Introduction
Kartagener syndrome (KS) is a rare autosomal
recessive genetic disorder. In 1933, Kartagener linked visceral
inversion, sinusitis and bronchiectasis as independent syndromes
(1). In 1976, Pedersen et
al (2) proposed that owing to
the congenital lack of axial arms in the cilia throughout the body,
there is a loss of motility, resulting in the retention of
secretions and pathogens. This condition predisposes individuals to
recurrent bronchiectasis and sinus lesions in the long term. In
1981, Sleigh (3) found that cilia
were not completely immobile but exhibited abnormal movement, which
was termed primary ciliary dyskinesia (PCD). PCD accompanied by
visceral reversal is known as KS, and ~50% of patients with PCD
have KS. KS is frequently accompanied by chronic respiratory
symptoms, and when it worsens, it is easily misdiagnosed as an
infection. In addition, atypical early imaging can easily lead to
missed diagnoses and misdiagnoses of pulmonary malignant tumors.
The present study reports a rare clinical case of KS complicated by
small-cell lung cancer, which was initially misdiagnosed as a lung
infection owing to atypical clinical symptoms and imaging findings.
After a precise diagnosis, the patient was ultimately diagnosed
with KS and received timely treatment. It is hoped that this case
will draw the attention of clinical respiratory physicians to KS
combined with lung cancer.
Case presentation
A 66-year-old male patient with a history of smoking
(the patient smoked 20 cigarettes per day x 40 years, and thus he
had a smoking index of 800), exhibited primary symptoms of cough,
chest pain and intermittent fever for 4 months, admitted to the
Department of Pulmonary and Critical Care Medicine of Jining No. 1
People's Hospital (Jining, China). Despite having received
intermittent antibiotics for a lung infection at a local hospital,
the patient's symptoms failed to improve and the chest pain
worsened. Physical examination revealed normal vital signs, coarse
breathing sounds in both lungs, and no dry or wet rales or pleural
friction sounds. On percussion, the boundary of the cardiac
dullness widened to the right and no pathological murmurs were
heard. Laboratory tests revealed a normal white blood cell count
(5.63x109/l; normal range, 4-10x109/l),
normal neutrophil levels (3.94x109/l; normal range,
2.04-7.5x109/l), a normal procalcitonin level (0.05
ng/ml; normal range, 0-0.05 ng/ml), an elevated neuron-specific
