Introduction
Tuberculosis (TB) is an infectious disease caused by
Mycobacterium tuberculosis. According to World Health
Organization (WHO), TB ranks among the 10 leading causes of death
globally and is the leading cause of mortality from a single
infectious agent (1). While
pulmonary tuberculosis is the most common manifestation, global
statistics from 2019 indicate that ~16% of global tuberculosis
cases were extrapulmonary (1).
Extrapulmonary tuberculosis can affect nearly any organ, most
frequently involving the lymph nodes, followed by pleural TB
(2). Studies have demonstrated
that the proportion of extrapulmonary tuberculosis cases among
total tuberculosis cases has been gradually increasing (3,4).
Pleural TB is prone to misdiagnosis due to its atypical clinical
symptoms, lack of characteristic imaging manifestations, challenges
in specimen acquisition, low etiology positivity rate, and
difficulties in diagnosis. In a number of cases, confirmation is
required through biopsy. Ultrasound offers portability and high
resolution and ultrasound-guided puncture biopsy provides real-time
imaging, safety and ease of operation. It is an effective method
for diagnosing pleural TB and is crucial for differentiating
pleural TB from other infectious and non-infectious diseases
(5). Conventional methods for
detecting tuberculosis include smear microscopy, culture and
cytology; however, these methods possess certain limitations.
Mycobacterial culture can serve as a reference standard but is
time-consuming and requires skilled personnel for operation.
Cytological methods for detecting lymphadenopathy necessitate
expert interpretation and smaller laboratories often lack the
necessary equipment, such as fluorescence or LED microscopes. These
factors hinder the accurate and prompt diagnosis of patients with
lymphatic tuberculosis in low-resource settings (6). Real-time fluorescence quantitative
nucleic acid amplification using GeneXpert M. tuberculosis
(MTB)/resistance to rifampin (RIF), a novel diagnostic technology
for tuberculosis (6), can detect
M. tuberculosis complex DNA. This method allows for the
detection of rifampicin resistance-related mutations in the rpoB
gene during the identification of M. tuberculosis complex,
facilitating early and rapid diagnosis while effectively minimizing
