Introduction
End-stage renal disease (ESRD) remains a global
health concern. Given the application of new immunosuppressive
agents and the continuous improvement of organ transplantation
technology, allogeneic kidney transplantation has become the best
alternative therapy for the treatment of ESRD (1), which may lead to improvements in the
survival rate and quality of life of patients compared with
continuous dialysis (2). However,
potential kidney transplant recipients often develop anti-human
leukocyte antigen (HLA) antibodies due to previous transplants,
blood transfusions and pregnancy. This HLA pre-sensitization status
not only decreases the success rate of transplant matching, but it
also markedly increases the risk of acute rejection (AR) following
transplantation. Antibody-mediated rejection is the main cause of
chronic kidney graft injury, which is characterized by the
existence of donor-specific antibodies (DSA) in kidney transplant
recipients and which greatly limits the functional recovery and
long-term survival of kidney grafts (3,4).
Therefore, it is essential to test the sensitivity of kidney
transplant patients prior to transplantation. The pre-transplant
immune risk assessment includes evaluations of pre-existing HLA
antibodies and non-HLA antibodies in the recipient, HLA
compatibility, complement-dependent cytotoxicity (CDC) crossmatch
(CDC-XM), immune memory cells, among other factors. CDC-XM and HLA
typing are critical for evaluating donor-recipient compatibility,
for minimizing graft rejection and for improving transplant
outcomes in solid organ transplantation.
CDC-XM is an important laboratory test that is
administered prior to organ transplantation to determine the
likelihood of AR through detecting the presence of antibodies in
the recipient's body that are able to bind to HLA on the surface of
lymphocytes, thereby activating the complement system. In CDC-XM,
CDC-positivity is a contraindication for kidney transplantation.
The HLA system has a crucial role in regulating the body's immune
response, which is mediated through presenting antigens and
distinguishing between ‘self’ and ‘non-self’, so as to effectively
resist the invasion of pathogens. It involves a set of cell-surface
antigen-presenting proteins categorized as class I and II major
histocompatibility complex molecules (MHC I/II). Humans possess
three class I (A, B and C) antigens that are present on all
nucleated cells and three class II (DP, DQ and DR) antigens that
are present only on antigen-presenting cells and endothelial cells.
HLA-A, -B and -DRB1 heterodimers are responsible for the majority
of the polymorphisms in HLA, making these heterodimers the primary
focus of HLA matching in the allocation of donor kidneys for
transplantation (5).
In kidney transplantation, lower levels of CDC-XM
and improved HLA matching have been shown to be associated with
improved graft survival, along with the post-operative
administration of lower dosages of immunosuppressive agents, a
reduced incidence of immunosuppressive side effects and a lower
degree of sensitization. Currently, recipients with CDC >10%
tend to be excluded from studies in Asia and relatively few studies
have been published on the short-term effects of CDC and HLA
matching in kidney transplantation. Therefore, the aim of the
present study was to analyze the effect of CDC-XM levels and HLA
matching on early clinical outcomes in kidney transplant
recipients.
