Introduction
Sclerosing angiomatoid nodular transformation (SANT)
is a rare benign inflammatory tumor-like lesion of the spleen,
characterized by vascular nodules and non-neoplastic stroma.
Initially described by Martel et al in 2004, SANT consists
of a series of benign vascular lesions exhibiting distinctive
morphological features (1). Most
non-lymphoid primary tumors of the spleen have a vascular origin
(2). Radiological differentiation
of SANT from other vascular splenic lesions, such as hamartoma,
hemangioma, and littoral cell angioma, especially metastatic
lesions, is often challenging because of their radiological
resemblance (2). Because SANT
sometimes has an ability to increase in size coupled with the
potential for 18F-fluorodeoxyglucose (18F-FDG) accumulation, which
is specifically a radiotracer used in the medical imaging modality
positron emission tomography (PET), it is frequently misidentified
as metastasis. With a differential diagnosis of malignancy, such as
lymphoma or metastasis, diagnostic studies are necessary, but
preoperative percutaneous procedures are frequently avoided because
of potential complications, such as hemorrhage and the risk of
dissemination. The possibility of metastasis remains, particularly
in patients with prior malignancies. Notably, the etiology of SANT
remains unclear, as does the precise pathogenesis of SANT, and it
remains to be determined if it is a de novo lesion or the
final common pathway of a variety of benign splenic conditions. To
emphasize the clinical significance of differential diagnoses for
splenic masses, we present our experience with a case wherein SANT
mimicked a metachronous splenic metastasis from rectal cancer that