the risk of cross-contamination. Additionally, GeneXpert MTB/RIF
(Cepheid) is less influenced by the presence of anti-tuberculosis
drugs (6). Additionally, enhanced
sensitivity, specificity and accuracy in detecting rifampicin
resistance are notable advantages of this method. The present study
aimed to investigate the value of ultrasound-guided pleural TB
puncture combined with GeneXpert MTB/RIF in diagnosing pleural TB.
However, among all the searches, there is only one study that
confirms the diagnosis of pleural TB by ultrasound-guided pleural
biopsy combined with GeneXpert MTB/RIF, indicating that this is
still not widely promoted or used and has not been recognized by
clinical doctors. The present study also aimed to further confirm
the diagnostic value and significance of this study for pleural TB
(7).
Materials and methods
Patients
The pathology, acid fast staining,
Mycobacterium culture and GeneXpert results of patients with
pleural lesions who underwent ultrasound-guided biopsy between
April 2018 and April 2021 at the Shandong Public Health Clinical
Center (Shandong Chest Hospital) were retrospectively analyzed.
Diagnosis of tuberculosis was conducted following the guidelines
set forth by the WHO (1) and the
clinical diagnostic standards established by the Chinese Medical
Association for tuberculosis (8).
Clinically diagnosed TB patients met the following criteria: i)
Presence of clinical symptoms consistent with tuberculosis; ii)
imaging highly suggestive of tuberculosis; and iii) satisfactory
response to anti-tuberculosis treatment (9). All lesions were routinely examined by
ultrasound prior to biopsy. Clinical case data were collected,
including patient age, sex, comorbidities, laboratory examination
results and treatment response. None of the patients received
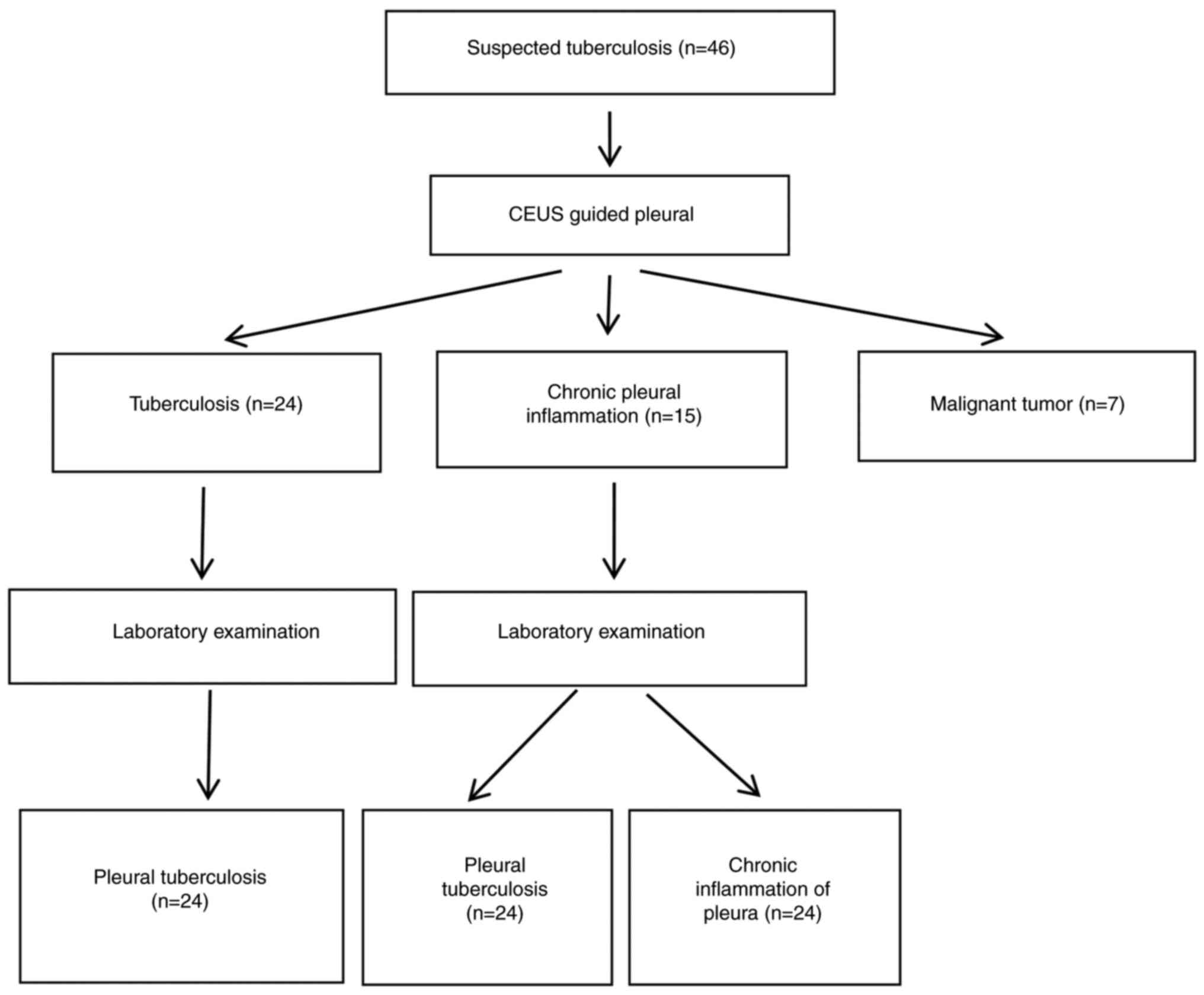
treatment before biopsy. A flow diagram illustrating the study
process is provided (Fig. 1). The
patients with TB included 19 males and 8 females, with ages ranging
from 16-56 years and a mean age of 23.7±14.1 years. Pulmonary
tuberculosis was present as a complication in 17 cases.
All methods were carried out in accordance with
relevant guidelines and regulations. The present study protocols
were approved by the Ethics Committee of Shandong Public Health
Clinical Center (Shandong Chest Hospital; approval no.
2021XKYYEC-33).
