Introduction
Pseudomonas aeruginosa (PA) is a
Gram-negative bacterium known as an opportunistic pathogen,
commonly found in water, soil and on human skin (1-4).
It poses a significant risk to individuals with weakened immune
systems, causing serious infections that are difficult to treat due
to their inherent resistance to antibiotics (1-4).
Lower respiratory tract infections (LRTIs) caused by PA are
particularly concerning, as they can lead to severe conditions,
such as pneumonia, which may be life-threatening if untreated
(5-9).
Patients who are immunocompromised, have cystic fibrosis, or suffer
from burn wounds, are notably vulnerable to PA-induced LRTIs
(5-9).
Globotriaosylceramide (Gb3) is a glycosphingolipid
found in cell membranes that plays a crucial role in various
medical conditions (10,11). It was initially recognized in the
context of Fabry disease, and its significance has expanded to
include tumor biology and infectious diseases (12-16).
Gb3 serves as a receptor for specific bacterial toxins associated
with gastrointestinal infections, such as enterotoxigenic and Shiga
toxin-producing Escherichia coli infections (16-19).
In the context of PA, previous research studies have
identified the importance of Gb3 in the uptake of the bacterium by
host cells (10,11). Gb3 acts as a key receptor by
interacting with the bacterial lectin LecA on host cell surfaces
(10,11). This interaction activates the
‘lipid zipper mechanism’, facilitating bacterial adhesion,
internalization and progression of the infection (20,21).
It forms a plasma membrane domain enriched in Gb3, cluster of
differentiation (CD)59, phosphatidylinositol (3,4,5)-triphosphate and flotillin, which
facilitates the efficient uptake of PA strain PAO1 into the host
cell (22,23). The depletion of flotillins and CD59
from the host cells significantly reduces PA invasiveness,
highlighting the importance of the Gb3-enriched domain in bacterial
invasion (23,24). However, the concentration of Gb3 in
the context of PA infections remains underexplored and its clinical
relevance is not well defined.
Despite the recognized role of Gb3 in PA
pathogenicity at cellular and molecular levels, there is a scarcity
of clinical data regarding Gb3 levels in patients with PA
infections. This scarcity can be attributed to several factors.
Firstly, the measurement of Gb3 requires specialized laboratory
techniques and equipment, such as ELISA assays, high-performance
liquid chromatography and mass spectrometry, which are not widely
available in various clinical settings, limiting large-scale
studies (25). Secondly, research
on Gb3 as a biomarker for PA infections is relatively new. While
Gb3 has been extensively studied in the context of Fabry disease
and certain cancer types, its specific role in PA-induced LRTIs
remains underexplored. Lastly, the involvement of Gb3 in multiple
pathological processes, including other bacterial infections and
cancer progression, complicates its measurement and interpretation.
This complexity necessitates careful study design and analysis,
which may have deterred extensive clinical research in this area
(26). These challenges have
limited the broad application of Gb3 measurements in clinical
practice and underscore the necessity for further research to
assess the role of Gb3 in PA infections.
In the present study, the concentration levels of
Gb3 in bronchoalveolar lavage fluid (BALF) and serum samples from
patients with PA infection were quantified and compared with the
levels noted in healthy individuals. It is proposed that elevated
Gb3 levels are associated with PA infections and may serve as a
valuable biomarker for detecting and assessing the severity of
these infections.
Materials and methods
Ethics statement
The present study received approval (approval no.
2024-045) from the Ethics Committee of The Affiliated People's
Hospital of Ningbo University (Ningbo, China). Written informed
consent was obtained from all participants or their guardians,
adhering to the principles outlined in the Declaration of
Helsinki.
Participant selection
The present prospective study was conducted from 1
June 2024 to 10 September 2024, at The Affiliated People's Hospital
of Ningbo University. A total of 108 participants were included and
divided into two groups as follows: Group PA included 54 patients
with PA-induced LRTIs (30 males and 24 females, mean age
67.54±10.33 years; Table I) and
Group H included 54 healthy individuals (31 males and 23 females,
mean age 51.47±7.71 years; Table
I) without chronic medical history or current medication use,
selected from the physical examination center of The Affiliated
People's Hospital of Ningbo University. The exclusion criteria
included patients on statins, aspirin, inhaled corticosteroids,
long-term macrolides or those who received anti-infective
medication prior to admission or could not tolerate bronchoscopy
and lavage.
 | Table IClinical characteristics of patients
with PA-induced lower respiratory tract infections and healthy
controls. |
Table I
Clinical characteristics of patients
with PA-induced lower respiratory tract infections and healthy
controls.
| | Unmatched
cohort | Matched cohort |
|---|
|
Characteristics | PA (n=54) | Healthy (n=54) |
t/z/χ2 | P-value | PA (n=15) | Healthy (n=15) |
t/z/χ2 | P-value |
|---|
| Age, years | 67.54±10.33 | 51.47±7.71 | 7.439 | <0.001 | 54.87±7.75 | 54.67±7.59 | 0.868 | 0.870 |
| BMI,
kg/m2 | 21.15±3.91 | 23.57±1.63 | 3.539 | <0.001 | 21.27±4.77 | 23.58±1.86 | 1.750 | 0.097 |
| Male, n (%) | 30 (55.6) | 31 (57.4) | 3.569 | 0.312 | 10 (66.7) | 7 (46.7) | 1.942 | 0.379 |
| Chronic obstructive
pulmonary diseases, n (%) | 16 (29.6) | 0 (0) | N/A | N/A | 3(20) | 0 (0) | N/A | N/A |
| Bronchiectasia,
% | 30 (55.6) | 0 (0) | N/A | N/A | 5 (33.3) | 0 (0) | N/A | N/A |
| Diffuse
panbronchiolitis, n (%) | 1 (1.8) | 0 (0) | N/A | N/A | 0 (0) | 0 (0) | N/A | N/A |
| Interstitial lung
disease, n (%) | 2 (3.7) | 0 (0) | N/A | N/A | 0 (0) | 0 (0) | N/A | N/A |
The diagnosis of PA-induced LRTIs followed the
Guidelines for Chinese Expert Consensus on the Management of Lower
Respiratory Tract Infections of Pseudomonas aeruginosa in
Adults (2022 revision) (27).
Diagnosis was based on clinical symptoms, such as pneumonia,
tracheobronchitis, lung abscess, or empyema, along with isolation
of PA from qualified lower respiratory tract specimens and
identification of high-risk factors for acute PA infection.
