Introduction
Traumatic brain injury (TBI) is a critical medical
condition that defined by structural damage and/or functional
impairment of the brain due to excessive external force (1). The yearly incidence of TBI is
estimated at 50 million cases worldwide; thus, ~50% of the global
population will have an episode of TBI in their life (2). Severe TBI, typically indicated by a
Glasgow Coma Scale score of 3-8, reflects a state where patients
are typically unconscious or progressively losing awareness
post-injury (3). The condition is
associated with a high mortality rate, with ~30% of patients not
surviving, while ~50% will experience moderate to severe disability
within 1year, although a minority (10-20%) exhibit clear recovery
(4). Due to its high risk of
disability and mortality, severe TBI has been garnering attention.
In data from 2014, the Centers for Disease Control and Prevention
in the United States noted 2.53 million emergency patients
hospitalized due to brain injuries. 288,000 patients admitted to
the hospital due to traumatic brain injury, and 56,800 patients
succumbed to TBI (5). Between
December 22, 2014 and August 1, 2017, 13,138 patients from 52
hospitals in 22 provinces of China were analyzed. Most patients
were male [9,782 (74%)], with a median age of 48 years
[interquartile range (IQR) 33-61]. Overall, 637 (5%) patients
succumbed to TBI, including 552 (20%) with severe TBI. The expected
14-day mortality was 1,116 (13%), but 544 (7%) mortalities within
14 days were observed (observed to expected ratio 0·49) (6). Globally, in 2019, TBI had 27.16
million new cases, 48.99 million prevalent cases and 7.08 million
years of life lived with disability. The global age-standardized
incidence rate of TBI decreased significantly by -5.5% [95%
confidence interval (CI) -8.9% to -3.0%] from 1990-2019.
Regionally, in 2019, Eastern Europe and high-income North America
had the highest burden of TBI. In 2019, Slovenia and Afghanistan
had the highest age-standardized incidence rates of TBI. Falls were
the leading cause of TBI in 74% (150/204) of countries/territories,
followed by pedestrian road injuries (14%; 29/204), motor vehicle
road injuries (5%; 11/204), and conflicts and terrorism (2%; 4/204)
(6). In Canada ~160,000
individuals suffer from brain injuries each year, resulting in
11.000 deaths and over 6.000 permanent disabilities (7). Nearly half of the TBI cases in Canada
are caused by falls and motor vehicle collisions, with an estimated
direct and indirect cost of approximately $3 billion annually
associated with TBI (8).
Therefore, the incidence, disability and mortality rates of TBI are
high in different countries and for different sexes and ages.
Studying the risk factors that lead to increased incidence and
mortality rates of TBI may help reduce the incidence and mortality
rates of TBI.
With continuous advancements in diagnosis and
treatment, patients with mild TBI are now more frequently
discharged without significant long-term neurological effects
(9). However, these improvements
have not sufficiently impacted the persistently high mortality
rates in patients with moderate to severe cranial-cerebral
injuries. A previous study has indicated that 60% patients with TBI
are vulnerable to emotional, cognitive, behavioral and physical
impairments of varying severity (10). The mortality rate for severe TBI
remains high, reaching 30-40% (11) and is frequently accompanied with
multiple complications. Survivors frequently experience cognitive,
motor, speech and psychological deficits, severely diminishing
their quality of life (12). This
deterioration imposes substantial economic costs and emotional
strain on both families and society (10). Therefore, developing novel
effective treatment strategies, improving prognostic accuracy and
enhancing post-treatment quality of life for patients with severe
TBI remain to be pressing challenges in the current clinical
landscape. As a result, research into the prognostic factors for
severe TBI is gaining increasing focus (9-12).
Research shows that country, income level, pre hospital treatment,
age, sex, anemia, diabetes, shock, hypotension, hypoxemia, trauma
score, GCS, coagulation characteristics, and cerebral hemorrhage
types are all incidence- and mobility-related risk factors in
patients with severe TBI (13,14).
However, these studies are relatively few and some of them are
inconsistent, leading to ongoing controversies, such as coagulation
characteristics, diffuse axonal injury (DAI), prehospital
intubation, and cerebral hemorrhage types (15). Comprehensive meta-analyses and
systematic reviews are beneficial tools for identifying mortality
risk factors, enabling early recognition, heightened clinical
vigilance and timely intervention. These measures are vital for
reducing the severe TBI mortality rates.
Materials and methods
Review design
The present study followed the Preferred Reporting
Items standards for both Systematic Reviews and Network
Meta-Analyses guidelines (16) for
conducting systematic reviews and meta-analyses, ensuring
compliance with the standards (16). The present systematic review and
meta-analysis were also conducted according to the Meta-analyses of
Observational Studies in Epidemiology criteria for observational
studies (17). The protocol was
registered at https://inplasy.com/, with a
registration number ofINPLASY202440111.
Literature search
Relevant data were systematically retrieved from
PubMed (https://pubmed.ncbi.nlm.nih.gov), Web of Science
(https://webofscience.clarivate.cn),
CINAHL (www.lib.cgu.edu.tw) and EMBASE
(www.embase.com), encompassing studies from
database inception until October 17, 2023. The search focused on
English-language keywords, such as ‘head trauma’, ‘brain trauma’,
‘mortality’, ‘death’, and ‘risk factor’ (Table I). To minimize potential omissions,
manual searches of references from selected studies and relevant
reviews were conducted to identify additional literature. Only
English-language studies were included.
 | Table ISearch strategy. |
Table I
Search strategy.
| Database | Search
strategy | Results |
|---|
| PubMed | (((Death
(Title/Abstract)) OR mortality (Title/Abstract))) AND
(((((((craniocerebral injuries (MeSH Terms)) OR brain injuries
(MeSH Terms)) OR head injur* (Title/Abstract)) OR brain injur*
(Title/Abstract)) OR head trauma (Title/Abstract)) OR brain trauma
(Title/Abstract)) OR cerebral injur*) AND (risk factor (MeSH
Terms)) Searches were limited to English language papers and human
population. | 2,171 |
| Web of | (((((TS=(‘brain
injur*’)) OR TS=(‘head injur*’)) OR TS=(‘brain trauma’)) OR | 583 |
| Science | TS=(‘head
trauma’))) AND (((TS=(death)) OR TS=(mortality))) AND (TS=(‘risk
factor’)) Searches were limited to English language. Refined by: *
(excluding): review, conferenceabstractsand editorial
material. | |
| CINAHL | S1 TI mortality OR
AB mortality OR TI death OR AB death | 1,020 |
| | S2 (MH ‘Brain
Injuries’) | |
| | S3 (MH ‘Head
Injuries’) | |
| | S4SU head injur* OR
AB head injur* | |
| | S5SU brain injur*
OR AB brain injur* | |
| | S6SU head trauma OR
AB head trauma | |
| | S7SU brain trauma
OR AB brain trauma | |
| | S8 S2 OR S3 ORS4 OR
S5 OR S6 OR S7 | |
| | S9 TX risk
factor | |
| | S9 S1 AND S8 AND
S9 | |
| EMBASE | ((((((‘head
injur*’) OR (‘brain injur*’)) OR (‘head trauma’)) OR (‘brain
trauma’)) OR (‘cerebral injur*’))):ti,ab,kw AND (‘risk factor’) AND
((((mortality) OR (death))):ti,ab,kw) | 2,614 |
Study selection and criteria for
consideration of studies
Based on the PICOS framework (18) and established grading criteria for
TBI (2,19), inclusion criteria were: i) Study
subjects comprise patients with severe TBI (3≤GCS≤8); ii) cohort
studies; iii) research content focuses on factors related to
mortality in patients with severe TBI; iv) relevant data was
extractable. Outcomes were drawn from studies reporting potential
risk factors associated with incidence and mortality in severe TBI,
including country, income level, pre-admission treatments, age,
sex, anemia, diabetes, shock, hypotension, hypoxemia, trauma
scores, GCS, coagulation profiles, cerebral hemorrhage types,
locations and DAI. The study design focused on observational cohort
studies with extractable data, excluding the following: i) Review
articles, conference papers and case reports; ii) studies involving
patients with mild (GCS ≥13) or moderate (8≤GCS≤12) TBI or mixed
cohorts of moderate-to-severe TBI; iii) non-cohort studies; and iv)
duplicate studies. In total, two independent reviewers, MWL and
WMC, conducted thorough screening of titles and abstracts,
retrieving full texts for studies that met the inclusion criteria.
Discrepancies were resolved through discussions, with third-party
arbitration by MYL when necessary.
Data collection, extraction, and
outcomes of interest
Information was independently extracted by two
researchers (BRZ and QJZ) from the selected studies, including the
lead author, year of publication, country of origin, study design,
sample size, subject age, criteria for severe TBI and reported
mortality rates. Discrepancies in data extraction were resolved
through either consensus or consultation with a third party (MWL).
The primary outcomes assessed were incidence and
mortality-associated risk factors in patients with severe TBI,
including age, anemia, diabetes, shock, hypotension, hypoxemia,
trauma scores, GCS and coagulation disorders. Secondary outcomes
focused on protective factors against mortality in these patients,
such as diffuse axonal injury, EDH and the use of intracranial
pressure monitors.
Quality assessment of literature
The quality of cohort studies was evaluated by YLZ
and SJG using the Newcastle-Ottawa Scale (NOS), which assessed
critical factors, such as population selection, group comparability
and outcome assessment. According to previous descriptions
(20), the NOS assigned scores
ranging from 0 to 9, where a score of ≥7 would suggest high study
quality, 4-6 stars suggesting moderate quality and <4 suggesting
low quality.
Statistical methods
Statistical analyses were performed using R version
4.2.2 (https://www.r-project.org; R Foundation)
to assess the incidence and mortality-related risk factors among
patients with severe TBI (21).
Dichotomous outcomes were evaluated using risk ratios (RR)
(22), whilst continuous outcomes
were presented as mean differences (MD). Meta-analyses were
performed to synthesize outcome data and pooled odds ratios (ORs)
with corresponding 95%CIs were calculated (23). Given that the present meta-analyses
incorporate data from patients who underwent various surgical
procedures from diverse geographic regions, all outcomes were
analyzed using the DerSimonian-Laird (DL) random-effects model
(24). According to the
recommendations provided by the Cochrane Handbook for Systematic
Reviews of Interventions (https://training.cochrane.org/handbook/current/chapter-10#section-10-10-4-1)
(25), a random-effects model was
utilized for all of our meta-analyses. Subgroup analyses were
performed based on country and varying definitions of severe
TBI.
Sensitivity analysis
Sensitivity analysis assessed the stability and
reliability of research outcomes by systematically removing
individual studies to identify potential sources of heterogeneity,
followed by an examination of the underlying causes. In the present
study, leave-one-out sensitivity analysis was performed to evaluate
mortality rates (26), using
random-effects model to assess risk factors associated mortality
(27).
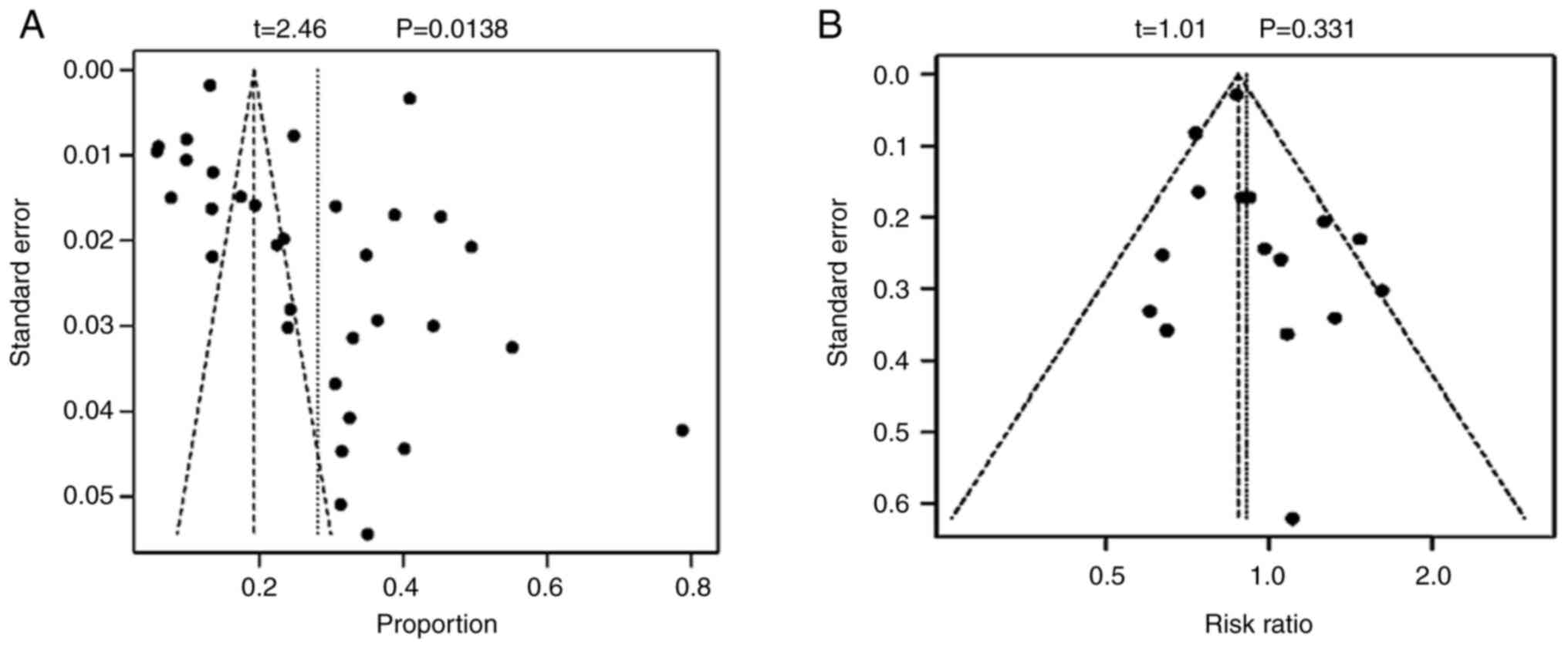
Publication bias
Publication bias, driven by the study's inherent
characteristics and the direction of its findings, influences
decisions on whether to publish or disseminate the research,
potentially resulting in unreported negative outcomes. Publication
bias was evaluated using the Begg's test and funnel plots.
P<0.05 was considered to indicate a statistically significant
difference (28).