Ultrasound puncture
Based on the location of pleural lesions, patients
were positioned differently, including sitting, supine, lateral or
prone. The optimal puncture pathway for accessing pleural lesions
was determined. This pathway was designed to avoid the ribs and to
run obliquely at a shallow angle to the pleural plane, thereby
facilitating the visualization of the puncture needle, enabling the
acquisition of a greater volume of pleural tissue, and minimizing
the risk of lung tissue puncture. Color Doppler flow imaging was
employed to assess the blood supply to the lesion, ensuring that
large blood vessels along the puncture pathway were avoided and
that the pleural tissue with blood supply was targeted. The needle
entry point was identified and marked accordingly. Routine
disinfection was performed and a sterile towel was placed over the
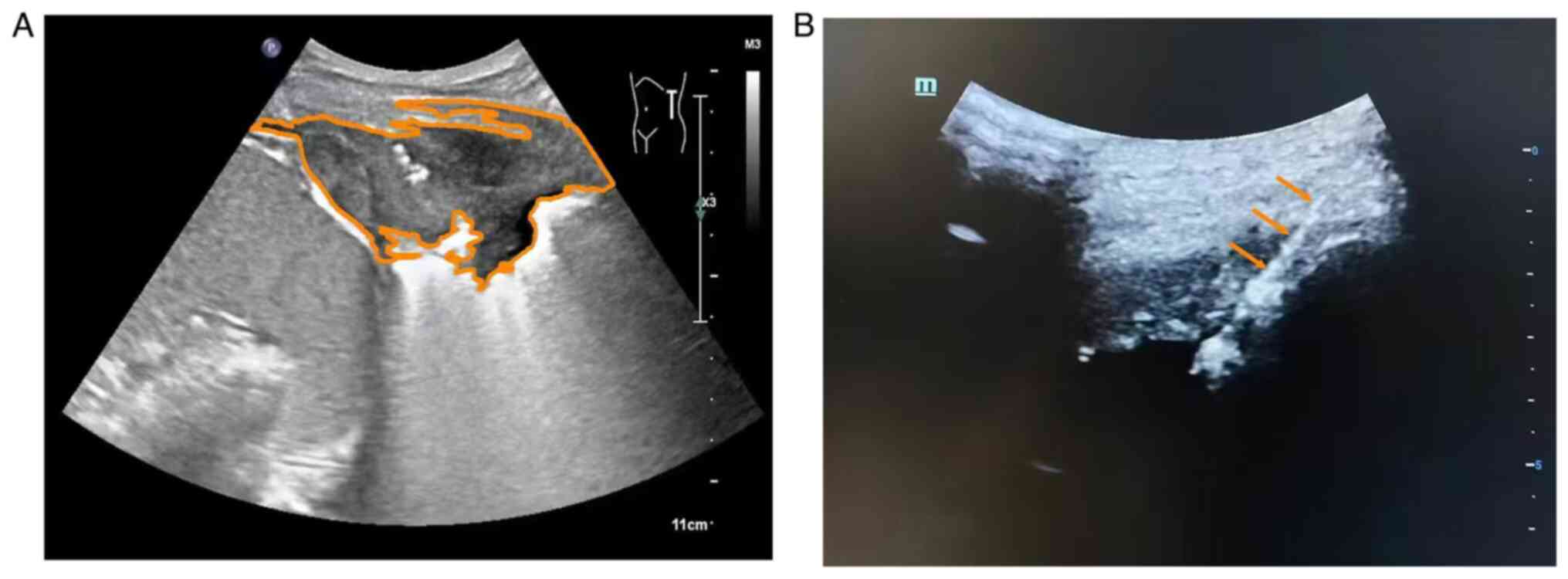
area. Local infiltration anesthesia was administered. An ultrasonic
diagnostic instrument (Philips Q5; Philips Healthcare) equipped
with a convex array probe (C5-1) operating at a frequency of 1 to 5
MHz was used. Puncture was carried out according to the
predetermined pathway using a No. 18 semi-automatic cutting biopsy
needle (18G; 10 cm; Becton, Dickinson and Company). The needle was
inserted under real-time ultrasound guidance (Fig. 2A and B). Once the needle tip was observed to
reach the target area, the biopsy gun was activated to obtain a
tissue sample. Depending on the specimen condition, tissue
acquisition was performed 2-3 times. The changes in chest pain,
dizziness and chest tightness were monitored during the procedure.
Postoperatively, ultrasonography was conducted to assess for
complications such as hemoptysis, pneumothorax and bleeding.
Pathological examination, acid fast staining, M.
tuberculosis culture and GeneXpert testing were performed in
all cases. Specimens were fixed in 10% formalin solution for 18-24
h at 20-35˚C and subsequently submitted for pathological
analysis.
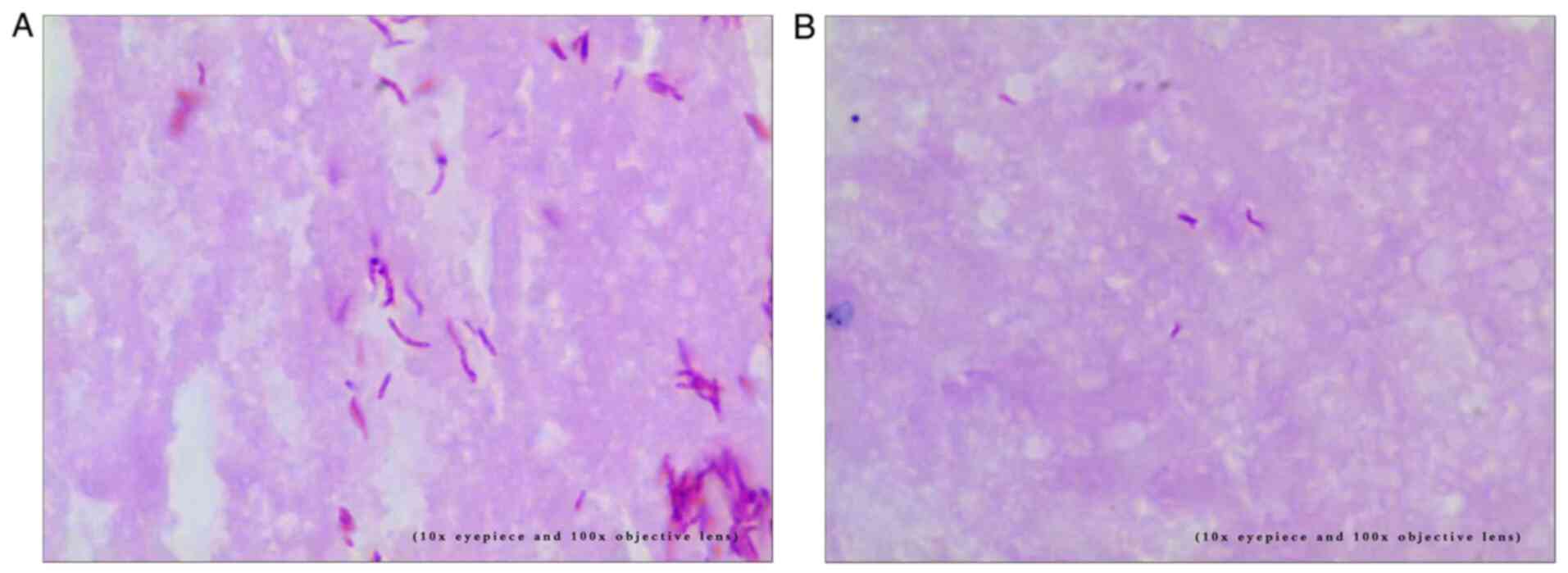
Histopathological examination
The specimens were dehydrated using a gradient of
ethanol from low to high concentration and embedded in high
concentration paraffin. Subsequently, they were sectioned at 15-30
µm. A 4 µm color band was prepared for staining (Fig. 3A and B), followed by pathological
diagnosis.
