Introduction
Oesophageal granular cell tumours (EGCTs) are common
benign tumours with potential for malignancy in clinical medicine
(1). These tumours are typically
found in the submucosal layer of the oesophagus. Although their
incidence is low, an increasing number of cases are being diagnosed
with the widespread use of endoscopic techniques and routine
screening practices (2-4).
EGCTs make up a relatively small proportion of all oesophageal
tumours, accounting for only 6-10% of all oesophageal tumours
(5). Accordingly, they are
relatively rare, where there is currently an insufficient
understanding of these tumours in clinical practice.
According to the existing literature, there is a
certain bias in the sex distribution of EGCTs, with a greater
proportion of female compared with male patients. EGCTs typically
occur in middle-aged and elderly individuals, with a reported
median age of 43 years and a mean age of 44.00±3.48 years based on
one case series (6). However, age
distribution may vary depending on the population studied. Further
research is required to better characterize the epidemiological
patterns of EGCTs.
The main origin of EGCTs is Schwann cells (7). These cells form part of the
peripheral nervous system and are responsible for forming nerve
sheaths, in addition to supporting and protecting the function of
nerve fibres (7). GCTs have been
proposed to result from the abnormal proliferation of Schwann cells
(8). Although the exact
pathological mechanism of this process remains to be clarified,
their abnormal proliferation may be associated with genetic
factors, environmental factors, immune escape and inflammatory
responses (2,9). Tumour cells may be influenced by
certain immune factors that contribute to the tumour's development,
such as eosinophils and transforming growth factor β, both of which
have been implicated in promoting tumourigenesis (10). In addition, certain oesophageal
diseases, such as oesophagitis, may cause changes in the local
inflammatory environment, thereby promoting the formation of GCTs
(11). However, the exact
mechanism of their interaction remains to be fully elucidated.
Pathological examination is key in diagnosing EGCTs.
This type of tumour is comprised of large polygonal cells with
granular cytoplasm that are positive for S-100 protein expression,
supporting the neurogenic origin of these tumours (12,13).
For symptomatic or growing tumours, endoscopic resection, which is
a minimally-invasive treatment with a favorable outcome, results in
high rates of complete tumour removal, minimal risk of recurrence
and excellent long-term survival (1,14).
Studies have suggested that <2% of GCTs, including those in the
esophagus, show signs of malignant transformation or metastasis.
While the risk of malignancy is extremely low, there is potential
for malignancy in a small subset of cases. This may be indicated by
factors such as rapid tumour growth, a tumour size >5 cm and
histopathological features such as nuclear pleomorphism or
increased mitotic activity (15,16).
Even asymptomatic tumours should be closely monitored through
regular follow-up to detect any potential changes in tumour
behavior at an early stage.
The present case report provided a case analysis,
contributing to the limited EGCT literature by giving a detailed
description of the clinical manifestations, diagnostic methods and
treatment plan for a 68-year-old male patient diagnosed with an
EGCT discovered during a routine physical examination.
Case report
Case presentation
The patient was a 68-year-old man who was admitted
to the Affiliated Hospital of Jiujiang University (Jiujiang, China)
in September 2024, after the discovery of an oesophageal submucosal
tumour (SMT) during a physical examination 1 day prior. The patient
had a history of type 2 diabetes for 30 years and was managed with
insulin, although the patient's blood glucose levels were not
regularly monitored. The patient had previously undergone
cholecystectomy 5 years ago due to cholelithiasis and
cholecystitis.
Physical examination upon admission revealed the
following: i) Body temperature, 36.6˚C (normal range, 36.1-37.2˚C);
ii) pulse, 85 beats/min (normal range, 60-100 beats/min); iii)
respiratory rate, 19 breaths/min (normal range, 12-20 breaths/min);
iv) blood pressure, 124/57 mmHg (normal value, 120/80 mmHg); v)
clear state of mind; and vi) no jaundice or sclera throughout the
body. No significant abnormalities were detected during
cardiopulmonary auscultation. The abdomen was flat and there were
no abdominal masses or varicose veins. There were also no
intestinal obstructions or gastrointestinal peristaltic waves, no
abdominal muscle tension in the liver, spleen or rib areas. In
addition, there were no tenderness or rebound pain in the entire
abdomen. The Murphy's sign was negative. There was no tenderness at
the MacLehose point, no abdominal percussion drum sounds, no
percussion pain in the liver area, no percussion pain in the area
of either kidney and no egophony of the lungs. Bowel sounds were
detected at a rate of 4 times/min (normal range, 3-4 times/min),
reflecting normal intestinal motility. No pathological reflexes,
such as the Babinski reflex (extension of the great toe in response
to stimulation of the sole) were present, suggesting there were no
signs of upper motor neuron involvement.
In September 2024, the patient went to Jiujiang
University Affiliated Hospital (Jiujiang, China) for a gastroscopy
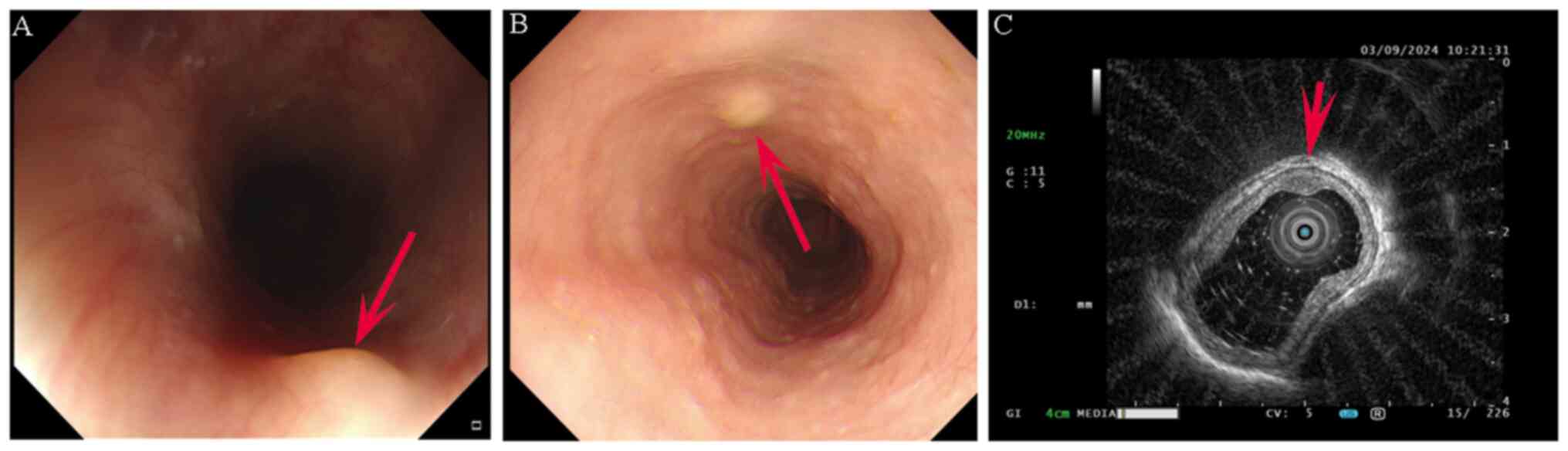
(Fig. 1A and B) and the results were as follows: i)
Multiple submucosal elevations in the oesophagus; and ii) chronic
nonatrophic gastritis with gastric antral erosion. The patient was
admitted to the hospital on the same day to receive further
treatment.
