Introduction
Periodontal diseases, particularly periodontitis,
pose a significant public health concern due to their high
prevalence and effect on both oral and systemic health (1,2). The
classification of periodontitis into stages I-IV and Grades A-C by
the American Academy of Periodontology (AAP) and the European
Federation of Periodontology (EFP) highlights the critical role of
risk factors, including genetic predisposition, systemic diseases,
lifestyle choices and environmental influences, in shaping disease
progression and treatment outcomes (3). A thorough understanding of these risk
factors, integrated with the standardized classification system, is
essential for accurately predicting individual disease trajectories
and optimizing therapeutic strategies.
Non-surgical periodontal therapy (NSPT), which
includes scaling and root planing, remains the foundational
treatment for patients with periodontitis. This therapy aims to
reduce probing depth (PD) and improve clinical attachment levels
(4). However, patient responses to
NSPT vary widely, underscoring the need for a deeper understanding
of the prognostic factors that influence treatment outcomes
(5). Identifying these factors is
essential for developing personalized and accurate prognostic and
therapeutic strategies.
The reduction in PD achieved through NSPT is
influenced by a multitude of factors, including microbial, host
immune, genetic and behavioral components (6). Although prior studies have suggested
factors such as smoking status, systemic health and periodontal
disease severity as potential predictors of treatment response, the
true effects of these factors are currently unclear due to
variations in study design and analytical methods. Additionally,
genetic susceptibility, microbiological characteristics and
periodontal conditions differ between Chinese and Caucasian
populations (7) and the existing
findings are inconsistent in their conclusions. These
inconsistencies highlight the need for rigorous investigation to
comprehensively elucidate the determinants of PD reduction.
Traditional statistical analysis methods such as
logistic regression and ANOVA can fail to adequately process
periodontal data due to their inability to account for the
hierarchical structures in these data, involving patients, teeth
and sites of disease (8). This
limitation can result in underestimated standard errors and
potentially misleading conclusions when data at lower levels are
aggregated to higher levels. Mixed-effects models, particularly
hierarchical linear models (HLM), address these shortcomings by
effectively handling multilevel nested data (9). These models decompose variation
across different levels and analyze interactions between them,
enabling a more precise separation of the effects of predictor
variables at each level. Mixed-effects models have been extensively
used in periodontal research (10). For instance, Jiao et al
(11) employed HLM to identify
predictors of periodontal disease progression at both the patient
and tooth levels. Beyond periodontics, mixed-effects models have
also been applied to nested data in endodontics (9) and prosthodontics and orthodontics
(12), underscoring their
versatility and robustness in dental research.
The null hypothesis of the present study was that
any observed changes in PD reduction were due to random variation,
rather than the influence of specific prognostic factors.
Therefore, the present study aimed to identify prognostic factors
associated with PD reduction following NSPT by using a linear mixed
effects model which analyzed decomposing variance contributions of
these factors across the patient, tooth and site levels. These
findings may have the potential to refine clinical decision-making
for the treatment of patients with periodontitis and improve
prognostic assessments in periodontal therapy.
Materials and methods
Study design and patients
The present retrospective observational study
evaluated prognostic factors influencing probing depth reduction
following NSPT in patients with periodontitis. A three-level nested
random-effects mixed-effects model was employed to assess the
effect of clinical characteristics and periodontal disease
classification on treatment outcomes.
Patients who received NSPT treatment at the
Department of Periodontology, School and Hospital of Stomatology at
Tianjin Medical University (Tianjin, China), were considered for
inclusion in the present study. Patients with ≥1 documented
periodontal re-evaluation record between January 2021 and January
2022 were included. The research protocol was approved by the
Ethics Committee of the Tianjin Medical University School and
Hospital of Stomatology (approval no. TMUhME20200307; Tianjin,
China). All subjects provided written informed consent for
participation in the present study.