Acid fast staining was performed using an acid fast
staining solution (Zhuhai Beso Biotechnology Co., Ltd.),
experienced laboratory doctors applied the modified alkaline
reddening method to conduct the procedure. The staining temperature
was 60˚C for 5 min. The results were determined according to the
reagent instructions.
M. tuberculosis culture was carried out using
the Mycobacterium culture monitoring system and reagents
provided by BACTEC MGIT 960 (Becton, Dickinson and Company) for
strain identification, following the manufacturer's instructions
and specifications. GeneXpert MTB/RIF detection was performed using
the GeneXpert MTB/RIF and an automated detection platform
[GeneXpert (Cepheid)]. Samples were pretreated according to the
operating procedures, and automatic detection and result
interpretation were conducted as per the guidelines.
Statistical analysis
The database was established and statistically
analyzed using SPSS 24.0 (IBM Corp.). Indicators for statistical
description include mean, standard deviation, frequency and
composition ratio. Data following a normal distribution were
expressed as mean and standard deviation, while non-normally
distributed data were reported as median and interquartile range
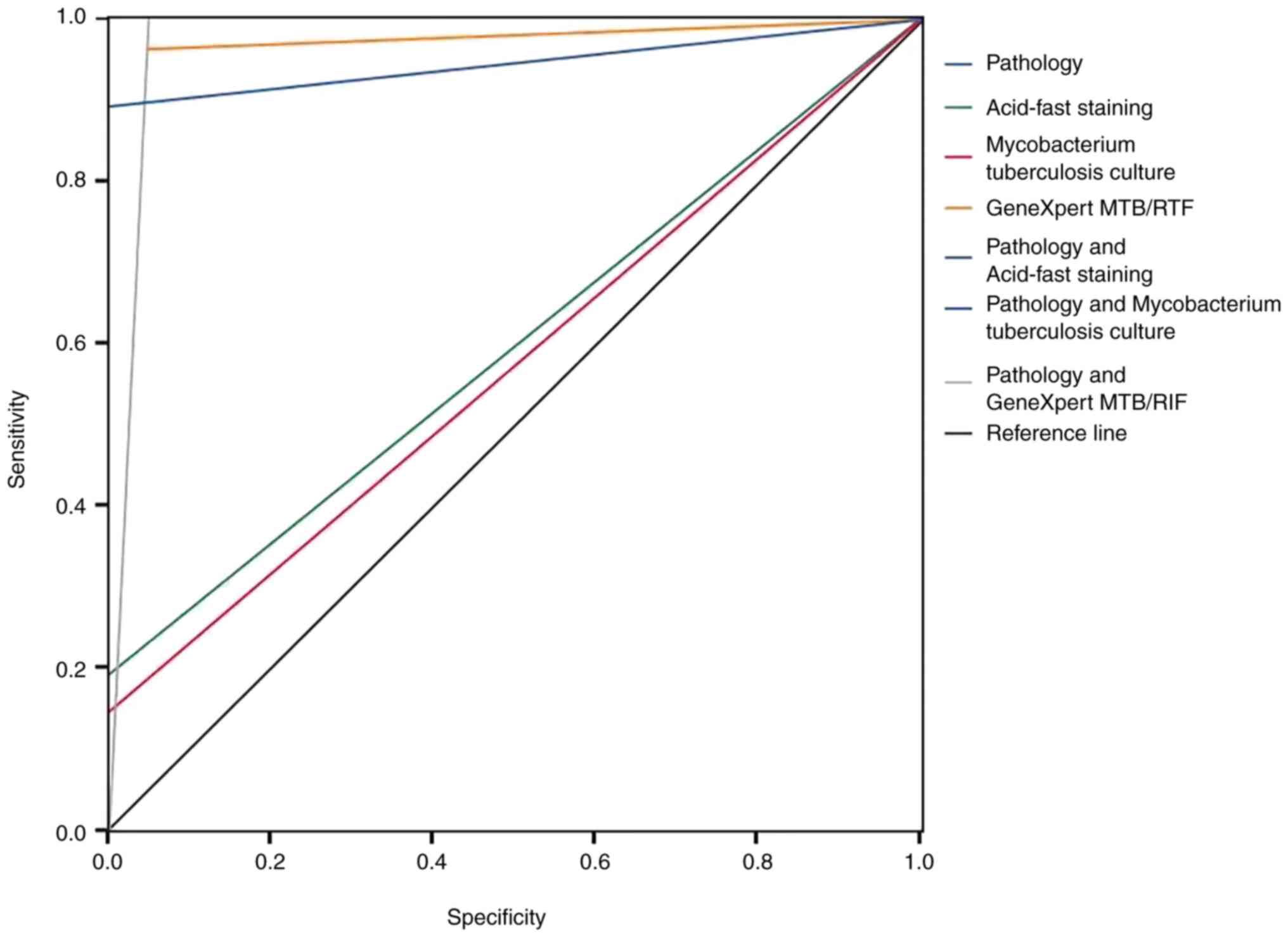
(m±IQR). Statistical inference was conducted using ROC curve
analysis, κ-value, sensitivity, specificity, positive predictive
value and negative predictive value. P<0.05 was considered to
indicate a statistically significant difference. The optimal
diagnostic threshold was determined using the Jordan index
(Fig. 4).