After admission, relevant laboratory tests were
completed and the results were as follows: i) White blood cell
count, 6.43x109/l (normal range,
4.0-11.0x109/l); ii) neutrophil percentage, 71.8%
(normal range, 40-75%); iii) red blood cell count,
3.85x1012/l (normal range, 4.3-5.9x1012/l);
iv haemoglobin, 116 g/l (normal range, 130-175 g/l for men and
120-160 g/l for women); v) haematocrit, 35.60% (normal range,
40-50% for men and 36-44% for women); vi) platelet count,
240x109/l (normal range, 150-400x109/l); vii)
liver and kidney function and electrolytes, normal; viii) blood
urea nitrogen, 8.40 mmol/l (normal range: 2.5-7.5 mmol/l); ix)
creatinine, 170.6 µmol/l (normal range, 44-133 µmol/l for men and
44-124 µmol/l for women); x) uric acid, 425 µmol/l (normal range,
150-420 µmol/l for men and 120-350 µmol/l for women); xi) fasting
glucose, 9.66 mmol/l (normal range, 3.9-5.5 mmol/l); xii) total
cholesterol, 4.72 mmol/l (normal value, <5.2 mmol/l); xiii)
triglycerides, 3.01 mmol/l (normal range, <1.7 mmol/l); xiv)
high-density lipoprotein, 1.00 mmol/l (normal range, >1.0 mmol/l
for men and >1.2 mmol/l for women); xv) low-density lipoprotein,
2.75 mmol/l (normal value, <3.4 mmol/l); and xvi) glycated
haemoglobin, 8.10% (normal value, <5.7%). The levels of tumour
markers carcinoembryonic antigen, α-fetoprotein, carbohydrate
antigen (CA)199 and CA724 were within normal ranges. No
abnormalities were detected in the pretransfusion tests, urinalysis
and hepatitis B panel.
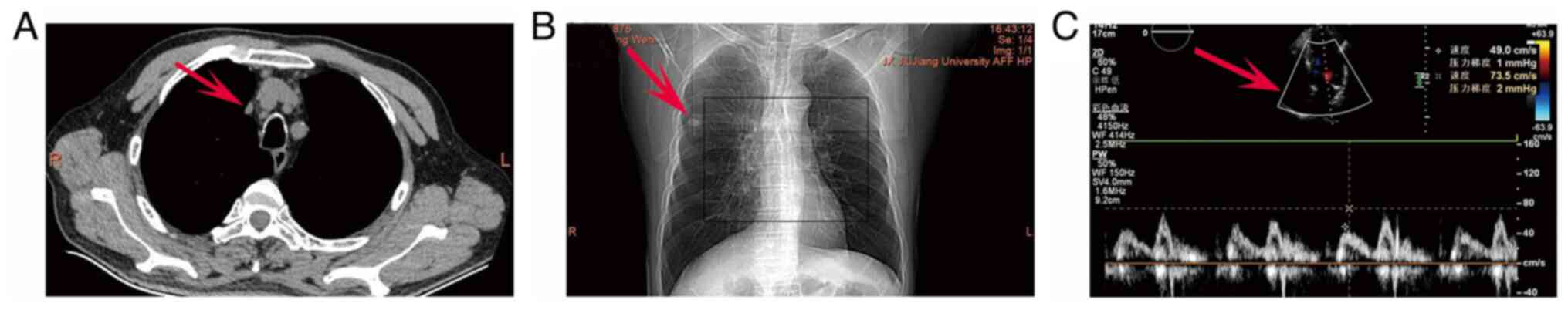
A plain chest X-ray and upper abdominal CT scan was
subsequently performed. The presence of low-density shadows in both
kidneys was detected, prompting further enhanced CT examination
(Fig. 2A). No obvious signal could
be found in the gallbladder or tail of the pancreas. Emphysema was
noted based on a ground-glass nodule in the upper lobe of the right
lung (the patient had no history of smoking), where a 3-month
follow-up examination was recommended. A small solid nodule in the
upper lobe of the right lung was also noted and an annual follow-up
examination was recommended. In addition, a calcified lesion in the
upper lobe of the right lung was also identified. Fibrous lesions
in the upper lobe of the right lung and lower lobe of the left
lung, arteriosclerosis of the aorta and coronary arteries and
multiple old fractures of the ribs on both sides were observed
(Fig. 2B). Routine 12-lead
electrocardiogram examination revealed results to be within the
normal range. Cardiac ultrasound revealed no significant
abnormalities in terms of cardiac structure or blood flow (Fig. 2C). However, decreased left
ventricular diastolic function (Grade I) was noted.