enolase level (48.96 ng/ml; normal range, 0-16.3 ng/ml) and an
elevated carcinoembryonic antigen level (13.52 ng/ml; normal range,
0-5 ng/ml), as well as normal liver and kidney function, blood
lipids and blood glucose. Electrocardiography showed a right-sided
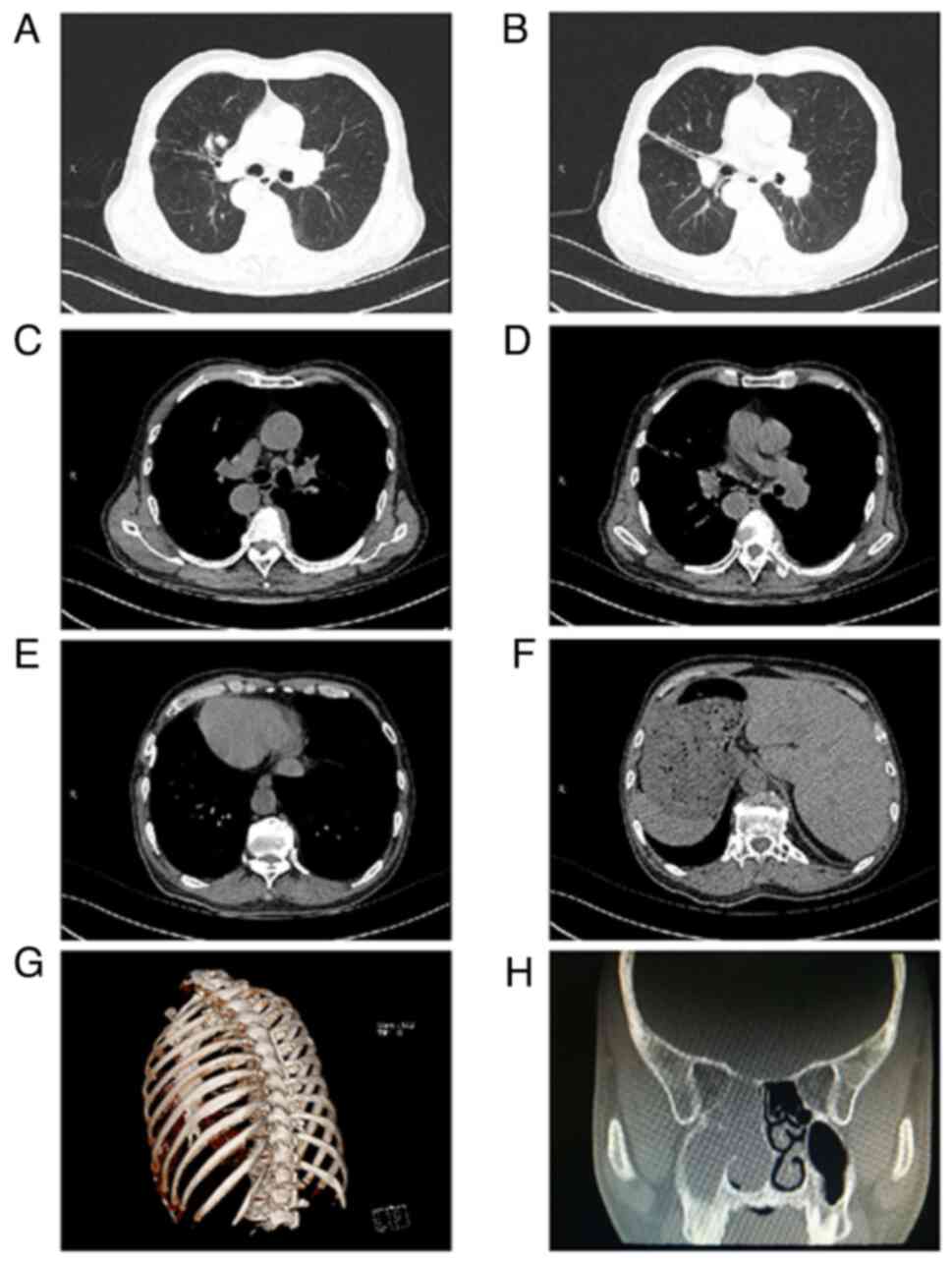
heart. Chest CT + 3D reconstruction of the ribs revealed chronic
inflammation of both lungs, multiple bronchiectasis in both lungs,
nodules in the upper lobe of the right lung, a flaky high-density
shadow in the right main bronchus, bilateral pleural thickening,
localized calcification of the right pleura and a right-sided heart
with transposition of thoracic and abdominal organs (Fig. 1A-G). Paranasal sinus CT revealed
right maxillary sinusitis, ethmoid sinus, nasal soft tissue shadow,
polyps, deviated nasal septum, and bilateral middle and lower
turbinate hypertrophy (Fig. 1H).
The patient presented with right locus coeruleus, visceral
inversion, sinusitis and bronchial dilatation and was diagnosed
with KS. Clinical consideration of the patient as having KS,
combined with the patient's previous long-term smoking history and
the presence of a flaky hyperdense shadow in the right main
bronchus, did not exclude the possibility of bronchial phlegm
embolism, foreign body or obstruction. Bronchoscopy revealed normal
bronchial tubes in the right lung. A polypoid neoplasm was observed
in the opening of the upper lobe of the left lung, incompletely
obstructing the lumen and spreading along the middle segment to the
opening of the middle and lower lobes of the left lung, with
necrosis on the surface, which was biopsied (Fig. 2A and B). Pathological and immunohistochemical
analysis was performed in the Department of Pathology, Jining NO. 1
People's Hospital. The specimens were fixed in 10% neutral
formalin, with conventional paraffin embedding, and sliced into
4-µm thick continuous sections. Light microscopy was used for
observation. Hematoxylin (5 min) and eosin (2 min) staining of the
pathological specimens was performed at room temperature. At the
same time, the EnVision two-step method of immunohistochemistry was
used according to standard protocols, with antibodies for
cytokeratin 7 (CK7; cat. no. Kit-0021), CD56 (cat. no. R-0148-03),
thyroid transcription factor 1 (TTF-1; cat. no. MAB-0599),
synaptophysin (Syn; cat. no. R-0482-03), chromogranin A (CgA; cat.
no. RMA-0548), aspartic peptidase A, (NapsinA; cat. no. MAB-0704)
and lymphocyte-specific protein tyrosine kinase (LCK; cat. no.