Results
Using Common Reporting Standard (CRS) as a
reference, 27 patients were diagnosed with tuberculous nodal
pleurisy (Fig. 1), including 19
males and eight females, with ages ranging from 16-56 years and a
mean age of 23.7±14.1 years. Pulmonary tuberculosis was present as
a complication in 17 cases.
Pathological results
Ultrasound-guided pleural biopsy was conducted based
on the microscopic observation of fibrous tissue or mesothelial
cells. Pleural tissue was successfully obtained from all 46
patients, with a success rate of 100% (46/46). The length of the
puncture tissue strips ranged from 0.5-1.8 cm. Pathological
diagnosis revealed malignancy in seven cases, tuberculosis in 24
cases and chronic inflammation in 15 cases. Among the 15 cases of
chronic pleural inflammation, three were ultimately diagnosed as
tuberculous pleurisy. The pathological diagnosis of malignancy was
consistent with the final clinical diagnosis, accounting for 15.21%
(7/46) of all biopsies. Using CRS as a reference, 27 patients were
ultimately diagnosed with tuberculous pleurisy, based on clinical
manifestations, imaging examination and diagnostic treatment
results.
Laboratory results
Among the 27 patients with pleural TB, M.
tuberculosis culture was positive in four cases, acid-fast
staining (Fig. 3A and B) was positive in 5 cases, and GeneXpert
MTB/RIF was positive in 26 cases (Table I). The positive diagnostic rates
for M. tuberculosis culture, acid-fast staining and
GeneXpert MTB/RIF were 14.81, 18.52 and 96.30%, respectively. Among
the 27 confirmed cases of pleural TB, GeneXpert MTB/RIF exhibited
the highest positive rate, with the combination of GeneXpert
MTB/RIF and pathology yielding a positive rate of 100%. GeneXpert
MTB/RIF was positive in all four culture-positive cases. In 15
cases where M. tuberculosis culture and acid-fast staining
were negative and GeneXpert MTB/RIF was positive. Finally, chest
wall tuberculosis was ultimately diagnosed. GeneXpert MTB/RIF
increased the pathogenic-positive detection rate of tissue biopsy
specimens by 55.56% (15/27). The κ-value for GeneXpert MTB/RIF
technology as shown in Table II
was 0.91; the κ-values for acid-fast staining and M.
tuberculosis culture were 0.16 and 0.13, respectively. The AUC
value for GeneXpert MTB/RIF technology was 0.96, while the AUC
values for acid-fast staining and M. tuberculosis culture
were 0.59 and 0.57, respectively. The positive diagnostic rate, AUC
and κ-values for the combination of pathology and GeneXpert MTB/RIF
were 100.00%, 0.94 and 0.92, respectively, all of which were higher
than those for the combination of pathology with acid-fast staining
or Mycobacterium tuberculosis culture.
 | Table IDiagnosis results of pathology and
three detection techniques for pleural tuberculosis. |
Table I
Diagnosis results of pathology and
three detection techniques for pleural tuberculosis.
| | Clinical
comprehensive diagnosis |
|---|
| Detection
techniques | Detection result | Chest wall
tuberculosis (n) | Non chest wall
tuberculosis (n) | Total (n) |
|---|
| Pathology | Positive | 24 | 0 | 24 |
| | Negative | 3 | 19 | 22 |
| | Total | 27 | 19 | 46 |
| Acid-fast
staining | Positive | 5 | 0 | 5 |
| | Negative | 22 | 19 | 41 |
| | Total | 27 | 19 | 46 |
| Mycobacterium
tuberculosis culture | Positive | 4 | 0 | 4 |
| | Negative | 23 | 19 | 42 |
| | Total | 27 | 19 | 46 |
| GeneXpert
MTB/RIF | Positive | 26 | 1 | 27 |
| | Negative | 1 | 18 | 19 |
| | Total | 27 | 19 | 46 |
| Pathology and
Acid-fast staining | Positive | 24 | 0 | 24 |
| | Negative | 3 | 19 | 22 |
| | Total | 27 | 19 | 46 |
| Pathology and
Mycobacterium tuberculosis culture | Positive | 24 | 0 | 24 |
| | Negative | 3 | 19 | 22 |
| | Total | 27 | 19 | 46 |
| Pathology and
GeneXpert MTB/RIF | Positive | 27 | 1 | 28 |
| | Negative | 0 | 18 | 18 |
| | Total | 27 | 19 | 46 |
 | Table IIDiagnostic value of pathology and
three detection techniques for pleural tuberculosis. |
Table II
Diagnostic value of pathology and
three detection techniques for pleural tuberculosis.