The criteria for inclusion were as follows: i)
Patients aged ≥18 years; ii) patients diagnosed with periodontitis
according to the classification of periodontitis into stages (I-IV)
and Grades (A-C) by the AAP and the EFP (13); iii) patients completed NSPT and
attended a follow-up evaluation at 3 months; and iv) full
availability of complete baseline and follow-up records in the
electronic periodontal charting system. The following exclusion
criteria were used: i) Patients with acquired immune deficiency
syndrome, nephrosis, hepatitis or pregnancy which affected
periodontal treatment outcomes; ii) recent use of antibiotics or
undergoing periodontal surgeries prior to the evaluation period;
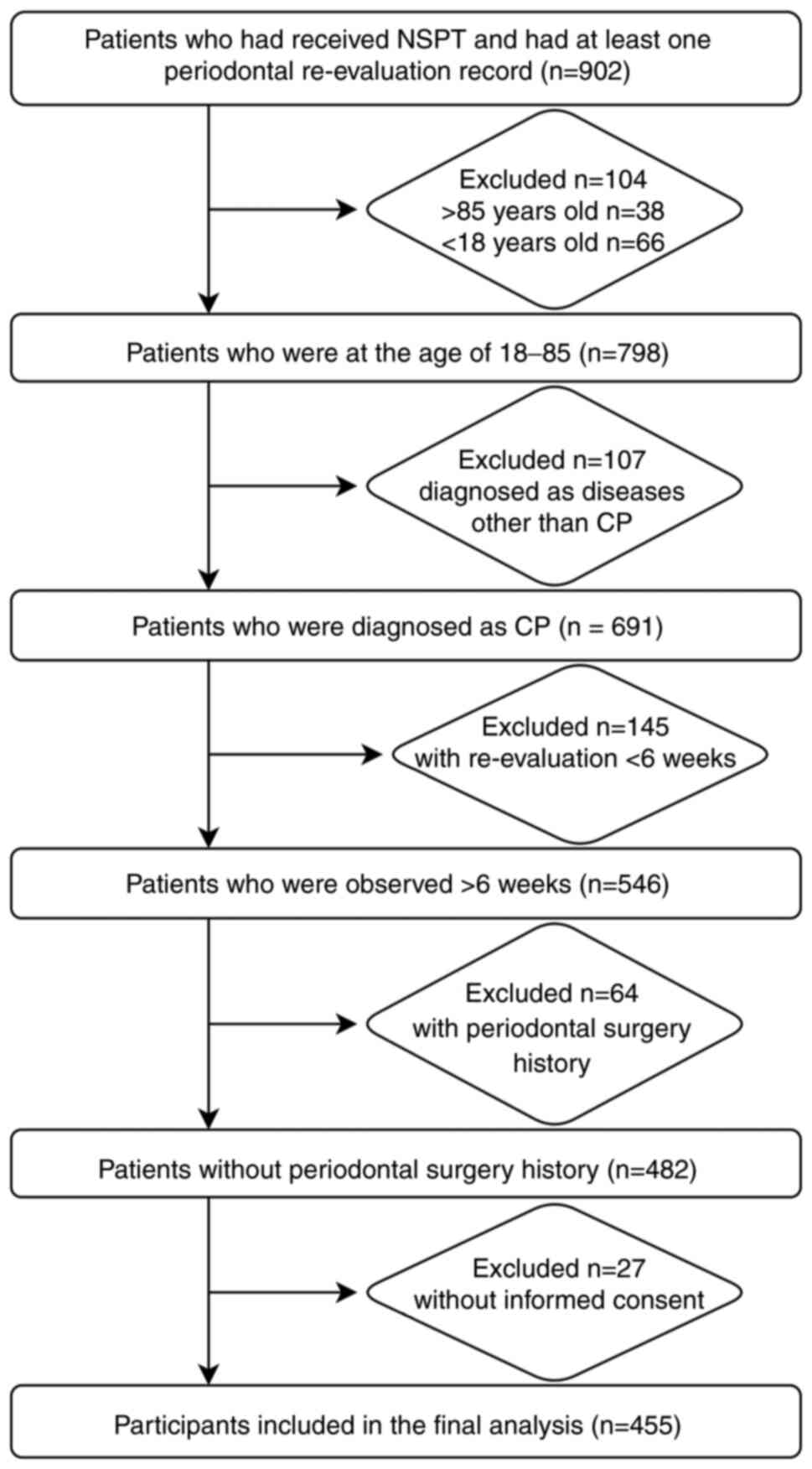
and iii) incomplete records or loss to follow-up. Fig. 1 illustrates the process of
selecting and screening patients.
Data extraction and variables
collected
Data were extracted from the electronic periodontal
charting record system at Tianjin Medical University. A detailed
assessment of patient-related parameters was conducted during the
first visit (T0) and final follow-up visit (T1).
The variables collected at the patient level were
demographic data (patient age and sex), frequency of daily tooth
brushing, diabetes mellitus status, smoking status and the stage
and Grade of periodontal disease classification. The variables
collected at the tooth level were the tooth type (central incisors,
lateral incisors, cuspid teeth, premolars or molars 1-28) and the
tooth mobility (0-III). The variables collected at the site level
were the results of the follow-up assessment, which was the PD at
the 3-month time point. PD was measured at six different sites:
Mesial, distal and middle sites of both the buccal and lingual
surfaces. The data from the third molars and teeth lost during NSPT
were excluded.
Primary outcome measure
The primary outcome measure of the present study was
the reduction in PD observed at the 3-month follow-up point after
the completion of NSPT. To assess PD reduction, baseline
measurements were recorded prior to the NSPT, followed by
subsequent evaluations conducted at a standardized 3-month
post-treatment timeframe. The difference between the mean baseline
PD and the mean PD recorded at the 3-month re-evaluation
constituted the PD reduction.
Periodontal examinations and
treatments
Standardized protocols for periodontal examinations
and treatments were adhered to, ensuring that all procedures were
uniformly applied by qualified clinical periodontists. This process
involved oral hygiene instruction (OHI) and scaling and root
planing (SRP) utilizing both ultrasonic scalers
(Cavitron® Ultrasonic Scaler; Dentsply Sirona) and hand
instruments (Gracey Curettes; Hu-Friedy) for sites demonstrating PD
≥4 mm following the initial evaluation. All patients were scheduled
for a re-evaluation at the 3-month time point post-treatment.
During the maintenance phase, comprehensive periodontal charting,
reinforcement of OHI, prophylactic scaling and SRP were also
performed. The probing depths at baseline (T0) and at the follow-up
assessment (T1), along with the reductions observed at various
sites, were analyzed.