R-0347-03) (all pre-diluted; Fuzhou Maixin and Shanghai Changdao
Biotechnology Co., Ltd.) The secondary antibody was from the
Universal DAB Detection Kit [cat. no. 760-500; Roche Diagnostics
(Shanghai) Co., Ltd.], which was a universal type. Cancer cells
were detected in the bronchoalveolar lavage fluid, and H&E
stain revealed heterogeneous epithelioid cells with dense cells,
deeply stained nuclei and little cytoplasm. Immunohistochemistry
revealed CK7(+), CD56(+), TTF-1(+), Syn(+), CgA(-), NapsinA(-) and
LCK(+) results, confirming the diagnosis of small cell lung cancer
(Fig. 2C-K). A complete
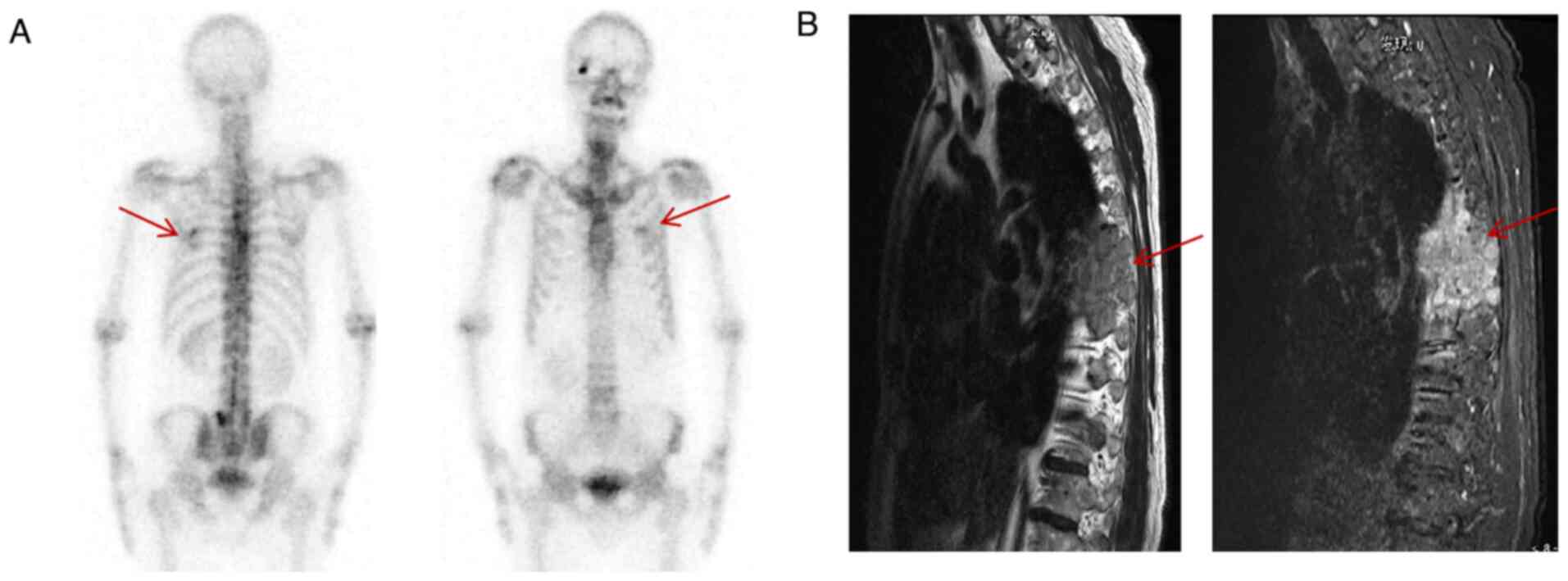
extrapulmonary auxiliary examination suggested bone metastasis
(Fig. 3A, B). Therefore, the following diagnoses
were made: i) Small cell lung cancer of the left lung (extensive
stage) and ii) KS. Small cell lung cancer is the most malignant
type of lung cancer, is incurable and combination chemotherapy can