had been successfully resected 2 years prior.
Case report
Case presentation
A 53-year-old woman with a history of hypertension
and three caesarean sections was referred to Sakai City Medical
Center (Osaka, Japan) for evaluation because of general fatigue,
fever, and anemia in October 2017. She reported no abdominal pain,
weight loss, hematochezia, or prior malignancies. Physical
examination revealed no abnormalities. Laboratory tests indicated
anemia (7.1 g/dl); elevated levels of lactate dehydrogenase (299
U/l), total bilirubin (1.51 mg/dl), and C-reactive protein (4.68
mg/dl); and normal levels of carcinoembryonic antigen (CEA) (3.0
ng/ml) and carbohydrate antigen 19-9 (CA 19-9) (11.8 U/ml).
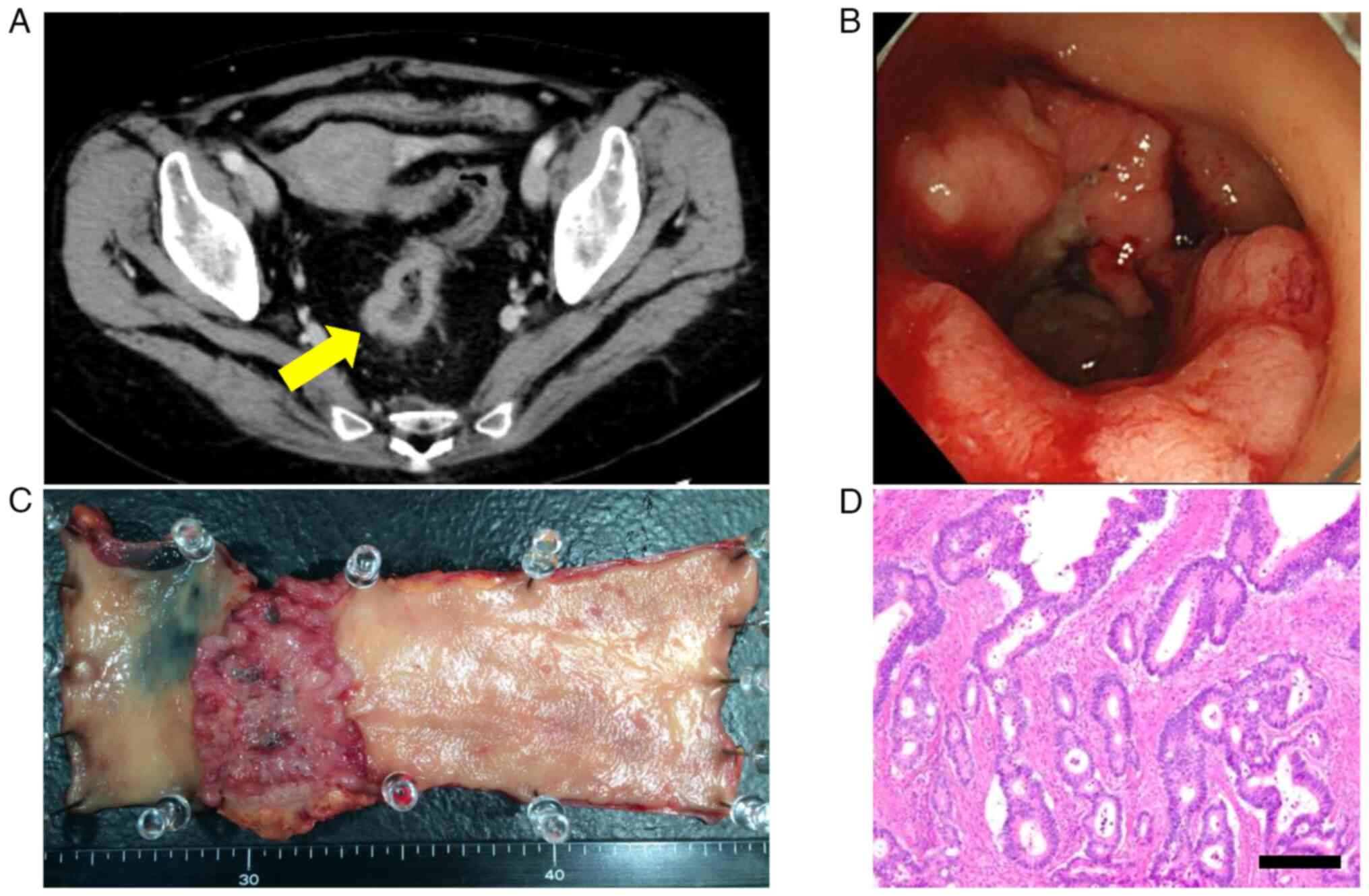
Contrast-enhanced computed tomography (CE-CT) was performed for
rectal cancer (Fig. 1A). A total
colonoscopy confirmed a circumferential rectal cancer (Fig. 1B). No distant metastases were
observed. Subsequently, the patient underwent laparoscopic low
anterior rectal resection and covering ileostomy. The resected
tumor measured approximately 70x55 mm (Fig. 1C). Pathological evaluation
indicated a moderately differentiated tumor with sub-serosal
invasion, with no lymph node metastasis (0/29), and the rectal
cancer was pathological stage II (Fig.
1D). The postoperative course included minor leakage at the
anastomosis requiring endoluminal drainage, and discharge on day
19. Adjuvant chemotherapy was not administered. Three months later,
the ileostomy was closed. Routine postoperative surveillance
included CE-CT and monitoring of CEA and CA 19-9 levels, which
remained within the normal range.