Diagnostic procedures
All participants underwent diagnostic tests,
including blood tests [white blood cell (WBC) counts, neutrophil
(NEU) counts, lymphocyte counts, monocyte counts,
neutrophil-to-lymphocyte ratio (NLR), C-reactive protein (CRP) and
procalcitonin (PCT) levels], computed tomography (CT) scans and
bronchoscopies. For patients with PA-induced LRTIs, peripheral
blood samples were collected on the 1st and 7th days following
diagnosis. BALF was collected on the 1st day post-diagnosis from
the infected lung segments identified by chest CT scans.
Gb3 level measurement
Peripheral blood and BALF samples were collected and
centrifuged within 3 h at 800 x g for 10 min at room temperature
(~20˚C). The supernatants of BALF and serum were separated and
stored at -80˚C for subsequent analysis. Gb3 levels were measured
using the Gb3 ELISA kit (cat no. BLL104146E9; Baililai Biological),
according to the manufacturer's protocol.
Statistical analysis
A power analysis was conducted prior to the study to
determine the necessary number of participants to achieve adequate
statistical power. Based on preliminary data, the positive rate of
Gb3 in the Group PA (case group) was anticipated to be 93.3, and
40.0% in the Group H (control group). The sample size calculation
formula for matched case-control studies was utilized: n=2pq x
(Zα +
Zβ)2/(ρ1-ρ0)2,
where α=0.05 (two-sided significance level), Zα=1.96
(Z-score corresponding to α), β=0.2 (for a power of 80%),
Zβ=0.842 (Z-score corresponding to β), ρ0=0.4
(positive rate in the control group), ρ1=0.933 (positive
rate in the case group), P=(ρ0 + ρ1)/2=0.665,
q=1-P=0.335.
Substituting these values into the formula, n=12.27,
~13 was obtained. This calculation indicated that at least 13
matched pairs were needed to achieve 80% power at a significance
level of 0.05. In the present study, 54 paired samples were
initially included. After propensity score matching (PSM) for age
and body mass index (BMI), 15 matched pairs were selected for
demographic and laboratory result analysis. This number exceeds the
calculated requirement and ensures sufficient statistical power.
The match was performed using a nearest neighbor algorithm with a
1:1 ratio and a caliper value of 0.03.
Categorical variables are presented as numbers or
percentages. Categorical variable comparisons between groups were
conducted using the Chi-square test. The Kolmogorov-Smirnov test
was used to assess the distribution of continuous variables.
Continuous variables with normal distributions are reported as the
mean ± standard deviation, while those with non-normal
distributions are reported as the median and interquartile range.
Group comparisons of non-normally distributed continuous variables
were carried out using the Wilcoxon signed rank test. Correlations
between variables, including Gb3 levels, CRP and PCT, were assessed
using Spearman's rank correlation coefficients. Univariate and
multivariate logistic regression analyses were performed to
evaluate the association between Gb3 levels and the incidence of
PA.
Prediction accuracy was evaluated using receiver
operating characteristic (ROC) curves, with the area under the ROC
curve (AUC) values calculated for each parameter. Prediction rates
were compared using the log-rank test. All statistical analyses
were performed using SPSS software version 26.0 (IBM Corp.),
GraphPad Prism software version 9.3.1 (GraphPad Software;
Dotmatics), MedCalc version 22.026 (MedCalc Software Ltd.) and R
software version 4.1.0 (https://www.R-project.org/). A P<0.05 was
considered to indicate a statistically significant difference.
Results
Participant composition
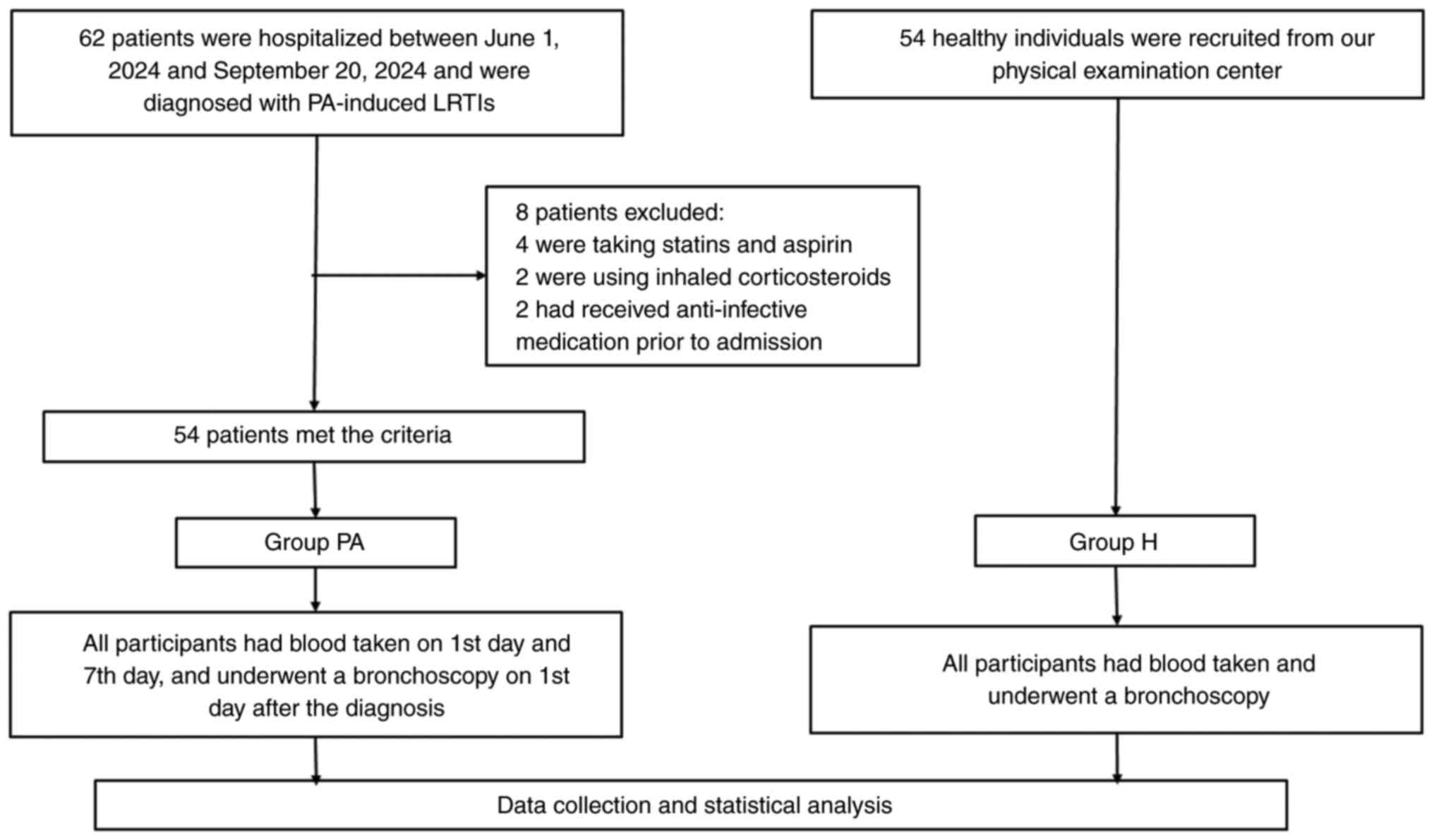
As revealed in Fig.