only control the disease temporarily; the cancer will eventually
progress (4). The patient had an
ECOG performance status score (5)
of 1, and according to the Chinese Primary Lung Cancer Diagnostic
and Treatment Guidelines (6), the
patient received a cisplatin/etoposide combination chemotherapy for
6 cycles and bisphosphonates treatment; the disease was controlled
and the patient was rated as being in partial remission after the
treatment according to Solid Tumor Response Assessment Criteria
(7). However, half a year later,
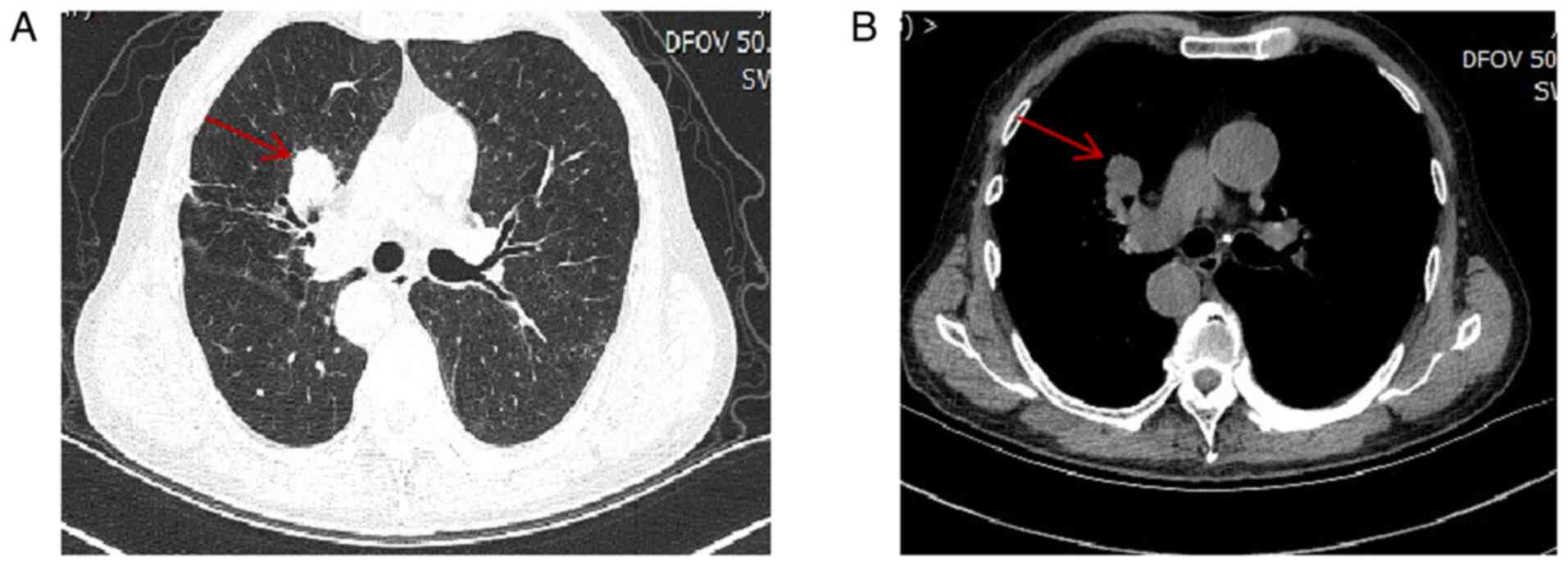
the patient's disease had progressed (Fig. 4), and the patient's family refused
to continue the antitumor treatment due to side effects and
financial concerns, and chose symptomatic supportive therapy
instead. Through telephone follow-ups, it was revealed that the
patient eventually passed away 1 year later owing to the
progression of lung cancer.