In September 2024, the patient underwent painless
endoscopic ultrasonography (EUS), which revealed submucosal
elevation in the lower oesophagus (suspected fibroma or GCT;
Fig. 1C). Ultrasound revealed
moderate hypoechoic changes originating from the submucosa, with
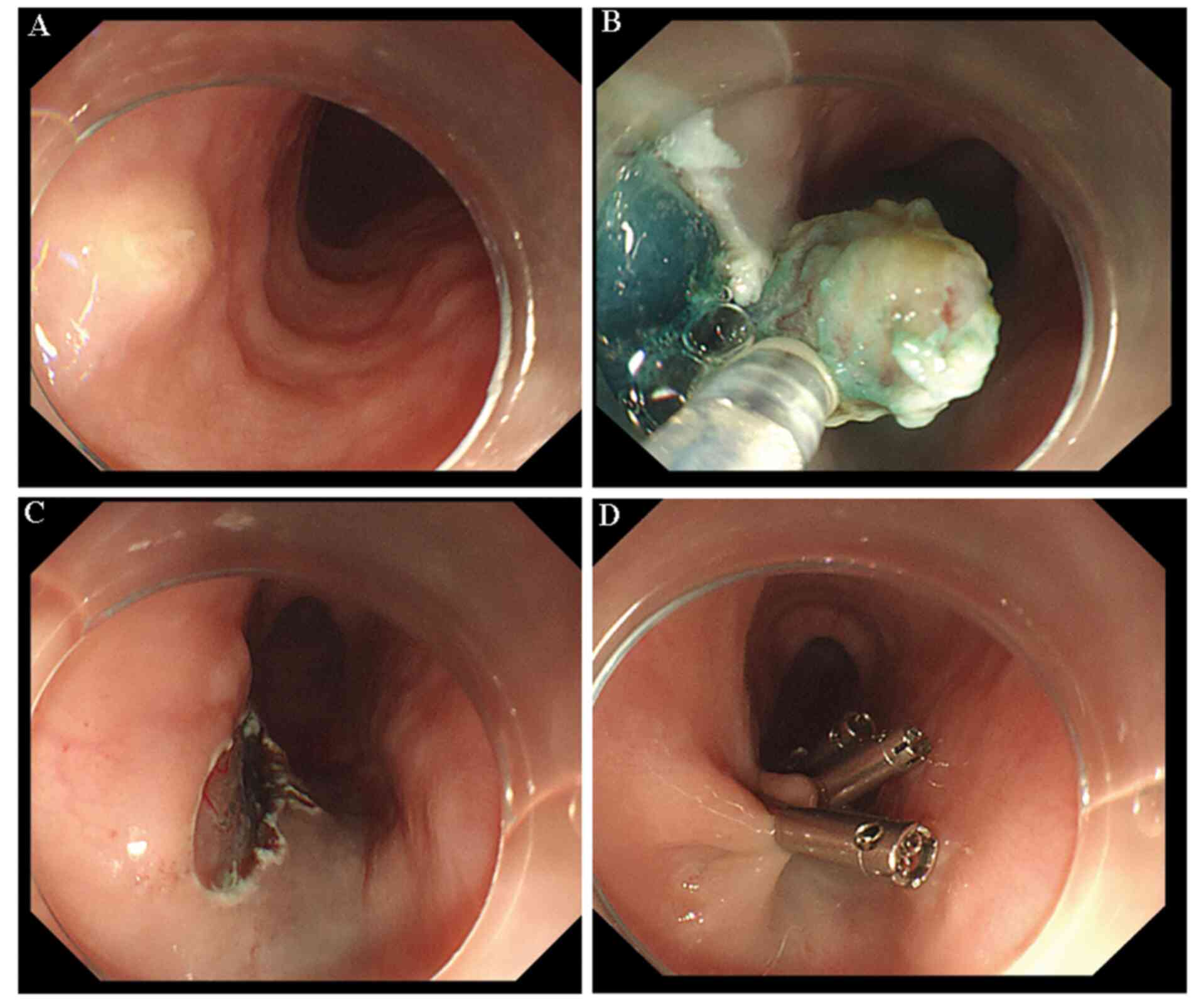
clear and regular boundaries and an oval shape. In total, 1 day
later, the patient underwent endoscopic submucosal dissection (ESD;
Fig. 3). The excised oesophageal
mucosa was submitted to the pathology department for
examination.
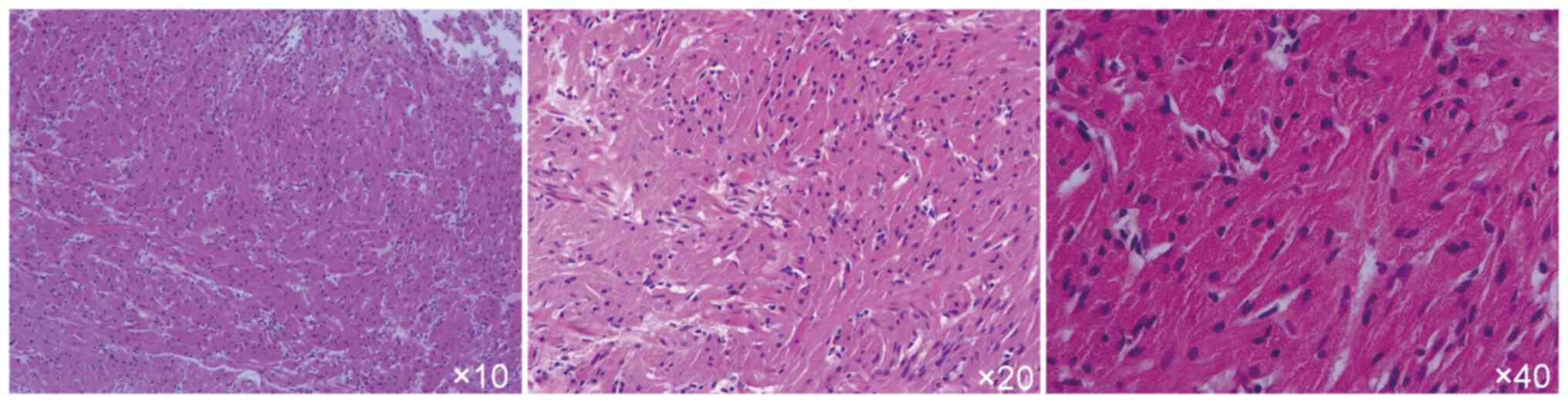
The histopathological results revealed mild
hyperplasia of the squamous epithelium, with no significant
cellular atypia. Spindle cell proliferation in the submucosa with
eosinophilic cytoplasm was noted. Mitosis was scarcely observed and
the cells contained abundant granular material. The nuclei were
small and centrally located, which was consistent with a diagnosis
of a GCT (Fig. 4). The
immunohistochemical (IHC) findings were as follows: Smooth muscle
actin (SMA; -); Desmin (-); S-100 positive (+); discovered on
gastrointestinal stromal tumours (GIST)-1 (Dog-1; -); CD117 (-);
succinate dehydrogenase B (SDHB; +); and Ki-67, ~2% positive
(Fig. 5). The lack of the smooth
muscle markers SMA and Desmin ruled out the possibility of
leiomyoma or other smooth muscle tumours such as leiomyosarcoma,
further supporting the diagnosis of a non-muscle origin of the
lesion. Strong positivity for S-100 confirmed the neural origin of
the tumour, consistent with a diagnosis of GCT, which is known to
originate from Schwann cells (17). The absence of both CD117 and DOG-1
markers effectively excluded GISTs, since these markers are
typically expressed in such tumours (18,19).