Sample size estimation
The sample size calculation was primarily guided by
the complexity of the model and the need to ensure robust parameter
estimation. A key factor in determining the required sample size
was the ratio of the number of observations (N) to the number of
parameters to be estimated (K). Following best practices outlined
in previously published literature (14), a conservative N/K ratio of 10 was
used to ensure robust parameter estimation and avoid
overparameterization. This approach accounted for the complexity of
the model, including fixed effects, random effects and interaction
terms. The parameters of the present study included the intercept,
fixed effects, random effects, nested effects and the residual
term, totaling ~15 items. A model with 15 estimated parameters
would require a ≥150 observations to meet this threshold. The final
dataset comprised 455 patients, which ensured that the sample size
was sufficiently large to provide reliable estimates for all
parameter estimates included in the model.
Statistical analysis
R (version 4.2.2; R Foundation for Statistical
Computing; https://cran.r-project.org/) for Windows was used for
all statistical analyses (15,16).
Categorical variables were expressed as absolute frequencies [n
(%)], whereas continuous variables were expressed as the mean (SD).
The linear mixed effects model (LMM) was adopted to explain the
hierarchical and clustered structure of the patient, tooth and side
periodontal data. The site, tooth and patient level were included
as nested random effects to help to account for non-independence
using the R package ‘lme4’ (15).
A multi-model inference procedure was applied using
the R package ‘MuMIn’ (version 1.47.5) (17). This method was used to select the
model by creating a set of models with all possible combinations of
the initial variables and sorting them according to the Akaike
Information Criterion (AIC) fitted with the Maximum Likelihood
(18). All models with ΔAIC <2
were selected and the model averaging approach with lmer was used
to estimate parameters and associated P-values, using the function
model.avg (19). Variance