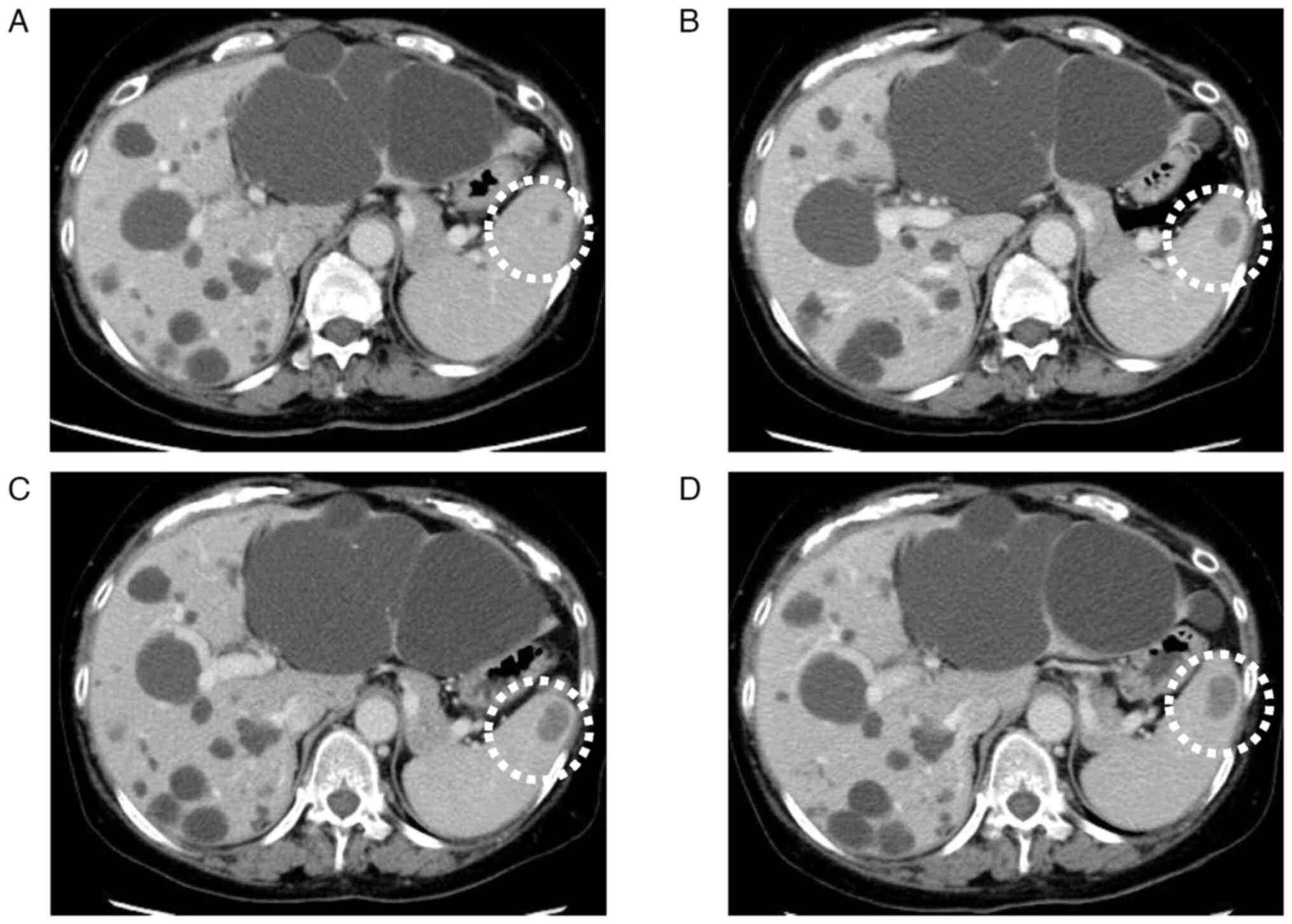
One year postoperatively, CE-CT revealed a
low-density lesion measuring 7 mm in diameter in the spleen
(Fig. 2A). While metachronous
metastasis was suspected, FDG-positron emission tomography (PET)
revealed no FDG accumulation in the lesion, and tumor marker levels
of CEA and CA 19-9 remained within normal range. The low-density
lesion was closely monitored via CE-CT for 3 years, during which it
gradually enlarged to 25 mm, with no other suspicious metastatic
lesions (Fig. 2B-D). The
differential diagnoses included metachronous metastasis,
inflammatory pseudotumors, and malignant lymphoma. Hence, the
patient opted for surgical intervention. Laparoscopic splenectomy
with five ports was performed successfully. The operative time was
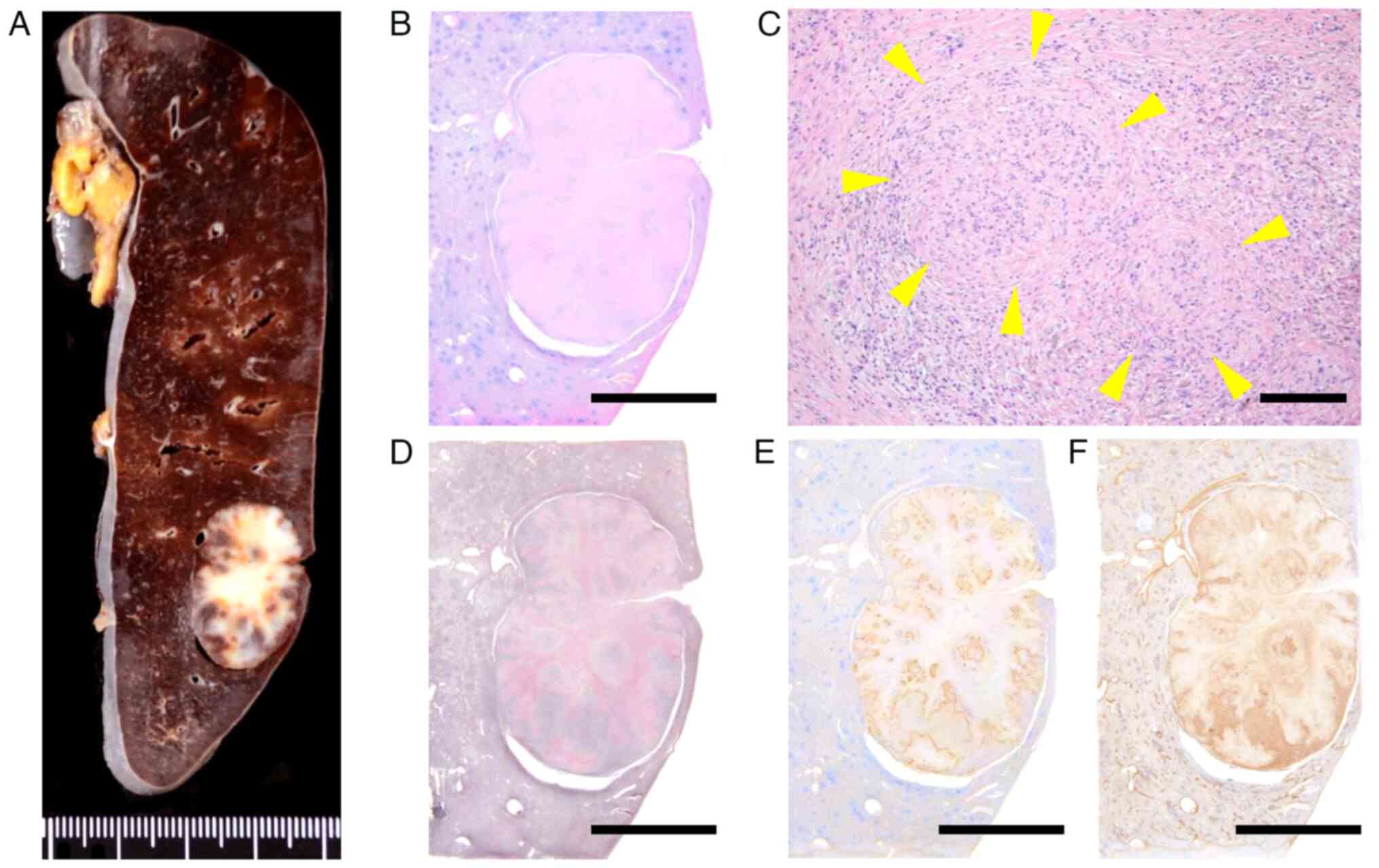
196 min, and only 5 ml of blood was lost. Macroscopically, the
resected specimen displayed a well-circumscribed, unencapsulated,
nodular mass measuring 25 mm, situated just beneath the splenic
capsule, with a whitish fibrotic stroma (Fig. 3A). Histological examination using
hematoxylin and eosin staining revealed multiple angiomatoid
nodules within the fibrosclerotic stroma (Fig. 3B and C). Each angiomatoid nodule comprised
slit-like, round, or irregularly shaped vascular spaces. Reticulin
staining revealed concentric layers of collagen bundles surrounding
the angiomatoid nodules (Fig. 3D).
Immunohistochemical analysis indicated positive expression of
α-smooth muscle actin (SMA) and factor VIII (3,4)
(Fig. 3E and F). Collectively, these findings supported
the diagnosis of SANT. The patient's recovery was uneventful, and
she was discharged 7 days postoperatively.