| Detection
techniques | AUC | Sensitivity
(%) | Specificity
(%) | Positive predictive
value (%) | Negative predictive
value (%) | κ-value | P-value | Total coincidence
rate (%) | Jordan index
(%) |
|---|
| Pathology | 0.944 | 88.89 | 100.00 | 100.00 | 86.36 | 0.869 | 0.000 | 93.48 | 88.89 |
| Acid-fast
staining | 0.593 | 18.52 | 100.00 | 100.00 | 46.34 | 0.158 | 0.047 | 52.17 | 18.52 |
| Mycobac-
terium tuberculosis culture | 0.574 | 14.81 | 100.00 | 100.00 | 45.24 | 0.126 | 0.079 | 50.00 | 14.81 |
| GeneXpert
MTB/RIF | 0.955 | 96.30 | 94.74 | 96.30 | 94.74 | 0.910 | 0.000 | 95.65 | 91.03 |
| Pathology and
Acid-fast staining | 0.944 | 88.89 | 100.00 | 100.00 | 86.36 | 0.869 | 0.000 | 93.48 | 88.89 |
| Pathology and
Mycobac- terium tuberculosis culture | 0.944 | 88.89 | 100.00 | 100.00 | 86.36 | 0.869 | 0.000 | 93.48 | 88.89 |
| Pathology and Gene
Xpert MTB/RIF | 0.974 | 100.00 | 94.74 | 96.43 | 100.00 | 0.955 | 0.000 | 97.83 | 94.74 |
Complications of ultrasound-guided
puncture
None of the 46 patients experienced complications
such as hemoptysis, pneumothorax and hemothorax; Only one patient
presented with a pleural reaction characterized by dizziness and
nausea, with an incidence rate of 1% (1/46). After bed rest, the
symptoms resolved and the puncture was successfully repeated,
allowing the procedure to be completed without further issues.
Discussion
Pleurisy is a common clinical condition, and
tuberculous pleurisy is a form caused by M. tuberculosis
infection. The underlying mechanism involves the entry of M.
tuberculosis and tuberculous proteins into the pleural space,
triggering a significant pleural reaction, primarily characterized
by pleural effusion and pleural thickening (9). The clinical symptoms of tuberculous
pleurisy are nonspecific, making diagnosis challenging. Routine
diagnostic methods often fail to achieve definitive results.
Imaging examinations play a crucial role in detecting pleural
lesions, however, diagnosis requires pathological and laboratory
confirmation. According to the 2010 guidelines of the British
Thoracic Society for the diagnosis and management of unilateral
pleural effusion in adults, when the nature of pleural effusion
cannot be determined through ultrasound-guided aspiration, it is
recommended that pleural biopsy be performed under imaging guidance
to further clarify the nature of the lesion (10). Historically, Cope biopsy needles
and Abrams biopsy needles were used for percutaneous pleural
biopsy; however, these methods provided limited tissue samples and
were associated with significant trauma, making complications such
as pneumothorax and bleeding more likely (11,12).
Defrancis et al (13) first
introduced closed pleural biopsy into clinical practice, and
through continuous refinement, it has become a primary diagnostic
method for pleural lesions. Nevertheless, variations in guiding
techniques have led to differences in the sensitivity and
specificity for diagnosing pleural diseases (13). Thoracoscopic biopsy has been shown
to enhance the diagnostic accuracy of GeneXpert MTB/RIF for
tuberculosis (14), but the
procedure is associated with greater trauma and carries a risk of
postoperative infection and tuberculosis transmission.
Ultrasound-guided biopsy, characterized by high precision, minimal
invasiveness and safety, allows for accurate specimen collection,
facilitating both pathological and laboratory examinations.
Pleural TB has been recognized for a long time. When
the duration of tuberculous pleurisy exceeds 4 weeks, the positive
rate of pleural biopsy is markedly reduced (15). Most cases of pleural TB involve
paucibacillary disease (caused by a small number of bacteria),
which reduces the sensitivity of traditional smear microscopy for
diagnosis. In resource-limited settings, Mycobacterium
culture and histological examination are not widely available due
to the long culture time and the need for fully equipped
laboratories. GeneXpert MTB/RIF is an automated PCR test capable of
accurately detecting tuberculosis and rifampicin resistance in
sputum samples (16). Based on a
systematic review (17), the World
Health Organization issued recommendations regarding extrapulmonary
tuberculosis, stating that GeneXpert MTB/RIF can be used as an
alternative to conventional methods (such as routine microscopy,
culture or histopathology) to detect specific non-respiratory
specimens from patients with suspected extrapulmonary
tuberculosis.
GeneXpert MTB/RIF is a diagnostic test used for
detecting the DNA of the M. tuberculosis complex. Upon
identification of the M. tuberculosis complex, mutations
related to rifampicin resistance in the rpoB gene are detected.
Test results can be obtained within 2 h after the initiation of the
test, requiring only minimal technical time. Unlike traditional
nucleic acid amplification tests, GeneXpert MTB/RIF integrates
sample processing, PCR amplification and detection into a single
self-contained test unit (18).
Following sample loading, all analytical steps are all fully
automated and self-sufficient. GeneXpert MTB/RIF employs molecular
beacon technology to detect rifampicin resistance. Molecular
beacons are nucleic acid probes that can identify and report the
presence or absence of normal, favorable and wild type sequences of
the rpoB gene.