decomposition was performed to determine the variation of PD
reduction within the patient, tooth and site levels using the R
package ‘ape’ (version 5.8) (20).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Baseline characteristic of
participants
A total of 72,688 sites distributed on 12,119 teeth
in the 455 patients were included in the final analysis (Fig. 1). Characteristics and
patient-related factors analyzed were described in Table I.
 | Table IBaseline clinical and periodontal
parameters by variables for all patients (n=455). |
Table I
Baseline clinical and periodontal
parameters by variables for all patients (n=455).
| Characteristic | Value |
|---|
| Mean age ± SD,
years | 49.36±12.34 |
| Male sex, n (%) | 197 (43.3) |
| Non-smoking, n
(%) | 397 (87.3) |
| Diabetes mellitus, n
(%) | 427 (93.8) |
| Daily brushing
frequency, n (%) | |
|
1 | 18 (4.0) |
|
2 | 356 (78.2) |
|
3 | 71 (15.6) |
|
4 | 6 (1.3) |
|
5 | 4 (0.9) |
| Stage of
periodontitis, n (%) | |
|
Ⅰ | 1 (0.2) |
|
Ⅱ | 57 (12.5) |
|
Ⅲ | 331 (72.7) |
|
Ⅳ | 66 (14.5) |
| Grade of
periodontitis, n (%) | |
|
A | 0 (0.0) |
|
B | 185 (40.7) |
|
C | 270 (59.3) |
| Patient-level,
n | 455 |
| Tooth-level, n | 12,119 |
| Site-level, n | 72,688 |
Model selection and PD reduction
The overall mean PD reduction at the patient level
across all sites was 0.88 mm. The model selection process for the
linear mixed-effects models, which estimated the influence of PD
reduction, is detailed in Table
II. A total of seven models were chosen based on an ΔAIC of
<2 from all possible combinations of the initial variables
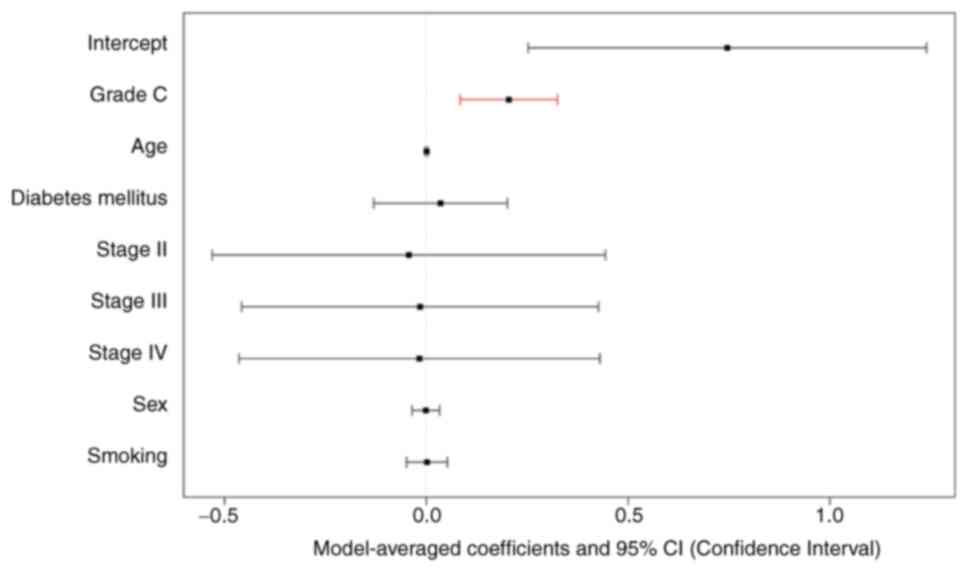
(Table II). Grade C periodontitis
was included, with a model-averaged coefficient of 0.20 [95%
confidence interval (CI), 0.08-0.33; P<0.001). The
model-averaged coefficients for fixed effects were measured
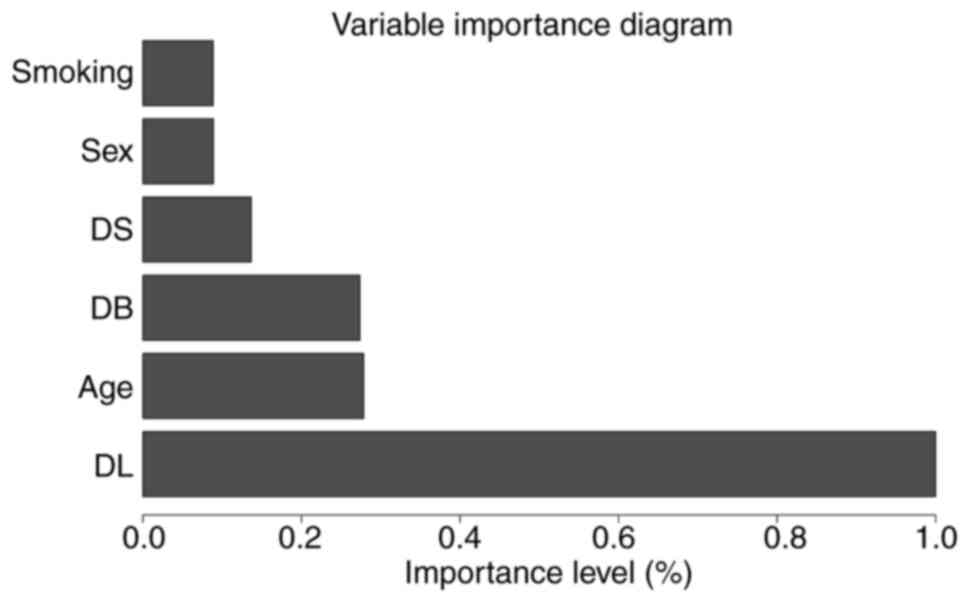
(Fig. 2) and the significance of
each independent fixed effect in the averaged model was analyzed
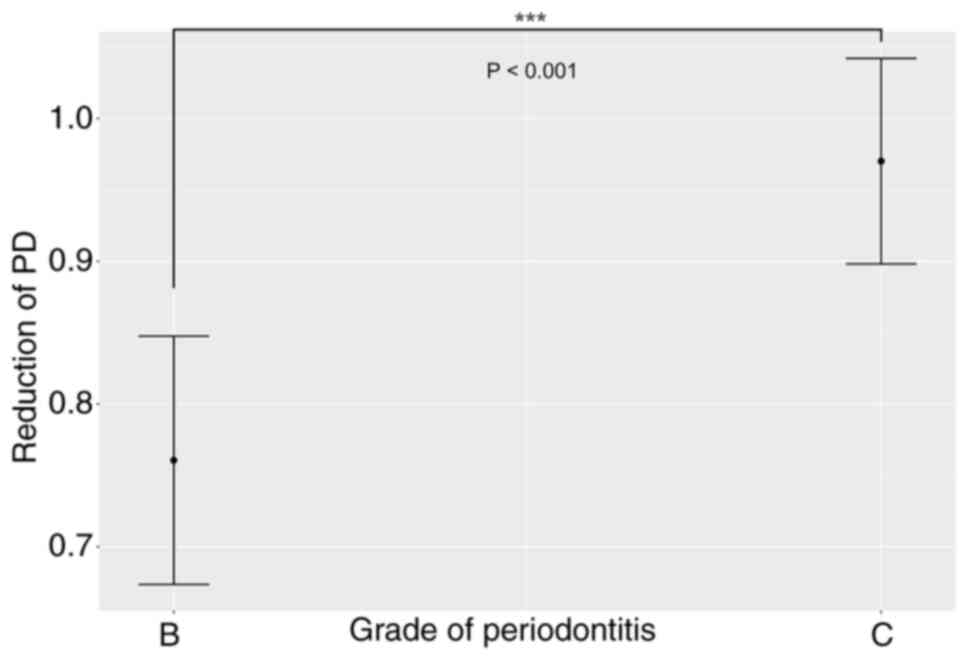
(Fig. 3). After adjusting for
other pertinent variables, the mixed-effects model demonstrated a
statistically significant effect of periodontitis Grade on PD
reduction following NSPT (Fig.