The positive staining for SDHB confirmed that the tumour was
benign, since SDH-deficient tumours (frequently associated with
malignancy) were excluded by this intact expression (20). The low Ki-67 index (~2%) indicated
a low proliferative rate, consistent with the benign nature of the
tumour and its low malignant potential (21,22).
These IHC findings collectively supported the diagnosis of EGCT,
distinguishing it from other potential SMTs, such as GISTs and
leiomyomas, further indicating its benign nature. Additionally, it
should be noted that SMA, Desmin, Dog-1 and CD117 were negative
specifically in tumour cells. Whilst these markers were not
expressed in the GCT itself, background positivity may have been
observed in non-tumour structures, such as vascular endothelial and
immune cells, which is a normal finding and not indicative of
tumour origin.
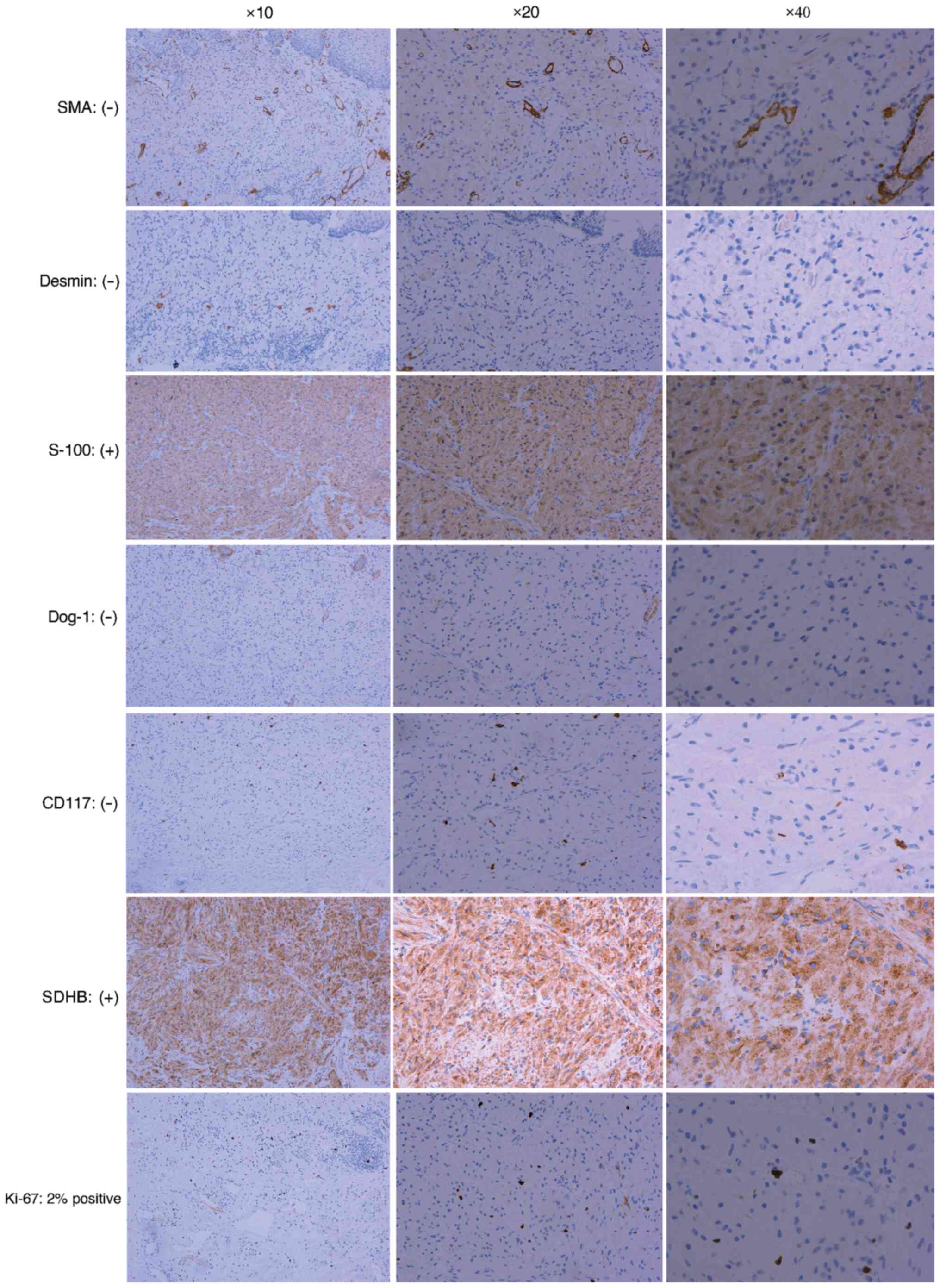 | Figure 5Immunohistochemical results. SMA:
Negative, indicating no smooth muscle differentiation. Desmin:
Negative, further supporting the absence of smooth muscle
differentiation. S-100: Positive, confirming the neural origin of
the tumour cells. Dog-1: Negative, ruling out the possibility of a
GIST. CD117: Negative, further excluding GIST and other related
tumours. SDHB: Positive, confirming the benign nature of the tumour
and excluding SDH-deficient malignancies. Ki-67: ~2% positive,
indicating a low proliferative index, which is consistent with the
benign nature of the tumour. These results collectively support the
diagnosis of an oesophageal granular cell tumour and help exclude
other potential diagnoses. The higher-magnification images are
derived from the same original image (magnification, x10, x20 and
x40). GIST, gastrointestinal stromal tumour; SMA, smooth muscle
actin; Dog-1, discovered on gastrointestinal stromal tumours; SDHB,
succinate dehydrogenase B. |
On the basis of the examination results and the
patient's medical history, the final diagnosis was EGCT. After
undergoing ESD, the patient was kept nil per os for 24 h,
followed by a liquid diet of water, congee and juice. The patient
was discharged on postoperative day 3 and was prescribed oral
omeprazole at a dose of 20 mg once daily for 2 weeks to facilitate
mucosal healing.