1, 62 patients were initially considered eligible between June
1, 2024 and September 20, 2024. A total of 8 patients were excluded
for the following reasons: 4 patients were on statins and aspirin,
2 patients were using inhaled corticosteroids and 2 patients had
received anti-infective medication prior to admission. A total of
54 patients with PA-induced LRTIs (Group PA) were included in the
study. Additionally, 54 healthy individuals without chronic medical
history or current medication use were selected from the physical
examination center to form the control group (Group H). This
resulted in a total of 108 participants in the present study.
Among the 54 participants in Group PA, 16 patients
(29.6%) were diagnosed with chronic obstructive pulmonary diseases,
30 patients (55.6%) had bronchiectasis, 1 patient (1.8%) had
diffuse panbronchiolitis and 2 patients (3.7%) had interstitial
lung disease, as shown in Table
I.
Basic characteristics of
participants
The clinical characteristics of Group PA and Group H
are presented in Table I. In the
unmatched cohort, significant differences were observed between the
two groups in terms of age and BMI. The mean age of Group PA was
significantly higher than that of Group H (67.54±10.33 years vs.
51.47±7.71 years; P<0.001). Similarly, the mean BMI of Group PA
was significantly lower than that of Group H (21.15±3.91 kg/m² vs.
23.57±1.63 kg/m²; P<0.001). Due to these significant differences
in age and BMI, which could act as confounding factors, PSM was
performed between Groups PA and H to create a matched cohort for
further analysis. This process resulted in 15 matched pairs for
follow-up analysis (as matched cohorts in Table I).
Gb3 levels and inflammatory indices of
participants
The results indicated that both Gb3 levels and the
inflammatory index were increased in Group PA compared with those
noted in Group H (P<0.001; Table
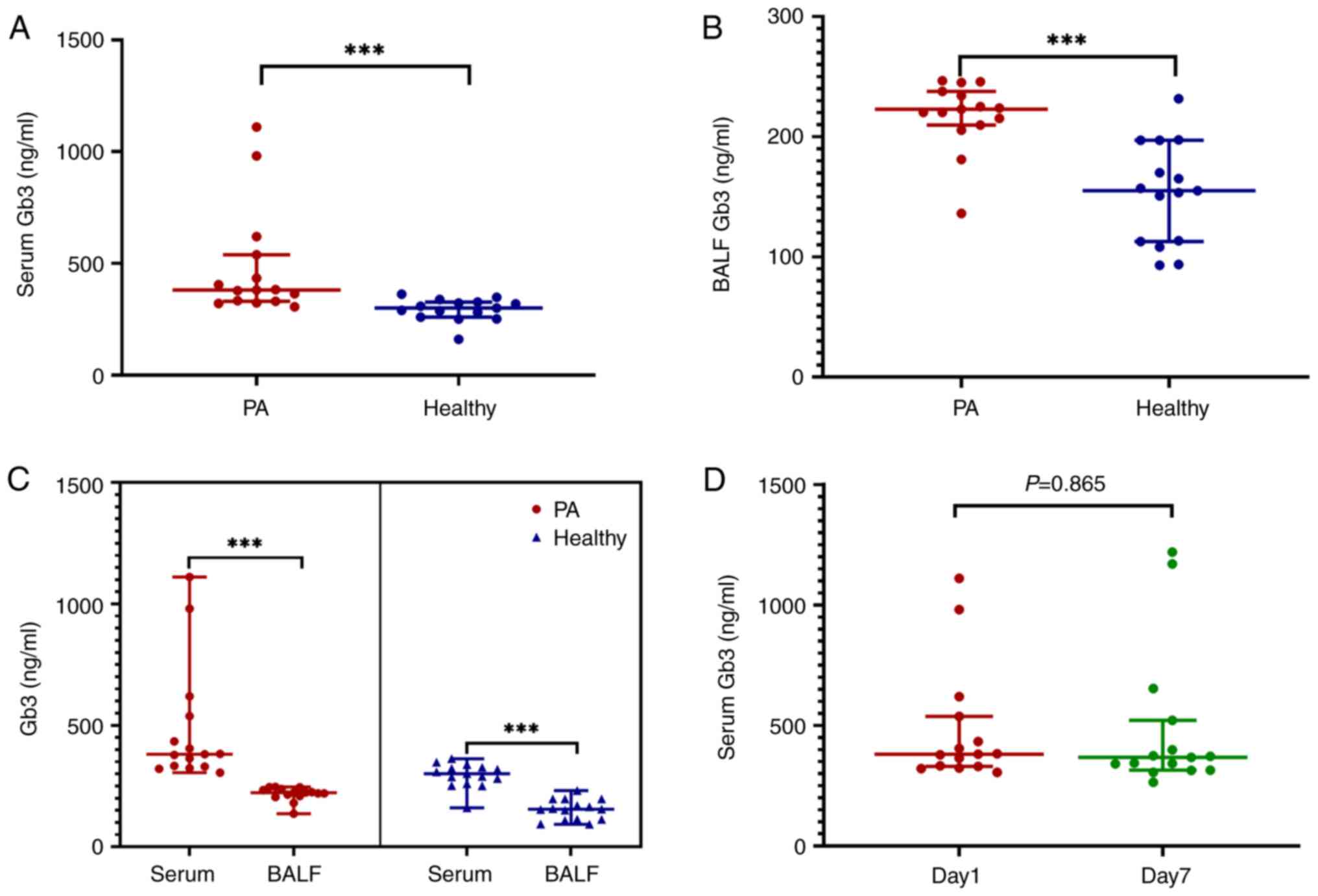
II). Specifically, on day 1, the median serum Gb3 level in
Group PA was significantly higher than that in Group H [380.95
ng/ml (IQR, 330.38-538.17 ng/ml) vs. 300.84 ng/ml (IQR,
259.89-326.79 ng/ml); P<0.001; Table II]. Similarly, the median BALF Gb3
level was significantly higher in Group PA compared with Group H
[222.82 ng/ml (IQR, 209.69-237.73 ng/ml) vs. 155.17 ng/ml (IQR,
112.77-197.09 ng/ml); P<0.001; Table II and Fig. 2A and B].