4).
 | Table IIModel selection process for the
linear mixed-effect models estimates the influence of PD reduction
with ΔAIC <2. |
Table II
Model selection process for the
linear mixed-effect models estimates the influence of PD reduction
with ΔAIC <2.
| Model | Model type | df | logLik | AIC | ΔAIC | AIC weight |
|---|
| Model 1 | DL +
(1|Patient/Tooth/Site) | 6 | -100402.8 | 200817.6 | 0.00 | 0.24 |
| Model 2 | DL + Age +
(1|Patient/Tooth/Site) | 7 | -100402.1 | 200818.1 | 0.59 | 0.18 |
| Model 3 | DL + DB +
(1|Patient/Tooth/Site) | 7 | -100402.1 | 200818.2 | 0.64 | 0.17 |
| Model 4 | DL + DS +
(1|Patient/Tooth/Site) | 9 | -100400.3 | 200818.7 | 1.09 | 0.14 |
| Model 5 | DL + Age + DB +
(1|Patient/Tooth/Site) | 8 | -100401.6 | 200819.2 | 1.67 | 0.10 |
| Model 6 | DL + Sex +
(1|Patient/Tooth/Site) | 7 | -100402.8 | 200819 5 | 1.95 | 0.09 |
| Model 7 | DL + Smoking +
(1|Patient/Tooth/Site) | 7 | -100402.8 | 200819.5 | 1.96 | 0.09 |
Prognostic factors for the reduction
of PD
The Grade of periodontitis demonstrated a
significant association with PD reduction following model
selection. A greater mean decrease in PD was observed in patients
with Grade C periodontitis compared with patients with Grade B
periodontitis (0.96 vs. 0.76 mm, respectively; z=3.32; P<0.001)
(Fig. 4). No significant
differences were observed concerning age, sex, diabetes mellitus or
daily brushing frequency.
Random effects and variance components
of PD reduction at the patient, tooth and site levels
Random effects analysis in the model-averaged
analysis indicated standard deviations of 59.4% for patient, 39.1%
for tooth and 73.8% for site levels. The marginal and conditional
R2 values in the model-average were 0.015 and 0.673,
respectively. The intraclass correlation coefficient was 0.67
(Table III). The R2
of the LMM was also calculated (Table III). Variance components for PD
reductions across all random effects were measured (Fig. 5).
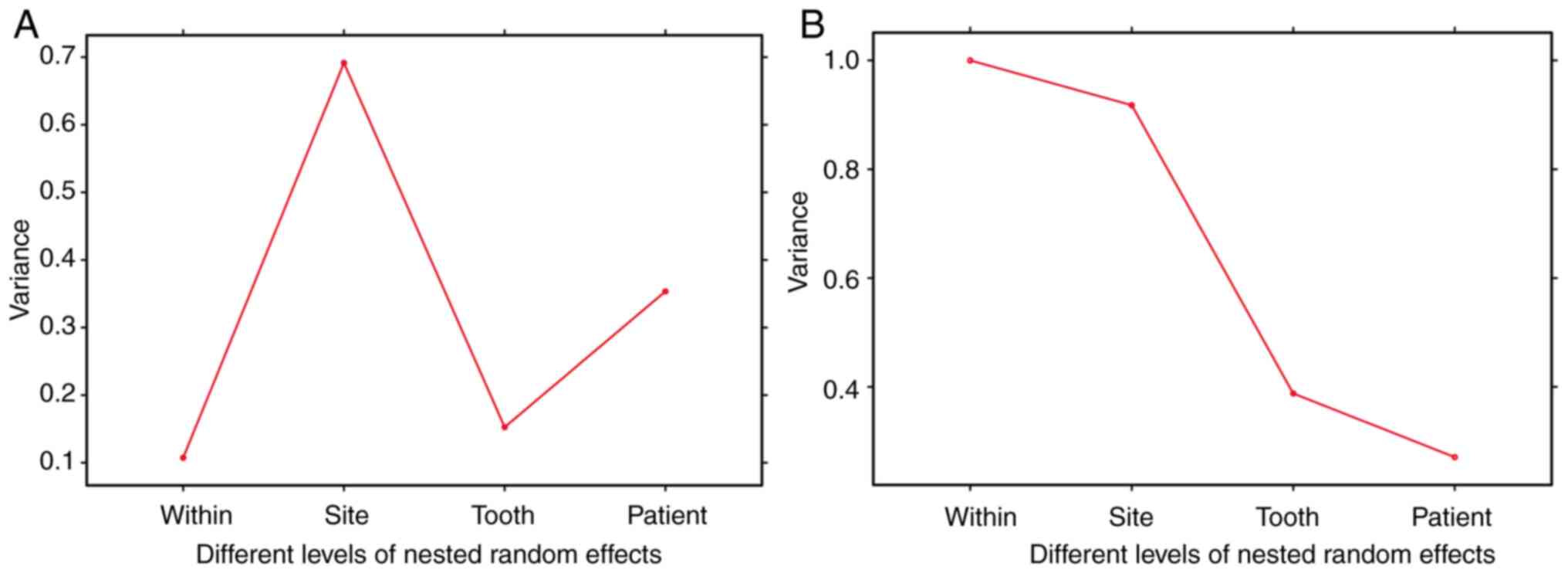 | Figure 5Different levels of nested random
effects account for the variance components in periodontal data.