Results
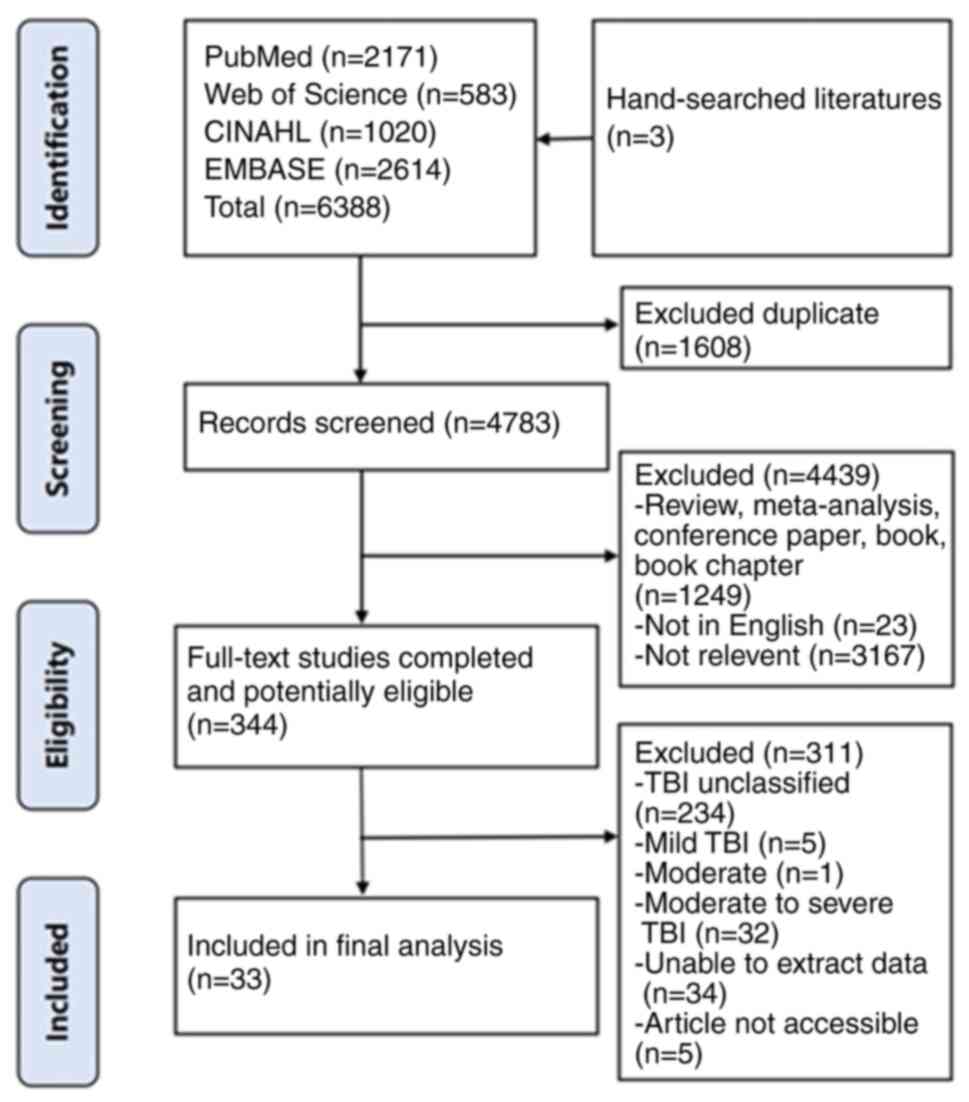
Literature search results
A total of 6,388 articles were retrieved through
database searches. After removing duplicates, 1,608 articles were
excluded, resulting in 4,783 articles for title and abstract
screening. Of these, 4,439 were excluded for the following reasons:
1,249 were reviews or conference papers, 23 were non-English
publications and 3,167 were unrelated to the research focus.
Following this, 344 articles were subjected to full-text review,
leading to the exclusion of additional studies, with 33 articles
ultimately included in the meta-analysis (Fig. 1) (29-61).
The median sample size was 439 cases, with a range of 77-34,175
cases, totaling 71,718 cases. The median mortality rate across the
studies was 30.57%, ranging 5.83-78.72%. Of the selected articles,
eight originated from developing countries with lower levels of
economy, technology and living standards, including medical
standards and 25 were from developed countries with higher levels
of economic and social development, as well as higher living
standards, including medical standards. Table II summarized the key
characteristics of the studies included in the present
meta-analysis.
 | Table IICharacteristics of studies included
in the meta-analysis. |
Table II
Characteristics of studies included
in the meta-analysis.
| First author/s,
year | Country | Patients type | Cohort design | Sample size | Age, years | Sex,
male/female | Severe traumatic
brain injury definition | Incidence (%) | (Refs.) |
|---|
| Jeremitsky et
al, 2005 | USA | NR | Retrospective | 77 | Survival, 39.8
±19.6; death, 55.9±19.0 | 51/26 | GCS≤8 | 27/77 (31.17) | (29) |
| van Wessem
etal, 2022 | Netherlands | NR | Prospective | 234 | Survival, 46
(28-59); death, 56 (32-73) | 157/77 | AIS head≥3 | 57/234 (24.36) | (30) |
| Shen et al,
2023 | China | NR | Retrospective | 269 | Median, 72.8
(60-89) | 209/60 | GCS≤8 | 98/269 (36.43) | (31) |
| Failla et
al, 2015 | USA | NR | Prospective | 244 | Survival,
32.72±13.54; death, 42.69±17.30 | 199/45 | GCS≤8 | 33/244 (13.52) | (32) |
| Tsai et al,
2022 | China | NR | Retrospective | 1,347 | Survival,
56.0±19.3; death,62.4±18.7 | 910/437 | AIS head≥3 | 134/1,347
(9.48) | (33) |
| Tang et al,
2021 | China | NR | Retrospective | 94 | Median, 44.5
(31-57) | 80/14 | GCS<5 | 74/94 (78.72) | (34) |
| Rodríguez et
al, 2019 | Colombia | NR | Retrospective | 83 | Survival,
37.8±17.0; death, 81.7±18.7 | 76/7 | Total AIS≥3;
GCS≤8 | 26/83 (31.33) | (35) |
| Choffat et
al, 2019 | Switzerland | NR | Prospective | 832 | Median, 54.3
(32.2-71.3) | 612/220 | AIS head>3 | 255/832
(30.65) | (36) |
| Hartl et al,
2008 | USA | NR | Prospective | 797 | Range, ≥16 | - | GCS≤8 | 79/797 (9.91) | (37) |
| Kumar et al,
2019 | USA | NR | Prospective | 157 | Survival,
33.54±1.35; death, 51.08±2.29 | 127/30 | GCS≤8 | 48/157 (30.57) | (38) |
| Lele et al,
2019 | India | NR | Prospective | 200 | Mean,
36.9±13.0 | 168/32 | AIS head≥3;
GCS<8 | 48/200 (24.00) | (39) |
| Dawes et al,
2015 | USA | NR | Prospective | 822 | - | 603/219 | GCS≤8 | 319/822
(38.81) | (40) |
| Boto et al,
2014 | Spain | NR | Prospective | 652 | Range, ≥15 | - | GCS≤8 | 114/652
(17.48) | (41) |
| Shibahashi et
al, 2021 | Japan | NR | Retrospective | 34,175 | Survival, 60
(40-74); death, 70 (54-81) | 23,607/10568 | AIS head≥3 | 4,513/34,175
(13.21) | (42) |
| Emami et al,
2019 | Germany | Isolated | Prospective | 21,242 | - | 15,044/6198 | AIS head≥3 |
8,691/21,242(40.91) | (43) |
| Corral et
al, 2012 | Spain | NR | Retrospective | 224 | Median, 35.6
(23-55) | 189/35 | GCS≤8 | 74/224 (33.04) | (44) |
| Catapano et
al, 2019 | USA | Isolated | Retrospective | 600 | Mean,
59.8±13.4 | - | AIS head≥3 | 35/600 (5.83) | (45) |
| Franschman et
al, 2011 | Netherlands | NR | Retrospective | 274 | Survival, 36±7;
death, 52±3 | - | GCS≤8 | 121/274
(44.16) | (46) |
| Cai et al,
2016 | USA | NR | Retrospective | 580 | Survival,
49.4±19.7; death, 63.3±20.2 | 410/169 | AIS head≥3;
GCS≤8 | 287/580
(49.48) | (47) |
| Lanzillo et
al, 2019 | Italy | NR | Prospective | 457 | Survival,
49.7±17.8; death, 61.9±13.6 | 290/167 | GCS≤8 | 107/457
(23.41) | (48) |
| Rahimi et
al, 2014 | Iran | NR | Retrospective | 108 | Survival,
12.32±5.24; death, 12.55±4.90 | 81/27 | GCS≤8 | 34/108 (31.48) | (49) |
| Yang et al,
2011 | China | NR | Retrospective | 234 | Survival,
47.6±16.9; death, 55.6±18.0 | - | GCS≤8 | 129/234
(55.13) | (50) |
| Talving et
al, 2011 | USA | Isolated | Retrospective | 320 | Mean, 10.7±5.1 | 234/86 | AIS head≥3 | 25/320 (7.81) | (51) |
| Catapano et
al, 2016 | USA | Isolated | Retrospective | 698 | Range, ≥40 | 475/223 | AIS head≥3 | 42/698 (6.02) | (52) |
| Mohseni et
al, 2014 | Sweden | NR | Retrospective | 622 | Mean, 64±13 | 423/199 | AIS head≥3 | 121/622
(19.45) | (53) |
| Talving et
al, 2010 | USA | Isolated | Retrospective | 815 | Range, 18-64 | 692/123 | AIS head≥3 | 111/815
(13.62) | (54) |
| Talving et
al, 2009 | USA | All | Retrospective | 436 | Mean, 37±20 | 339/97 | AIS head≥3 | 93/414 (22.46) | (55) |