Patients and methods
Patients and study cohort
This retrospective study included 112 recipients who
underwent kidney transplantation between June 2022 and June 2023 at
The First Affiliated Hospital of Anhui Medical University (Anhui,
China). Ethical approval for The present study was granted by The
Ethics Committee of The First Affiliated Hospital of Anhui Medical
University (Anhui, China; approval no. PJ2024-05-30). The inclusion
criteria for the patients were as follows: i) The patient had
received kidney transplantation for the first time; ii) the patient
was aged 18-60 years; and iii) the patient was administered a
triple immunosuppressive regimen of tacrolimus (TAC) +
mycophenolate mofetil (MMF) + glucocorticoids. The exclusion
criteria were: i) The patient had already received multiple kidney
transplants or combined multi-organ transplants; ii) the patient
was <18 or >60 years of age; and iii) patients were with
combined infectious diseases, such as viral hepatitis, tuberculosis
or acquired immunodeficiency syndrome. All enrolled kidney
transplant recipients were screened pre-operatively for
low-resolution HLA typing, anti-HLA antibodies and anti-MHC class
I-associated molecule A (MICA) antibodies and CDC-XM. The present
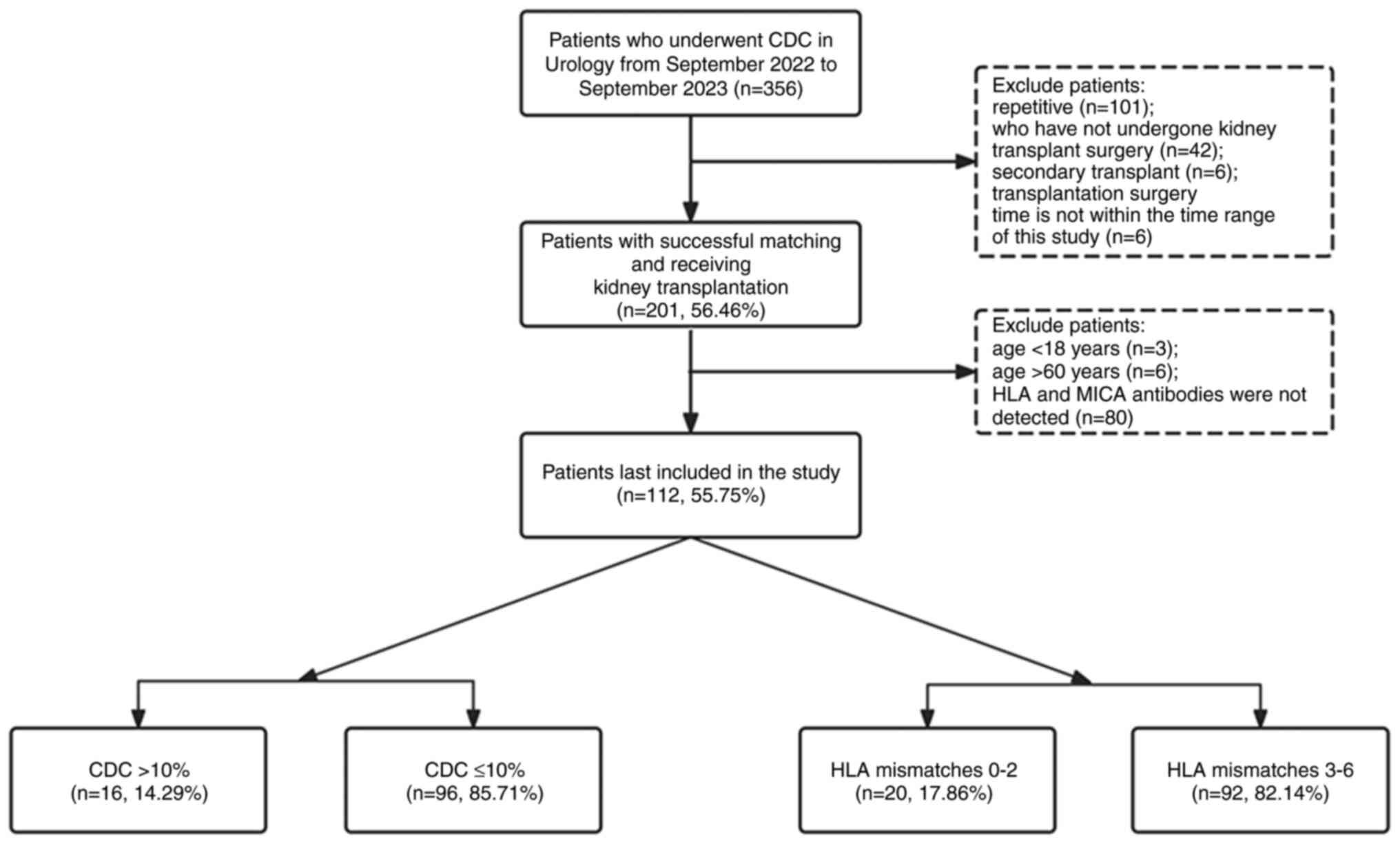
study was divided into two parts, the first being the prognostic
effect on kidney transplant patients based on CDC-XM levels and/or
HLA matching and the second part was to identify risk factors for
DGF after kidney transplantation. The results were statistically
analyzed independently and details of the patients selected from
the database are shown in Fig.