Variance decomposition was conducted to ascertain the variation in
PD reduction using linear mixed-effects models with nested random
effects at the patient, tooth, and site levels. The analysis
involved two key aspects: (A) the estimation of random effects
variance parameters within residuals, sites, teeth, and patients,
and (B) the cumulative magnitudes of these random effects variance
parameters within residuals, sites, teeth, and patients. PD,
probing depth. |
 | Table IIIStatistics of random effects
(standard device) for seven models with ∆AIC <2 and
model-averaged value. |
Table III
Statistics of random effects
(standard device) for seven models with ∆AIC <2 and
model-averaged value.
| Model | SD (patient) | SD (tooth) | SD (site) | SD (residual) | Marginal R2 | Conditional R2 | ICC |
|---|
| Model 1 | 0.595 | 0.391 | 0.685 | 0.574 | 0.013 | 0.611 | 0.61 |
| Model 2 | 0.594 | 0.391 | 0.685 | 0.574 | 0.014 | 0.611 | 0.61 |
| Model 3 | 0.594 | 0.391 | 0.757 | 0.476 | 0.016 | 0.696 | 0.69 |
| Model 4 | 0.593 | 0.391 | 0.769 | 0.456 | 0.020 | 0.714 | 0.71 |
| Model 5 | 0.594 | 0.391 | 0.756 | 0.478 | 0.017 | 0.694 | 0.69 |
| Model 6 | 0.595 | 0.391 | 0.756 | 0.478 | 0.014 | 0.694 | 0.69 |
| Model 7 | 0.595 | 0.391 | 0.755 | 0.478 | 0.014 | 0.694 | 0.69 |
| Model-average | 0.594 | 0.391 | 0.738 | 0.502 | 0.015 | 0.673 | 0.67 |
Discussion
The present retrospective analysis of 455 patients
provided findings which could potentially determine the effect of
periodontitis grade on treatment outcomes. The mean reduction in PD
observed in the present study was 0.88 mm at the patient level
across all sites measured. Notably, patients with Grade C
periodontitis demonstrated a markedly greater reduction in PD
compared with patients with Grade B periodontitis. The
multivariable coefficient for Grade C periodontitis was 0.20,
indicating an independent association with enhanced treatment
efficacy. These findings were consistent with the hypothesis that
Grade C periodontitis, characterized by rapid progression and a
higher inflammatory burden, may respond more robustly to intensive
NSPT measures.
The findings of the present study were consistent
with a study that investigated the factors predicting responses to
NSPT in 40 Chinese patients over a 1-year observation period
(21). The mean PD reductions in
the aforementioned study were 0.62, 0.66 and 0.60 mm at 3, 6 and 12
months, respectively. Additionally, a systematic review assessing
the clinical efficacy of NSPT reported a weighted mean PD reduction
of 0.64 mm in pockets initially >5 mm in size (22). These results are generally aligned
with the present study, with minor variations probably due to
ethnographic differences. For instance, studies have demonstrated
differences between Caucasian and Asian populations (23). Previous research has used 16S
pyrosequencing to analyze bacterial profiles in patients with
chronic periodontitis demonstrated a relatively higher abundance of
Porphyromonas gingivalis in the Chinese population (17.85%)
and compared with other groups (11.26%) (24,25).
This may be one of the reasons for the difference in the results of
the present study.
The present study further corroborated previous
findings. Nascimento et al (26) suggested that individuals with
severe periodontitis at the baseline experienced more significant
treatment effects, whereas those with moderate periodontitis had a
limited benefit. Jiao et al (10,11)
reported that PD reduction was primarily influenced by baseline PD
and baseline attachment loss, which are both pivotal factors in
determining the stage of chronic periodontitis. In addition, Chen
et al (27) proposed that a
wider radiographic angle in teeth might predict improved outcomes
from NSPT.
The present study identified that neither sex nor
age were confounding factors, aligning with prior research. In the
multivariable analysis, the brushing frequency used did not
markedly influence PD reduction, which contradicts the results of a
previous study (28). This
discrepancy may be attributed to differences in sample size,
ethnicity and certain population characteristics. It is also
important to consider that definitions of adherence varied across
studies, potentially contributing to the observed differences.
Therefore, the results of the present study may require further
validation.
The variance decomposition analysis underscored the
substantial influence of patient-level and site-level factors on PD
reduction. The standard deviations attributable to random effects
at the patient, tooth and site levels were 59.4, 39.1 and 73.8%,
respectively. These results highlighted the complexity and
multi-faceted nature of periodontal disease and its response to
therapy. The significant variance at the site level suggested that
local factors, potentially including site-specific bacterial load,
local immune response and anatomical considerations, serve a
crucial role in therapeutic outcomes (29). The findings of the present study
aligned with existing literature, which emphasized that localized
bacterial load and the immune response were critical determinants
of periodontal healing as sites with higher bacterial loads often
demonstrated poorer patient outcomes despite effective treatment
(30). Additionally, anatomical
factors such as root morphology and pocket depth markedly influence
the accessibility and efficacy of non-surgical therapy,
underscoring the challenges posed by site-specific characteristics
(31). Future studies should
investigate recently introduced preventive treatments such as ozone
(32), photobiomodulation
(33) and paraprobiotics (34) to understand their potential effects
on periodontal tissues.
The nested random-effects model provides a robust
framework for evaluating hierarchical data (35). By accounting for the nested
structure of periodontal data, such as sites within teeth and teeth
within patients, the model allowed for a more nuanced understanding
of how different levels of variation contributed to treatment
efficacy. This methodology aligned with contemporary analytic
approaches aimed at disentangling the complex interrelationships
inherent in clinical periodontal data.
The present study adopted an observational design,
wherein all participants underwent a uniform NSPT. Instead of using
a randomized methodology, the study's primary aim was to document
the clinical characteristics and prognostic outcomes of patients in
real-world settings who are subjected to a standardized treatment
protocol. Although baseline differences among participants may be
present, these were systematically addressed through a linear
mixed-effects model that incorporated relevant covariates and
adjusted for both measured and unmeasured confounding variables.
