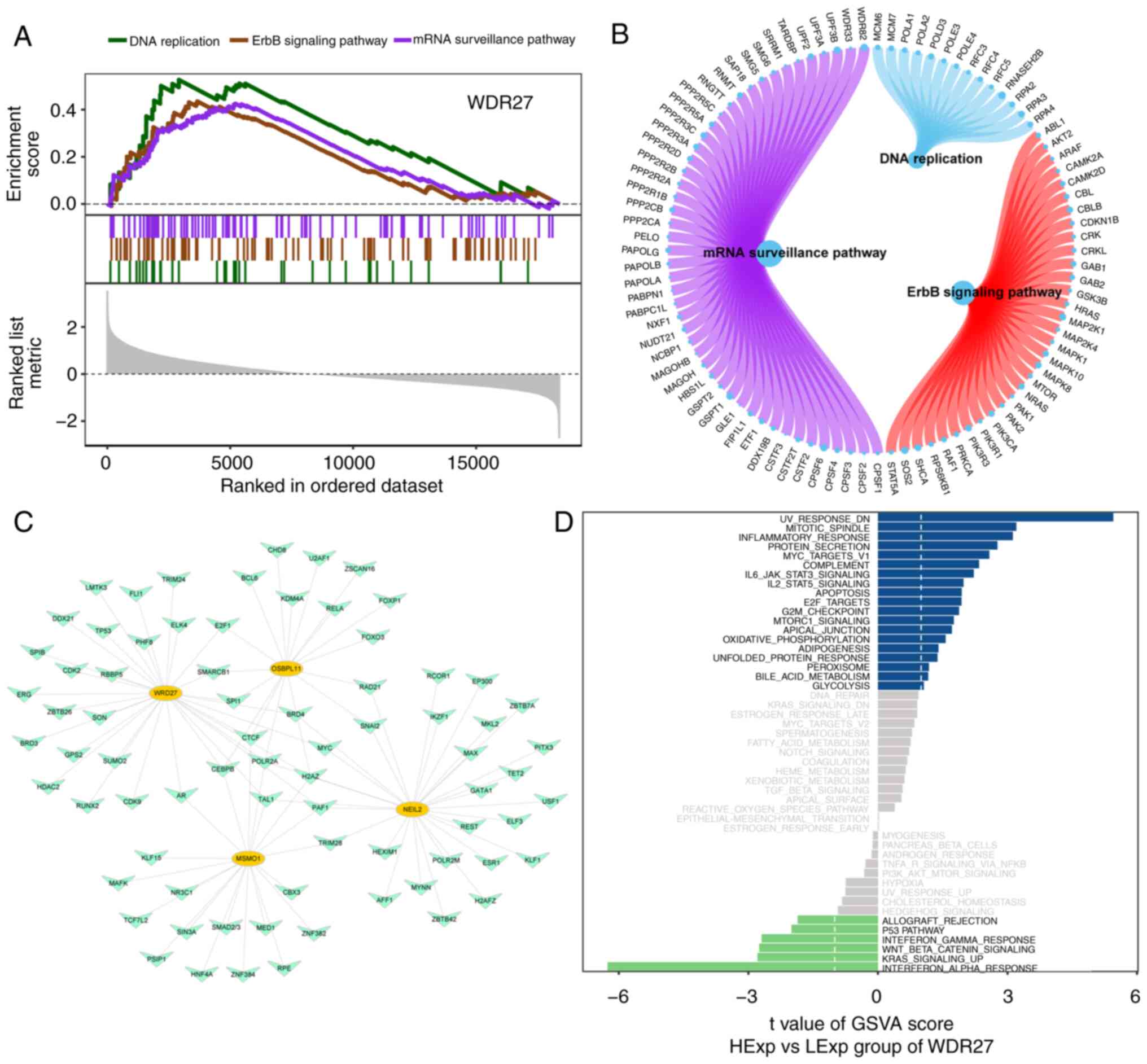
4F). WDR27 enriched pathways included DNA replication
(P=0.002), ErbB signaling pathway (P=0.002) and mRNA surveillance
pathway (P=0.001; Fig. 5A and
B). The GSVA results showed that
high expression of WDR27 was enriched in the inflammatory
(GSVA score=3.12) and IL6 JAK STAT3 signaling pathways (GSVA
score=2.22; Fig. 5D).