In the present study, the positive rate of GeneXpert
MTB/RIF combined with pathology was found to be 100%, while the
positive rate of GeneXpert MTB/RIF alone was 96.30% (26/27), both
of which were higher than the rates observed for acid-fast staining
and tuberculous culture. The three detection methods were analyzed
in conjunction with pathology for data evaluation. The diagnostic
value for pleural TB was determined to be as follows: GeneXpert
MTB/RIF technology combined with pathology (AUC value=0.97) >
tuberculosis culture combined with pathology (AUC
value=0.94)=acid-fast staining combined with pathology (AUC
value=0.94). The positive rate of GeneXpert MTB/RIF in the present
study was higher than that reported in previous studies involving
ultrasound-guided or closed pleural biopsy (19).
Some researchers consider the clinical diagnosis of
tuberculous pleurisy and tuberculous pericarditis to be the gold
standard. GeneXpert MTB/RIF demonstrates high sensitivity (90.0 and
72.0%) and specificity (100.0% for both) (20), which is consistent with the
findings of the present study. Additionally, research has been
conducted regarding the value of GeneXpert MTB/RIF in detecting
drug-resistant tuberculous pleurisy. Among 60 patients with
tuberculous pleurisy, GeneXpert MTB/RIF confirmed the presence of
rifampicin resistance genes in 10 cases, while only five cases were
identified using the proportional method (21).
In the present study, four cases tested positive
using the M. tuberculosis culture method. The colonies were
evaluated using the proportional method, and the results were
consistent with those obtained from the GeneXpert MTB/RIF method. A
limitation of this study may be attributed to the small volume of
positive tuberculosis culture data included. Numerous studies have
demonstrated that GeneXpert MTB/RIF is highly valuable for
diagnosing extrapulmonary tuberculosis, including lymphatic
tuberculosis, spinal tuberculosis, urinary tuberculosis and nervous
system tuberculosis (22-25).
Additionally, there are studies confirming its diagnostic value in
pleural TB (26). The findings of
the present study align with these previous results. The positive
rate of GeneXpert MTB/RIF, along with its AUC and κ-values were
higher than those of acid-fast staining and tuberculosis culture.
Furthermore, in the analysis of all combined experiments, the
positive rate, AUC and κ-values for GeneXpert MTB/RIF combined with
pathology were also the highest.
The consistency analysis indicated that GeneXpert
MTB/RIF technology demonstrated good consistency (κ=0.91), whereas
acid-fast staining (κ=0.16) and tuberculosis culture (κ=0.13)
exhibit poor consistency. This may be attributed to the limited
number of cases included. Although GeneXpert MTB/RIF facilitates
the detection of M. tuberculosis and rifampicin resistance,
it cannot fully replace traditional methods for assessing
rifampicin resistance. The total number of cases included in the
present study was not particularly large. The diagnosis of
tuberculosis relies not only on pathological findings but also on
etiological assessments. Most patients included in the present
study were clinically suspected of having tuberculosis, resulting
in a specificity of 100%, which is consistent with numerous
research findings and does not impact the generalizability of the
results.
In conclusion ultrasound-guided percutaneous biopsy,
in conjunction with laboratory examination, is recognized as a safe
and effective method for diagnosing pleural TB. The combination of
ultrasound-guided puncture and GeneXpert MTB/RIF exhibits high
sensitivity and specificity, demonstrating significant value in the
diagnosis of pleural TB and the detection of rifampicin
resistance.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the Shandong medical
and health science and technology development plan project (grant
no. 202309020993).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
QY and YQ made substantial contributions to
conception and design. QY and JC made substantial contributions to
acquisition of data. FX, JC and SJ made substantial contributions
to analysis and interpretation of data. YQ and QY wrote the
manuscript. QY and FX constructed figures. JC and QY constructed
the tables. YQ and SJ confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study met the conditions of the Helsinki
declaration. All methods were carried out in accordance with
relevant guidelines and regulations and all experimental protocols
were approved by the Ethics Committee of Shandong Public Health
Clinical Center (Shandong Chest Hospital; approval no.
2021XKYYEC-33). Informed consent was waived by the Ethics Committee
of Shandong Public Health Clinical Center (Shandong Chest
Hospital)/2021XKYYEC-33 for this study due to retrospective
nature.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
World Health Organization. Global
tuberculosis report 2020. Geneva, World Health Organization,
2020.
|
|
2
|
Peto HM, Pratt RH, Harrington TA, LoBue PA
and Armstrong LR: Epidemiology of extrapulmonary tuberculosis in
the United States, 1993-2006. Clin Infect Dis. 49:1350–1357.
2009.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Sandgren A, Hollo V and van der Werf MJ:
Extrapulmonary tuberculosis in the European Union and European
Economic Area, 2002 to 2011. Euro Surveill.
18(20431)2013.PubMed/NCBI
|
|
4
|
Wang X, Yang Z, Fu Y, Zhang G and Wang X,
Zhang Y and Wang X: Insight to the epidemiology and risk factors of
extrapulmonary tuberculosis in Tianjin, China during 2006-2011.
PLoS One. 9(e112213)2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Nachiappan AC, Rahbar K, Shi X, Guy ES,
Mortani Barbosa EJ Jr, Shroff GS, Ocazionez D, Schlesinger AE, Katz
SI and Hammer MM: Pulmonary tuberculosis: Role of radiology in
diagnosis and management. Radiographics. 37:52–72. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bholla M, Kapalata N, Masika E, Chande H,
Jugheli L, Sasamalo M, Glass TR, Beck HP and Reither K: Evaluation
of Xpert® MTB/RIF and Ustar EasyNAT™ TB IAD for
diagnosis of tuberculous lymphadenitis of children in Tanzania: A
prospective descriptive study. BMC Infect Dis.