| Wafaisade et
al, 2010 | Germany | Isolated | Retrospective | 3,114 | Range ≥16 | 2,150/964 | AIS head≥3 | 773/3,114
(24.82) | (56) |
| Davis et al,
2003 | USA | NR | Prospective | 836 | Range ≥18 | 677/159 | GCS:3~8 | 378/836
(45.22) | (57) |
| Lustenberger et
al, 2010 | USA | Isolated | Retrospective | 132 | Mean,34.9±1.6 | 106/26 | AIS head≥3 | 43/132 (32.58) | (58) |
| Saadat et
al, 2012 | Iran | NR | Retrospective | 122 | Median, 13
(7.75-17) | 91/31 | GCS≤8 | 49/122 (40.16) | (59) |
| Lustenberger et
al, 2011 | USA | Isolated | Retrospective | 439 | Mean,37.7±16.4 | 369/70 | AIS head≥3 | 59/439 (13.44) | (60) |
| Salim et al,
2009 | USA | NR | Retrospective | 482 | Mean,38.6±16.9 | 400/82 | AIS head≥3 | 168/482
(35.06) | (61) |
Quality assessment of included
studies
Table III
presented the NOS ratings for the cohort studies, which yielded
either 7 or 8 points, with 20 studies achieving a score of 7 and 13
studies receiving a score of 8. A detailed assessment of the
overall quality of these studies was provided in Table III.
 | Table IIIRisk of bias assessment according to
the NOS score. |
Table III
Risk of bias assessment according to
the NOS score.
| Author, year | Selection | Comparability | Outcome | NOS score | (Refs.) |
|---|
| Jeremitsky et
al, 2005 | 4 | 1 | 2 | 7 | (29) |
| van Wessem et
al, 2022 | 4 | 1 | 3 | 8 | (30) |
| Shen et al,
2023 | 4 | 1 | 3 | 8 | (31) |
| Failla et
al, 2015 | 4 | 1 | 3 | 8 | (32) |
| Tsai et al,
2022 | 4 | 1 | 2 | 7 | (33) |
| Tang et al,
2021 | 4 | 1 | 3 | 8 | (34) |
| Rodríguez et
al, 2019 | 4 | 1 | 2 | 7 | (35) |
| Choffat et
al, 2019 | 4 | 1 | 3 | 8 | (36) |
| Hartl et al,
2008 | 4 | 1 | 3 | 8 | (37) |
| Kumar et al,
2019 | 4 | 1 | 3 | 8 | (38) |
| Lele et al,
2019 | 4 | 1 | 3 | 8 | (39) |
| Dawes et al,
2015 | 4 | 1 | 2 | 7 | (40) |
| Boto et al,
2014 | 4 | 1 | 3 | 8 | (41) |
| Shibahashi et
al, 2021 | 4 | 1 | 3 | 8 | (42) |
| Emami et al,
2019 | 4 | 1 | 2 | 7 | (43) |
| Corral et
al, 2012 | 4 | 1 | 2 | 7 | (44) |
| Catapano et
al, 2019 | 4 | 1 | 2 | 7 | (45) |
| Franschman et
al, 2011 | 4 | 1 | 2 | 7 | (46) |
| Cai et al,
2016 | 4 | 1 | 2 | 7 | (47) |
| Lanzillo et
al, 2019 | 4 | 1 | 3 | 8 | (48) |
| Rahimi et
al, 2014 | 4 | 1 | 2 | 7 | (49) |
| Yang et al,
2011 | 4 | 1 | 2 | 7 | (50) |
| Talving et
al, 2011 | 4 | 1 | 3 | 8 | (51) |
| Catapano et
al, 2016 | 4 | 1 | 2 | 7 | (52) |
| Mohseni et
al, 2014 | 4 | 1 | 2 | 7 | (53) |
| Talving et
al, 2010 | 4 | 1 | 2 | 7 | (54) |
| Talving et
al, 2009 | 4 | 1 | 2 | 7 | (55) |
| Wafaisade et
al, 2010 | 4 | 1 | 2 | 7 | (56) |
| Davis et al,
2003 | 4 | 1 | 2 | 7 | (57) |
| Lustenberger et
al, 2010 | 4 | 1 | 2 | 7 | (58) |
| Saadat et
al, 2012 | 4 | 1 | 2 | 7 | (59) |
| Lustenberger et
al, 2011 | 4 | 1 | 2 | 7 | (60) |
| Salim et al,
2009 | 4 | 1 | 2 | 7 | (61) |
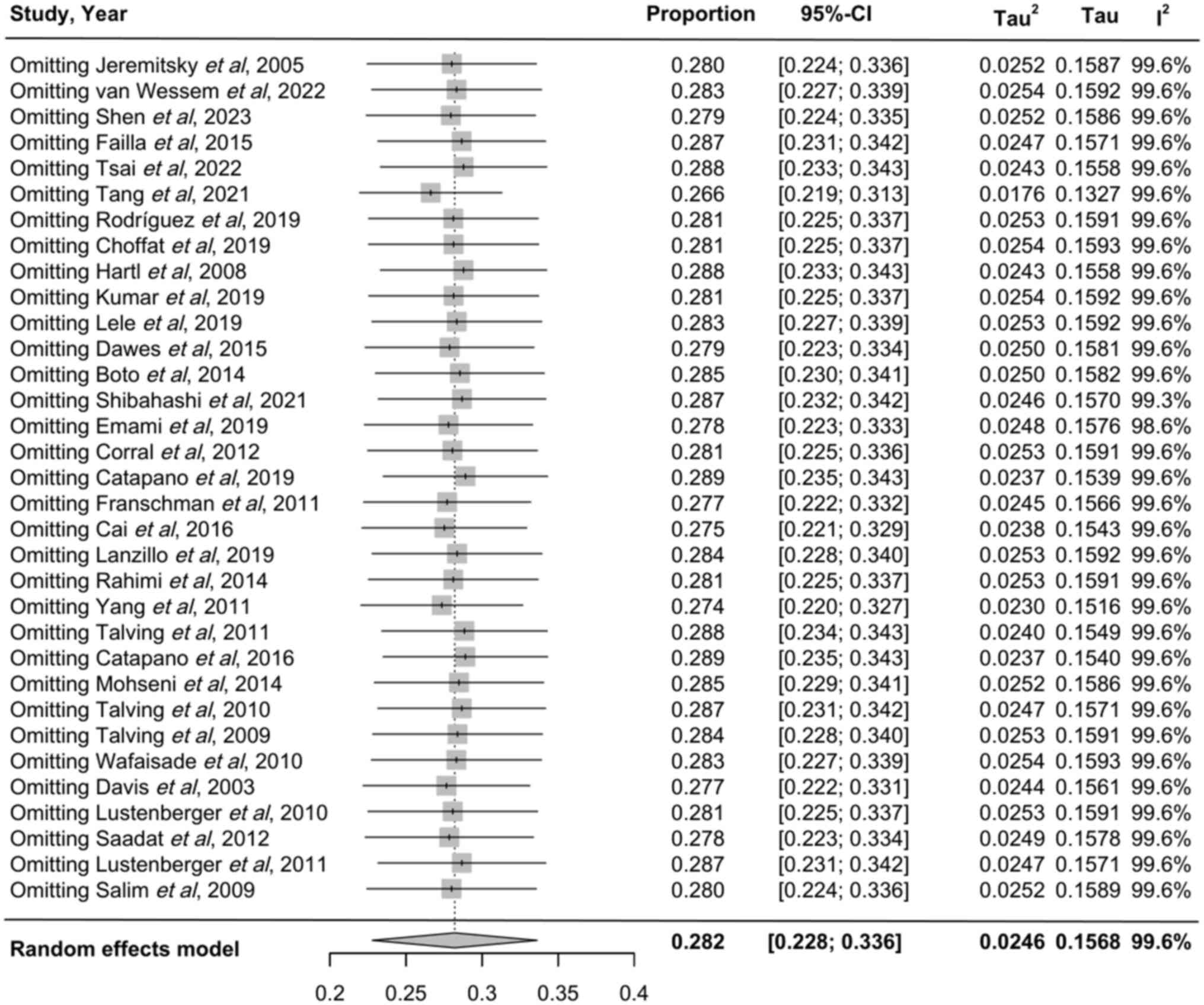
Meta-analysis results
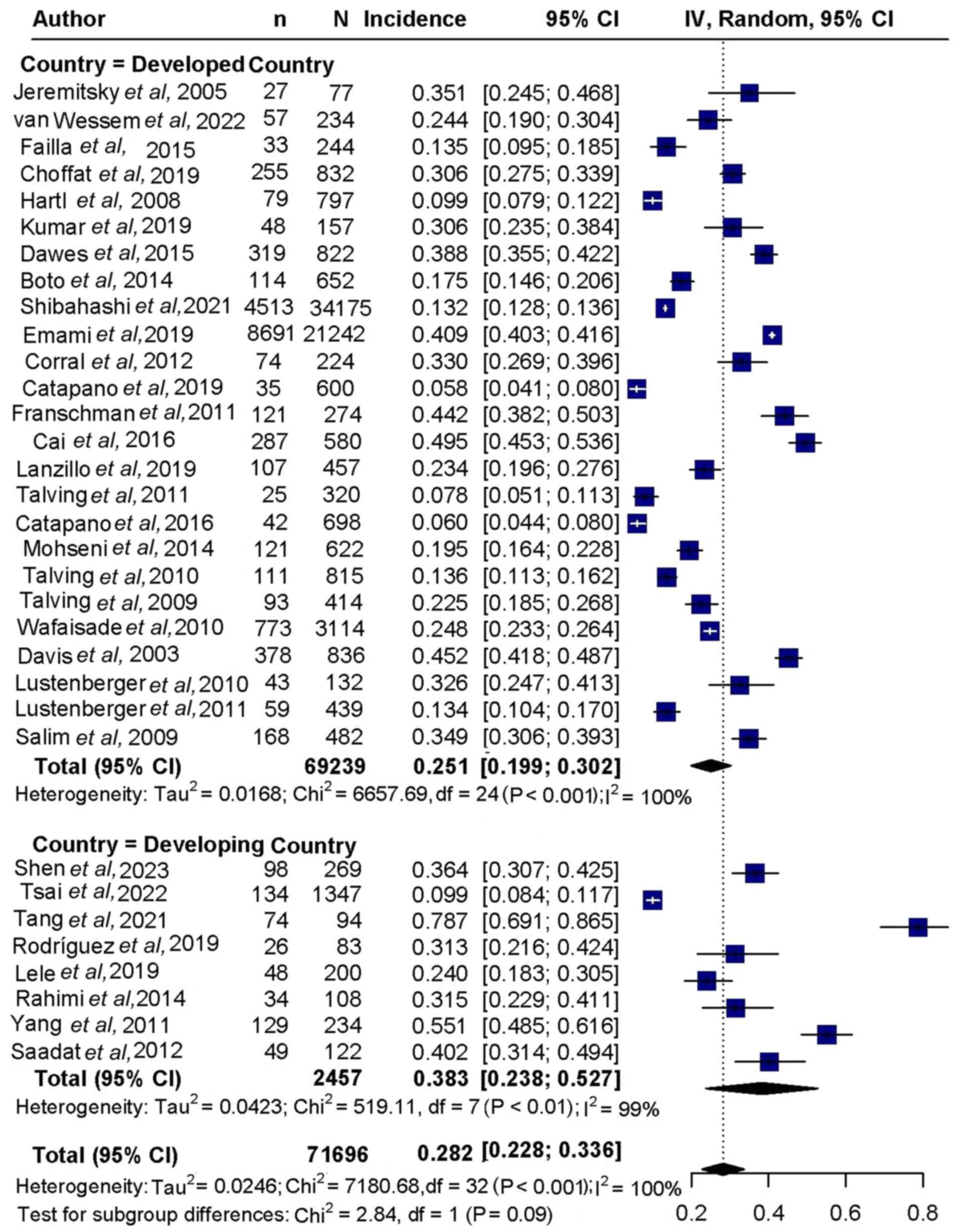
The pooled analysis of patient mortality yielded a
rate of 28.2% (95%CI, 22.8-33.6%; Figs. 2 and 3). Subgroup analysis indicated a
mortality rate of 25.1% (95%CI,19.9-30.2%) in developed countries,
compared with 38.3% (95%CI,23.8-52.7%) in developing countries
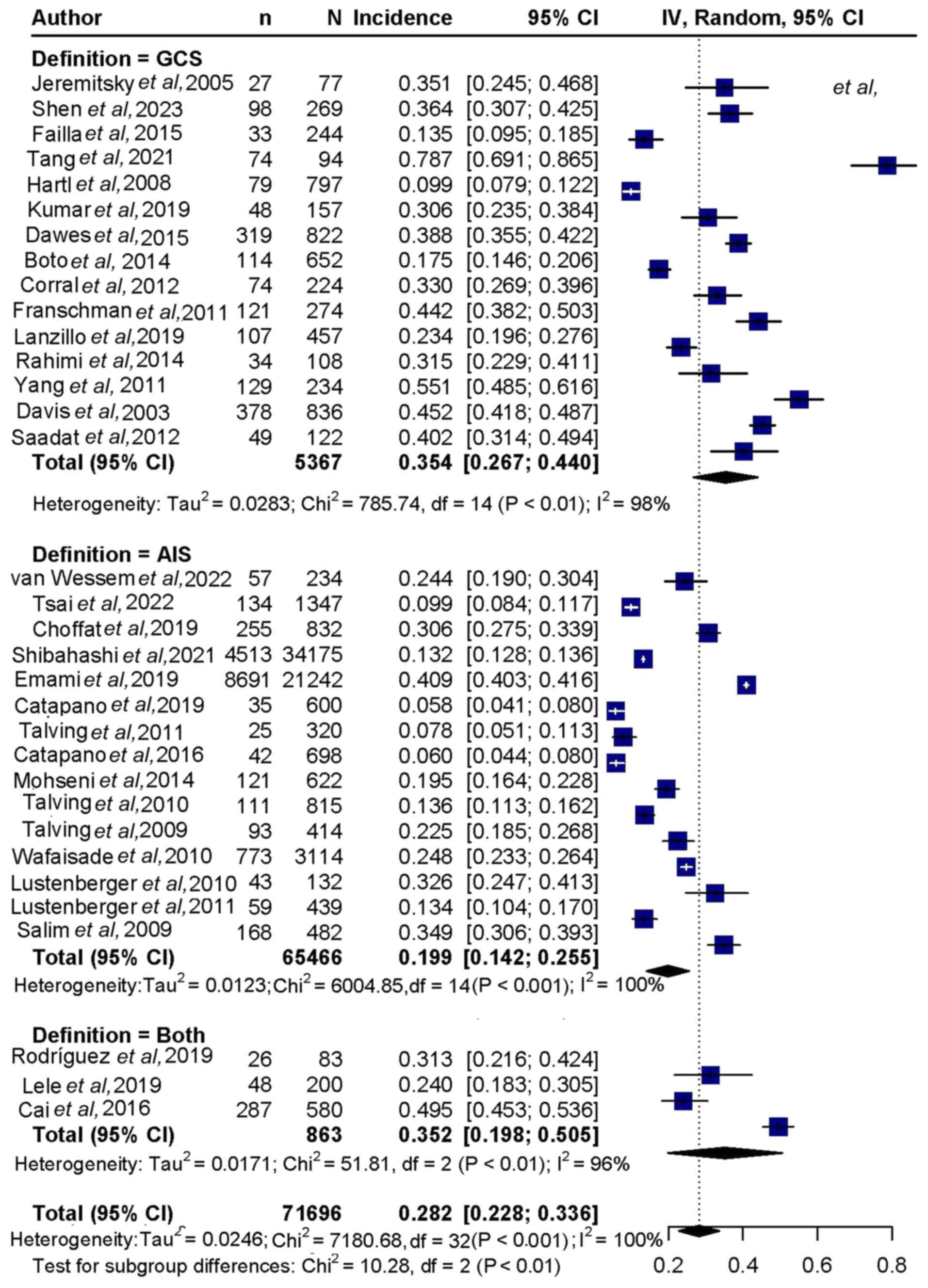
(Fig. 2). Regarding clinical
scoring, mortality was 35.4% (95%CI, 26.7-44.0%) for the GCS score,
19.9% (95%CI, 14.2-25.5%) for the Abbreviated Injury Scale (AIS)
score and 35.2% (95%CI,19.8-50.5%) for both scores combined
(Fig. 3).
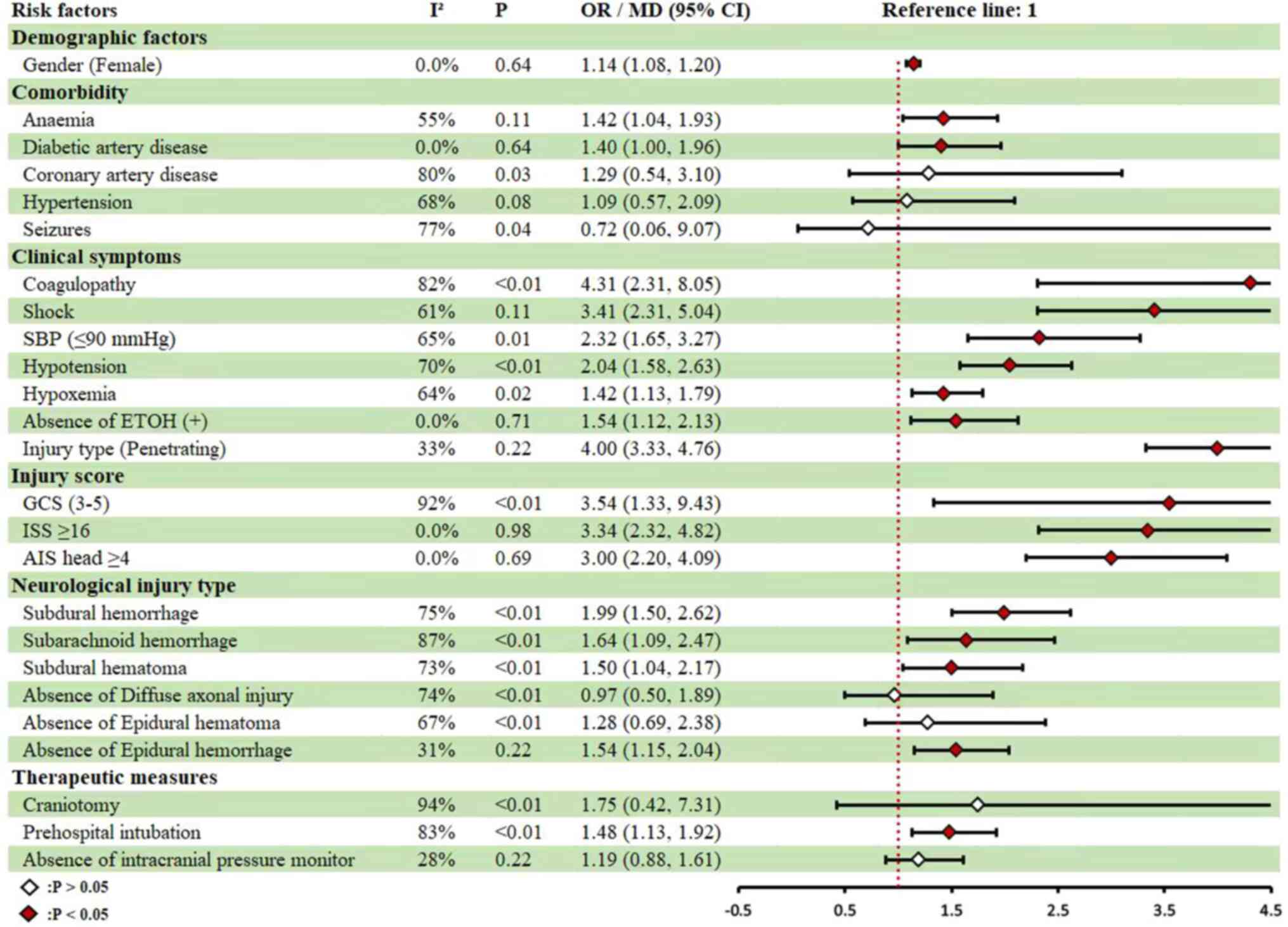
Among the general demographic characteristics,
deceased patients with severe TBI were significantly older compared
with survivors (49.02±20.09). Female sex (RR, 1.14; 95%CI,
1.08-1.20) and comorbidities such as anemia (RR, 1.42; 95%CI,
1.04-1.93) and diabetes mellitus (RR, 1.40; 95%CI, 1.00-1.96) were
identified as significant risk factors for mortality from severe
TBI in this cohort. In terms of clinical symptoms, coagulopathy
(RR, 4.31; 95%CI, 2.31-8.05), shock (RR, 3.41; 95%CI, 2.31-5.04),
systolic blood pressure (SBP) ≤90 mmHg(RR, 2.32; 95%CI, 1.65-3.27),
hypotension (RR, 2.04; 95%CI, 1.58-2.63), and hypoxemia (RR,1.42;
95%CI, 1.13-1.79) were all associated with an increased risk of
mortality. Furthermore, negative ethanol status (RR, 1.54; 95%CI,
1.13-2.13) and penetrating injury (RR, 4.00; 95%CI, 3.33-4.76) were
also significantly associated with increased mortality risk. Injury
severity scores further highlighted risk factors for mortality,
including GCS 3-5 (RR, 3.54; 95%CI, 1.33-9.43), Injury Severity
Score (ISS)≥16 (RR, 3.34; 95%CI, 2.32-4.82) and AIS head ≥4 (RR,
3.00; 95%CI, 2.20-4.09; Fig. 4 and
Table III). Neurological injury
type also contributed to mortality risk, with subdural hemorrhage
(RR, 1.99; 95%CI, 1.50-2.62), subarachnoid hemorrhage (RR, 1.64;
95%CI, 1.09-2.47), and subdural hematoma (SDH) (RR, 1.50; 95%CI,
1.04-2.17) being notable risk factors. Additionally, absence of
epidural hemorrhage (EDH; RR, 1.54; 95%CI, 1.09-2.17) was
significantly associated with an increased risk of mortality.