Transcriptional regulation of hub gene
analysis
Four hub genes were used as gene sets for this
analysis to further explore the transcriptional regulatory networks
involved in the hub genes. Using the Cistrome DB database, 69
transcription factors were predicted using MSMO1, 104 using
NEIL2, 88 transcription factors were predicted using
OSBPL11 and 107 transcription factors were predicted using
WDR27. Visualization of a comprehensive transcriptional
regulatory network of hub genes for disulfide ptosis in MMD was
constructed using Cytoscape (Fig.
5C).
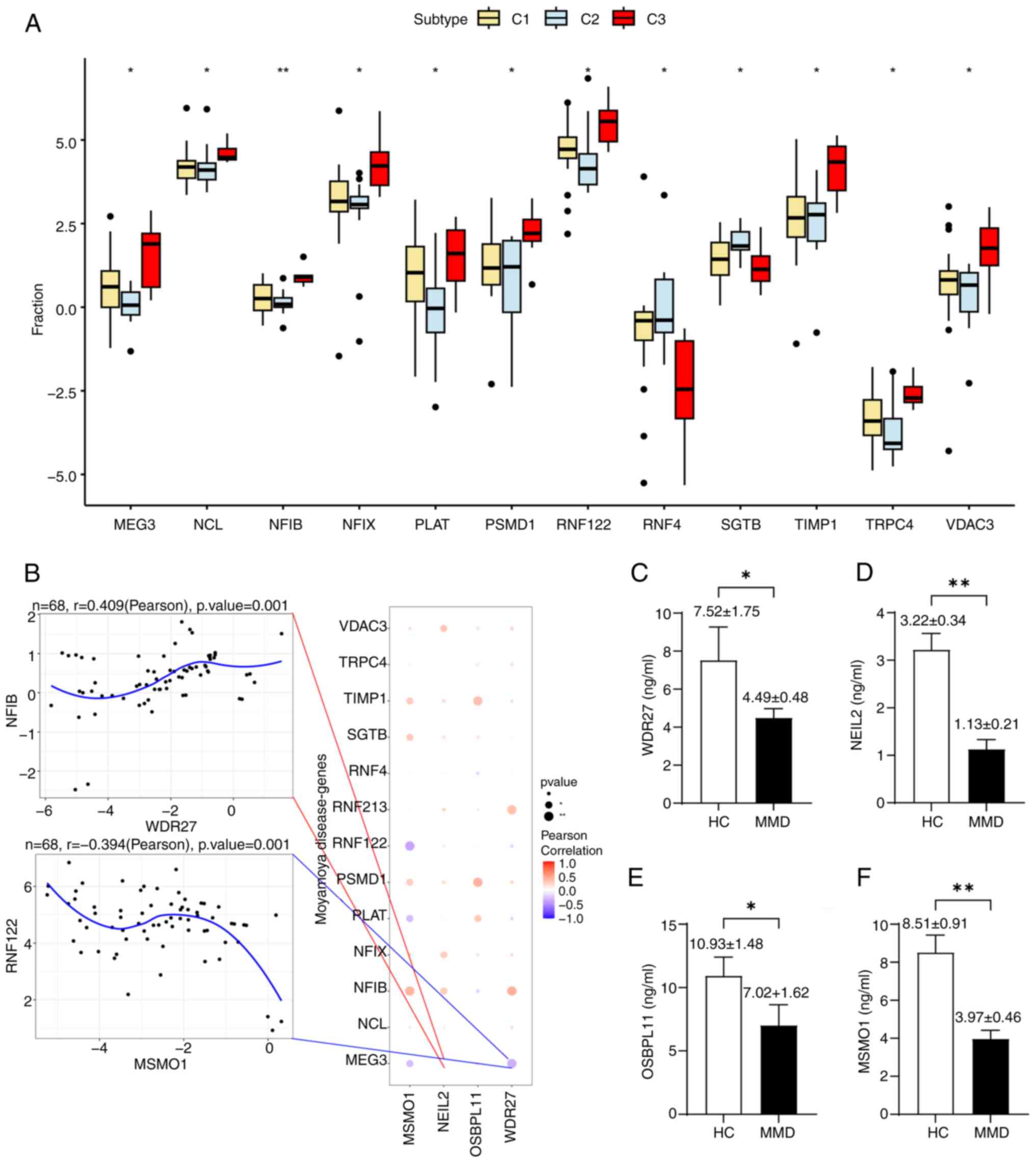
MMD-related genes correlation
analysis
Differential analysis of MMD-related genes revealed
that MEG3, NCL, NFIB and others were
significantly differentially expressed in different molecular
groups of MMD, which indicated different gene phenotypes in the
molecular classification based on disulfidptosis-related genes
(Fig. 6A). The expression levels
of hub genes were also significantly correlated with those of
various MMD-related genes (Fig.