Immunohistochemical techniques
Tissue sections were mounted on slides and then
processed for immunohistochemistry using a BOND III automated
staining system (Leica, Heidelberg, Germany). After
deparaffinization, epitope retrieval, and peroxidase blocking, they
were incubated with an anti-human-SMA antibody (1:300; DAKO, Santa
Clara, CA, USA) or an anti-human-factor-VIII antibody (1:2;
Nichirei, Tokyo, Japan). Bound antibodies were detected using
biotin-conjugated secondary antibodies, which reacted with
diaminobenzidine (Vector Laboratories, Burlingame, CA, USA), and
the sections were counterstained with hematoxylin.
Discussion
SANT is a benign lesion that is usually incidentally
identified (1). Most patients with
SANT are asymptomatic, primarily presenting in middle-aged adults,
with a female-to-male ratio of 2:1(1). Laboratory investigations generally
yield no specific findings, including those for biomarker levels.
Histologically, SANT is characterized by multiple vascular nodules
of angiomatoid appearance embedded in a fibrosclerotic stroma. The
thick endothelial cells with spindly or ovoid cells surround the
angiomatoid nodules with thin, round, or irregularly shaped
vessels. In these angiomatoid nodules, three distinct types of
vessels are usually seen: CD34+/CD8-/CD31+ capillaries,
CD34-/CD8+/CD31+ sinusoids, and small CD34-/CD8-/CD31+ veins.
Immunohistochemically, SANT typically shows positive expression of
α-SMA, and factor VIII (3,4). The precise pathogenesis of SANT is
unclear, and it remains to be determined if it is a de novo
lesion or the final common pathway of a variety of benign splenic
conditions. Passive congestion of the red pulp may cause metabolic
changes in those areas, damaging the sinus endothelial cells. Such
damage may lead to fibrin deposition and inflammation, culminating
in granulation within the red pulp (5). Martel et al hypothesized that
SANT is a response to stromal proliferation and that the
internodular zones resemble an inflammatory pseudotumor (1). The plasma cells and stromal sclerosis
present in SANT have been connected to IgG4-related sclerosing
disease (6,7). Recently, Epstein–Barr virus-related
sclerosing conditions have been associated with SANT (8). Unfortunately, the values of IL-2 or
IgG4 were not measured in our case. To achieve a preoperative
radiological diagnosis, standard imaging techniques, including
abdominal ultrasound, CT, and magnetic resonance imaging, typically
describe SANT as a hypodense, occasionally multinodular splenic
mass (2,9). In our case, MRI was not performed for
splenic lesions. The differential diagnosis of a hypodense mass in
the spleen includes malignant lesions, such as lymphoma or
metastasis, and benign lesions, such as hemangioma, littoral cell
angioma, hemangioendothelioma, and splenic hamartoma. Splenic
hemangioma is often composed of cavernous capillary vessels
expressing CD31 and CD34, but not CD8. Hemangioma actually shows
regressive changes, such as infarction, fibrosis, and cystic
degeneration, while hemangioma does not display the distinctive
angiomatoid nodular appearance of SANT (10). Littoral cell angioma is composed of
sinusoid lining cells. Although these cells normally represent
CD31+/CD34-/CD8+ cells as a putative counterpart, some cells that
do not fully reproduce show an immunophenotype such as
CD31+/CD34-/CD8-. Littoral cell angioma is distinctive from SANT,
because it shows monotonous blood vessel composition without
sclerosis. Hemangioendothelioma is a very rare tumor with
borderline malignant potential, showing clinical and morphological
features intermediate between those of hemangioma and angiosarcoma.
It is composed of ill-defined vascular spaces lined by cells with
mild-to-moderate cellular atypia and generally a low mitotic index
(11). SANT can be distinguished
from hemangioendothelioma because of its low proliferative index
and because it lacks cytologic atypia. Splenic hamartoma has been
defined as a tumor-like lesion composed of structurally
disorganized red pulp tissue. Hamartoma, however, lacks an
angiomatoid nodular pattern, and does not show a clear border, in
contrast to SANT.
Splenic metastasis is rare and generally occurs in
the context of multi-visceral metastatic cancer at the terminal
stage, although solitary metastasis has been reported (12). Given the propensity of SANT to
increase in size coupled with the potential for FDG accumulation,
it is frequently misidentified as metastasis (9,13).