Regarding treatment interventions, prehospital intubation (RR,
1.48;95%CI, 1.13-1.92) was associated with an increased risk of
mortality, whereas intracranial pressure monitoring (RR,
0.84;95%CI, 0.62-1.14) did not show a statistically significant
effect (Fig. 4 and Table III).
Table IV presented
the results of the subgroup analysis, identifying several factors
associated with an elevated mortality risk in patients with severe
TBI across different countries. These factors included age
(MD=14.28; 95%CI, 10.45-18.10), anemia (RR, 2.20; 95%CI,
1.20-4.04), diabetes mellitus (RR, 1.70; 95%CI, 1.01-2.88), SBP ≤90
mmHg (RR, 2.58; 95%CI, 1.85-3.59), hypotension (RR, 2.03; 95%CI,
1.40-2.94), hypoxemia (RR, 1.58; 95%CI, 1.21-2.07), subdural
hemorrhage (RR, 2.46; 95%CI, 2.33-2.60), and subarachnoid
hemorrhage (RR, 1.97; 95%CI, 1.27-3.04) in developed countries. GCS
scores of 3-5 (RR, 3.17; 95%CI, 0.82-12.28) and subdural hemorrhage
(RR, 2.46; 95%CI, 2.33-2.60) were also found to be significant
mortality risk factors in developed countries. Additionally, female
sex (RR, 1.10; 95%CI, 0.91-1.25) and absence of EDH (RR, 2.08;
95%CI, 0.81-5.26) demonstrated a positive association with
mortality. Under GCS criteria (8≥GCS≥3) (2), age (MD, 10.41; 95%CI, 5.06-15.77),
diabetes mellitus (RR, 1.70; 95%CI, 1.01-2.88), coagulopathy (RR,
4.86; 95%CI, 2.16-10.91) and subdural hemorrhage (RR, 1.98; 95%CI,
1.34-2.92) posed heightened mortality risks. SBP ≤90 mmHg (RR,
2.58; 95%CI, 1.85-3.59) was a key risk factor under AIS criteria
(AIS≥3) (62). The two criteria
identified hypotension (RR, 2.74; 95%CI, 2.26-3.33), hypoxemia (RR,
1.45; 95%CI, 1.14-1.83), subarachnoid hemorrhage (RR, 2.31; 95%CI,
1.90-2.81) and prehospital intubation (RR, 2.28; 95%CI, 1.84-2.84)
as significant mortality risks. Additionally, protective effects
were observed for female sex (RR, 1.35; 95%CI, 1.00-1.82) and
absence of intracranial pressure monitoring (RR, 1.59; 95%CI,
1.12-2.22) under both criteria. Furthermore, absence of diffuse
axonal injury (RR, 2.63; 95%CI, 1.47-4.76) and absence of EDH (RR,
2.27; 95%CI, 0.84-5.88) were more strongly associated with
increased mortality in cases with GCS scores (8≥GCS≥3).
 | Table IVResults of pooled analysis on for
risk factors in patients with sTBI. |
Table IV
Results of pooled analysis on for
risk factors in patients with sTBI.
| | Country | sTBI
definition | |
|---|
| Risk factor | Developed | Developing | GCS | AIS | Both | Total |
|---|
| Demographic
factors | | | | | | |
|
Age years,
(mean ± SD) | Deaths: 55.78
(15.21) | Deaths: 55.78
(15.21) | Deaths: 51.83
(19.91) | Deaths: 62.40
(18.70) | - | Deaths: 54.59
(20.103) |
| | Alive: 41.53
(16.47) | Alive: 53.04
(20.69) | Alive: 39.88
(18.08) | Alive: 56.00
(19.3) | | Alive: 49.02
(20.09) |
|
Sex (Female)
(female vs. male) | 1.14
(1.09,1.22) | 0.96
(0.73,1.25) | 1.14
(1.06,1.20) | 1.06
(0.97,1.37) | 1.35
(1.00,1.82) | 1.10
(0.97,1.25) |
| Comorbidity | | | | | | |
|
Anemia (yes
vs. no) | 2.20
(1.20,4.04) | 1.29
(0.99,1.68) | - | - | - | 1.42
(1.04,1.93) |
|
Diabetic
mellitus (yes vs. no) | 1.70
(1.01,2.88) | 1.22
(0.79,1.89) | 1.70
(1.01,2.88) | 1.22
(0.79,1.89) | - | 1.40
(1.00,1.96) |
|
Coronary
artery disease (yes vs. no) | - | - | - | - | - | 1.29
(0.54,3.10) |
|
Hypertension
(yes vs. no) | - | - | - | - | - | 1.09
(0.57,2.09) |
|
Seizures
(yes vs. no) | - | - | - | - | - | 0.72
(0.06,9.07) |
|
Clinical
symptoms | | | | | | |
|
Coagulopathy
(yes vs. no) | - | - | 4.86
(2.16,10.91) | 4.49
(1.89,10.66) | - | 4.31
(2.31,8.05) |
|
Shock (yes
vs. no) | - | - | - | - | - | 3.41
(2.31,5.04) |
|
Systolic
blood pressure (≤90 mmHg) (yes vs. no) | 2.58
(1.85,3.59) | 1.39
(0.86,2.25) | 1.39
(0.86,2.25) | 2.58
(1.85,3.59) | - | 2.32
(1.65,3.27) |
|
Hypotension
(yes vs. no) | 2.03
(1.40,2.94) | 1.94
(1.64,2.29) | 1.90
(1.68,2.16) | - | 2.74
(2.26,3.33) | 2.04
(1.58,2.63) |
|
Hypoxemia
(yes vs. no) | 1.58
(1.21,2.07) | 1.15
(0.92,1.42) | 1.40
(1.02,1.91) | - | 1.45
(1.14,1.83) | 1.42
(1.13,1.79) |
|
ETOH (+)
(yes vs. no) | - | - | - | - | - | 0.65
(0.47,0.89) |
|
Injury type
(Blunt) (blunt vs. penetrating) | - | - | - | - | - | 0.25
(0.21,0.30) |
| Injury score | | | | | | |
|
GCS
(3-5) | 3.17
(0.82,12.28) | 5.02
(3.06,8.24) | - | - | - | 3.54
(1.33,9.43) |
|
ISS ≥16 | - | - | - | - | - | 3.34
(2.32,4.82) |
|
AIS head
≥4 | - | - | - | - | - | 3.00
(2.20,4.09) |
| Neurological injury
type | | | | | | |
|
Subdural
hemorrhage (yes vs. no) | 2.46
(2.33,2.60) | 1.48
(1.10,1.97) | 1.98
(1.34,2.92) | 1.95
(1.16,3.27) | - | 1.99
(1.50,2.62) |
|
Subarachnoid
hemorrhage (yes vs. no) | 1.97
(1.27,3.04) | 0.89
(0.68,1.17) | 2.25
(0.96,5.29) | 1.15
(0.75,1.76) | 2.31
(1.90,2.81) | 1.64
(1.09,2.47) |
|
Subdural
hematoma (yes vs. no) | 1.53
(0.96,2.42) | 1.48
(1.03,2.14) | 1.50
(1.13,1.99) | 1.59
(0.73,3.48) | - | 1.50
(1.04,2.17) |
|
Diffuse
axonal injury (no vs. yes) | - | - | 2.63
(1.47,4.76) | 0.65
(0.38,1.11) | - | 0.97
(0.50,1.89) |
|
Epidural
hematoma (no vs. yes) | - | - | 1.61
(0.74,3.45) | 0.93
(0.30,2.78) | - | 1.54
(1.09,2.17) |
|
Epidural
hemorrhage (no vs. yes) | 2.08
(0.81,5.26) | 1.39
(0.89,2.17) | 2.27
(0.92,5.88) | 1.41
(1.27,1.56) | - | 1.54
(1.15,2.04) |
| Therapeutic
measures | | | | | | |
|
Craniotomy
(yes vs. no) | - | - | - | - | - | 1.75
(0.42,7.31) |
|
Prehospital
intubation (yes vs. no) | - | - | 1.20
(0.91,1.58) | 1.41
(1.32,1.50) | 2.28
(1.84,2.84) | 1.48
(1.13,1.92) |
|
Intracranial
pressure monitor (no vs. yes) | 0.97
(0.58,1.61) | 1.49
(0.93,2.44) | 1.25
(0.91,1.69) | 0.45
(0.15,1.30) | 1.59
(0.89,2.78) | 1.19
(0.88,1.61) |
Publication bias
Fig. 5 illustrated
the results of the Begg's test for studies with a sample size of
≥10, indicating significant publication bias for incidence (t=2.46;
P=0.0138), however, no significant publication bias for incidence
of sex was found (t=1.01; P=0.331).
Sensitivity analysis
Table V presented
the sensitivity analysis performed using the leave-one-out method,
confirming the stability of the mortality rate results. For
mortality-related risk factors, aside from sex, epidural hematoma
and intracranial pressure monitoring, all other factors remained
consistent following the application of the random-effects model
(Fig. 6).
 | Table VSensitivity analysis for risk factors
in patients with sTBI. |
Table V
Sensitivity analysis for risk factors
in patients with sTBI.
| | Country | sTBI
Definition | |
|---|
| Risk factor | Developed | Developing | GCS | AIS | Both | Total |
|---|
| Demographic
factors | | | | | | |
| Age years, (mean ±
SD) | Deaths: 56.82
(17.8) | Deaths: 51.06
(19.48) | Deaths: 52.42
(21.61) | Deaths: 59.71
(23.12) | - | Deaths: 53.81
(18.93) |
| | Alive: 43.26
(16.9) | Alive: 52.31
(23.6) | Alive: 40.35
(18.75) | Alive: 55.39
(21.04) | | Alive: 50.69
(23.27) |
| Sex (Female)
(female vs. male) | 1.19 (1.08,
1.32) | 0.93 (0.75,
1.15) | 1.08 (0.83,
1.33) | 1.14 (1.08,
1.20) | 1.35
(1.15,1.59) | 1.10
(0.97,1.25) |
| Comorbidity | | | | | | |
|
Anemia (yes
vs. no) | 2.20
(1.20,4.04) | 1.35
(1.09,1.66) | - | - | - | 1.64
(1.25,2.14) |
|
Diabetic
mellitus (yes vs. no) | 1.70
(1.18,2.44) | 1.22
(0.83,1.80) | 1.70
(1.18,2.44) | 1.22
(0.83,1.80) | - | 1.46
(1.09,1.94) |
|
Coronary
artery disease (yes vs. no) | - | - | - | - | - | 1.34
(0.39,1.95) |
|
Hypertension
(yes vs. no) | - | - | - | - | - | 1.26
(0.95,1.69) |
|
Seizures | - | - | - | - | - | 0.50
(0.16,1.51) |
| Clinical symptoms
(yes vs. no) | | | | | | |
|
Coagulopathy
(yes vs. no) | - | - | 4.86
(2.16,10.91) | 3.21
(2.88,3.57) | - | 3.27
(2.93,3.65) |
|
Shock (yes
vs. no) | - | - | - | - | - | 3.90
(2.87,5.31) |
|
SBP (≤90
mmHg) | 2.34
(2.18,2.51) | 1.39
(0.86,2.25) | 1.39
(0.86,2.25) | 2.34
(2.18,2.51) | - | 2.32
(2.16,2.48) |
|
Hypotension
(yes vs. no) | 2.06
(1.75,2.43) | 1.94
(1.64,2.29) | 1.88
(1.60,2.20) | - | 2.74
(2.26,3.33) | 2.03
(1.78,2.32) |
|
Hypoxemia
(yes vs. no) | 1.62
(1.39,1.89) | 1.15
(0.90,1.46) | 1.53
(1.31,1.79) | - | 1.45
(1.14,1.83)a | 1.51
(1.32,1.72) |
|
ETOH (+)
(yes vs. no) | - | - | - | - | - | 0.65
(0.51,0.83) |
|
Injury type
(blunt) (blunt vs. penetrating) | - | - | - | - | - | 0.26
(0.21,0.33) |
| Injury score | | | | | | |
|
GCS
(3-5) | 3.06
(2.30,4.07) | 5.02
(3.06,8.24) | - | - | - | 3.47
(2.71,4.43) |
|
ISS ≥16 | - | - | - | - | - | 3.34
(2.31,4.81) |
|
AIS head
≥4 | - | - | - | - | - | 2.98
(2.19,4.06) |
| Neurological injury
type | | | | | | |
|
Subdural
hemorrhage (yes vs. no) | 2.46
(2.33,2.60) | 1.47
(1.09,1.98) | 2.12
(1.59,2.82) | 2.43
(2.30,2.57) | - | 2.42
(2.29,2.56) |
|
Subarachnoid
hemorrhage (yes vs. no) | 2.15
(1.87,2.47) | 0.83
(0.62,1.10) | 2.47
(1.95,3.13) | 1.03
(0.38,1.29) | 2.31
(1.90,2.81) | 1.75
(1.55,1.98) |
|
Subdural
hematoma (yes vs. no) | 1.28
(1.03,1.59) | 1.48
(1.03,2.14) | 1.50
(1.13,1.99) | 1.20
(0.93,1.54) | - | 1.32
(1.10,1.59) |
|
DDiffuse
axonal injury (no vs. yes) | - | - | 2.63
(1.47,4.76) | 0.97
(0.85,1.11) | - | 1.03
(0.91,1.18) |
|
Epidural
hematoma (no vs. yes) | - | - | 1.72
(1.14,2.70) | 1.20
(0.67,2.13) | - | 1.54
(1.09,2.17) |
|
Epidural
hemorrhage (no vs. yes) | 2.08
(0.81,5.56) | 1.43
(1.06,1.96) | 2.17
(0.88,5.26) | 1.41
(1.28,1.56) | - | 1.43
(1.30,1.56) |
| Therapeutic
measures | | | | | | |
|
Craniotomy
(yes vs. no) | - | - | - | - | - | 0.94
(0.67,1.31) |
|
Prehospital
intubation (yes vs. no) | - | - | 1.23
(1.02,1.48) | 1.41
(1.32,1.50) | 2.28
(1.84,2.84) | 1.44
(1.36,1.53) |
| Therapeutic
measures | | | | | | |
|
Craniotomy
(yes vs. no) | - | - | - | - | - | 0.94
(0.67,1.31) |
|
Prehospital
intubation (yes vs. no) | - | - | 1.23
(1.02,1.48) | 1.41
(1.32,1.50) | 2.28
(1.84,2.84) | 1.44
(1.36,1.53) |
|
Intracranial
pressure monitor (no vs. yes) | 0.92
(0.53,1.59) | 1.52
(1.12,2.04) | 1.23
(0.90,1.69) | 0.45
(0.17,1.14) | 1.59
(1.12,2.22) | 1.22
(0.92,1.59) |
Discussion
A total of 33 articles were included in the present
meta-analysis to examine the mortality incidence and risk factors
associated with severe TBI. The NOS scores for cohort studies
varied between 7 and 8, with 20 studies receiving a score of 7 and
13 studies scoring 8. According to previous literature (20), this reflects a considerable amount
of literature analyzed, contributing to a relatively robust set of
research outcomes. TBI is a non-degenerative, non-reproductive
brain injury that is typically caused by excessive external force.