The patient underwent endoscopic submucosal
dissection (ESD) on September 4, 2024 and was discharged on
September 15. A follow-up endoscopic examination was recommended 6
months post-procedure to assess for recurrence or residual disease.
As of now, the patient remains asymptomatic, and there have been no
reported complications or new symptoms. We are aware of the
patient's condition based on their medical records and follow-up
advice provided at the time of discharge. Given that the follow-up
date has not yet passed, we are unable to provide further updates
at this time.
Histopathology and
immunohistochemistry
For histopathological examination, the tissue
specimens were initially fixed in 10% neutral-buffered formalin at
room temperature for 24-48 h. Following fixation, the tissues were
embedded in paraffin. The paraffin blocks were then sectioned at a
thickness of 5 µm. Dewaxing of the sections was performed by
immersing them in xylene, followed by rehydration through a graded
alcohol series (100, 95 and 70%). The sections were stained using
hematoxylin and eosin. The staining procedure involved staining
with hematoxylin at room temperature for 10 min, followed by eosin
for 3 min. The stained slides were examined under a light
microscope with a magnification of x400.
Antigen retrieval was performed on the
paraffin-embedded tissues using the Heat-Induced Epitope Retrieval
technique in citrate buffer (pH 6.0) at 95-100˚C, and then cooled
at room temperature for 10 min. The sections were subsequently
washed with xylene and rehydrated in a graded alcohol series.
Permeabilization was carried out using 0.1% Triton X-100 at room
temperature for 10 min, followed by washing with phosphate-buffered
saline (PBS) for intracellular antigen detection. To block
non-specific binding, a blocking reagent (5% BSA) was applied at
room temperature for 30 min. Primary antibodies were diluted
according to the manufacturer's recommendations: Rabbit antibodies
against SMA (1:1,000), Desmin (1:1,000), S-100 (1:1,000), Dog-1
(1:1,000), CD117 (1:1,000), SDHB (1:1,000) and Ki-67 (1:1,000) (all
Cell Signaling Technology, Inc.). The primary antibodies were
incubated at 4˚C overnight, after which the sections were washed in
PBS. Secondary goat anti-rabbit antibodies (cat no. RGAR011;
Proteintech Group, Inc.), were used at a dilution of 1:2,000 and
incubated at room temperature for 1 h, followed by another wash in
PBS. Visualization was achieved using 3,3'-diaminobenzidine (DAB)
as the chromogen with HRP/DAB detection. The HRP/DAB reaction was
allowed to proceed for 5-10 min at room temperature, and the
sections were counterstained with hematoxylin for 2 min.
Observations were made under an optical microscope at x400
magnification, with a scale bar of 50 µm included in the
legend.
Discussion
EGCTs are rare, benign lesions originating from
Schwann cells (23). Other
submucosal oesophageal lesions, such as leiomyomas, GISTs and
malignant oesophageal tumours, while also uncommon, their unique
clinical and pathological features render it essential for
clinicians to accurately identify and differentiate these
conditions. The clinical presentation of GCTs is typically
non-specific. In the majority of cases, these tumours are small and
asymptomatic, where numerous lesions are discovered incidentally
during endoscopic examinations. In rare instances, clinical
symptoms only appear when the tumour reaches a relatively large
size (24,25). Endoscopic biopsy frequently fails
to obtain adequate deep-tissue samples, complicating the
preoperative diagnosis and making accurate identification
challenging (26,27). Consequently, misdiagnosis is not
uncommon (28).
The patient in the present case report was a
68-year-old male who was asymptomatic. The oesophageal lesion was
discovered incidentally during a routine health check. This
highlights the potential for GCTs to remain undiagnosed in clinical
practice, with diagnoses frequently made during unrelated
examinations. EUS revealed a submucosal mass in the lower
oesophagus, which was confirmed as a GCT through histopathological
examination. The characteristic findings included large polygonal
cells with abundant granular cytoplasm. The positive
immunohistochemical staining for S-100 further confirmed that the
tumour originated from Schwann cells. EGCTs originate from Schwann
cells, which are derivatives of neural crest cells (29). The S-100 protein is a specific
marker widely expressed in tissues associated with neural crest
derivatives (30). Its positive
staining strongly supports the Schwann-cell origin of the tumour
and provides direct evidence for the diagnosis of EGCT. S-100
positivity effectively distinguishes EGCTs from other SMTs, such as
GISTs, leiomyomas and lipomas (15,31).
Unlike GISTs, which typically express CD117 and Dog-1, EGCTs are
negative for these markers but are consistently positive for
S-100(31). This characteristic is
crucial in establishing a definitive diagnosis and ruling out other
entities with overlapping morphological features. EGCTs are
histologically characterized by eosinophilic granules within the
cytoplasm, which are consistent with lysosomal accumulation
(32). The positive staining for
S-100 protein, in conjunction with the histological findings,
significantly enhances the diagnostic accuracy of EGCTs (10). In summary, positive S-100 protein
expression is not only a hallmark feature for the pathological
diagnosis of EGCTs, but also a key element in differentiating these
tumours from other morphologically similar SMTs. This highlights
the critical role of IHC in accurately diagnosing EGCTs and guiding
clinical decision-making.