 | Table IILaboratory results of patients with
PA-induced lower respiratory tract infections and healthy
controls. |
Table II
Laboratory results of patients with
PA-induced lower respiratory tract infections and healthy
controls.
| Laboratory
results | PAa (n=15) | Healthy (n=15) |
t/z/χ2 | P-value |
|---|
| White blood cell
count, x109/l | 14.90 (10.68,
20.63) | 6.50 (6.00,
7.30) | 3.776 | <0.001 |
| Neutrophil count,
x109/l | 12.96 (9.57,
17.68) | 4.40 (3.64,
5.18) | 4.015 | <0.001 |
| Lymphocyte count,
x109/l | 0.75 (0.54,
1.82) | 1.80 (1.37,
1.93) | 1.922 | 0.057 |
| Monocytes,
x109/l | 0.61 (0.35,
0.78) | 0.47 (0.40,
0.64) | 0.809 | 0.425 |
|
Neutrophil-to-lymphocyte ratio | 12.59 (7.90,
21,13) | 1.85 (2.02,
3.42) | 4.102 | <0.001 |
| C-reactive protein,
mg/l | 40.20 (33.30,
74.70) | N/A | N/A | N/A |
| Procalcitonin,
ng/ml | 0.53 (0.35,
0.88) | N/A | N/A | N/A |
| Serum Gb3,
ng/ml | 380.95 (330.38,
538.17) | 300.84 (259.89,
326.79) | 3.754 | <0.001 |
| Bronchoalveolar
lavage fluid Gb3, ng/ml | 222.82 (209.69,
237.73) | 155.17 (112.77,
197.09) | 3.754 | <0.001 |
When comparing serum and BALF Gb3 levels within each
group, serum Gb3 levels surpassed BALF Gb3 levels in both Group PA
and Group H (P<0.001; Fig. 2C).
No significant difference was noted in serum Gb3 levels between
days 1 and 7 post-diagnosis in Group PA (P=0.865; Fig. 2D).
In addition to Gb3 levels, inflammatory indices were
markedly elevated in Group PA. Specifically, the median WBC count
was significantly higher in Group PA compared with Group H
[14.90x109/l (IQR, 10.68-20.63x109/l) vs.
6.50x109/l (IQR, 6.00-7.30x109/l),
P<0.001; Table II]. The median
NEU count in Group PA was significantly higher than that in Group H
[12.96x109/l (IQR, 9.57-17.68x109/l) vs.
4.40x109/l (IQR, 3.64-5.18x109/l);
P<0.001; Table II].
Additionally, the NLR was significantly higher in Group PA compared
with Group H [median, 12.59 (IQR, 7.90-21.13) vs. 1.85 (IQR,
2.02-3.42); P<0.001; Table
II].
Further analysis indicated a negative correlation
between serum Gb3 levels on day 7 and the changes in serum PCT
levels over the 7 days post-diagnosis (rs=-0.736;
P=0.002; Table III). A similar
trend was observed between changes in Gb3 levels during the
observation period and changes in PCT levels (rs=-0.507;
P=0.054; Table III). However,
the correlation between serum Gb3 levels on day 7 and CRP changes
was not statistically significant (rs=-0.375; P=0.168;
Table III). Similarly, the
correlation between Gb3 changes and CRP changes was not
statistically significant (rs=-0.211; P=0.451; Table III). In addition, a significant
correlation between serum Gb3 and BALF Gb3 concentration levels was
observed, as well as a strong association with CRP and PCT levels
in patients on day 1 post-diagnosis (Fig. 3).
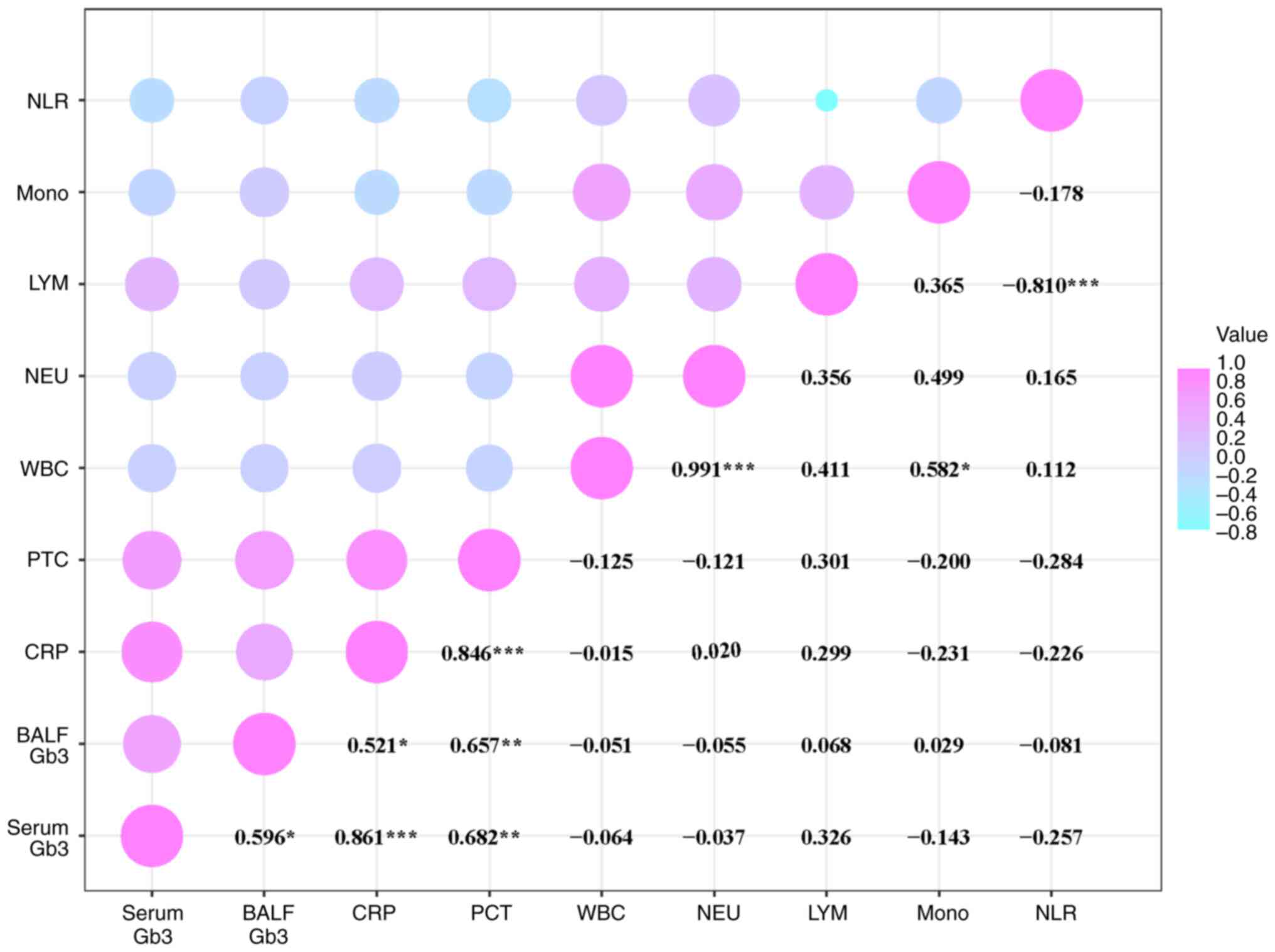 | Figure 3Correlation analysis of Gb3, CRP and