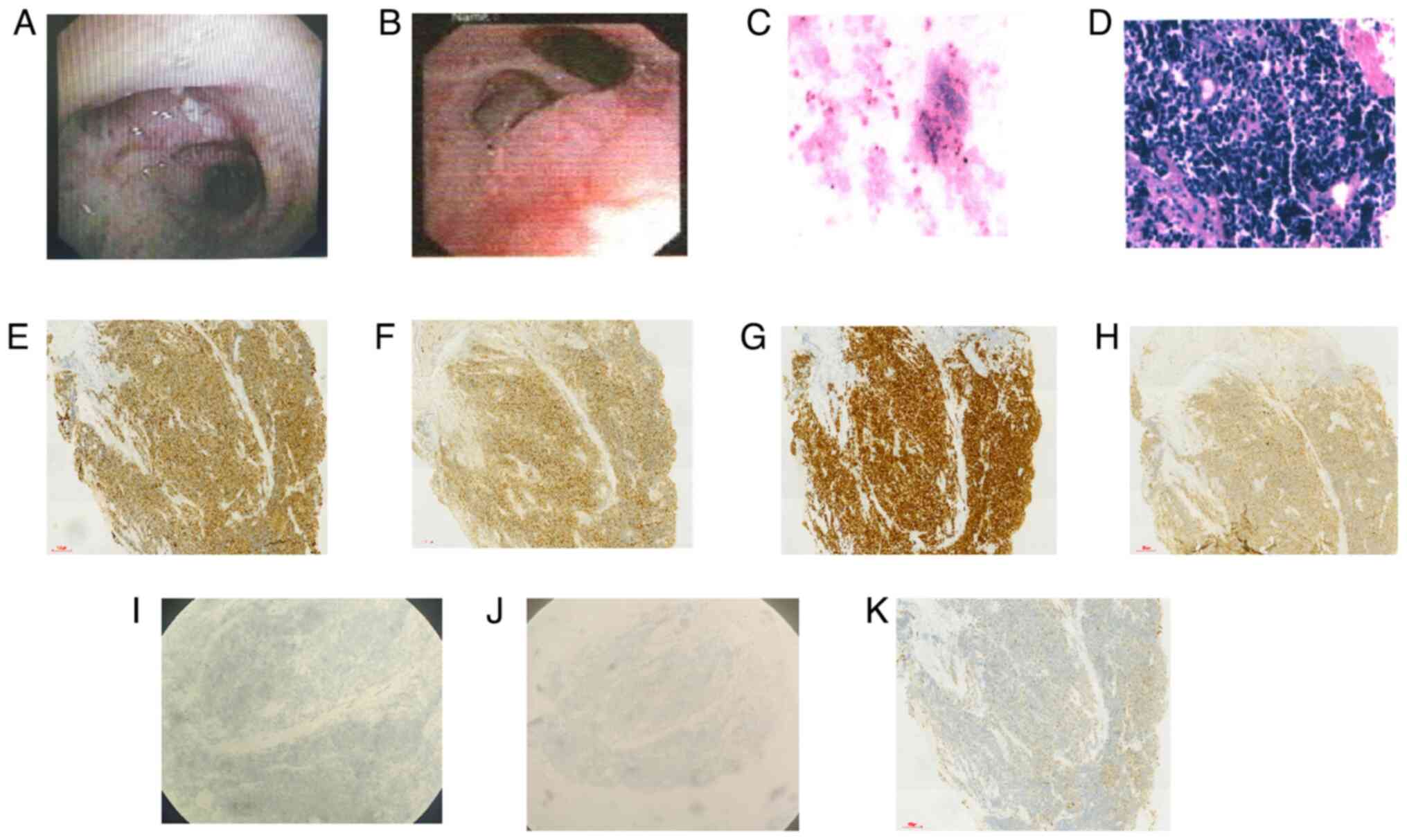 | Figure 2Bronchoscopy clarified the pathology.