Ki-67 is a nuclear protein intimately associated
with cell proliferation and serves as a pivotal marker for
evaluating the biological behaviour of tumours (33,34).
Its expression is particularly important in GCTs, including EGCTs,
which are characterized by a low Ki-67 proliferation index
(22,35). This low index is indicative of
their benign nature and indolent biological behaviour, offering a
valuable criterion for distinguishing benign GCTs from more
aggressive SMTs. The Ki-67 index is also instrumental in prognostic
assessments (36). Although the
majority of GCTs are benign, rare cases of malignant GCTs have been
documented. In these instances, an elevated Ki-67 index is
associated with increased aggressiveness, an increased risk of
metastasis and poorer clinical outcomes (37,38).
Consequently, monitoring Ki-67 expression can serve a crucial role
in identifying atypical or malignant features in GCTs. The
incorporation of Ki-67 into a comprehensive diagnostic and
pathological evaluation provides critical insights into tumour
behaviour, enhancing the ability to differentiate between benign
and malignant variants and informing optimal clinical management
strategies.
Whilst EGCTs are generally benign and exhibit slow
growth and low malignant potential, they can appear similar to SMTs
with greater malignant potential, such as GISTs, on imaging and
endoscopic examination (39,40).
Therefore, accurate differentiation between benign and malignant
lesions is essential for developing appropriate treatment
strategies. SMTs, such as GISTs, frequently display increased
proliferative activity (elevated Ki-67 index) and the expression of
specific IHC markers, such as CD117 and Dog-1 positivity. By
contrast, GCTs typically show S-100 positivity but are negative for
CD117 and Dog-1(41). Therefore,
precise identification through differential diagnosis helps prevent
the unnecessary overtreatment of benign lesions whilst enabling the
early detection and timely intervention for malignant tumours.
ESD is a minimally invasive procedure that allows
for the complete resection of tumours with well-defined margins
(42,43). ESD facilitates the en bloc
resection of lesions, providing high-quality specimens for
comprehensive histopathological and IHC evaluation, which would
otherwise be unattainable with less invasive biopsy methods, such
as fine-needle aspiration (44).
In addition, ESD allows for simultaneous diagnosis and treatment,
particularly for unclear lesions, such as GCTs. Complete
histological examination allows for the precise differentiation
between benign GCTs and malignant or potentially malignant SMTs,
such as GISTs (45,46). As a minimally invasive technique,
ESD reduces unnecessary interventions and surgical trauma,
particularly when GCTs are confirmed to be benign. Compared with
traditional surgical methods, including laparotomy and laparoscopic
surgery, which can lead to complications such as infection, blood
loss and adhesions, ESD results in less morbidity and faster
recovery. In brief, differential diagnosis is pivotal in the
management of oesophageal tumours, particularly in distinguishing
benign GCTs from malignant lesions, such as GISTs. ESD serves as a
valuable tool in achieving a precise diagnosis by providing
adequate tissue for analysis whilst simultaneously enabling
effective treatment. Its minimally invasive nature further enhances
patient outcomes and optimizes clinical management strategies.
In a previous study, amongst 330 patients with
oesophageal tumours, 12 patients with GCTs underwent treatment with
ESD. The results revealed that all 12 patients achieved complete
tumour resection, with no significant postoperative complications
(1). ESD has a high complete
resection rate, indicating that the majority of patients can have
their tumours entirely removed, thereby reducing the risk of
recurrence. Furthermore, patients in the ESD group demonstrated
increased postoperative survival rates and improved quality of life
compared to those undergoing traditional surgical procedures, such
as laparotomy or laparoscopic surgery. No significant cases of
recurrence were observed during the postoperative follow-up period,
highlighting the advantages of ESD in terms of faster recovery,
fewer complications and better long-term outcomes when compared to
more invasive surgical methods. The pathological results of the
endoscopic resections indicated that both endoscopic mucosal
resection and ESD can achieve complete resection rates of ≤92.9%.
This suggests that the majority of patients do not have residual
tumour tissue postoperatively, further reducing the risk of tumour
recurrence. In addition, another previous study reported that
~48.6% of patients with GCTs who underwent ESD experienced no
discomfort postoperatively, demonstrating the overall safety and
efficacy of ESD for the treatment of oesophageal GCTs (1). In a separate study, follow-up
endoscopic examinations conducted 9 months postoperatively revealed
that all patients who underwent endoscopic resection for
oesophageal GCTs had achieved complete mucosal healing at the
resection sites, with no signs of recurrence, metastasis or
oesophageal stenosis, demonstrating the effectiveness and safety of
the procedure (47).
Although the likelihood of malignant transformation
in EGCTs is rare, a thorough histological assessment remains
necessary. Malignant GCTs exhibit specific characteristics, such as
increased mitotic activity, nuclear pleomorphism and tumour
necrosis. However, these features were not observed in the case of
the present study. Therefore, the benign nature of the tumour was
confirmed, indicating a good prognosis. However, owing to the rare
possibility of malignant transformation or incomplete resection,
regular follow-up is recommended to monitor for recurrence.
In conclusion, the present case emphasizes the
importance of considering GCT in the differential diagnosis of
submucosal lesions in the oesophagus. The use of endoscopic
techniques, such as ESD, provides a safe and effective means of
diagnosis and treatment, with the lowest incidence rate of
postoperative complications, such as bleeding, infection and
oesophageal stenosis, compared to more invasive surgical
procedures. ESD serves an important role in improving patients'
quality of life through high-integrity resection and favourable
postoperative recovery. Early detection and intervention (even for
asymptomatic patients) are key to ensuring optimal outcomes and
preventing complications.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant no. 32360888), the Jiujiag
Science and Technology Program project (grant no. S2024ZDYFN0004)
and the Jiangxi Students' Platform for Innovation and
Entrepreneurship Training Program (grant no. 202411843023).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
RJ, YQS and NHZ contributed to the drafting of the
manuscript and the design of the study. YQS, TL and XJW provided
clinical data and performed relevant diagnoses and surgery. TL and
YQS conducted the literature review, revised the manuscript and
obtained medical images. SL was responsible for conceptualization,
visualization and funding acquisition. SL reviewed and edited the
manuscript for final submission. All authors have read and approved
the final manuscript. YQS and TL confirm the authenticity of all
the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent for publication of their
clinical details and/or clinical images was obtained from the
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ryu DG, Choi CW, Kim SJ, Hwang CS, Kang
DH, Kim HW, Park SB and Son BS: Clinical outcomes of esophageal
granular cell tumors with different endoscopic resection methods.