6B). NEIL2 showed a significant positive correlation
with MEG3 (Pearson's r=0.4), whereas WDR27 was
negatively correlated with MEG3 (Pearson's r=0.415).
Endothelial migration and
proliferation-related genes correlation analysis
The expression levels of the hub genes were
significantly correlated with various endothelial migration-related
genes (Table SIII). In
particular, OSBPL11 showed a significant negative
correlation with GIPC1 (Pearson's r=-0.613) and NEIL2
was significantly correlated with SOX18 (Pearson's
r=-0.575). Hub genes were also significantly related to various
endothelial proliferation-related genes (Table SIV). MSMO1 showed a
significant positive correlation with SEMA5A (Pearson's
r=0.582) and WDR27 was significantly correlated with
MSMO1 (Pearson's r=0.737).
ELISA
The results showed that WDR27 (P=0.045),
NEIL2 (P=0.0008), OSBPL11 (P=0.037) and MSMO1
(P=0.0015) were all significantly decreased in patients with MMD
compared to healthy controls, as shown in Fig. 6C-F, which correlated with the
results of previous bioinformatics analyses.
Discussion
The potential etiology and pathogenesis of MMD have
long been studied. Reduced RNF213 expression increases
endothelial cell proliferation, migration and tube formation by
inducing vascular endothelial growth factor receptor 2
overexpression (16). Takagi et
al (17) indicated that
caspase-3-dependent apoptosis occurred in the MCA media of MMD. The
cell death mechanisms may be important for exploring the potential
molecular pathogenesis of MMD. Recent studies have discovered a new
type of disulfide-induced cell death in human cells known as
disulfidptosis. Dysregulated disulfidptosis in target cells may
lead to the abnormal proliferation of endothelial cells, which
could lead to chronic vascular stenosis during MMD pathogenesis. In
the present study, the effects of disulfidptosis on the expression
profiles of arteries occluded by MMD were explored.
The current study identified 29 significantly
differentially expressed DRGs and four hub genes, namely
WDR27, OSBPL11, MSMO1 and NEIL2, were
selected. It was hypothesized that these hub genes may be potential
biomarkers for the molecular classification of MMD based on
disulfidptosis. The expression levels of these four hub genes were
validated using ELISA performed using serum samples from patients
with MMD and healthy controls. Thereby, an additional clinical
cohort was recruited to verify the differentially expressed hub
genes, to validate the results obtained from the bioinformatics
analyses. Disulfidptosis plays an important role in cancer and is
reported to be significantly different in most cancers, such as
lung adenocarcinoma and uterine corpus endometrial carcinoma, and
it is also related to physiological discrepancies in different
parts of the body (18).
Neurological tumors are more likely to experience glucose
starvation, leading to high disulfidptosis rates, affecting the
response to antitumor drugs and prognosis (18). MMD, which affects the brain, may
have a similar status to neurological tumors and may express
abnormal levels of disulfidptosis.
A clinical trial from Japan identified the
significant difference between a surgical and nonsurgical group,
which suggested the preventive effect of the direct bypass against
rebleeding in MMD. Furthermore, the results showed that the
subgroup with perforating arteries from the choroidal artery or
posterior cerebral artery had a higher risk of rebleeding, which
suggested the association of hemorrhage with different vascular
phenotypes in patients with MMD (19). The current study focused on the
differential expression of disulfidptosis in patients with MMD and
explored the effects of disulfidptosis on MMD pathogenesis. Using
bioinformatic analyses of this novel cell death mechanism, a novel
molecular classification of MMD was discovered, which may be
validated in the future to explain the different risks in clinical
MMD subgroups identified in previous studies from a molecular
perspective.