PCT levels in patients with PA-LRTIs. The horizontal and vertical
axes represent different variables. The numerical values correspond
to the Spearman's rank correlation coefficients between the pairs
of variables. The size of the bubbles reflects the magnitude of
these coefficients, while the color indicates the nature of the
correlation: Violet signifies a positive correlation, and blue
denotes a negative correlation (*P<0.05;
**P<0.01; ***P<0.001). Gb3,
globotriaosylceramide; CRP, C-reactive protein; PCT, procalcitonin;
PA, Pseudomonas aeruginosa; LRTIs, lower respiratory tract
infections; BALF, bronchoalveolar lavage fluid; WBC, white blood
cell count; NEU, neutrophil count; LYM, lymphocyte count; NLR,
neutrophil-to-lymphocyte ratio. |
 | Table IIICorrelation between Gb3 levels and
changes in serum CRP and PCT levels. |
Table III
Correlation between Gb3 levels and
changes in serum CRP and PCT levels.
| | Serum day 7
Gb3 | Gb3 change |
|---|
| Changes in serum
levels | rs | P-value | rs | P-value |
|---|
| CRP | -0.375 | 0.168 | -0.211 | 0.451 |
| PCT | -0.736 | 0.002 | -0.507 | 0.054 |
Predictive value of serum and BALF Gb3
levels
Univariate logistic regression analysis identified
serum Gb3 [Odds ratio (OR), 1.045; 95% confidence interval (CI),
1.009-1.083; P=0.015; Table IV]
and BALF Gb3 (OR, 1.050; 95% CI, 1.016-1.085; P=0.004; Table IV) as risk factors for PA
infections. Multivariate logistic regression analysis further
confirmed that serum Gb3 (OR, 1.048; 95% CI, 1.010-1.087; P=0.013;
Table IV) and BALF Gb3 (OR,
1.056; 95% CI, 1.016-1.098; P=0.006; Table IV) as independent risk factors for
PA infections. ROC curve analysis was conducted to assess the
predictive performance of serum and BALF Gb3 levels in
differentiating between Groups PA and H. The AUC for serum Gb3
levels was 0.899 (95% CI, 0.814-0.955; P<0.001; Table V), with a sensitivity of 87.04% and
a specificity of 80.65%. For BALF Gb3 levels, the AUC was 0.812
(95% CI, 0.711-0.889; P<0.001; Table V), with a sensitivity of 72.22% and
a specificity of 73.33%.
 | Table IVBinary logistic regression analysis
of the incidence of Pseudomonas aeruginosa-induced lower
respiratory tract infections. |
Table IV
Binary logistic regression analysis
of the incidence of Pseudomonas aeruginosa-induced lower
respiratory tract infections.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variable | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Serum Gb3 | 1.045
(1.009-1.083) | 0.015 | 1.048
(1.010-1.087) | 0.013 |
| BALF Gb3 | 1.050
(1.016-1.085) | 0.004 | 1.056
(1.016-1.098) | 0.006 |
 | Table VAUC values and thresholds of
different parameters for predicting the incidence of Pseudomonas
aeruginosa-induced lower respiratory tract infections. |
Table V
AUC values and thresholds of
different parameters for predicting the incidence of Pseudomonas
aeruginosa-induced lower respiratory tract infections.