This methodological approach facilitated an integrated analysis of
the interactions among various prognostic factors and their
influence on therapeutic outcomes, thereby offering a comprehensive
understanding of the determinants of NSPT efficacy. Nonetheless,
future prospective studies employing randomized designs could
further validate and refine these findings.
Despite the strengths of the present study,
including a large sample size and rigorous statistical analysis,
certain limitations warrant consideration. The retrospective design
inherently introduced potential biases related to medical record
accuracy and completeness. Additionally, the follow-up period was
limited to 3 months, precluding long-term assessment of PD
reduction sustainability. The research sample was obtained from the
Tianjin area of China and did not adequately represent populations
from different regions, ethnicities or socioeconomic backgrounds.
Moreover, the lack of consistent data on eating habits and details
of oral hygiene maintenance habits, which are known to influence
periodontal disease progression and treatment response. The absence
of these variables may have introduced residual confounding,
potentially affecting the observed associations and outcomes.
Future studies incorporating comprehensive data on these parameters
are essential to improved understand how external factors interact
with clinical characteristics.
In conclusion, the present study demonstrated that
Grade C periodontitis was independently associated with a greater
PD reduction following NSPT, emphasizing the importance of
individualized treatment approaches. Moreover, the prominent role
of site-specific factors underscores the necessity for targeted
therapeutic strategies that address local periodontal conditions.
These insights may contribute to the growing body of evidence
supporting tailored periodontal care aimed at optimizing clinical
outcomes.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
PK and CW designed the clinical study and
coordinated the research activities. CW and XB conducted patient
recruitment and managed clinical data collection. YY and FQ
performed the statistical analysis and contributed to data
interpretation. FQ, XL and MZ were responsible for the histological
examination and provided critical insights into the findings. PK
drafted the manuscript and incorporated comments from all authors.
CL made substantial contributions to the conception and design of
the study, critically revised the manuscript for important
intellectual content, approved the final version for publication
and agreed to be accountable for all aspects of the work. XB and MZ
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The research protocol was approved by the Ethics
Committee of the Tianjin Medical University School and Hospital of
Stomatology (approval no. TMUhME20200307).
Patient consent for publication
Written informed consent for publication was
obtained from all patients involved in the study.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Professor Changyi Li ORCID ID:
0000-0003-1444-4852.
References
|
1
|
Kapila YL: Oral health's inextricable
connection to systemic health: Special populations bring to bear
multimodal relationships and factors connecting periodontal disease
to systemic diseases and conditions. Periodontol 2000. 87:11–16.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Genco RJ and Sanz M: Clinical and public
health implications of periodontal and systemic diseases: An
overview. Periodontol 2000. 83:7–13. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tonetti MS, Greenwell H and Kornman KS:
Staging and grading of periodontitis: Framework and proposal of a
new classification and case definition. J Periodontol. 89 (Suppl
1):S159–S172. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Trombelli L, Franceschetti G and Farina R:
Effect of professional mechanical plaque removal performed on a
long-term, routine basis in the secondary prevention of
periodontitis: A systematic review. J Clin Periodontol. 42 (Suppl
16):S221–S236. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liu HJ, Wang B, Wang AC, Zhang DH, Mao C
and Li QH: Prognostic factors affecting the short-term efficacy of
non-surgical treatment of chronic periodontitis: A multilevel
modeling analysis. Eur J Med Res. 26(50)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Corbella S, Calciolari E, Alberti A, Donos
N and Francetti L: Systematic review and meta-analysis on the
adjunctive use of host immune modulators in non-surgical
periodontal treatment in healthy and systemically compromised
patients. Sci Rep. 11(12125)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Goh EXJ and Ong MMA: Anatomical,
microbiological, and genetic considerations in treatment of Chinese
periodontal patients. J Investig Clin Dent.
10(e12381)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hannigan A and Lynch CD: Statistical
methodology in oral and dental research: Pitfalls and
recommendations. J Dent. 41:385–392. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Masood M, Masood Y and Newton J: The
clustering effects of surfaces within the tooth and teeth within
individuals. J Dent Res. 94:281–288. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jiao J, Zhang L, Meng HX, Shi D, Lu RF, Xu
L, Feng XH and Cao ZQ: Clinical performance of non-surgical
periodontal therapy in a large Chinese population with generalized
aggressive periodontitis. J Clin Periodontol. 45:1184–1197.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jiao J, Shi D, Cao ZQ, Meng HX, Lu RF,
Zhang L, Song Y and Zhao JR: Effectiveness of non-surgical
periodontal therapy in a large Chinese population with chronic
periodontitis. J Clin Periodontol. 44:42–50. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pandis N, Walsh T, Polychronopoulou A and
Eliades T: Cluster randomized clinical trials in orthodontics:
Design, analysis and reporting issues. Eur J Orthod. 35:669–675.