Sci Rep. 13(10738)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lu W, Xu MD, Zhou PH, Zhang YQ, Chen WF,
Zhong YS and Yao LQ: Endoscopic submucosal dissection of esophageal
granular cell tumor. World J Surg Oncol. 12(221)2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shibagaki K, Ishimura N and Kinoshita Y:
Endoscopic submucosal dissection for duodenal tumors. Ann Transl
Med. 5(188)2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dogom SA, Thongpiya J, Elmassry M, Feist
M, Sharma M and Rateb G: Successful endoscopic submucosal
dissection of an esophageal granular cell tumor in a pediatric
patient: A case report and a therapeutic insight. JPGN Rep.
5:384–388. 2024.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tipirneni K, Mehl A, Bowman B and Joshi V:
Esophageal granular cell tumor: A benign tumor or an insidious
cause for concern? Ochsner J. 16:558–561. 2016.PubMed/NCBI
|
|
6
|
Wang HQ and Liu AJ: Esophageal granular
cell tumors: Case report and literature review. World J
Gastrointest Oncol. 7:123–127. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Benchekroun Z, Akammar A, Bennani H,
Haloua M, Lamrani YA, Boubbou M, Chbani L, Maâroufi M and Alami B:
Atypical esophageal granular cell tumor: Case report. Radiol Case
Rep. 16:3995–3999. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Barat M, Pellat A, Dohan A, Hoeffel C,
Coriat R and Soyer P: CT and MRI of gastrointestinal stromal
tumors: New trends and perspectives. Can Assoc Radiol J.
75:107–117. 2024.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Çolak S, Gürbulak B, Çelik G, Bektaş H and
Dursun N: Gastrointestinal tract schwannomas and brief review of
literature. Turk J Surg. 37:408–412. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Riffle ME, Polydorides AD, Niakan J and
Chehade M: Eosinophilic esophagitis and esophageal granular cell
tumor: An unexpected association. Am J Surg Pathol. 41:616–621.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Malik F, Bernieh A and Saad AG: Esophageal
granular cell tumor in children: A clinicopathologic study of 11
cases and review of the literature. Am J Clin Pathol. 160:106–112.
2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yu-Fong Chang J, Hwang MJ, Sun A and
Chiang CP: Granular cell tumor: Case report. J Dent Sci.
16:1018–1019. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Stefansson K and Wollmann RL: S-100
protein in granular cell tumors. (Granular cell myoblastomas).
Cancer. 49:1834–1838. 1982.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhong N, Katzka DA, Smyrk TC, Wang KK and
Topazian M: Endoscopic diagnosis and resection of esophageal
granular cell tumors. Dis Esophagus. 24:538–543. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Stašek M, Aujeský R, Škarda J, Švébišová
H, Vrba R, Szkorupa M and Neoral C: Malignant granular cell tumor
of the esophagus: A case report. Ann Thorac Cardiovasc Surg.
26:359–364. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Menon L and Buscaglia JM: Endoscopic
approach to subepithelial lesions. Therap Adv Gastroenterol.
7:123–130. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Solomon LW and Velez I: S-100 negative
granular cell tumor of the oral cavity. Head Neck Pathol.
10:367–373. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chelliah A, Kalimuthu S, Szentgyorgyi E
and Chetty R: Granular cell tumour of the colon with extensive
sclerosis. Diagn Histopathol. 22:279–281. 2016.
|
|
19
|
Wei J, Wang D, Zhang R and Xu C: Granular
cell tumor of the ampulla of Vater: Report of a unique case with
emphasis on immunohistochemistry for TFE3 antigen expression. Int J
Clin Exp Pathol. 9:318–323. 2016.
|
|
20
|
Ding CKC, Chan S, Mak J, Umetsu SE, Simko
JP, Ruiz-Cordero R, Saunders T and Chan E: An exploration in
pitfalls in interpreting SDHB immunohistochemistry. Histopathology.
81:264–269. 2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ferreira JCB, Oton-Leite AF, Guidi R and
Mendonça EF: Granular cell tumor mimicking a squamous cell
carcinoma of the tongue: A case report. BMC Res Notes.
10(14)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chrysomali E, Nikitakis NG, Tosios K, Sauk
JJ and Papanicolaou SI: Immunohistochemical evaluation of cell
proliferation antigen Ki-67 and apoptosis-related proteins Bcl-2
and caspase-3 in oral granular cell tumor. Oral Surg Oral Med Oral
Pathol Oral Radiol Endod. 96:566–572. 2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Duvuru S, Sanker V, Pandit D, Khan S,
Alebrahim S and Dave T: Granular cell tumor of the brain: Case
report and review of literature. J Surg Case Rep.
2023(rjad701)2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Matsuzawa N, Nishikawa T, Ohno R, Inoue M,
Nishimura Y, Okamoto T, Shimizu T, Shinagawa T, Nishizawa Y and
Kazama S: Paraganglioma of the urinary bladder initially diagnosed
as gastrointestinal stromal tumor requiring combined resection of
the rectum: A case report. World J Surg Oncol.
20(185)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gagliardi F, Spina A, Barzaghi LR, Bailo
M, Losa M, Terreni MR and Mortini P: Suprasellar granular cell
tumor of the neurohypophysis: Surgical outcome of a very rare
tumor. Pituitary. 19:277–285. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Koizumi E, Goto O, Nakagome S, Habu T,
Ishikawa Y, Kirita K, Noda H, Higuchi K, Onda T, Akimoto T, et al:
Technical outcomes and postprocedural courses of mucosal
incision-assisted biopsy for possible gastric gastrointestinal
stromal tumors: A series of 48 cases (with video). DEN Open.