It can lead to impaired or altered consciousness and resulting in
temporary or permanent cognitive and physical disabilities
(63). In the USA, TBI contributes
to ~40% of injury-related fatalities (64). According to the Centers for Disease
Control and Prevention, ~1.72 million individuals in the USA
sustain TBIs annually, with 275,000 requiring hospitalization
(65). These injuries lead to
50,000 deaths and 70,000 cases of long-term disability each year,
with 5.3 million individuals living with these disabilities,
creating significant emotional and socio-economic impact on society
and corresponding families (66).
A previous study has indicated that TBI is prevalent, which
frequently results in neurological deficits, behavioral changes and
cognitive decline, particularly among survivors of sports-related
injuries (67). The broader public
health implications of TBI are considerable (4,5,6,66).
Severe TBI is associated with high rates of mortality and
disability, with a small proportion of patients remaining in a
vegetative state for extended periods (68). Previous studies have reported a
median mortality rate of 30.57%, ranging from 5.83 to 78.72%
(69-71).
In the present analysis, the pooled mortality rate was 27.8%
(95%CI: 22.5-33.2%), with subgroup analysis revealing 25.2% (95%CI:
20.2-30.1%) in developed countries and 38.0% (95%CI: 21.4-54.7%) in
developing nations, aligning with previous studies (72,73).
The significantly lower mortality rate in developed countries is
likely attributed to superior economic resources, advanced
emergency systems and timely medical interventions, which
corroborates earlier findings (74,75).
By contrast, in developing countries, such as China, due to
insufficient investment in medical equipment, emergency medical
systems and training of medical personnel, patients with TBI tended
to have a higher mortality rate (76). To change this situation, trauma
centers have been established in hospitals at different levels.
Concurrently, manpower, material resources and funding have all
been increased, which is coupled with the drive of purchasing
various advanced medical equipment related to trauma and training
medical personnel to enable patients with TBI to receive timely and
effective treatment in turn improving prognosis (77,78).
The overall incidence and mortality rates of
moderate and severe TBI in Central Norway have remained low, but
are significantly increased from the age of 70 years onwards, with
the majority of individuals aged ≥80 years succumbing to severe TBI
(79,80). These estimates are heavily
influenced by the elevated rates in the elderly population
(79), aligning with the present
findings. This trend is largely attributed to age-related
degenerative organ conditions, diminished compensatory mechanisms,
reduced physiological resilience and the presence of comorbidities,
such as heart and lung failure (81). Additionally, poor regulatory
function, coupled with frequent complications, further compounds
the risk (81). Elderly patients
with intracranial hemorrhage will typically struggle to endure the
combined stress of trauma and surgery, worsening pre-existing
conditions, lowering the body's tolerance to brain injury and in
turn heightening the likelihood of complications, sequelae and
mortality (82). These elements
critically affect prognosis. Studies (83,84)
previously highlighted that patients aged ≥65 years had a higher
mortality rate and poorer neurological recovery compared with those
in younger patients with TBI. Anemia (RR, 1.42; 95%CI, 1.04-1.93)
and diabetes mellitus (RR, 1.41; 95%CI, 1.07-1.84) have been
identified as significant mortality risk factors in severe TBI.
Hemoglobin, essential for oxygen transport, becomes compromised
during anemia, leading to reduced cerebral oxygenation and
secondary brain injury (85). A
previous study using animal models of TBI has demonstrated that
anemia can aggravate brain injury (86). In another study using a rat TBI
model, Hare et al (87)
observed that anemia enlarged the contusion area and accelerated
apoptotic neuron death in cerebral tissue injury following acute
neurotrauma, thereby aggravating secondary damage. Decreased
hematocrit has been associated with poorer discharge prognosis
scores in patients with TBI (88),
while hemoglobin levels ≤10 g/dl are associated with reduced
cerebral oxygenation and an increased risk of unfavorable outcomes
(89). By contrast, transfusion of
red blood cell suspension has been documented to enhance cerebral
oxygen metabolism in patients with TBI (89). Blood glucose levels have also been
implicated in patient prognosis (90-92),
with previous studies identifying a relationship between elevated
blood glucose upon admission and adverse outcomes in patients with
severe cranial injuries the following morning. Post-trauma insulin
resistance frequently emerges, impeding the biological efficacy of
insulin and contributing to hyperglycemia (90). Ischemia and hypoxia following brain
injury trigger anaerobic metabolism, leading to substrate
production and the accumulation of acidic metabolites, which
exacerbate the ischemia, edema and neuronal necrosis (93). This in turn increases the risk of
mortality among patients with severe brain injury. Effective blood
glucose regulation has been reported to improve patient outcomes
(94). However, early blood sugar
reduction alone is insufficient. Instead, sustained stabilization
of blood glucose post-reduction creates optimal conditions for a
positive prognosis. Zhu et al (95) report that intensive glycemic
control (IGC) plays a protective role in improving neurological
outcome, decreasing infection rate and reducing the length of stay
in intensive care units (ICU). However, IGC therapy can also
remarkably increase the risk of hypoglycemia, but it will not
affect the mortality in TBI patients. Previous studies on the
glucose lability index have indicated that blood glucose
variability in patients with severe brain injury constitutes an
independent mortality risk factor (96-98).
In total, ~66% patients with severe TBI present with abnormal
coagulation profiles upon emergency admission, whereas ~50% of
those with coagulopathies experience early brain contusion
exacerbation or progression of hemorrhage within 48 h post-injury
(99). In addition, the severity
of the initial brain injury is directly associated the onset of
coagulation dysfunction, with more severe injuries precipitating
earlier occurrences (100).
Post-TBI coagulopathy is commonly associated with poor prognosis, a
finding that aligns with the present study (100). Numerous studies have reported an
association between coagulation function and patient prognosis
(99,101,102). The more severe the TBI, the worse
the coagulation function and the worse patient prognosis. The cause
may be the abnormal coagulation function caused by the activation
of endogenous coagulation pathways after acute trauma, leading to
secondary TBI and worsening of patient condition (103). Therefore, timely supplementation
of low-dose fresh frozen plasma may improve the coagulation
function and prognosis of patients with severe TBI. Previous
studies have shown that hypotension (defined as systolic pressure
<90 mmHg) and hypoxia (PaO2≤60 mmHg) can double the
mortality risk in patients with craniocerebral injuries (104,105). Studies from the 1970s have
identified ‘systemic injury’, involving hypotension, hypoxia and
hypercapnia, to be predictors of increased mortality (106-109),
consistent with the present study. In the present study, diffuse
axonal injury (RR, 0.38; 95%CI, 0.21-0.68) and EDH (RR, 0.45;
95%CI, 0.30-0.69) exhibited a more pronounced protective effect
against mortality in patients with severe TBI, as measured using
the GCS. However, clinical evidence of diffuse axonal injury on MRI
appears to be primarily beneficial for predicting short-term
functional outcomes during hospitalization, without a clear
association with long-term prognosis (110,111). EDH is less likely to cause brain
herniation and coagulation dysfunction, which may explain why the
mortality rate of EDH is also lower (112).
Previous studies have indicated that EDH is
associated with favorable outcomes, defined as a Glasgow Outcome
Scale (GOS) of 4 or 5 (good recovery or moderate disability) at
discharge or follow-up, with rates ranging from 69 to 95% (mean,
84.3±14.6%; median 88.9%) (113,114). By contrast, the outcomes for
subdural hematoma (SDH) during the perioperative period vary
significantly, with rates between 9 and 76% (mean 32.1±13.6%,
median 26.5%) (115,116). In the present study, subdural
hemorrhage (RR, 1.99; 95%CI, 1.50-2.62), subarachnoid hemorrhage
(RR, 1.64; 95%CI, 1.09-2.47) and SDH (RR, 1.50; 95%CI, 1.04-2.17)
were identified to be mortality risk predictors in patients with
severe TBI, whilst epidural hemorrhage (RR, 0.69; 95%CI, 0.63-0.76)
was observed to be protective, being associated with improved
prognosis and lower mortality rate of episodic hemorrhage. Subdural
hemorrhage, subarachnoid hemorrhage and subdural subatoma can not
only lead to progressive hemorrhagic injury (PHI), but also to
brain herniation and coagulation dysfunction, which can increase
the mortality rate of patients with TBI (117). PHI following TBI represents a key
secondary injury mechanism that significantly contributes to both
disability and mortality in affected patients (118). In total, ~20% patients with TBI
patients require surgical intervention due to PHI, with a notably
poorer prognosis observed in those who develop PHI compared with
those who do not (119). The rate
of in-hospital mortality for patients with TBI with adverse
outcomes stands at 29%, with mortality rates of 17% for patients
without PHI and 44% for those with PHI (119). Stein et al (120) previously identified SDH as having
the highest likelihood of PHI among various TBI types. Further
analysis of 782 TBI cases revealed a significant association
between SDH and the occurrence of PHI (121). Di et al (122) previously identified a
significantly higher risk of PHI in patients with EDH compared with
those with other types of TBI lesions, with the risk of PHI in
patients with EDH being ~4X greater compared with that of patients.
The requirement for prehospital intubation (RR, 1.48; 95%CI,
1.13-1.92) found in the present study suggests a severe clinical
condition, frequently involving impaired consciousness or
respiratory failure, thereby contributing to increased mortality.
Despite previous studies reporting that prehospital intubation does
not reduce mortality in patients with severe TBI (123,124), the skill level of medical
personnel has emerged as a critical factor. Poorly executed
intubation by inadequately trained personnel significantly elevates
the risk of mortality, suggesting that in the absence of properly
trained providers, routine prehospital intubation should be
reconsidered for patients with TBI (125). Conversely, recent data indicate
that when performed by experienced and well-trained practitioners
following current TBI guidelines, prehospital endotracheal
intubation can be beneficial (126). However, prehospital intubation
remains to be a risk factor for mortality in patients with severe
TBI in the present study, whilst the use of intracranial pressure
monitors (RR, 0.75; 95%CI, 0.65-0.86) has been shown to be
protective.
Trauma scoring quantifies injury severity through
objective criteria, serving a fundamental role in trauma management
(127). The GCS score remains a
widely accepted tool for assessing both the severity and prognosis
of patients with TBI. It mainly evaluates three parameters, eye,
verbal and motor responses, with the total score reflecting the
patient's level of consciousness. A score of 15 represents normal
consciousness, whilst lower scores correspond to increasing levels
of impairment (128). TBI
severity can be categorized into three levels based on GCS scores:
Mild (13≤GCS<15); moderate (9≤GCS<12); and severe
(3≤GCS<8) (129).
Nevertheless, the GCS has its limitations, particularly in its
subjectivity and inability to accurately evaluate patients
requiring endotracheal intubation or those unable to communicate
verbally (128). Studies have
demonstrated that the GCS-Full Outline of Unresponsiveness (FOUR)
score are comparable in predicting TBI outcomes, including
mortality, length of hospital stay and morbidity (130,131). The FOUR score, however, offers a
more comprehensive neurological assessment with higher specificity
and positive predictive value, making it a valuable clinical tool
(130). In patients with TBI, the
FOUR score has been reported to surpass GCS in predicting hospital
stay duration and morbidity (132). Additionally, injury severity, low
GCS and polytrauma are key predictors of early in-hospital
mortality, while surgical interventions such as head, chest,
abdomen, limb, blood vessels and other surgical procedures are
associated with extended hospitalizations (133). The AIS divides the body into nine
compartments, which are then reclassified into six regions for
calculating the ISS. The AIS code consists of 6 ‘front points’ for
injury location and 1 ‘rear point’ for injury severity. Severity is
rated from 1 to 5, with a score of 6 indicating a non-survivable
injury (134). A previous study
has shown that patients with severe TBI in the unfavorable outcome
group have an average AIS score of 4.39±0.82 and a higher frequency
of AIS scores of 5. Therefore, an AIS head score of five
significantly raises the likelihood of an unfavorable prognosis
(135). In the present study, GCS
scoring contributed 35.0% (95%CI: 25.8-44.3%) and AIS scoring
contributed 19.9% (95%CI: 14.2-25.5%), whereas their combined use
accounted for 33.4% (95%CI: 21.8-45.0%), indicating that GCS
remains a widely employed tool for assessing patients with severe
TBI. The present study identified several factors that were
significantly associated with increased mortality risk in patients
with TBI with GCS scores: Age (MD, 10.41; 95%CI, 5.06-15.77),
diabetes mellitus (RR, 1.70; 95%CI, 1.18-2.45), coagulopathy (RR,
4.86; 95%CI, 2.16-10.91) and subdural hemorrhage (RR, 1.98; 95%CI,
1.34-2.92). In severe TBI cases with AIS scores, SBP ≤90 mmHg (RR,
2.58; 95%CI, 1.85-3.59) was associated with a higher mortality
risk. Additionally, for severe TBI cases evaluated using both GCS
and AIS scores, hypotension (RR, 2.74; 95%CI, 2.26-3.33), hypoxemia
(RR, 1.42; 95%CI, 1.13-1.79), subarachnoid hemorrhage (RR, 2.31;
95%CI, 1.90-2.81) and prehospital intubation (RR, 2.28; 95%CI,
1.84-2.84) were associated with an elevated risk of mortality.
These results indicated that GCS provides a more accurate
prediction of mortality risk in elderly patients and those with
diabetes, coagulopathy or subdural hemorrhage, whereas AIS
demonstrates greater predictive value in cases involving SBP ≤90
mmHg. In patients experiencing hypotension, hypoxemia, subarachnoid
hemorrhage or requiring prehospital intubation, both GCS and AIS
offer improved predictive capabilities for assessing mortality risk
in severe TBI. Furthermore, diffuse axonal injury (DAI; RR, 0.38;
95%CI, 0.21-0.68) was found to have a notable protective effect
against mortality in severe TBI cases with GCS scores, which may be
related to the relatively small effect of TBI combined with DAI on
the mortality rate of TBI patients and clinical evidence of DAI on
MRI may only be useful for predicting short-term in-hospital
functional outcome (136).
However, a previous study has indicated that neither clinical nor
anatomic DAI associates with survival, Glasgow Outcome
Scale-Extended or Quality of Life after Brain Injury-Overall Scale
outcomes (136). A single-point
reduction in GCS score nearly doubles the risk of in-hospital
mortality, whilst coagulopathy increases the risk of in-hospital
mortality ~6 times (137).
In the general population across all age groups
(0-65 years), TBI incidence is typically higher in male compared
with female patients (138).
However, certain studies have suggested that female patients tend
to exhibit a greater frequency and longer duration of post-TBI
symptoms, such as headaches, anxiety and depression, compared with
those in male patients (139,140), which is consistent with the
results of the present study. Additionally, in the present study,
male sex and intracranial pressure monitoring appear to be
protective factors in severe TBI cases. This association may result
from the early detection of intracranial pressure changes and
timely therapeutic interventions. Psychological resilience may also
serve a role, with male patients exhibiting greater resilience
during TBI recovery (141,142),
whilst female patients may face higher risks of anemia, hypotension
and immune disorders (141,143).