Soluble CD163 and CXCL5 are reportedly increased in
patients with MMD and correlated with CD163+ M2-polarized
macrophages, which may be implicated in the pathogenesis of MMD
(4). Wang et al (20) identified through proteomics that
certain expressed proteins that were significantly related to the
immune response may lead to increased endothelial proliferation in
MMD. Therefore, in the present study, specific immune cells were
chosen for the correlation analysis with hub genes by performing an
immune infiltration analysis in MMD, to identify which cell types
may be involved in MMD pathogenesis.
WDR27 is a scaffold protein that contains multiple
WD repeats. It has been demonstrated that WDR27 is involved in the
anti-tumor necrosis factor response in rheumatoid arthritis
(21). Furthermore, WDR27 was also
found to be related to immunity and cell movement in monkeypox
infection (22). In the present
study, WDR27 was also found to be significantly correlated with
immune responses, such as resting CD4 memory T cells and memory B
cells in MMD, which could lead to immune dysregulation related to
chronic vascular stenosis. Of note, genome-wide association
analyses of sleep disturbances revealed that WDR27 is related to
insomnia symptoms (23).
OSBPL11 has a similar structure and sequence to
OSBP, leading to similar biological functions. OSBP binds to
oxysterols, which inhibit cholesterol synthesis (24). Bouchard et al (25) identified that OSBPL11 is related to
cholesterol and glucose metabolism in obesity, which could lead to
high cardiovascular disease risk. In the present study, KEGG
enrichment analysis also revealed that glycan degradation pathways
were significantly different between patients with MMD and
controls, which may be due to abnormal glucose metabolism induced
by downregulated OSBPL11 in MMD. A characteristic glucose
hypometabolic pattern has been reported in patients with MMD with
vascular cognitive impairment, and it has been indicated that
abnormal brain glucose metabolism is related to cognitive
impairment in MMD (26).
MSOM1 plays an important role in cholesterol
synthesis (27). Previous studies
have reported that MSMO1 promotes the development of different
cancers, such as liver, breast and oligodendrogliomas (28-30).
It was identified that downregulation of MSMO1 in pancreatic cancer
is associated with advanced progression and poor prognosis.
Epithelial-mesenchymal transition-like cell morphology and cell
mobility were activated by MSMO1 knockout, indicating that MSMO1
may induce cell motility and cell migration (31). The present results also showed a
similar downregulation of MSMO1 in MMD compared to controls, which
may be involved in the abnormal endothelial migration in MMD.
NEIL2 is generally required to protect against
oxidative DNA damage and maintain gene stability (32). A previous study has demonstrated
that NEIL2-deficient mice have an increased susceptibility to
inflammation with pro-inflammatory mediators (33). It has also been suggested that
NEIL2 inhibits the inflammatory response in bacterial and viral
infections (34). The present
results also showed a significant downregulation of NEIL2 in
MMD, and NEIL2 was significantly correlated with
neutrophils, which indicated abnormal inflammation in the arterial
wall that may lead to chronic occlusion in MMD. NEIL2 may have a
tumor-suppressive role in various cancers, including lung
adenocarcinoma, cervical cancer and breast cancer (35,36).
The current study has several limitations. First,
more participants with MMD should be enrolled in studies on
disulfidptosis in MMD. As the annual incidence of MMD is 0.5-1.5
per 100,000 individuals in East Asian countries but as low as 0.1
per 100,000 in other regions, including North America, the datasets
retrieved with a sample size >6 yielded only four microarray
datasets (2). The small sample
size may increase the risk of overfitting the data and lead to bias
in the results, which means that the results may not be generalized
to larger populations. Second, the datasets included in the present
study lacked the clinical prognosis of patients with MMD, and a
prognostic model could not be constructed for different subtypes of
MMD. Third, the roles of disulfidptosis in MMD were studied using
only bioinformatics analyses, which were not verified with in
vitro experiments. The cell models could be constructed using
gene knockdown/overexpression to validate the effects of
differentially expressed genes in MMD. The cell viability, cell
migration and ability of vascular formation could be explored
between different cell groups with various cell functional assays.
Bioinformatic analysis could lead to bias in the results. The
connection between disulfidptosis and MMD needs to be examined in
future studies. Fourth, the CIBERSORT method, a type of
deconvolution algorithm, was used to perform immune infiltration
analysis, which can predict the proportion of cell types that are
not actually present and decrease the accuracy of the results. A
high correlation may exist in the gene expression of different cell
types during analysis, which might lead to unstable analysis
results and decrease their accuracy.