4(e264)2023.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Krutsri C, Iwai T, Kida M, Imaizumi H,
Kawano T, Tadehara M, Watanabe M, Okuwaki K, Yamauchi H and
Wasaburo K: Pancreatic granular cell tumor diagnosed by endoscopic
ultrasound-guided fine needle aspiration biopsy. Clin J
Gastroenterol. 12:347–354. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Inokuchi Y, Watanabe M, Hayashi K, Kaneta
Y, Furuta M, Machida N and Maeda S: A case of esophageal granular
cell tumor diagnosed by mucosal incision-assisted biopsy. Clin J
Gastroenterol. 15:53–58. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Finnegan DK, Cartoceti AN, Hauck AM and
LaDouceur EEB: Meningeal granular cell tumour in a green tree
python (morelia viridis). J Comp Pathol. 174:54–57. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yamada S, Katayama Y, Fujimoto Y, Kobori
I, Kusano Y, Soga K, Sato T, Matsushima J, Ban S and Tamano M: A
case of a granular cell tumor arising in a patient with
long-segment Barrett's esophagus. Intern Med. 64:557–561.
2025.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Martin-Broto J, Martinez-Marín V, Serrano
C, Hindi N, López-Guerrero JA, Bisculoa M, Ramos-Asensio R,
Vallejo-Benítez A, Marcilla-Plaza D and González-Cámpora R:
Gastrointestinal stromal tumors (GISTs): SEAP-SEOM consensus on
pathologic and molecular diagnosis. Clin Transl Oncol. 19:536–545.
2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Saito R, Chambers JK and Uchida K:
Immunohistochemical study of autophagy associated molecules and
cell adhesion molecules in canine intracranial granular cell
tumors. J Vet Med Sci. 84:1474–1479. 2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang M, Sun ZQ and Zou XP: Esophageal
granular cell tumor: Clinical, endoscopic and histological features
of 19 cases. Oncol Lett. 8:551–555. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Maekawa H, Maekawa T, Yabuki K, Sato K,
Tamazaki Y, Kudo K, Wada R and Matsumoto M: Multiple
esophagogastric granular cell tumors. J Gastroenterol. 38:776–780.
2003.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lazǎr D, Tǎban S, Sporea I, Dema A,
Cornianu M, Lazăr E, Goldiş A and Vernic C: Ki-67 expression in
gastric cancer. Results from a prospective study with long-term
follow-up. Rom J Morphol Embryol. 51:655–661. 2010.PubMed/NCBI
|
|
36
|
Sun X and Kaufman PD: Ki-67: More than a
proliferation marker. Chromosoma. 127:175–186. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ocal I, Avci A, Cakalagaoglu F and Can H:
Lack of correlations among histopathological parameters, Ki-67
proliferation index and prognosis in pheochromocytoma patients.
Asian Pac J Cancer Prev. 15:1751–1755. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Graefe C, Eichhorn L, Wurst P, Kleiner J,
Heine A, Panetas I, Abdulla Z, Hoeft A, Frede S, Kurts C, et al:
Optimized Ki-67 staining in murine cells: A tool to determine cell
proliferation. Mol Biol Rep. 46:4631–4643. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Trinidad CM, Wangsiricharoen S, Prieto VG
and Aung PP: Rare variants of dermatofibrosarcoma protuberans:
Clinical, histologic, and molecular features and diagnostic
pitfalls. Dermatopathology (Basel). 10:54–62. 2023.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Sandeford J, Anderson L, Burling M and
Carter J: Vulvar granular cell tumour in a recently post-partum
woman: A case report. Eur J Gynaecol Oncol. 43:96–100. 2022.
|
|
41
|
Fahim S, Aryanian Z, Ebrahimi Z,
Kamyab-Hesari K, Mahmoudi H, Alizadeh N, Heidari N, Livani F,
Ghanadan A and Goodarzi A: Cutaneous granular cell tumor: A case
series, review, and update. J Family Med Prim Care. 11:6955–6958.
2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Okada M, Kitaoka M, Sakaeda H, Aono R,
Satake T, Ohkawa Y, Umeshita J, Yano K, Tashima M and Nakashima J:
A case report of esophageal granular cell tumor complicated with
esophageal intramural pseudodiverticulosis successfully resected by
ESD. Gastroenterol Endosc. 66:29–35. 2024.
|
|
43
|
Takaya H, Kawaratani H, Kaneko M, Takeda
S, Sawada Y, Kitade M, Moriya K, Namisaki T, Sawai M, Mitoro A, et
al: Gastric granular cell tumor in a youth excised by endoscopic
submucosal dissection: A case report and literature review. Acta
Gastroenterol Belg. 80:317–319. 2017.PubMed/NCBI
|
|
44
|
Li QL, Zhang YQ, Chen WF, Xu MD, Zhong YS,
Ma LL, Qin WZ, Hu JW, Cai MY, Yao LQ and Zhou PH: Endoscopic
submucosal dissection for foregut neuroendocrine tumors: An initial
study. World J Gastroenterol. 18:5799–5806. 2012.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sarker S, Gutierrez JP, Council L,
Brazelton JD, Kyanam Kabir Baig KR and Mönkemüller K:
Over-the-scope clip-assisted method for resection of full-thickness
submucosal lesions of the gastrointestinal tract. Endoscopy.
46:758–761. 2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kono Y, Hirata I, Katayama T, Uemura H,
Hirata T, Gotoda T, Miyahara K, Moritou Y and Nakagawa M: Current
evidence and issues of endoscopic submucosal dissection for gastric
neoplasms during antithrombotic therapy. Clin J Gastroenterol.
13:650–659. 2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Fan X, Jiao J, Luo L, Zhu L, Zheng Z, Chen
X, Wang T, Liu W and Wang B: Role of endoscopic ultrasound and
endoscopic resection in the diagnosis and treatment of esophageal
granular cell tumors. Scand J Gastroenterol. 57:1264–1271.
2022.PubMed/NCBI View Article : Google Scholar
|