Neuroinflammation and neurodegeneration triggered by
TBI can disrupt intestinal function through the bidirectional
brain-gut axis, leading to motility disorders and increased mucosal
permeability (144,145). Alterations in microbiota
composition, along with activation of resident and recruited immune
cells, intensify both systemic and neuroinflammation, further
aggravating brain damage. Children with TBI commonly experience
gastrointestinal dysfunction, which can be evaluated using the
gastrointestinal failure (GIF) score. This score provides a
critical assessment of gastrointestinal function, where its average
over the first 3 days holds significant prognostic value for
predicting ICU mortality in surgical intensive care units (146). As such, gastrointestinal
dysfunction and the GIF score may represent potential risk factors
for mortality in patients with severe brain injury, though further
research is required. Previous studies have also indicated that
patients with severe TBI exhibit fluid overload in brain tissue
(147,148). However, fluid overload has not
been significantly associated with mortality, prolonged ventilator
use, increased risk of acute kidney injury or extended pediatric
intensive care unit stay (147),
suggesting it may not be a major determinant of mortality in this
context. In moderate to severe TBI cases, hypocalcemia occurs most
frequently, followed by hypomagnesemia, hypokalemia, hypernatremia,
hyponatremia and hypermagnesemia (149). Hypocalcemia, hypomagnesemia and
hypokalemia generally present early after TBI, whereas
hypernatremia typically arises during the intracranial pressure
(ICP) phase and hyponatremia appears predominantly after ICP
reduction (149). The concurrent
presence of hypernatremia and hypocalcemia has been associated with
prognostic outcomes (150).
Furthermore, early post-traumatic seizures have been associated
with extended ICU and hospital stays, prolonged mechanical
ventilation and worse 24-month outcomes, including mortality and
the development of post-traumatic epilepsy (151).
The present meta-analysis examined risk factors
possibly contributing to the incidence and mortality of patients
with severe TBI, encompassing variables, such as age, geographic
region, income level, anemia, diabetes, shock, hypotension,
hypoxemia, trauma severity scores and specific types of brain
injury. The cohort studies included were of high quality, as
reflected by NOS scores ranging from 7 to 8, with 20 studies
scoring 7 and 13 studies scoring 8.
However, limitations in the present study must be
acknowledged. Evaluation standards for TBI vary significantly among
hospitals and countries, which may have affected the uniformity of
assessment criteria. In addition, the prognosis of severe TBI is
shaped by medical expertise, economic resources, available
emergency systems and medical infrastructure, which can lead to
disparities between developed and developing nations. The type,
location and presence of associated organ damage in brain injuries
can further contribute to outcome variability. Furthermore, it
should also be noted that GCS scores in patients with TBI may be
skewed by confounding factors, such as hypoxia, hypotension and
shock. Publication bias in the Begg test for the incidence of
severe brain trauma may be due to the heterogeneity in the study
population included in the meta-analysis. In the included studies,
there may be significant differences in basic diseases (such as
anemia and diabetes), physiological status (such as shock,
hypotension and hypoxemia), trauma scores, and brain injury types
in each patient. These factors may have varying degrees of effect
on the incidence of severe brain trauma, leading to biased results.
In addition, there may be differences in the criteria for defining
and grading brain injury among different studies, which may also
increase heterogeneity between studies and affect the results of
the meta-analysis.
The present study identified age, anemia, diabetes,
shock, hypotension, hypoxemia, trauma score, brain injury type and
coagulation dysfunction as significant risk factors for both the
onset of TBI and mortality in severe TBI. Immediate correction of
anemia, diabetes, shock, hypotension, hypoxemia and coagulation
dysfunction in patients with severe TBI is therefore recommended to
improve prognosis.
TBI with a GCS score of <8 associated with a poor
outcome (2). Prognosis can vary
depending on the specific nature of the TBI (152). Additionally, various factors,
such as anemia, hypoxemia and shock, may influence GCS scores,
potentially leading to inaccurate assessments. Nevertheless, recent
advances in comprehensive treatment have resulted in improved
outcomes for some patients.
The present study found that the GCS and AIS have
their own advantages and disadvantages in evaluating severe TBI.
GCS provides a more accurate prediction of mortality risk in
elderly patients and those with diabetes, coagulopathy or subdural
hemorrhage, whereas AIS demonstrates a greater predictive value in
cases involving SBP ≤90 mmHg. In patients experiencing hypotension,
hypoxemia, subarachnoid hemorrhage or requiring prehospital
intubation, both GCS and AIS offer improved predictive capabilities
for assessing the mortality risk in severe TBI (153).
In the present study, prehospital intubation was
identified to be a risk factor for mortality in severe TBI, though
its role in treatment remains a subject of debate. Despite this,
prehospital intubation is recommended for patients in deep coma or
with respiratory and circulatory instability (154). In such cases, intubation by
trained and experienced practitioners is essential to manage
ventilation, optimize oxygenation and secure the airway, providing
critical time for more advanced interventions upon hospital
admission.
The present study also identified ICP as a
protective factor in severe TBI, though it remains widely
recognized that ICP is strongly associated with both preoperative
and postoperative mortality (155,156). Consequently, continuous
monitoring of ICP in patients with severe TBI, both before and
after surgery, is essential to ensure prompt and effective
intervention.
Previous studies have indicated that neither
clinical nor anatomical DAI significantly influenced survival,
Glasgow Outcome Scale-Extended or Quality of Life after Brain
Injury-Overall Scale outcomes (135,157). However, EDH was associated with a
higher survival rate (113). In
the present analysis, both DAI and EDH demonstrated a stronger
protective effect against mortality in severe TBI cases compared
with that with lower GCS scores, which is inconsistent with
previous studies and requires further research to confirm. While
prior studies did not identify sex as an independent predictor of
poor outcomes in severe TBI (158), the present results suggest that
male sex exhibits a more pronounced mortality-preventing effect in
such cases.
Future iterations of the present study are
anticipated to include a broader range of high-quality literature.
Whilst the present study has examined the risk factors influencing
both the incidence and mortality in severe TBI patients, more
robust trial designs and high-quality clinical studies are needed
to generate more comprehensive and higher-tier evidence. Such
advancements will deepen the understanding of factors contributing
to the occurrence and mortality of severe TBI. Effective management
of various factors that can be effectively treated outside the
hospital by the ambulance team, such as diabetes, shock,
hypotension, hypoxemia and prehospital intubation, can eventually
result in a decreased mortality rate. By contrast, coagulopathy,
subdermal hemorrhage, subarachnoid hemorrhage and subatoma can only
be effectively treated in a hospital setting. Therefore, prompt
transportation to the hospital is of paramount importance because
the treatment for coagulopathy, coagulopathy, subdermal hemorrhage,
subarachnoid hemorrhage and subatoma should be introduced as soon
as possible. With the continuous improvement in the economy,
medical system, medical level, and medical equipment, whether in
developed countries or not, the level of treatment and prevention
of TBI brain injury is also constantly improving, and the mortality
and disability rates of severe TBI will further decrease.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Nature Science
Foundation of China (grant no. 81960350) and Union Foundation
Yunnan Provincial Science and Technology Department and Kunming
Medical University of China (grant no. 202201AY070001-091).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MWL, BRZ, RLL and WMC had full access to all the
data in the study and take responsibility for the integrity of the
data and the accuracy of the data analysis. ZQM, BRZ, RLL, YEC and
QJZ contributed to the conceptualization, data curation, formal
analysis and writing original draft. YEC, SJG, YLZ and MWL
contributed to the methodological design, writing reviewing and
editing. MWL, YLZ and SJG verified research data. MWL, RLL, WC, ZQM
and YLZ contributed to the investigation (data collection). MWL and
YEC confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Khellaf A, Khan DZ and Helmy A: Recent
advances in traumatic brain injury. J Neurol. 266:2878–2889.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Robinson CP: Moderate and severe traumatic
brain injury. Continuum (Minneap Minn). 27:1278–1300.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bazarian JJ, McClung J, Cheng YT, Flesher
W and Schneider SM: Emergency department management of mild
traumatic brain injury in the USA. Emerg Med J. 22:473–477.
2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Capizzi A, Woo J and Verduzco-Gutierrez M:
Traumatic brain injury: An overview of epidemiology,
pathophysiology, and medical management. Med Clin North Am.
104:213–238. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Traumatic Brain Injury and Spinal Cord
Injury Collaborators. Global, regional, and national burden of
traumatic brain injury and spinal cord injury, 1990-2016: A
systematic analysis for the Global Burden of Disease Study 2016 GBD
2016. Lancet Neurol. 18:56–87. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gao G, Wu X, Feng J, Hui J, Mao Q, Lecky
F, Lingsma H, Maas AIR and Jiang J: China CENTER-TBI Registry
Participants. Clinical characteristics and outcomes in patients
with traumatic brain injury in China: A prospective, multicentre,
longitudinal, observational study. Lancet Neurol. 19:670–677.
2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Guan B, Anderson DB, Chen L, Feng S and
Zhou H: Global, regional and national burden of traumatic brain
injury and spinal cord injury, 1990-2019: A systematic analysis for
the Global Burden of Disease Study 2019. BMJ Open.
13(e075049)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Juengst SB, Perrin PB, Klyce DW,
O'Neil-Pirozzi TM, Herrera S, Wright B, Lengenfelder J, Lercher K,
Callender L and Arango-Lasprilla JC: Caregiver Characteristics of
Adults with Acute Traumatic Brain Injury in the United States and
Latin America. Int J Environ Res Public Health.
19(5717)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pundlik J, Perna R and Arenivas A: Mild
TBI in interdisciplinary neurorehabilitation: Treatment challenges
and insights. NeuroRehabilitation. 46:227–241. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang KK, Yang Z, Zhu T, Shi Y, Rubenstein
R, Tyndall JA and Manley GT: An update on diagnostic and prognostic
biomarkers for traumatic brain injury. Expert Rev Mol Diagn.
18:165–180. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dang B, Chen W, He W and Chen G:
Rehabilitation treatment and progress of traumatic brain injury
dysfunction. Neural Plast. 2017(1582182)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dijkland SA, Foks KA, Polinder S, Dippel
DWJ, Maas AIR, Lingsma HF and Steyerberg EW: Prognosisin moderate
and severe traumatic brain injury: A systematic review of
contemporary models and validation studies. J Neurotrauma. 37:1–13.
2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kamabu LK, Bbosa GS, Lekuya HM, Cho EJ,
Kyaruzi VM, Nyalundja AD, Deng D, Sekabunga JN, Kataka LM, Obiga
DOD, et al: Burden, riskfactors, neurosurgical evacuation outcomes,
and predictors of mortality among traumatic brain injury patients
with expansive intracranial hematomas in Uganda: A mixed methods
study design. BMC Surg. 23(326)2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tunthanathip T, Phuenpathom N, Saehaeng S,
Oearsakul T, Sakarunchai I and Kaewborisutsakul A: Traumatic
cerebrovascular injury: Prevalence and risk factors. Am J Emerg
Med. 38:182–186. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kolakowsky-Hayner SA, Bellon K and Yang Y:
Unintentional injuries after TBI: Potential risk factors, impacts,
and prevention. NeuroRehabilitation. 39:363–370. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Brooke BS, Schwartz TA and Pawlik TM:
MOOSE reporting guidelines for Meta-analyses of Observational
studies. JAMA Surg. 156:787–788. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Amir-Behghadami M and Janati A:
Population, intervention, comparison, outcomes and Study (PICOS)
design as a framework to formulate eligibility criteria in
systematic reviews. Emerg Med J. 37(387)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Silver JM, McAllister TW and Yudofsky SC:
Textbook of traumatic brain injury. 293. 2005.
|
|
20
|
Lo CK, Mertz D and Loeb M:
Newcastle-Ottawa Scale: Comparing reviewers' to authors'
assessments. BMC Med Res Methodol. 14(45)2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Balduzzi S, Rücker G and Schwarzer G: How
to perform a meta-analysis with R: A practical tutorial. Evid Based
Ment Health. 22:153–160. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hancock M and Kent P: Interpretation of
dichotomous outcomes: Risk, odds, risk ratios, odds ratios and
number needed to treat. J Physiother. 62:172–174. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Alavi M, Hunt GE, Visentin DC, Watson R,
Thapa DK and Cleary M: Using risk and odds ratios to assess effect
size for meta-analysis outcome measures. J Adv Nurs. 76:3231–3234.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cumpston M, Li T, Page MJ, Chandler J,
Welch VA, Higgins JP and Thomas J: Updated guidance for trusted
systematic reviews: A new edition of the Cochrane Handbook for
Systematic Reviews of Interventions. Cochrane Database Syst Rev.
10(ED000142)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ancona N, Maestri R, Marinazzo D, Nitti L,
Pellicoro M, Pinna GD and Stramaglia S: Leave-one-out prediction
error of systolic arterial pressure time series under paced
breathing. Physiol Meas. 26:363–372. 2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Burgess S and Thompson SG: Interpreting
findings from Mendelian randomization using the MR-egger method.
Eur J Epidemiol. 32:377–389. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mavridis D and Salanti G: How to assess
publication bias: Funnel plot, trim-and-fill method and selection
models. Evid Based Ment Health. 17(30)2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Jeremitsky E, Omert LA, Dunham CM,
Wilberger J and Rodriguez A: The impact of hyperglycemia on
patients with severe brain injury. J Trauma. 58:47–50.
2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
van Wessem KJP, Jochems D and Leenen LPH:
The effect of prehospital tranexamic acid on outcome in polytrauma
patients with associated severe brain injury. Eur J Trauma Emerg
Surg. 48:1589–1599. 2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shen H, Liu H, He J, Wei L and Wang S:
Risk factors of prognosis in older patients with severe brain
injury after surgical intervention. Eur J Med Res.
28(479)2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Failla MD, Kumar RG, Peitzman AB, Conley
YP, Ferrell RE and Wagner AK: Variation in the BDNF gene interacts
with age to predict mortality in a prospective, longitudinal cohort
with severe TBI. Neurorehabil Neural Repair. 29:234–246.
2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Tsai CH, Rau CS, Chou SE, Su WT, Hsu SY
and Hsieh CH: Delta de Ritis ratio is associated with worse
mortality outcomes in adult trauma patients with moderate-to-severe
traumatic brain injuries. Diagnostics (Basel).
12(3004)2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tang Z, Yang R, Zhang J, Huang Q, Zhou X,
Wei W and Jiang Q: Outcomes of traumatic brain-injured patients
with Glasgow coma scale <5 and bilateral dilated pupils
undergoing decompressive craniectomy. Front Neurol.
12(656369)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Rodríguez-Triviño CY, Torres Castro I and
Dueñas Z: Hypochloremia in patients with severe traumatic brain
injury: A possible risk factor for increased mortality. World
Neurosurg. 124:e783–e788. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Choffat C, Delhumeau C, Fournier N and
Schoettker P: Effect of Pre-hospital intubation in patients with
severe traumatic brain injury on outcome: A prospective cohort
study. J Clin Med. 8(470)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Härtl R, Gerber LM, Ni Q and Ghajar J:
Effect of early nutrition on deaths due to severe traumatic brain
injury. J Neurosurg. 109:50–56. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kumar RG, DiSanto D, Awan N, Vaughan LE,
Levochkina MS, Weppner JL, Wright DW, Berga SL, Conley YP, Brooks
MM and Wagner AK: Temporal acute serum estradiol and tumor necrosis
factor-α associations and risk of death after severe traumatic
brain injury. J Neurotrauma. 37:2198–2210. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lele A, Kannan N, Vavilala MS, Sharma D,
Mossa-Basha M, Agyem K, Mock C, Pandey RM, Dash HH, Mahapatra A and
Gupta D: Patients who benefit from intracranial pressure monitoring
without cerebrospinal fluid drainage after severe traumatic brain
injury. Neurosurgery. 85:231–239. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Dawes AJ, Sacks GD, Cryer HG, Gruen JP,
Preston C, Gorospe D, Cohen M, McArthur DL, Russell MM,
Maggard-Gibbons M and Ko CY: Intracranial pressure monitoring and
inpatient mortality in severe traumatic brain injury: A propensity
score-matched analysis. J Trauma Acute Care Surg. 78:492–501.