(A) Polypoid neoplasm seen in the opening of the upper lobe of the
left lung. (B) The neoplasm spreading along the mid-section to the
opening of the middle and lower lobes of the left lung. (C) Cancer
cells were detected in the bronchoalveolar lavage fluid by HE
staining (magnification, x200); (D) Hematoxylin and eosin staining
revealed heterogeneous epithelioid cells with dense cells, deeply
stained nuclei and little cytoplasm, denoting small cell lung
cancer (magnification, x200). Immunohistochemistry staining for (E)
cytokeratin 7(+), (F) CD56(+), (G) thyroid transcription
factor-1(+), (H) synaptophysin(+), (I) chromogranin A(-), (J)
aspartic peptidase A(-) and (K) lymphocyte-specific protein
tyrosine kinase(+) (magnification, x100). |
Discussion
The patient was clinically suspected of having KS
owing to recurrent cough, sputum production and fever, symptoms
commonly attributed to infectious factors. However, the
exacerbation of chest pain prompted admission to our hospital for
further evaluation. Based on the clinical symptoms, the following
differential diagnoses were considered: Pulmonary embolism,
myocardial infarction, tension pneumothorax and aortic coarctation.
3D reconstruction of the ribs was performed with the aim of
excluding fractures and other conditions. However, upon chest CT
examination, small nodules and hyperdense shadows were observed in
the patient's airways, suggesting the possibility of sputum
embolus, foreign bodies and possible lung cancer. The patient
lacked the typical pathognomonic features of lung cancer and no
obvious mass on CT. The patient's condition progressed despite
anti-infective treatment and the patient developed chest pain. This
was different from the previously reported cases (8-10),
and therefore, it is required to reconsider whether there was a
combination of other diseases on the basis of KS. Thus, the
patient's chest CT was repeatedly scrutinized and slight
irregularities in the left bronchial wall, partial stenosis and
characteristic wave-like changes were found. Therefore, it was
deemed necessary to perform a bronchoscopy, revealing an
abnormality in the left bronchus and a tissue biopsy was taken.
Finally, pathological examination confirmed the diagnosis of small
cell lung cancer. The patient's chest pain was attributed to a
combination of small cell lung cancer and rib metastasis.
In conclusion, KS is typically characterized by
recurrent respiratory infections. The present case highlights the
importance of prompt action when anti-infective treatments do not
yield satisfactory results in clinical settings and CT scans do not
fully explain clinical symptoms, particularly in heavy smokers.
Intensified examinations, including bronchoscopy, should be
promptly pursued. This case serves as a reminder of the need for
timely diagnosis of KS combined with small cell lung cancer,
offering valuable guidance for clinical practice.
A literature review was then performed. KS is a
subset of PCD, a rare autosomal recessive genetic disease with an
incidence of ~ 1 in 30,000 (11,12).
The pathogenesis of KS is not fully understood, but several studies
have shown that the majority of causative mutations involve two
genes, DNA H5 and DNAI1(13).
Typical clinical manifestations include visceral transposition,
bronchiectasis and chronic infectious sinusitis. Patients with KS
may present with respiratory symptoms since early childhood, with
recurrent fever, cough, sputum and blood in the sputum. The right
heart and visceral transposition do not usually cause significant
clinical symptoms. Imaging is the primary method used to confirm
the diagnosis of KS. CT examination of the chest and abdomen can
reveal visceral inversion and bronchial dilatation and determine
whether they are combined with infection; additionally, CT of the
sinus can identify sinus lesions. Treatment mainly focuses on
preventing respiratory infections, including the use of antibiotics
and pulmonary physiotherapy to prevent complications (12,14).
KS is not associated with age, sex or smoking
history (11,12), whereas lung cancer, especially
squamous lung cancer, is more common in older adults, men and
smokers. There are limited reports on KS combined with small-cell
lung cancer. In KS, the congenital absence of axial arms of the
cilia lead to loss of mobility, causing the retention of secretions
and pathogens, dysfunction of airway clearance, long-term
stimulation of pathogenic microorganisms, chronic inflammation, and
long-term and repeated exposure to chronic carcinogens, which
ultimately result in the development of lung cancer (8-10).
This is the main factor in lung cancer development reported in the
previous literature. In the present case, the patient was at high
risk of developing lung cancer due to being an elderly male and
having a long history of smoking, which, combined with the presence
of KS, likely contributed to the development of lung cancer.