In conclusion, in the present study, a novel
molecular classification of MMD based on disulfidptosis gene
expression was constructed and 348 upregulated genes and 801
downregulated genes were identified in MMD compared to controls. A
total of four hub genes (WDR27, OSBPL11, MSOM1
and NEIL2) were selected as biomarkers for the different
subtypes of MMD. The DRG results identified that disulfidptosis may
affect the progression of MMD pathogenesis. Based on this, MMD
molecular subtypes were established and four hub genes were
selected. Immune infiltration analysis indicated that the
correlation between hub genes and immune dysfunction could lead to
abnormal migration and proliferation of endothelial cells in MMD.
The results of GSEA and GSVA correlated with the results of immune
dysfunction. Furthermore, the expression of hub genes was validated
in an internal cohort using ELISA, which was in agreement with
previous analyses. To the best of our knowledge, this study was the
first to explore the relationship between MMD and disulfidptosis,
which may provide a new perspective to indicate a novel subtype
classification of MMD and new biomarkers for the diagnosis of
MMD.
Supplementary Material
Detailed clinical information of the
participants.
Clinical and demographic
characteristics of MMD participants.
Correlation analysis between hub genes
and endothelial migration-related genes.
Correlation analysis between hub genes
and endothelial proliferation-related genes.
Acknowledgements
Not applicable.
Funding
Funding: This research was funded by the National Natural
Science Foundation of China (grant no. 82371296 to RW), which
covered the expenses related to testing and processing, data
collection, analysis and interpretation of the experimental
data.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SH and RW conceived the study and designed the
experiments. SH, YW, YS, JZ, ZZ and YZ collected data, performed
bioinformatics analysis, performed experiments and discussed the
contents. YW and YS checked and confirmed the authenticity of the
raw data. All authors wrote and revised the manuscript. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Ethics
Committee of Beijing Tiantan Hospital (Beijing, China). The
bioinformatics study was approved (approval no. KY 2023-2024-02) in
2023, while the blood samples were obtained from July 2021 to
December 2022 which was approved (approval no. KY 2020-045-02) in
May 2020. Written informed consent was obtained from all
participants in accordance with the Declaration of Helsinki and the
study protocol was approved by the Ethics Committee of Beijing
Tiantan Hospital (approval no. KY 2020-045-02).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Suzuki J and Takaku A: Cerebrovascular
‘moyamoya’ disease. Disease showing abnormal net-like vessels in
base of brain. Arch Neurol. 20:288–299. 1969.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ihara M, Yamamoto Y, Hattori Y, Liu W,
Kobayashi H, Ishiyama H, Yoshimoto T, Miyawaki S, Clausen T, Bang
OY, et al: Moyamoya disease: Diagnosis and interventions. Lancet
Neurol. 21:747–758. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mertens R, Graupera M, Gerhardt H, Bersano
A, Tournier-Lasserve E, Mensah MA, Mundlos S and Vajkoczy P: The
genetic basis of moyamoya disease. Transl Stroke Res. 13:25–45.
2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fujimura M, Fujimura T, Kakizaki A,
Sato-Maeda M, Niizuma K, Tomata Y, Aiba S and Tominaga T: Increased
serum production of soluble CD163 and CXCL5 in patients with
moyamoya disease: Involvement of intrinsic immune reaction in its
pathogenesis. Brain Res. 1679:39–44. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sun H, Li W, Xia C, Ren Y, Ma L, Xiao A,
You C, Liu Y and Tian R: Angiographic and hemodynamic features in
asymptomatic hemispheres of patients with moyamoya disease. Stroke.
53:210–217. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Takekawa Y, Umezawa T, Ueno Y, Sawada T
and Kobayashi M: Pathological and immunohistochemical findings of
an autopsy case of adult moyamoya disease. Neuropathology.
24:236–242. 2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
He S, Zhang J, Liu Z, Wang Y, Hao X, Wang
X, Zhou Z, Ye X, Zhao Y, Zhao Y and Wang R: Upregulated
cytoskeletal proteins promote pathological angiogenesis in moyamoya
disease. Stroke. 54:3153–3164. 2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu X, Nie L, Zhang Y, Yan Y, Wang C,
Colic M, Olszewski K, Horbath A, Chen X, Lei G, et al: Actin
cytoskeleton vulnerability to disulfide stress mediates
disulfidptosis. Nat Cell Biol. 25:404–414. 2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Koppula P, Zhuang L and Gan B: Cystine
transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient
dependency, and cancer therapy. Protein Cell. 12:599–620.