2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Boto GR, Gómez PA, De La Cruz J and Lobato
RD: Severe head injury and the risk of early death. J Neurol
Neurosurg Psychiatry. 77:1054–1059. 2006.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Shibahashi K, Hoda H, Okura Y and Hamabe
Y: Acceptable blood pressure levels in the prehospital setting for
patients with traumatic brain injury: A multicenter observational
study. World Neurosurg. 149:e504–e511. 2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Emami P, Czorlich P, Fritzsche FS,
Westphal M, Rueger JM, Lefering R and Hoffmann M: Observed versus
expected mortality in pediatric patients intubated in the field
with Glasgow Coma Scale scores <9. Eur J Trauma Emerg Surg.
45:769–776. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Corral L, Javierre CF, Ventura JL, Marcos
P, Herrero JI and Mañez R: Impact of non-neurological complications
in severe traumatic brain injury outcome. Crit Care.
16(R44)2012.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Catapano JS, Chapman AJ, Dull M,
Abbatematteo JM, Horner LP, Godzik J, Brigeman S, Morgan CD,
Whiting AC, Lu M, et al: Association of Angiotensin-converting
enzyme inhibitors with increased mortality among patients with
isolated severe traumatic brain injury. Neurocrit Care. 31:507–513.
2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Franschman G, Peerdeman SM, Andriessen TM,
Greuters S, Toor AE, Vos PE, Bakker FC, Loer SA and Boer C: Effect
of secondary prehospital risk factors on outcome in severe
traumatic brain injury in the context of fast access to trauma
care. J Trauma. 71:826–832. 2011.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Cai SS, Bonds BW, Hu PF and Stein DM: The
role of cardiac troponin I in prognostication of patients with
isolated severe traumatic brain injury. J Trauma Acute Care Surg.
80:477–483. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Lanzillo B, Piscosquito G, Marcuccio L,
Lanzillo A and Vitale DF: Prognosis of severe acquired brain
injury: Short and long-term outcome determinants and their
potential clinical relevance after rehabilitation. A comprehensive
approach to analyze cohort studies. PLoS One.
14(e0216507)2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Rahimi S, Bidabadi E, Mashouf M, Seyed
Saadat SM and Rahimi S: Prognostic value of arterial blood gas
disturbances for in-hospital mortality in pediatric patients with
severe traumatic brain injury. Acta Neurochir (Wien). 156:187–192.
2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Yang CJ, Hsiao KY, Su IC and Chen IC: The
association between anemia and the mortality of severe traumatic
brain injury in emergency department. J Trauma. 71:E132–E135.
2011.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Talving P, Lustenberger T, Lam L, Inaba K,
Mohseni S, Plurad D, Green DJ and Demetriades D: Coagulopathy after
isolated severe traumatic brain injury in children. J Trauma.
71:1205–1210. 2011.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Catapano JS, Chapman AJ, Horner LP, Lu M,
Fraser DR and Fildes JJ: Pre-injury polypharmacy predicts mortality
in isolated severe traumatic brain injury patients. Am J Surg.
213:1104–1108. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Mohseni S, Talving P, Wallin G, Ljungqvist
O and Riddez L: Preinjury β-blockade is protective in isolated
severe traumatic brain injury. J Trauma Acute Care Surg.
76:804–808. 2014.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Talving P, Plurad D, Barmparas G, Dubose
J, Inaba K, Lam L, Chan L and Demetriades D: Isolated severe
traumatic brain injuries: Association of blood alcohol levels with
the severity of injuries and outcomes. J Trauma. 68:357–362.
2010.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Talving P, Benfield R, Hadjizacharia P,
Inaba K, Chan LS and Demetriades D: Coagulopathy in severe
traumatic brain injury: A prospective study. J Trauma. 66:55–61.
2009.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Wafaisade A, Lefering R, Tjardes T,
Wutzler S, Simanski C, Paffrath T, Fischer P, Bouillon B and
Maegele M: Acute coagulopathy in isolated blunt traumatic brain
injury. Neurocrit Care. 12:211–219. 2010.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Davis DP, Hoyt DB, Ochs M, Fortlage D,
Holbrook T, Marshall LK and Rosen P: The effect of paramedic rapid
sequence intubation on outcome in patients with severe traumatic
brain injury. J Trauma. 54:444–453. 2003.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Lustenberger T, Talving P, Kobayashi L,
Barmparas G, Inaba K, Lam L, Branco BC and Demetriades D: Early
coagulopathy after isolated severe traumatic brain injury:
Relationship with hypoperfusion challenged. J Trauma. 69:1410–1414.
2010.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Seyed Saadat SM, Bidabadi E, Seyed Saadat
SN, Mashouf M, Salamat F and Yousefzadeh S: Association of
persistent hyperglycemia with outcome of severe traumatic brain
injury in pediatric population. Childs Nerv Syst. 28:1773–1777.
2012.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Lustenberger T, Inaba K, Barmparas G,
Talving P, Plurad D, Lam L, Konstantinidis A and Demetriades D:
Ethanol intoxication is associated with a lower incidence of
admission coagulopathy in severe traumatic brain injury patients. J
Neurotrauma. 28:1699–1706. 2011.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Salim A, Teixeira P, Ley EJ, DuBose J,
Inaba K and Margulies DR: Serum ethanol levels: Predictor of
survival after severe traumatic brain injury. J Trauma. 67:697–703.
2009.PubMed/NCBI View Article : Google Scholar
|
|
62
|
van Wessem KJP, Niemeyer MJS and Leenen
LPH: Polytrauma patients with severe cervical spine injuries are
different than with severe TBI despite similar AIS scores. Sci Rep.
12(21538)2022.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Pavlovic D, Pekic S, Stojanovic M and
Popovic V: Traumatic brain injury: Neuropathological,
neurocognitive and neurobehavioral sequelae. Pituitary. 22:270–282.
2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Marin JR, Weaver MD and Mannix RC: Burden
of USA hospital charges for traumatic brain injury. Brain Inj.
31:24–31. 2017.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Thurman DJ, Alverson C, Dunn KA, Guerrero
J and Sniezek JE: Traumatic brain injury in the United States: A
public health perspective. J Head Trauma Rehabil. 14:602–615.
1999.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Lee E, Jayasinghe N, Swenson C and
Dams-O'Connor K: Dispositional optimism and cognitive functioning
following traumatic brain injury. Brain Inj. 33:985–990.
2019.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Ansari A, Zoghi S, Tavanaei R, Payman AA,
Lu VM, Niakan A, Taheri R and Khalili H: Characteristics and
recovery trends of severe TBI patients with a favorable functional
outcome at 6-month follow-up. Neurosurg Rev. 48(41)2025.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Maegele M, Lefering R, Sakowitz O, Kopp
MA, Schwab JM, Steudel WI, Unterberg A, Hoffmann R, Uhl E and Marzi
I: The incidence and management of moderate to severe head injury.
Dtsch Arztebl Int. 116:167–173. 2019.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Chang HYM, Flahive J, Bose A, Goostrey K,
Osgood M, Carandang R, Hall W and Muehlschlegel S: Predicting
mortality in moderate-severe TBI patients without early withdrawal
of life-sustaining treatments including ICU complications: The
MYSTIC-score. J Crit Care. 72(154147)2022.PubMed/NCBI View Article : Google Scholar
|
|
70
|
O'Reilly GM, Afroz A, Curtis K, Mitra B,
Kim Y, Solly E, Ryder C, Hunter K, Hendrie DV, Rushworth N, et al:
The determinants for death in hospital following moderate to severe
traumatic brain injury in Australia. Emerg Med Australas.
37(e14562)2025.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Strnad M, Borovnik Lesjak V, Vujanović V
and Križmarić M: Predictors of mortality in patients with isolated
severe traumatic brain injury. Wien Klin Wochenschr. 129:110–114.
2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Arango JI, George L, Griswold DP, Johnson
ED, Suarez MN, Caquimbo LD, Molano M, Echeverri RA, Rubiano AM and
Adelson PD: Severe pediatric TBI management in a Middle-income
country and a high-income country: A comparative assessment of two
centers. Front Surg. 8(670546)2021.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Teasell R, Flores-Sandoval C, Janzen S,
MacKenzie HM, Mehrabi S, Sequeira K, Bayley M and Bateman EA:
Comparing randomized controlled trials of moderate to severe
traumatic brain injury in lower to middle income countries versus
high income countries. J Neurotrauma. 41:1271–1281. 2024.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Rivera-Lara L, Videtta W, Calvillo E,
Mejia-Mantilla J, March K, Ortega-Gutierrez S, Obrego GC, Paranhos
JE and Suarez JI: Reducing the incidence and mortality of traumatic
brain injury in Latin America. Eur J Trauma Emerg Surg.
49:2381–2388. 2023.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Tropeano MP, Spaggiari R, Ileyassoff H,
Park KB, Kolias AG, Hutchinson PJ and Servadei F: A comparison of
publication to TBI burden ratio of low- and middle-income countries
versus high-income countries: How can we improve worldwide care of
TBI? Neurosurg Focus. 47(E5)2019.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Naik A, Bederson MM, Detchou D,
Dharnipragada R, Hassaneen W, Arnold PM and Germano IM: Traumatic
brain injury mortality and correlates in low- and middle-income
countries: A meta-epidemiological study. Neurosurgery. 93:736–744.
2023.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Shakir M, Altaf A, Irshad HA, Hussain N,
Pirzada S, Tariq M, Trillo-Ordonez Y and Enam SA: Factors delaying
the continuum of care for the management of traumatic brain injury
in low- and Middle-income countries: A systematic review. World
Neurosurg. 180:169–193. 2023.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Wooldridge G, Hansmann A, Aziz O and
O'Brien N: Survey of resources available to implement severe
pediatric traumatic brain injury management guidelines in low and
middle-income countries. Childs Nerv Syst. 36:2647–2655.
2020.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Pantelatos RI, Rahim S, Vik A, Rao V,
Müller TB, Nilsen TIL and Skandsen T: The epidemiology of moderate
and severe traumatic brain injury in central norway.
Neuroepidemiology. 57:185–196. 2023.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Rahim S, Laugsand EA, Fyllingen EH, Rao V,
Pantelatos RI, Müller TB, Vik A and Skandsen T: Moderate and severe
traumatic brain injury in general hospitals: A ten-year
population-based retrospective cohort study in central Norway.
Scand J Trauma Resusc Emerg Med. 30(68)2022.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Guindo-Martínez M, Amela R, Bonàs-Guarch
S, Puiggròs M, Salvoro C, Miguel-Escalada I, Carey CE, Cole JB,
Rüeger S, Atkinson E, et al: The impact of non-additive genetic
associations on age-related complex diseases. Nat Commun.
12(2436)2021.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Diaz J, Rooney A, Calvo RY, Benham DA,
Carr M, Badiee J, Sise CB, Sise MJ, Bansal V and Martin MJ:
Isolated intracranial hemorrhage in elderly patients with
pre-injury anticoagulation: Is full trauma team activation
necessary? J Surg Res. 268:491–497. 2021.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Toida C, Muguruma T, Gakumazawa M,
Shinohara M, Abe T, Takeuchi I and Morimura N: Age- and
severity-related in-hospital mortality trends and risks of severe
traumatic brain injury in Japan: A nationwide 10-year retrospective
study. J Clin Med. 10(1072)2021.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Wågberg S, Stålnacke BM and Magnusson BM:
Gender and age differences in outcomes after mild traumatic brain
injury. J Clin Med. 12(4883)2023.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Desjardins P, Turgeon AF, Tremblay MH,
Lauzier F, Zarychanski R, Boutin A, Moore L, McIntyre LA, English
SW, Rigamonti A, et al: Hemoglobin levels and transfusions in
neurocritically ill patients: A systematic review of comparative
studies. Crit Care. 16(R54)2012.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Vanhala H, Junttila E, Kataja A, Huhtala
H, Luostarinen T and Luoto T: Incidence and associated factors of
anemiain patients with acute moderate and severe traumatic brain
injury. Neurocrit Care. 37:629–637. 2022.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Hare GM, Mazer CD, Hutchison JS, McLaren
AT, Liu E, Rassouli A, Ai J, Shaye RE, Lockwood JA, Hawkins CE, et
al: Severe hemodilutional anemia increases cerebral tissue injury
following acute neurotrauma. J Appl Physiol (1985). 103:1021–1029.
2007.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Acker SN, Partrick DA, Ross JT, Nadlonek
NA, Bronsert M and Bensard DD: Head injury and unclear mechanism of
injury: Initial hematocrit less than 30 is predictive of abusive
head trauma in young children. J Pediatr Surg. 49:338–340.
2014.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Komurcu O, Dost B, Ozdemir E, Aras M and
Ulger F: Red blood cell transfusion and hemoglobin level on
neurological outcome in the first 24 hours of traumatic brain
injury. Am J Emerg Med. 59:74–78. 2022.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Gul HF, Dolanbay T, Simsek AT and Aras M:
Evaluation of blood urea, creatinine, and glucose levels as
biochemical indicators of the type and severity of traumatic brain
injury. Turk Neurosurg. 31:333–338. 2021.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Hajikarimloo B, Jabbaripour S, Tohidinia
AM, Valinejad Qanati A, Fahim F, Javadpour P and Ghasemi R: Insulin
potential in preventing brain damage after traumatic brain injury:
What we know. J Neuroendocrinol. 37(e13458)2025.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Lozano A, Franchi F, Seastres RJ, Oddo M,
Lheureux O, Badenes R, Scolletta S, Vincent JL, Creteur J and
Taccone FS: Glucose and lactate concentrations in cerebrospinal
fluid after traumatic brain injury. J Neurosurg Anesthesiol.
32:162–169. 2020.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Tregub P, Malinovskaya N, Hilazheva E,
Morgun A and Kulikov V: Permissive hypercapnia and hypercapnic
hypoxia inhibit signaling pathways of neuronal apoptosis in
ischemic/hypoxic rats. Mol Biol Rep. 50:2317–2333. 2023.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Gribnau A, van Zuylen ML, Coles JP,
Plummer MP, Hermanns H and Hermanides J: Cerebral glucose
metabolism following TBI: Changes in plasma glucose, glucose
transport and alternative pathways of Glycolysis-A translational
narrative review. Int J Mol Sci. 25(2513)2024.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Zhu C, Chen J, Pan J, Qiu Z and Xu T:
Therapeutic effect of intensive glycemic control therapy in
patients with traumatic brain injury: A systematic review and
meta-analysis of randomized controlled trials. Medicine
(Baltimore). 97(e11671)2018.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Hanna M, Balintescu A, Glassford N,
Lipcsey M, Eastwood G, Oldner A, Bellomo R and Mårtensson J:
Glycemic lability index and mortality in critically ill patients-A
multicenter cohort study. Acta Anaesthesiol Scand. 65:1267–1275.
2021.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Kurtz P and Rocha EEM: Nutrition therapy,
glucose control, and brain metabolism in traumatic brain injury: A
multimodal monitoring approach. Front Neurosci.