However, the exact effects of KS on tumorigenesis require further
investigation. For the patient in this case study, respiratory
infection symptoms were the primary concern, leading to ongoing
anti-infective treatment. Owing to the prominence of these
symptoms, the clinical signs of lung cancer were not obvious,
potentially resulting in delayed diagnosis. Furthermore, small cell
lung cancer is highly malignant, and despite a timely diagnosis,
the prognosis of the patient in this case was poor, with the
patient surviving only 1 year after diagnosis.
Acknowledgements
Not applicable.
Funding
Funding: This research was supported by the Key R&D Program
of Jining (grant no. 2023YXNS243) and the Medical System Staff
Science and Technology Innovation Program Project of Shandong
(grant no. SDYWZGKCJHLH2023068).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JZ, LW and CQ contributed to the conception and
design of the study. Data collection and analysis were performed by
CB, JM and LH. The manuscript was written by JZ and CB. CQ confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
As the patient had passed away when the case report
was prepared, written informed consent was obtained from the
patient's daughter for the publication of potentially identifying
images or data included in this article.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kartagener M: Zur Pathogenese der
Bronchiedtasien. I. Mitte lung Bron chiektasien bei Situs viscerum
inversus. Beitr Klin Tuberk. 83:489–501. 1933.
|
|
2
|
Pedersen H and Mygind N: Absence of
axonemal arms in nasal mucosa cilia in Kartagener's syndrome.
Nature. 5:494–495. 1976.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Sleigh MA: Ciliary function in mucus
transport. Chest. 80 (Suppl 6):S791–S795. 1981.PubMed/NCBI
|
|
4
|
Torre LA, Siegel RL and Jemal A: Lung
cancer statistics. Adv Exp Med Biol. 893:1–19. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Schnipper LE, Davidson NE, Wollins DS,
Tyne C, Blayney DW, Blum D, Dicker AP, Ganz PA, Hoverman JR,
Langdon R, et al: American Society of Clinical Oncology Statement:
A conceptual framework to assess the value of cancer treatment
options. J Clin Oncol. 33:2563–2577. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhi X, Shi Y and Yu J: Standards for the
diagnosis and treatment of primary lung cancer (2015 version) in
China. Zhonghua Zhong Liu Za Zhi. 37:67–78. 2015.PubMed/NCBI(In Chinese).
|
|
7
|
Schwartz LH, Litière S, de Vries E, Ford
R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J,
et al: RECIST 1.1-update and clarification: From the RECIST
committee. Eur J Cancer. 62:132–137. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Horie M, Arai H, Noguchi S, Suzuki M,
Sakamoto Y and Oka T: Kartagener syndrome with lung cancer and
mediastinal tumor. Nihon Kokyuki Gakkai Zasshi. 48:375–378.
2010.PubMed/NCBI(In Japanese).
|
|
9
|
Zhou D, Tian Y, Lu Y and Yang X:
Anatomical variants of pulmonary segments and uni-portal
thoracoscopic segmentectomy for lung cancer in a patient with
Kartagener syndrome: A case report. Gen Thorac Cardiovasc Surg.
69:1432–1437. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang J, Xiao Y, Bai M and Bian J: A rare
case of Kartagener syndrome combined with lung cancer. Asian J
Surg. 46:4801–4802. 2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ibrahim R and Daood H: Kartagener
syndrome: A case report. Can J Respir Ther. 57:44–48.
2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tadesse A, Alemu H, Silamsaw M and
Gebrewold Y: Kartagener's syndrome: A case report. J Med Case Rep.
12(5)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pandey AK, Maithani T and Bhardwaj A:
Kartagener's syndrome: A clinical reappraisal with two case
reports. Egypt J Ear Nose Throat Allied Sci. 15:271–274. 2014.
|
|
14
|
Flume PA, O'Sullivan BP, Robinson KA, Goss
CH, Mogayzel PJ Jr, Willey-Courand DB, Bujan J, Finder J, Lester M,
Quittell L, et al: Cystic fibrosis pulmonary guidelines: Chronic
medications for maintenance of lung health. Am J Respir Crit Care
Med. 176:957–969. 2007.PubMed/NCBI View Article : Google Scholar
|