In our case, the patient was asymptomatic, and with no increase of
tumor marker levels. During a 3-year follow-up period after the
initial detection of the splenic lesion following curative rectal
cancer surgery, the low-density lesion gradually enlarged to 25 mm,
despite FDG-PET showing no FDG accumulation. The possibility of
metastasis remains, particularly in patients with prior
malignancies (12). With a
differential diagnosis of malignancy, such as lymphoma or
metastasis, diagnostic studies are necessary, but preoperative
percutaneous procedures are frequently avoided because of potential
complications, such as hemorrhage and the risk of dissemination
(14). In our case, a preoperative
biopsy was also avoided because of concerns regarding bleeding and
needle-tract seeding. In such cases, splenectomy may be
performed.
To date, nine cases of SANT, including the case
presented here, have been reported in patients with a history of
cancer treatment (14-21).
These nine cases involved five males and four females. The tumors
ranged from 2 to 7 cm in diameter, and the patients had histories
of melanoma; retroperitoneal sarcoma; and rectal, breast, uterine,
ovarian, and renal cancers. In two instances, preoperative biopsies
were conducted. One patient was diagnosed with SANT, which was
managed with 9 years of surveillance, during which, there was no
malignant transformation or recurrence (14). In another case, preoperative biopsy
erroneously indicated metastasis, leading to splenectomy, which
confirmed SANT on pathological analysis (17). In two cases, splenectomy was
performed alongside the initial surgeries for malignancies where
metastasis was suspected, whereas in the remaining cases,
splenectomy was performed following a period of observation lasting
up to 5 years. SANT is a benign splenic condition with good
prognosis. To date, there have been no reports of recurrence after
splenectomy (22).
When a splenic lesion is incidentally identified,
particularly during the follow-up of a previous cancer treatment,
SANT should be considered as a differential diagnosis. In cases
connected to prior malignancies, splenectomy is essential for both
diagnostic and therapeutic purposes. Incorporating data from
multicenter studies or additional cases for retrospective and
statistical analysis or a comprehensive study using a larger sample
size are required.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and material
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HT, MN, YY, AK and AM contributed to the diagnosis
and treatment of the patient. HT and MM made substantial
contributions to conception and design. HT, AK, YY and MN made
substantial contributions to acquisition of data. HT, MM, TY, NO,
TT, HH, AN, RK, SN and AM made substantial contributions to
analysis and interpretation of data. HT wrote the first draft of
the manuscript. HT and MM confirm the authenticity of all the raw
data. All of the authors are accountable for all aspects of the
work, and read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case report and any
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Martel M, Cheuk W, Lombardi L,
Lifschitz-Mercer B, Chan JK and Rosai J: Sclerosing angiomatoid
nodular transformation (SANT): Report of 25 cases of a distinctive
benign splenic lesion. Am J Surg Pathol. 28:1268–1279.
2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Abbott RM, Levy AD, Aguilera NS, Gorospe L
and Thompson WM: From the archives of the AFIP: Primary vascular
neoplasms of the spleen: Radiologic-pathologic correlation.
Radiographics. 24:1137–1163. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kashiwagi S, Kumasaka T, Bunsei N,
Fukumura Y, Yamasaki S, Abe K, Mitani K, Abe H, Matsumoto T and
Suda K: Detection of Epstein-Barr virus-encoded small RNA-expressed
myofibroblasts and IgG4-producing plasma cells in sclerosing
angiomatoid nodular transformation of the spleen. Virchows Archiv.
453:275–282. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kim HH, Hur YH, Koh YS, Kim JC, Kim HJ,
Kim JW, Kim Y, Lee JH and Cho CK: Sclerosing angiomatoid nodular
transformation of the spleen related to IgG4-associated disease:
Report of a case. Surg Today. 43:930–936. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Diebold J, Le Tourneau A, Marmey B, Prevot
S, Müller-Hermelink HK, Sevestre H, Molina T, Billotet C, Gaulard
P, Knopf JF, et al: Is sclerosing angiomatoid nodular
transformation (SANT) of the splenic red pulp identical to
inflammatory pseudotumour? Report of 16 cases. Histopathology.