| | | 95% CI | |
|---|
| Variable | AUC | Lower limit | Upper limit | Sensitivity
(%) | Specificity
(%) | Threshold | P-value |
|---|
| Serum Gb3,
ng/ml | 0.899 | 0.814 | 0.955 | 87.04 | 80.65 | >322.70 | <0.001 |
| Bronchoalveolar
lavage fluid Gb3, ng/ml | 0.812 | 0.711 | 0.889 | 72.22 | 73.33 | >165.15 | <0.001 |
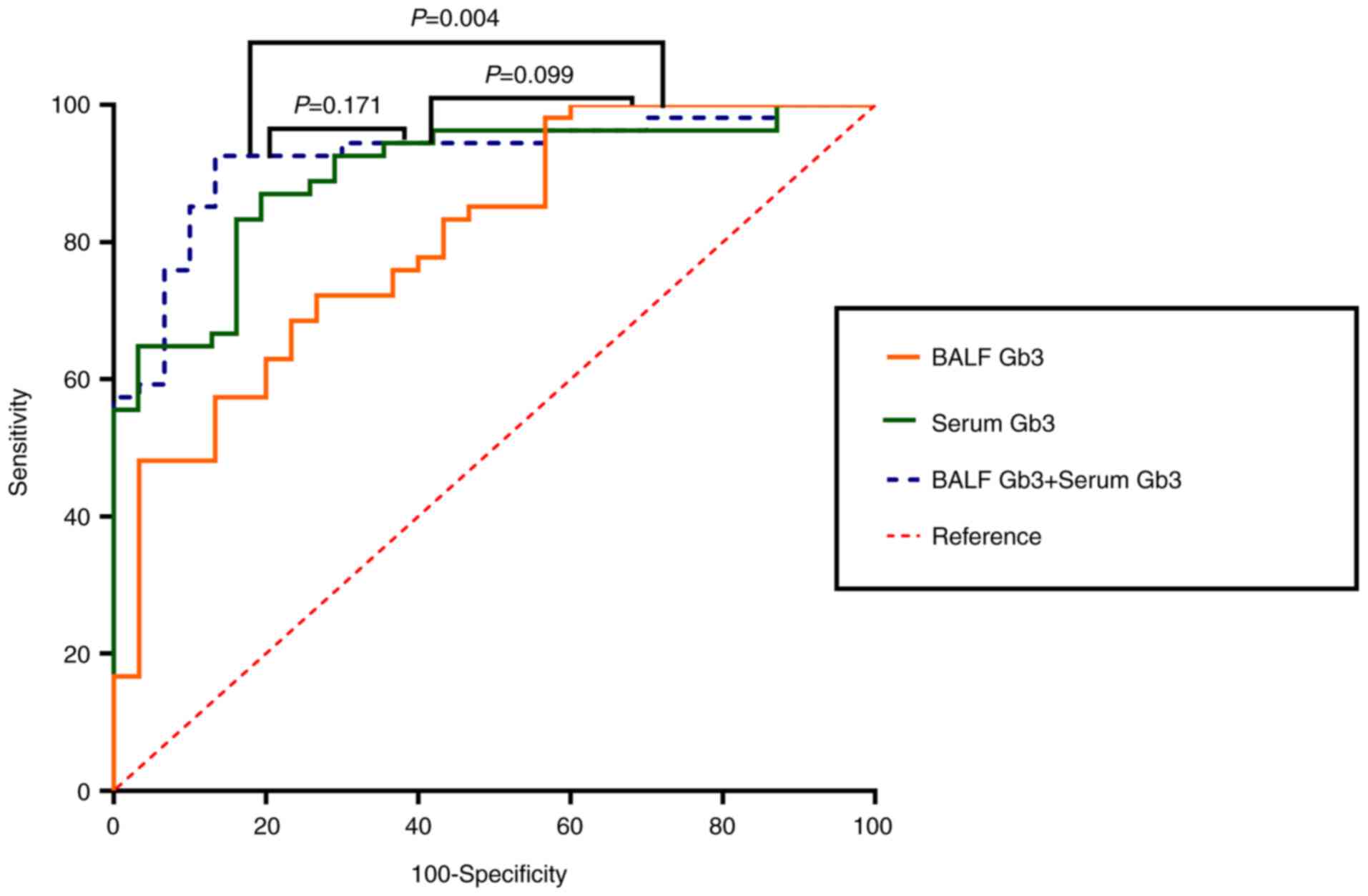
Subsequently, a log-rank test was used to compare
the ROC curves of patients with PA-induced LRTIs and those of
healthy individuals. The combined use of serum and BALF Gb3 levels
indicated the highest predictive capability (Fig. 4). Serum Gb3 alone demonstrated
strong predictive ability, followed by BALF Gb3 (Fig. 4). However, the difference between
serum Gb3 and BALF Gb3 levels was not statistically significant
(P=0.099; Fig. 4).
Discussion
In the present study, it was found that patients
with PA-induced LRTIs had significantly higher concentrations of
Gb3 in both serum and BALF samples on the first day following
diagnosis compared with healthy controls. This increase (notably in
BALF) suggests that Gb3 may serve as a specific biomarker for
PA-induced LRTIs. Multivariate logistic regression analysis
identified Gb3 levels as independent risk factors for PA infection,
highlighting the potential predictive role of Gb3 in the disease.
ROC curve analysis further supported the clinical utility of Gb3,
with serum levels indicating high sensitivity and specificity for
PA-induced LRTIs. Although BALF Gb3 also exhibited significant
predictive capacity, it was slightly less effective than serum Gb3.
Notably, the combination of serum and BALF Gb3 levels provided the
strongest predictive capabilities, indicating a synergistic effect
in diagnosing PA-induced LRTIs.
The higher predictive ability of serum Gb3 compared
with BALF Gb3 may be attributed to several factors. Gb3 is
expressed on various immune cells, such as dendritic cells and
macrophages, which are more prevalent in the systemic circulation
than in the alveoli (28,29). During inflammatory responses, the
activation and mobilization of these immune cells can lead to
increased Gb3 levels in the serum, reflecting both local pulmonary
and systemic immune reactions to PA infection (28,29).
In contrast to these observations, BALF-based analysis of Gb3
levels is confined to the lung environment and may not reflect
systemic changes (28,29). Furthermore, the bronchoalveolar
lavage procedure can dilute the concentration of Gb3 in BALF due to
the instillation and retrieval of fluid, potentially reducing its
measurable levels and predictive capacity. The differences in Gb3
metabolism and turnover rates between the bloodstream and lung
tissue may also contribute to higher and more stable Gb3 levels in
serum (28,29). These factors suggest that serum Gb3
may serve as a more robust and reliable biomarker for PA-induced
LRTIs compared with BALF Gb3.
Despite the slightly lower predictive ability of
BALF Gb3, the elevated levels observed reinforce the association
between Gb3 and PA infections in the lung environment, which is the
primary site of infection. Increased Gb3 levels in the lungs may
provide more binding sites for PA, promoting the initial bacterial
adhesion and internalization. In addition, elevated Gb3 levels
could affect the immune response of the host, potentially affecting
B-cell activation and antibody production. In PA infections, high
Gb3 levels may alter the immune response, impacting the ability of
the body to clear the pathogen.
The marked difference in Gb3 levels between patients
with PA and healthy controls suggests that Gb3 could serve as an
auxiliary marker for PA-induced LRTIs. Implementing Gb3 measurement
could aid in the early identification and clinical assessment of
these infections, which is crucial for initiating prompt and
effective treatment. Given the prognostic significance of BALF Gb3
levels, timely bronchoscopy with alveolar lavage may provide
valuable insights into the infection and guiding management.
The present research study demonstrated an optimal
correlation between Gb3 and these markers on the 1st day
post-diagnosis, suggesting that Gb3 may reflect infection severity.
CRP and PCT are general markers of inflammation commonly used to
evaluate suspected infections (30-33).
The combination of Gb3 with these markers could provide a more
comprehensive assessment of the initial condition of a patient.