2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang L, Rashad S, Zhou Y, Niizuma K and
Tominaga T: RNF213 loss of function reshapes vascular transcriptome
and spliceosome leading to disrupted angiogenesis and aggravated
vascular inflammatory responses. J Cereb Blood Flow Metab.
42:2107–2122. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang M, Zhang B, Jin F, Li G, Cui C and
Feng S: Exosomal MicroRNAs: Biomarkers of moyamoya disease and
involvement in vascular cytoskeleton reconstruction. Heliyon.
10(e32022)2024.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mamiya T, Kanamori F, Yokoyama K, Ota A,
Karnan S, Uda K, Araki Y, Maesawa S, Yoshikawa K and Saito R: Long
noncoding RNA profile of the intracranial artery in patients with
moyamoya disease. J Neurosurg. 138:709–716. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kanamori F, Yokoyama K, Ota A, Yoshikawa
K, Karnan S, Maruwaka M, Shimizu K, Ota S, Uda K, Araki Y, et al:
Transcriptome-wide analysis of intracranial artery in patients with
moyamoya disease showing upregulation of immune response, and
downregulation of oxidative phosphorylation and DNA repair.
Neurosurg Focus. 51(E3)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Newman AM, Liu CL, Green MR, Gentles AJ,
Feng W, Xu Y, Hoang CD, Diehn M and Alizadeh AA: Robust enumeration
of cell subsets from tissue expression profiles. Nat Methods.
12:453–457. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fukui M: Guidelines for the diagnosis and
treatment of spontaneous occlusion of the circle of Willis
(‘moyamoya’ disease). Research committee on spontaneous occlusion
of the circle of Willis (Moyamoya disease) of the ministry of
health and welfare, Japan. Clin Neurol Neurosurg. 99
(Suppl):238–240. 1997.PubMed/NCBI
|
|
16
|
Ye F, Niu X, Liang F, Dai Y, Liang J, Li
J, Wu X, Zheng H, Qi T and Sheng W: RNF213 loss-of-function
promotes pathological angiogenesis in moyamoya disease via the
Hippo pathway. Brain. 146:4674–4689. 2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Takagi Y, Kikuta K, Sadamasa V, Nozaki K
and Hashimoto N: Caspase-3-dependent apoptosis in middle cerebral
arteries in patients with moyamoya disease. Neurosurgery.
59:894–900. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhao D, Meng Y, Dian Y, Zhou Q, Sun Y, Le
J, Zeng F, Chen X, He Y and Deng G: Molecular landmarks of tumor
disulfidptosis across cancer types to promote disulfidptosis-target
therapy. Redox Biol. 68(102966)2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Miyamoto S, Yoshimoto T, Hashimoto N,
Okada Y, Tsuji I, Tominaga T, Nakagawara J and Takahashi JC:
Effects of extracranial-intracranial bypass for patients with
hemorrhagic moyamoya disease: Results of the Japan adult moyamoya
trial. Stroke. 45:1415–1421. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang X, Han C, Jia Y, Wang J, Ge W and
Duan L: Proteomic profiling of exosomes from hemorrhagic moyamoya
disease and dysfunction of mitochondria in endothelial cells.
Stroke. 52:3351–3361. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Honne K, Hallgrímsdóttir I, Wu C, Sebro R,
Jewell NP, Sakurai T, Iwamoto M, Minota S and Jawaheer D: A
longitudinal genome-wide association study of anti-tumor necrosis
factor response among Japanese patients with rheumatoid arthritis.