16(246)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sun W, Zhou Y, Li W, Wang Y, Xiong K,
Zhang Z and Fan L: Diagnostic yield of Xpert MTB/RIF on
contrast-enhanced ultrasound-guided pleural biopsy specimens for
pleural tuberculosis. Intl J Infect Dis. 108:89–95. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rice JP, Seifert M, Moser KS and Rodwell
TC: Performance of the Xpert MTB/RIF assay for the diagnosis of
pulmonary tuberculosis and rifampin resistance in a low-incidence,
high-resource setting. PLoS One. 12(e0186139)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chinese Medical Association. Clinical
diagnosis standardof TB for
clinicaltechnologyoperation(TBvol-umes). People's Medical
Publishing House, 2005.
|
|
10
|
Jian S, Rong ZL and Baohua S: Diagnostic
value of pleural histopathology and pleural effusion in tuberculous
pleurisy. Western Med. 27:27–28. 2015.
|
|
11
|
Hooper C, Lee YC and Maskell N: BTS
Pleural Guideline Group. Investigation of a unilateral pleural
effusion in adults: British Thoracic Society pleural disease
guideline 2010. Thorax. 65:4–17. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Barnes TW, Morgenthaler TI, Olson EJ,
Hesley GK, Decker PA and Ryu JH: Sonographically guided
thoracentesis and rate of pneumothorax. J Clin Ultrasound.
33:442–446. 2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Defrancis N, Klosk E and Albano E:
Needlebiopsy of theparietalpleura. N Engl J Med. 252:948–949.
1995.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Benamore RE, Scott K, Richards CJ and
Entwisle JJ: Image guided pleura biopsy: Diagnostic yield and
complications. Clin Radiol. 61:700–705. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Christopher DJ, Schumacher SG, Michael JS,
Luo R, Balamugesh T, Duraikannan P, Pollock NR, Pai M and Denkinger
CM: Performance of Xpert MTB/RIF on pleural tissue for the
diagnosis of pleural tuberculosis. Eur Respir J. 42:1427–1429.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Diacon AH, Van de Wal BW, Wyser C, Smedema
JP, Bezuidenhout J, Bolliger CT and Walzl G: Diagnostic tools in
tuberculous pleurisy: A direct comparative study. Eur Respir J.
22:589–591. 2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Steingart KR, Schiller I, Horne DJ, Pai M,
Boehme CC and Dendukuri N: Xpert® MTB/RIF assay for
pulmonary tuberculosis and rifampicin resistance in adults.
Cochrane Database Syst Rev. 2014(CD009593)2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Blakemore R, Story E, Helb D, Kop J,
Banada P, Owens MR, Chakravorty S, Jones M and Alland D: Evaluation
of the analytical performance of the Xpert MTB/RIF assay. J Clin
Microbiol. 48:2495–2501. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Denkinger CM, Schumacher SG, Boehme CC,
Dendukuri N, Pai M and Steingart KR: Xpert MTB/RIF assay for the
diagnosis of extrapulmonary tuberculosis: A systematic review and
meta-analysis. Eur Respir J. 44:435–446. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Koegelenberg CF, Irusen EM, von
Groote-Bidlingmaier F, Bruwer JW, Batubara EM and Diacon AH: The
utility of ultrasound-guided thoracentesis and pleural biopsy in
undiagnosed pleural exudates. Thorax. 70:995–997. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Saeed M, Ahmad M, Iram S, Riaz S, Akhtar M
and Aslam M: A breakthrough for the diagnosis of tuberculous
pericarditis and pleuritisin less than 2 hours. Saudi Med J.
38:699–705. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Haijing Z, Chengyong L and Dongqing Z:
Application value of GeneXpert MTB/RIF system in rapid diagnosis of
tuberculous pleurisy). Beijing Med J. 38:739–741. 2016.
|
|
23
|
Sun W, Gu J, Bi K, Zhang Y, Shen MJ, Wang
Y and Fan L: Clinical performance of Xpert MTB/RIF on
contrast-enhanced ultrasound-guided core biopsy specimens for rapid
diagnosis of superficial tuberculous lymphadenitis in high TB
burden settings. Infection. 49:653–660. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gu Y, Wang G, Dong W, Li Y, Ma Y, Shang Y,
Qin S and Huang H: Xpert MTB/RIF and GenoType MTBDRplus assays for
the rapid diagnosis of bone and joint tuberculosis. Infect Dis.
36:27–30. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yu C, Xuhui L and Liang F: Diagnostic
value of genexp ERT m TB/RIF in HIV negative urinary tuberculosis.
Chin J Tuberculosis. 39:1100–1106. 2017.
|
|
26
|
Bahr NC, Marais S, Caws M, van Crevel R,
Wilkinson RJ, Tyagi JS, Thwaites GE and Boulware DR: Tuberculous
Meningitis International Research Consortium. GeneXpert MTB/Rif to
diagnose tuberculous meningitis: Perhaps the first test but not the
last. Clin Infect Dis. 62:1133–1135. 2016.PubMed/NCBI View Article : Google Scholar
|