No significant differences were observed in serum
Gb3 levels between pre-treatment and post-treatment periods. This
lack of change may be due to an insufficient observation time or
the poor overall health of the patients. A previous study by Müller
et al (34) reported that
lysophosphatidylcholine levels significantly decrease during the
acute phase of pneumonia (within 48 h), with normalization
potentially taking up to 60 days post-treatment (34). The study of Nan et al
(35) has also shown a significant
association between lipid levels and infection severity, indicating
that the effects of treatment on lipid levels may not be
immediately apparent. In the present study, a negative correlation
was observed between serum Gb3 concentrations and the changes in
serum CRP and PCT levels on days 1 and 7. This suggests that high
Gb3 levels are associated with a slower decrease in these
inflammatory markers, potentially indicating longer recovery
times.
The present study exhibits several limitations that
need to be addressed prior to the applications of these findings in
clinical diagnosis. The relatively small sample size, single-center
design and the presence of underlying conditions in various
patients may affect the generalizability of the current findings.
Large-scale, multicenter clinical trials involving more diverse
patient populations are necessary to validate these findings and
ensure broader clinical applicability. In addition, the changes in
BALF Gb3 concentration levels were not monitored over time.
Understanding the temporal patterns of Gb3 levels is crucial for
assessing its role as a biomarker throughout the infection process,
including disease progression and response to treatment. Long-term
follow-up studies are required to better elucidate these
dynamics.
While it was found that Gb3 levels were
significantly elevated in patients with PA-induced LRTIs, Gb3 has
also been shown to be implicated in infections caused by other
bacterial pathogens, such as Shiga toxin-producing Escherichia
coli (16-19).
This suggests that elevated Gb3 levels may not be exclusive to PA
infections, potentially limiting the specificity of Gb3 as a
biomarker for PA-induced LRTIs. Consequently, the diagnostic
utility of Gb3 may be affected by its involvement in other
bacterial infections and elevated Gb3 levels could indicate
infections beyond PA. Future research should determine whether the
increase in Gb3 levels is specific to PA-induced LRTIs or
represents a general response to certain bacterial pathogens.
Finally, there is currently no standardized method
for measuring Gb3 levels or established clinical cutoff values. The
measurement of Gb3 requires specialized laboratory techniques and
equipment, which may limit its practicality in routine clinical
settings (25,26). Developing reliable, reproducible
measurement techniques and determining clinically relevant
thresholds are essential steps prior to the implementation of Gb3
as a diagnostic biomarker. By acknowledging these limitations, the
present study aims to guide future research efforts to address
these challenges and facilitate the practical application of Gb3 in
diagnosing PA-induced LRTIs.
In conclusion, the present study highlights a
significant association between elevated levels of Gb3 and
PA-induced LRTIs. Patients with PA-induced LRTIs exhibited
significantly higher Gb3 concentration levels in both serum and
BALF compared with those of healthy controls, with serum Gb3 levels
indicating the highest predictive capability. These findings
suggest that Gb3 may serve as a promising biomarker for the early
detection and diagnosis of PA-induced LRTIs. Although Gb3 indicates
a potential as a diagnostic tool, further research is necessary to
validate its specificity for PA infections and to overcome current
limitations prior to its clinical application. By addressing these
challenges, Gb3 can contribute to improved diagnostic accuracy and
patient outcomes in PA-induced LRTIs.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LY conceived the study and supervised the research.
TX drafted the manuscript. JL, YD and ZZ acquired and analyzed the
data. TX, JL, YD and ZZ participated in data interpretation. TX and
LY confirm the authenticity of all the raw data. All authors
provided critical revision of the manuscript for important
intellectual content. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
This study received the ethics approval (approval
no. 2024-045) of biomedical research involving humans from the
Ethics Committee of The Affiliated People's Hospital of Ningbo
University (Ningbo, China). Written informed consent was obtained
from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Crone S, Vives-Flórez M, Kvich L, Saunders
AM, Malone M, Nicolaisen MH, Martínez-García E, Rojas-Acosta C,
Catalina Gomez-Puerto M, Calum H, et al: The environmental
occurrence of Pseudomonas aeruginosa. APMIS. 128:220–231.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Berger C, Rückert C, Blom J, Rabaey K,
Kalinowski J and Rosenbaum MA: Estimation of pathogenic potential
of an environmental Pseudomonas aeruginosa isolate using
comparative genomics. Sci Rep. 11(1370)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Diggle SP and Whiteley M: Corrigendum:
Microbe profile: Pseudomonas aeruginosa: Opportunistic
pathogen and lab rat. Microbiology (Reading).
167(001073)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Shi Y, Cao Q, Sun J, Hu X, Su Z, Xu Y,
Zhang H, Lan L and Feng Y: The opportunistic pathogen
Pseudomonas aeruginosa exploits bacterial biotin synthesis
pathway to benefit its infectivity. PLoS Pathog.
19(e1011110)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sathe N, Beech P, Croft L, Suphioglu C,
Kapat A and Athan E: Pseudomonas aeruginosa: Infections and
novel approaches to treatment ‘Knowing the enemy’ the threat of
Pseudomonas aeruginosa and exploring novel approaches to
treatment. Infect Med (Beijing). 2:178–194. 2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang S and Stallforth P: Biofilms and
exopolysaccharides in Pseudomonas aeruginosa: Pathogenesis,
immune evasion, and lung-brain signaling during pneumonia. Signal
Transduct Target Ther. 9(204)2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Huang Q, Duan C, Ma H, Nong C, Zheng Q,
Zhou J, Zhao N, Mou X, Liu T, Zou S, et al: Structural and
functional characterization of itaconyl-CoA hydratase and
citramalyl-CoA lyase involved in itaconate metabolism of
Pseudomonas aeruginosa. Structure. 32:941–952.e3.
2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Maruri-Aransolo A, López-Causapé C,
Hernández-García M, García-Castillo M, Caballero-Pérez JD, Oliver A
and Cantón R: In vitro activity of cefiderocol in Pseudomonas
aeruginosa isolates from people with cystic fibrosis recovered
during three multicentre studies in Spain. J Antimicrob Chemother.
79:1432–1440. 2024.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ashworth EA, Wright RCT, Shears RK, Wong
JKL, Hassan A, Hall JPJ, Kadioglu A and Fothergill JL: Exploiting
lung adaptation and phage steering to clear pan-resistant
Pseudomonas aeruginosa infections in vivo. Nat Commun.
15(1547)2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Siukstaite L, Imberty A and Römer W:
Structural diversities of lectins binding to the glycosphingolipid
Gb3. Front Mol Biosci. 8(704685)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Schubert T, Sych T, Madl J, Xu M, Omidvar
R, Patalag LJ, Ries A, Kettelhoit K, Brandel A, Mely Y, et al:
Differential recognition of lipid domains by two Gb3-binding
lectins. Sci Rep. 10(9752)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Üçeyler N, Böttger J, Henkel L, Langjahr
M, Mayer C, Nordbeck P, Wanner C and Sommer C: Detection of blood
Gb3 deposits as a new tool for diagnosis and therapy monitoring in
patients with classic Fabry disease. J Intern Med. 284:427–438.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dinu IR and Firu ŞG: Fabry disease-current
data and therapeutic approaches. Rom J Morphol Embryol. 62:5–11.