Arthritis Res Ther. 18(12)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Anuraga G, Lang J, Xuan DTM, Ta HDK, Jiang
JZ, Sun Z, Dey S, Kumar S, Singh A, Kajla G, et al: Integrated
bioinformatics approaches to investigate alterations in
transcriptomic profiles of monkeypox infected human cell line
model. J Infect Public Health. 17:60–69. 2024.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lane JM, Liang J, Vlasac I, Anderson SG,
Bechtold DA, Bowden J, Emsley R, Gill S, Little MA, Luik AI, et al:
Genome-wide association analyses of sleep disturbance traits
identify new loci and highlight shared genetics with
neuropsychiatric and metabolic traits. Nat Genet. 49:274–281.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Diercks AH, Podolskaia IS, Murray TA, Jahn
AN, Mai D, Liu D, Amon LM, Nakagawa Y, Shimano H, Aderem A and Gold
ES: Oxysterol binding protein regulates the resolution of
TLR-induced cytokine production in macrophages. Proc Natl Acad Sci
U S A. 121(e2406492121)2024.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bouchard L, Faucher G, Tchernof A,
Deshaies Y, Marceau S, Lescelleur O, Biron S, Bouchard C, Pérusse L
and Vohl MC: Association of OSBPL11 gene polymorphisms with
cardiovascular disease risk factors in obesity. Obesity (Silver
Spring). 17:1466–1472. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Weng R, Ren S, Su J, Ni W, Yang C, Gao X,
Xiao W, Zhang X, Jiang H, Guan Y, et al: 18F-FDG PET and a
classifier algorithm reveal a characteristic glucose metabolic
pattern in adult patients with moyamoya disease and vascular
cognitive impairment. Brain Imaging Behav. 17:185–199.
2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kalay Yildizhan I, Gökpınar İli E,
Onoufriadis A, Kocyigit P, Kesidou E, Simpson MA, McGrath JA,
Kutlay NY and Kundakci N: New homozygous missense MSMO1 mutation in
two siblings with SC4MOL deficiency presenting with psoriasiform
dermatitis. Cytogenet Genome Res. 160:523–530. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Xu P, Wu M, Chen H and Xu J, Wu M, Li M,
Qian F and Xu J: Bioinformatics analysis of hepatitis C virus
genotype 2a-induced human hepatocellular carcinoma in Huh7 cells.
Onco Targets Ther. 9:191–202. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Simigdala N, Gao Q, Pancholi S,
Roberg-Larsen H, Zvelebil M, Ribas R, Folkerd E, Thompson A, Bhamra
A, Dowsett M and Martin LA: Cholesterol biosynthesis pathway as a
novel mechanism of resistance to estrogen deprivation in estrogen
receptor-positive breast cancer. Breast Cancer Res.
18(58)2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
He P, Sun L, Zhu D, Zhang H, Zhang L, Guo
Y, Liu S, Zhou J, Xu X and Xie P: Knock-down of endogenous
bornavirus-like nucleoprotein 1 inhibits cell growth and induces
apoptosis in human oligodendroglia cells. Int J Mol Sci.
17(435)2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cao R, Zhang Z, Tian C, Sheng W, Dong Q
and Dong M: Down-regulation of MSMO1 promotes the development and
progression of pancreatic cancer. J Cancer. 13:3013–3021.
2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tapryal N, Chakraborty A, Saha K, Islam A,
Pan L, Hosoki K, Sayed IM, Duran JM, Alcantara J, Castillo V, et
al: The DNA glycosylase NEIL2 is protective during SARS-CoV-2
infection. Nat Commun. 14(8169)2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chakraborty A, Wakamiya M, Venkova-Canova
T, Pandita RK, Aguilera-Aguirre L, Sarker AH, Singh DK, Hosoki K,
Wood TG, Sharma G, et al: Neil2-null mice accumulate oxidized DNA
bases in the transcriptionally active sequences of the genome and
are susceptible to innate inflammation. J Biol Chem.
290:24636–24648. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sayed IM, Sahan AZ, Venkova T, Chakraborty
A, Mukhopadhyay D, Bimczok D, Beswick EJ, Reyes VE, Pinchuk I,
Sahoo D, et al: Helicobacter pylori infection downregulates the DNA
glycosylase NEIL2, resulting in increased genome damage and
inflammation in gastric epithelial cells. J Biol Chem.
295:11082–11098. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sarker AH, Chatterjee A, Williams M, Lin
S, Havel C, Jacob P III, Boldogh I, Hazra TK, Talbot P and Hang B:
NEIL2 protects against oxidative DNA damage induced by sidestream
smoke in human cells. PLoS One. 9(e90261)2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ye F, Liu J, Wang H, Chen X, Cheng Q and
Chen H: Cervical carcinoma risk associate with genetic
polymorphisms of NEIL2 gene in Chinese population and its
significance as predictive biomarker. Sci Rep.
10(5136)2020.PubMed/NCBI View Article : Google Scholar
|