2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Papapanou PN, Sanz M, Buduneli N, Dietrich
T, Feres M, Fine DH, Flemmig TF, Garcia R, Giannobile WV, Graziani
F, et al: Periodontitis: Consensus report of workgroup 2 of the
2017 World Workshop on the classification of periodontal and
peri-implant diseases and conditions. J Periodontol. 89 (Suppl
1):S173–S182. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Harrison XA, Donaldson L, Correa-Cano ME,
Evans J, Fisher DN, Goodwin CE, Robinson BS, Hodgson DJ and Inger
R: A brief introduction to mixed effects modelling and multi-model
inference in ecology. PeerJ. 6(e4794)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bates D, Mächler M, Bolker B and Walker S:
Fitting linear mixed-effects models using lme4 arXiv: Jun 23, 2014
(Epub ahead of print).
|
|
16
|
Team RC: R: A language and environment for
statistical computing. R Foundation for Statistical Computing,
Vienna. Available from: http://www.R-project.org/2013.
|
|
17
|
Barton K: MuMIn: Multi-Model Inference.
Available from: http://r-forge.r-project.org/projects/mumin/2009.
|
|
18
|
Posada D and Buckley TR: Model selection
and model averaging in phylogenetics: Advantages of akaike
information criterion and bayesian approaches over likelihood ratio
tests. Syst Biol. 53:793–808. 2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Awodutire PO, Ilori OR, Uwandu C and
Akadiri OA: Pilot study of new statistical models for prognostic
factors in short term survival of oral cancer. Afr Health Sci.
22:310–317. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nakagawa S, Schielzeth H and O'Hara RB: A
general and simple method for obtaining R2 from generalized linear
mixed-effects models. Methods in Ecology and Evolution. Available
from: https://doi.org/10.1111/j.2041-210x.2012.00261.x,
2012.
|
|
21
|
Wan CP, Leung WK, Wong MC, Wong RM, Wan P,
Lo EC and Corbet EF: Effects of smoking on healing response to
non-surgical periodontal therapy: A multilevel modelling analysis.
J Clin Periodontol. 36:229–239. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Van Der Weijden GA and Timmerman MF: A
systematic review on the clinical efficacy of subgingival
debridement in the treatment of chronic periodontitis. J Clin
Periodontol. 29 (Suppl 3):55–71. 2002.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Feng X, Zhu L, Xu L, Meng H, Zhang L, Ren
X, Lu R, Tian Y, Shi D and Wang X: Distribution of 8 periodontal
microorganisms in family members of Chinese patients with
aggressive periodontitis. Arch Oral Biol. 60:400–407.
2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li Y, Feng X, Xu L, Zhang L, Lu R, Shi D,
Wang X, Chen F, Li J and Meng H: Oral microbiome in chinese
patients with aggressive periodontitis and their family members. J
Clin Periodontol. 42:1015–1023. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhou M, Rong R, Munro D, Zhu C, Gao X,
Zhang Q and Dong Q: Investigation of the effect of type 2 diabetes
mellitus on subgingival plaque microbiota by high-throughput 16S
rDNA pyrosequencing. PLoS One. 8(e61516)2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Nascimento GG, Dahlén G, López R and
Baelum V: Periodontitis phenotypes and clinical response patterns
to non-surgical periodontal therapy: Reflections on the new
periodontitis classification. Eur J Oral Sci. 128:55–65.
2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen MH, Yin HJ, Chang HH, Kao CT, Tu CC
and Chen YW: Baseline probing depth and interproximal sites predict
treatment outcomes of non-surgical periodontal therapy. J Dent Sci.
15:50–58. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Song J, Zhao H, Pan C, Li C, Liu J and Pan
Y: Risk factors of chronic periodontitis on healing response: A
multilevel modelling analysis. BMC Med Inform Decis Mak.
17(135)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tomasi C, Leyland AH and Wennström JL:
Factors influencing the outcome of non-surgical periodontal
treatment: A multilevel approach. J Clin Periodontol. 34:682–690.
2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lu RF, Xu L, Feng XH, Wang XE and Meng HX:
Multilevel analysis of non-surgical periodontal treatment of
patients with generalised aggressive periodontitis. Chin J Dent
Res. 24:191–198. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Rahim-Wöstefeld S, Kronsteiner D, El-Sayed
S, El-Sayed N, Eickholz P and Pretzl B: Development of a prognostic
tool: Based on risk factors for tooth loss after active periodontal
therapy. Clin Oral Investig. 26:813–822. 2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Scribante A, Gallo S, Pascadopoli M, Frani
M and Butera A: Ozonized gels vs chlorhexidine in non-surgical
periodontal treatment: A randomized clinical trial. Oral Dis.
30:3993–4000. 2024.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Elbay M, Elbay ÜS, Kaya E and Kalkan ÖP:
Effects of photobiomodulation with different application parameters
on injection pain in children: A randomized clinical trial. J Clin
Pediatr Dent. 47:54–62. 2023.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Butera A, Pascadopoli M, Nardi MG, Ogliari
C, Chiesa A, Preda C, Perego G and Scribante A: Clinical use of
paraprobiotics for pregnant women with periodontitis: Randomized
clinical trial. Dent J (Basel). 12(116)2024.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mdala I, Haffajee AD, Socransky SS, de
Blasio BF, Thoresen M, Olsen I and Goodson JM: Multilevel analysis
of clinical parameters in chronic periodontitis after root
planing/scaling, surgery, and systemic and local antibiotics:
2-year results. J Oral Microbiol. 4:2012.PubMed/NCBI View Article : Google Scholar
|