2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bichet DG, Aerts JM, Auray-Blais C,
Maruyama H, Mehta AB, Skuban N, Krusinska E and Schiffmann R:
Assessment of plasma lyso-Gb3 for clinical monitoring of
treatment response in migalastat-treated patients with Fabry
disease. Genet Med. 23:192–201. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nowak A, Beuschlein F, Sivasubramaniam V,
Kasper D and Warnock DG: Lyso-Gb3 associates with adverse long-term
outcome in patients with Fabry disease. J Med Genet. 59:287–293.
2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang T, de Waard AA, Wuhrer M and Spaapen
RM: The Role of glycosphingolipids in immune cell functions. Front
Immunol. 10(90)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Freedman SB, van de Kar N and Tarr PI:
Shiga toxin-producing escherichia coli and the hemolytic-uremic
syndrome. N Engl J Med. 389:1402–1414. 2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Park JY, Kim CH and Cho SH:
Glycan-adhering lectins and experimental evaluation of a lectin
fimh inhibitor in enterohemorrhagic Escherichia coli (EHEC)
O157:H7 strain EDL933. Int J Mol Sci. 23(9931)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Arenas-Mosquera D, Pinto A, Cerny N,
Berdasco C, Cangelosi A, Geoghegan PA, Malchiodi EL, De Marzi M and
Goldstein J: Cytokines expression from altered motor thalamus and
behavior deficits following sublethal administration of Shiga toxin
2a involve the induction of the globotriaosylceramide receptor.
Toxicon. 216:115–124. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zheng S, Eierhoff T, Aigal S, Brandel A,
Thuenauer R, de Bentzmann S, Imberty A and Römer W: The
Pseudomonas aeruginosa lectin LecA triggers host cell
signalling by glycosphingolipid-dependent phosphorylation of the
adaptor protein CrkII. Biochim Biophys Acta Mol Cell Res.
1864:1236–1245. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kociurzynski R, Makshakova ON, Knecht V
and Römer W: Multiscale molecular dynamics studies reveal different
modes of receptor clustering by Gb3-binding lectins. J Chem Theory
Comput. 17:2488–2501. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Feitz WJC, Bouwmeester R, van der Velden
TJAM, Goorden S, Licht C, van den Heuvel LPJW and van de Kar NCAJ:
The shiga toxin receptor globotriaosylceramide as therapeutic
target in Shiga Toxin E. coli Mediated HUS. Microorganisms.
9(2157)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Brandel A, Aigal S, Lagies S, Schlimpert
M, Meléndez AV, Xu M, Lehmann A, Hummel D, Fisch D, Madl J, et al:
The Gb3-enriched CD59/flotillin plasma membrane domain regulates
host cell invasion by Pseudomonas aeruginosa. Cell Mol Life
Sci. 78:3637–3656. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gao S, Ni C, Huang W, Hao H, Jiang H, Lv
Q, Zheng Y, Liu P, Kong D and Jiang Y: The interaction between
flagellin and the glycosphingolipid Gb3 on host cells contributes
to Bacillus cereus acute infection. Virulence. 11:769–780.
2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Szymczak-Kulus K, Czerwinski M and
Kaczmarek R: Human Gb3/CD77 synthase: A glycosyltransferase at the
crossroads of immunohematology, toxicology, and cancer research.
Cell Mol Biol Lett. 29(137)2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Geyer PE, Maak M, Nitsche U, Perl M,
Novotny A, Slotta-Huspenina J, Dransart E, Holtorf A, Johannes L
and Janssen KP: Gastric adenocarcinomas express the
glycosphingolipid Gb3/CD77: Targeting of gastric cancer cells with
Shiga Toxin B-subunit. Mol Cancer Ther. 15:1008–1017.
2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Pulmonary Infection Assembly of Chinese
Thoracic Society. Chinese expert consensus on the management of
lower respiratory tract infections of Pseudomonas aeruginosa
in adults (2022). Zhonghua Jie He He Hu Xi Za Zhi. 45:739–752.
2022.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
28
|
Sharma P, Zhang X, Ly K, Zhang Y, Hu Y, Ye
AY, Hu J, Kim JH, Lou M, Wang C, et al: The lipid
globotriaosylceramide promotes germinal center B cell responses and
antiviral immunity. Science. 383(eadg0564)2024.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Weinbergerova B, Kabut T, Kocmanova I,
Lengerova M, Pospisil Z, Kral Z and Mayer J: Bronchoalveolar lavage
fluid and serum 1,3-β-D-glucan testing for invasive pulmonary
aspergillosis diagnosis in hematological patients: The role of
factors affecting assay performance. Sci Rep.
10(17963)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gan Q, Li Z, Li X, Huang Y and Deng H:
Analysis of the effects of early screening combined with blood
lactate on the severity of patients with sepsis. Heliyon.
10(e31907)2024.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Formenti P, Gotti M, Palmieri F, Pastori
S, Roccaforte V, Menozzi A, Galimberti A, Umbrello M, Sabbatini G
and Pezzi A: Presepsin in critical illness: Current knowledge and
future perspectives. Diagnostics (Basel). 14(1311)2024.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hu Y, Ren J, Lv Z, Liu H and Qiu X:
Procalcitonin and C-reactive protein as early predictors in
patients at high risk of colorectal anastomotic leakage. J Int Med
Res. 52(3000605241258160)2024.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zeng Y, Xie Y, Chen D and Wang R: Related
factors of euthyroid sick syndrome in patients with sepsis. Beijing
Da Xue Xue Bao Yi Xue Ban. 56:526–532. 2024.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
34
|
Müller DC, Kauppi A, Edin A, Gylfe Å,
Sjöstedt AB and Johansson A: Phospholipid levels in blood during
community-acquired pneumonia. PLoS One. 14(e0216379)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nan W, Xiong F, Zheng H, Li C, Lou C, Lei
X, Wu H, Gao H and Li Y: Myristoyl lysophosphatidylcholine is a
biomarker and potential therapeutic target for community-acquired
pneumonia. Redox Boil. 58(102556)2022.PubMed/NCBI View Article : Google Scholar
|