53:299–310. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kuo TT, Chen TC and Lee LY: Sclerosing
angiomatoid nodular transformation of the spleen (SANT):
Clinicopathological study of 10 cases with or without abdominal
disseminated calcifying fibrous tumors, and the presence of a
significant number of IgG4+ plasma cells. Pathol Int. 59:844–850.
2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nagai Y, Hayama N, Kishimoto T, Furuya M,
Takahashi Y, Otsuka M, Miyazaki M and Nakatani Y: Predominance of
IgG4+ plasma cells and CD68 positivity in sclerosing angiomatoid
nodular transformation (SANT). Histopathology. 53:495–498.
2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Weinreb I, Bailey D, Battaglia D, Kennedy
M and Perez-Ordoñez B: CD30 and Epstein-Barr virus RNA expression
in sclerosing angiomatoid nodular transformation of spleen.
Virchows Archiv. 451:73–79. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lee D, Wood B, Formby M and Cho T: F-18
FDG-avid sclerosing angiomatoid nodular transformation (SANT) of
the spleen: Case study and literature review. Pathology.
39:181–183. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kutok JL and Fletcher CD: Splenic vascular
tumors. Semin Diagn Pathol. 20:128–139. 2003.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Budke HL, Breitfeld PP and Neiman RS:
Functional hyposplenism due to a primary epithelioid
hemangioendothelioma of the spleen. Arch Path Lab Med. 119:755–757.
1995.PubMed/NCBI
|
|
12
|
Compérat E, Bardier-Dupas A, Camparo P,
Capron F and Charlotte F: Splenic metastases: Clinicopathologic
presentation, differential diagnosis, and pathogenesis. Arch Path
Lab Med. 131:965–969. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Feng YM, Huang YC, Tu CW, Kao WS and Tu
DG: Distinctive PET/CT features of splenic SANT. Clin Nucl Med.
38:e465–e466. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Dutta D, Sharma M, Mahajan V and Chopra P:
Sclerosing angiomatoid nodular transformation of spleen
masquerading as carcinoma breast metastasis: Importance of splenic
biopsy in obviating splenectomy. Indian J Pathol Microbiol.
59:223–226. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Langer R, Dinges J, Dobritz M, Brauer RB,
Perren A, Becker K and Kremer M: Sclerosing angiomatoid nodular
transformation of the spleen presenting as a rapidly growing tumour
in a patient with rectal cancer. BMJ Case Rep.
2009(bcr11.2008)2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sitaraman LM, Linn JG, Matkowskyj KA and
Wayne JD: Sclerosing angiomatoid nodular transformation of the
spleen masquerading as a sarcoma metastasis. Rare Tumors.
2(e45)2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Demirci I, Kinkel H, Antoine D, Szynaka M,
Klosterhalfen B, Herold S and Janßen H: Sclerosing angiomatoid
nodular transformation of the spleen mimicking metastasis of
melanoma: A case report and review of the literature. J Med Case
Rep. 11(251)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lapa C, Steger U, Ritter CO, Wild V and
Herrmann K: Differentiation of an unclear splenic lesion in a
patient with cholangiocarcinoma. Clin Nucl Med. 39:470–471.
2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Efared B, Sidibé IS, Erregad F, Hammas N,
Chbani L and El Fatemi H: Sclerosing angiomatoid nodular
transformation of the spleen (SANT) in a patient with clear cell
carcinoma of the uterus: A case report. J Med Case Rep.
12(377)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Koyama R, Minagawa N, Maeda Y, Shinohara T
and Hamada T: A sclerosing angiomatoid nodular transformation
(SANT) mimicking a metachronous splenic metastasis from
endometrioid cancer and ovarian cancer. Int J Surg Case Rep.
65:292–295. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang ZB and Li L: Splenic sclerosing
angiomatoid nodular transformation in a patient with right renal
carcinoma: A case report. Asian J Surg. 44:396–397. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xia L, Li Z, Jiang P, Zhang Y, Bu Z and
Meng N: Sclerosing angiomatoid nodular transformation of the
spleen: Case reports and literature review. Medicine (Baltimore).
103(e38466)2024.PubMed/NCBI View Article : Google Scholar
|