14(190)2020.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Xue B, Ruan S, Xie P, Yan K, Gu Z, Meng N,
Zhang J, Liu H, Lu J, Zuo S and Zhang H: Prospective analysis of
glycemic variability in patients with severe traumatic brain
injury: Modified Leuven's adjustment process versus conventional
adjustment process. J Int Med Res. 46:1505–1516. 2018.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Böhm JK, Schaeben V, Schäfer N, Güting H,
Lefering R, Thorn S, Schöchl H, Zipperle J, Grottke O, Rossaint R,
et al: Extended coagulation profiling in isolated traumatic brain
injury: A CENTER-TBI Analysis. Neurocrit Care. 36:927–941.
2022.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Dong JF, Zhang F and Zhang J: Detecting
traumatic brain injury-induced coagulopathy: What we are testing
and what we are not. J Trauma Acute Care Surg. 94 (Suppl
1):S50–S55. 2023.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Nakae R, Fujiki Y, Takayama Y, Kanaya T,
Igarashi Y, Suzuki G, Naoe Y and Yokobori S: Time course of
coagulation and fibrinolytic parameters in pediatric traumatic
brain injury. J Neurosurg Pediatr. 28:526–532. 2021.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Maegele M, Aversa J, Marsee MK, McCauley
R, Chitta SH, Vyakaranam S and Walsh M: Changes in coagulation
following brain injury. Semin Thromb Hemost. 46:155–166.
2020.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Zhang LM, Li R, Sun WB, Wang XP, Qi MM,
Bai Y, Bai J and Zheng WC: Low-dose, early fresh frozen plasma
transfusion therapy after severe trauma brain injury: A clinical,
prospective, randomized, controlled study. World Neurosurg.
132:e21–e27. 2019.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Chesnut RM, Marshall LF, Klauber MR, Blunt
BA, Baldwin N, Eisenberg HM, Jane JA, Marmarou A and Foulkes MA:
The role of secondary brain injury in determining outcome from
severe head injury. J Trauma. 34:216–222. 1993.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Jeremitsky E, Omert L, Dunham CM, Protetch
J and Rodriguez A: Harbingers of poor outcome the day after severe
brain injury: Hypothermia, hypoxia, and hypoperfusion. J Trauma.
54:312–319. 2003.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Vella MA, Crandall ML and Patel MB: Acute
management of traumatic brain injury. Surg Clin North Am.
97:1015–1030. 2017.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Rice AD, Hu C, Spaite DW, Barnhart BJ,
Chikani V, Gaither JB, Denninghoff KR, Bradley GH, Howard JT, Keim
SM and Bobrow BJ: Correlation between prehospital and in-hospital
hypotension and outcomes after traumatic brain injury. Am J Emerg
Med. 65:95–103. 2023.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Rahaman P and Del Bigio MR: Histology of
brain trauma and Hypoxia-ischemia. Acad Forensic Pathol. 8:539–554.
2018.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Reddy P, Izzetoglu K, Shewokis PA,
Sangobowale M and Diaz-Arrastia R: Differences in time-frequency
characteristics between healthy controls and TBI patients during
hypercapnia assessed via fNIRS. Neuroimage Clin.
40(103504)2023.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Hazwani T, Khalifa AM, Azzubi M, Alhammad
A, Aloboudi A, Jorya A, Alkhuraiji A, Alhelabi S and Shaheen N:
Diffuse axonal injury on magnetic resonance imaging and its
relation to neurological outcomes in pediatric traumatic brain
injury. Clin Neurol Neurosurg. 237(108166)2024.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Johnson VE, Stewart W and Smith DH: Axonal
pathology in traumatic brain injury. Exp Neurol. 246:35–43.
2013.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Zhang S, Wang S, Wan X, Liu S, Shu K and
Lei T: Clinical evaluation of post-operative cerebral infarction in
traumatic epidural haematoma. Brain Inj. 31:215–220.
2017.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Behzadnia MJ, Anbarlouei M, Hosseini SM
and Boroumand AB: Prognostic factors in traumatic brain injuries in
emergency department. J Res Med Sci. 27(83)2022.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Gutowski P, Meier U, Rohde V, Lemcke J and
von der Brelie C: Clinical outcome of epidural hematoma treated
surgically in the era of modern resuscitation and trauma care.
World Neurosurg. 118:e166–e174. 2018.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Karnjanasavitree W, Phuenpathom N and
Tunthanathip T: The optimal operative timing of traumatic
intracranial acute subdural hematoma correlated with outcome. Asian
J Neurosurg. 13:1158–1164. 2018.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Aromatario M, Torsello A, D'Errico S,
Bertozzi G, Sessa F, Cipolloni L and Baldari B: Traumatic epidural
and subdural hematoma: Epidemiology, outcome, and dating. Medicina
(Kaunas). 57(125)2021.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Chen L, Xia S, Lin Y, Chen Y, Xian L, Yang
Y, Qiu X, Xu L, Xingshu Z, Chen D, et al: The role of coagulopathy
and subdural hematoma thickness at admission in predicting the
prognoses of patients with severe traumatic brain injury: A
multicenter retrospective cohort study from China. Int J Surg.
110:5545–5562. 2024.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Folkerson LE, Sloan D, Cotton BA, Holcomb
JB, Tomasek JS and Wade CE: Predicting progressive hemorrhagic
injury from isolated traumatic brain injury and coagulation.
Surgery. 158:655–661. 2015.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Chen T, Chen S, Wu Y, Chen Y, Wang L and
Liu J: A predictive model for postoperative progressive
haemorrhagic injury in traumatic brain injuries. BMC Neurol.
22(16)2022.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Stein SC, Young GS, Talucci RC, Greenbaum
BH and Ross SE: Delayed brain injury after head trauma:
Significance of coagulopathy. Neurosurgery. 30:160–165.
1992.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Powers AY, Pinto MB, Aldridge AM, Tang OY,
Chen JS, Berube RL, Doberstein C, Fox JM, Carnevale JA and Asaad
WF: Factors associated with the progression of conservatively
managed acute traumatic subdural hemorrhage. J Crit Care.
48:243–250. 2018.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Di G and Liu H, Jiang X, Dai Y, Chen S,
Wang Z and Liu H: Clinical predictors of progressive hemorrhagic
injury in children with mild traumatic brain injury. Front Neurol.
8(560)2017.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Dumont TM, Visioni AJ, Rughani AI, Tranmer
BI and Crookes B: Inappropriate prehospital ventilation in severe
traumatic brain injury increases in-hospital mortality. J
Neurotrauma. 27:1233–1241. 2010.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Haltmeier T, Benjamin E, Siboni S,
Dilektasli E, Inaba K and Demetriades D: Prehospital intubationfor
isolated severe blunt traumatic brain injury: Worse outcomes and
higher mortality. Eur J Trauma Emerg Surg. 43:731–739.
2017.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Bossers SM, Schwarte LA, Loer SA, Twisk
JW, Boer C and Schober P: Experience in prehospital endotracheal
intubation significantly influences mortality of patients with
severe traumatic brain injury: A systematic review and
Meta-analysis. PLoS One. 10(e0141034)2015.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Anderson J, Ebeid A and Stallwood-Hall C:
Pre-hospital tracheal intubation in severe traumatic brain injury:
A systematic review and meta-analysis. Br J Anaesth. 129:977–984.
2022.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Eichelberger MR, Gotschall CS, Sacco WJ,
Bowman LM, Mangubat EA and Lowenstein AD: A comparison of the
trauma score, the revised trauma score, and the pediatric trauma
score. Ann Emerg Med. 18:1053–1058. 1989.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Ambesi V, Miller C, Fitzgerald MC and
Mitra B: The GCS-Pupils (GCS-P) score to assess outcomes after
traumatic brain injury: A retrospective study. Br J Neurosurg.
23:1–4. 2024.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Anand KV, Shahid PT and Shameel KK:
Evaluating GCS and FOUR score in predicting mortality of traumatic
brain injury patients (TBI): A prospective study in a tertiary
hospital of south malabar. J Pharm Bioallied Sci. 16 (Suppl
1):S598–S600. 2024.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Anand KV, Shahid PT and Shameel KK:
Assessment of GCS and FOUR score as prognostic indicators for
hospital stay and morbidity in traumatic brain injury patients: An
observational study. J Pharm Bioallied Sci. 16 (Suppl 1):S601–S604.
2024.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Ahmadi S, Sarveazad A, Babahajian A,
Ahmadzadeh K and Yousefifard M: Comparison of Glasgow Coma Scale
and Full Outline of UnResponsiveness score for prediction of
in-hospital mortality in traumatic brain injury patients: A
systematic review and meta-analysis. Eur J Trauma Emerg Surg.
49:1693–1706. 2023.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Shestopalov AE, Yakovleva AV, Yadgarov MY,
Sergeev IV and Kuzovlev AN: Prevalence and impact of malnutrition
risk on outcomes in critically Ill patients with traumatic brain
injury and stroke: A retrospective cohort study using electronic
health records. Nutrients. 16(2396)2024.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Pastor IS, Para I, Vesa ȘC and Florian IȘ:
Identifying predictive factors for mortality in patients with TBI
at a neurosurgery department. J Med Life. 16:554–558.
2023.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Khormali M, Payandemehr P, Zafarmandi S,
Baigi V, Zafarghandi M and Sharif-Alhoseini M: Predictive utility
of the glasgow coma scale and the head abbreviated injury scale in
traumatic brain injury: Results from the national trauma registry
of iran. Arch Iran Med. 25:496–501. 2022.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Jeong TS, Choi DH and Kim WK: The
relationship between trauma scoring systems and outcomes in
patients with severe traumatic brain injury. Korean J Neurotrauma.
18:169–177. 2022.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Humble SS, Wilson LD, Wang L, Long DA,
Smith MA, Siktberg JC, Mirhoseini MF, Bhatia A, Pruthi S, Day MA,
et al: Prognosis of diffuse axonal injury with traumatic brain
injury. J Trauma Acute Care Surg. 85:155–159. 2018.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Kulesza B, Mazurek M, Nogalski A and Rola
R: Factors with the strongest prognostic value associated with
in-hospital mortality rate among patients operated for acute
subdural and epidural hematoma. Eur J Trauma Emerg Surg.
47:1517–1525. 2021.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Turkstra LS, Mutlu B, Ryan CW, Despins
Stafslien EH, Richmond EK, Hosokawa E and Duff MC: Sex and gender
differences in emotion recognition and theory of mind after TBI: A
narrative review and directions for future research. Front Neurol.
11(59)2020.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Sandhu MRS, Schonwald A, Boyko M, Jafar
TD, Freedman IG, Woeste J, Kurup A, Funaro MC, Zlotnik A, Gruenbaum
SE, et al: The association between female sex and depression
following traumatic brain injury: A systematic review and
meta-analysis. Neurosci Biobehav Rev. 168(105952)2025.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Cogan AM, McCaughey VK and Scholten J:
Gender differences in outcomes after traumatic brain injury among
service members and veterans. PM R. 12:301–314. 2020.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Oliverio R, Karelina K and Weil ZM: Sex,
Drugs, and TBI: The role of sex in substance abuse related to
traumatic brain injuries. Front Neurol. 11(546775)2020.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Mollayeva T, Mollayeva S and Colantonio A:
Traumatic brain injury: Sex, genderand intersecting
vulnerabilities. Nat Rev Neurol. 14:711–722. 2018.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Giordano KR, Rojas-Valencia LM, Bhargava V
and Lifshitz J: Beyond binary: Influence of sex and gender on
outcome after traumatic brain injury. J Neurotrauma. 37:2454–2459.
2020.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Hanscom M, Loane DJ and Shea-Donohue T:
Brain-gut axis dysfunction in the pathogenesis of traumatic brain
injury. J Clin Invest. 131(e143777)2021.PubMed/NCBI View Article : Google Scholar
|
|
145
|
Weaver JL: The brain-gut axis: A prime
therapeutic target in traumatic brain injury. Brain Res.
1753(147225)2021.PubMed/NCBI View Article : Google Scholar
|
|
146
|
Zhou Y, Lu W and Tang W: Gastrointestinal
failure score in children with traumatic brain injury. BMC Pediatr.
21(219)2021.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Stulce C, Reisner A, Kane JM, Shin HS,
McCracken C, Williamson J, Walson K and Paden M: Fluid overload in
pediatric severe traumatic brain injury. Pediatr Crit Care Med.
21:164–169. 2020.PubMed/NCBI View Article : Google Scholar
|
|
148
|
Jha RM, Kochanek PM and Simard JM:
Pathophysiology and treatment of cerebral edema in traumatic brain
injury. Neuropharmacology. 145:230–246. 2019.PubMed/NCBI View Article : Google Scholar
|
|
149
|
Mekkodathil A, El-Menyar A, Hakim S, Al
Jogol H, Parchani A, Peralta R, Rizoli S and Al-Thani H: Initial
serum levels of magnesium and calcium as predictors of mortality in
traumatic brain injury patients: A retrospective study. Diagnostics
(Basel). 13(1172)2023.PubMed/NCBI View Article : Google Scholar
|
|
150
|
Wang GH, Yan Y, Shen HP and Chu Z: The
clinical characteristics of electrolyte disturbance in patients
with moderate and severe traumatic brain injury who underwent
craniotomy and its influence on prognosis. J Korean Neurosurg Soc.
66:332–339. 2023.PubMed/NCBI View Article : Google Scholar
|
|
151
|
Laing J, Gabbe B, Chen Z, Perucca P, Kwan
P and O'Brien TJ: Risk factors and prognosis of early posttraumatic
seizures in moderate to severe traumatic brain injury. JAMA Neurol.
79:334–341. 2022.PubMed/NCBI View Article : Google Scholar
|
|
152
|
Forssten SP, Ahl Hulme R, Forssten MP,
Ribeiro MAF Jr, Sarani B and Mohseni S: Predictors of outcomes in
geriatric patients with moderate traumatic brain injury after
ground level falls. Front Med (Lausanne).
10(1290201)2023.PubMed/NCBI View Article : Google Scholar
|
|
153
|
Al-Salihi MM, Ayyad A, Al-Jebur MS and
Rahman MM: The ‘Talk and Die’ phenomenon in traumatic brain injury:
A meta-analysis. Clin Neurol Neurosurg. 218(107262)2022.PubMed/NCBI View Article : Google Scholar
|
|
154
|
Couret M, Gendre G, Magnusson L,
Schoettker P and Ribordy V: Out-of-hospital tracheal intubation and
alternatives in the prehospital setting. Rev Med Suisse.
16:325–330. 2020.PubMed/NCBI
|
|
155
|
Sun G, Shi L, Pan T, Li X and Zhang S:
Technique of ICP monitored stepwise intracranial decompression
effectively reduces postoperative complications of severe bifrontal
contusion. Front Neurol. 7(56)2016.PubMed/NCBI View Article : Google Scholar
|
|
156
|
McHugh DC, Fiore SM, Strong N and Egnor
MR: Bifrontal biparietal cruciate decompressive craniectomy in
pediatric traumatic brain injury. Pediatr Neurosurg. 54:6–11.
2019.PubMed/NCBI View Article : Google Scholar
|
|
157
|
Ma J, Zhang K, Wang Z and Chen G: Progress
of research on diffuse axonal injury after traumatic brain injuryy.
Neural Plast. 9746313:2016.PubMed/NCBI View Article : Google Scholar
|
|
158
|
Kennedy L, Nuno M, Gurkoff GG, Nosova K
and Zwienenberg M: Moderate and severe TBI in children and
adolescents: The effects of age, sex, and injury severity on
patient outcome 6 months after injury. Front Neurol.
13(741717)2022.PubMed/NCBI View Article : Google Scholar
|