1.
CDC-XM analysis
Lymphocytes isolated from the peripheral blood of
live kidney donors or the spleen of cadaveric kidney donors were
co-incubated with recipient serum on Terasaki plates (One Lambda,
Inc.) using Ficoll-Hypaque density gradient centrifugation (400 x
g, room temperature, 22 min) and positive and negative controls
were set up on Terasaki plates for quality control. Rabbit
complement was added to each well for co-incubation for 60 min at
37˚C, subsequently followed by the addition of 5 µl fluorescence
terminator FluoroQuench™ (One Lambda, Inc.), the plates
were placed under an inverted phase-contrast fluorescence
microscope to count the number of dead lymphocytes from a total of
300 lymphocytes in order to assess cell death in the presence of
complement and then the results were expressed as percentages (%).
The CDC-XM data collected in the present study were based on the
results of the final pre-operative examinations of the kidney
transplant recipients.
HLA genotyping
Recipient and donor HLA typing was performed
according to the method of DNA PCR amplification and typing, using
molecular sequence-specific priming methods for low-resolution
typing for the HLA-A, -B, -C, -DRB1 and -DQB1 genetic loci (cat.
nos. LTPPSD2, LTPPSD3, LTPPSD6, LTPPSD1, LTPPSD19; One Lambda,
Inc). A combination of amplification primers, phosphorylated
deoxynucleotides (dNTPs) and Taq DNA polymerase (total
volume: 20 µl) were mixed with pre-coordinated DNA samples. After
denaturation and neutralization, the material was first homogenized
with a hybridization buffer and microbeads and subsequently labeled
with Streptavidin, R-Phycoerythrin conjugate (SAPE; Thermo Fisher
Scientific, Inc.) The fluorescence intensity of phycoerythrin in
each microsphere was determined by reading the fluorescence signal
with LABScan™ 100 (Luminex®; One Lambda,
Inc.). The generated files were then imported into HLA
fusion® software version 2.0 (One Lambda, Inc.) for
analysis. PCR reactions were performed for 40 cycles, with
denaturation at 93˚C for 30 sec, annealing at 65˚C for 30 sec and
extension at 72˚C for 30 sec using a GeneAmp9700 thermal cycler
(Applied Biosystems, Inc.).
Anti-HLA and anti-MICA screening
Patient serum was collected prior to
transplantation. Anti-HLA and anti-MICA antibodies were
subsequently detected using multiplexed microsphere-based flow
cytometry (Luminex Technology; a LABScreen Mixed kit; One Lambda,
Inc.). The microbeads were covered with the major HLA class I and
II antigens and MICA antigens. Patients' sera were subsequently
incubated for 30 min with beads covered with HLA antigens and MICA
antigens produced by recombinant technology. After three washes,
the beads were incubated for 30 min with 100 µl 1:100
phycoerythrin-labelled goat anti-human IgG (One Lambda, Inc.).
After two further washes, the mean fluorescence intensity (MFI) of
each microbead was measured using LABScan™ 100 flow
cytometry (One Lambda, Inc.) and subsequently analyzed using HLA
Fusion® software version 2.0 (One Lambda, Inc.). Samples
with an MFI ≥500 were considered to be positive.
Induction protocol and maintenance
immunosuppression
All included recipients were administered an oral
triple immunosuppressive regimen comprising TAC, MMF and
glucocorticoids starting on admission. The initial dose of TAC was
0.16 mg/kg/day orally, with a target trough level of 8-12 ng/ml for
the first 3 months and 3-8 ng/ml thereafter. Start 0.5 g of MMF on
the day of surgery and use 1 g per day postoperatively (0.5 g bid)
and adjust as tolerated by the patient. High-dose
methylprednisolone shock therapy was used in the early
postoperative period, and the prednisone dose was reduced to 5-10
mg/day depending on the patient's condition. Anti-thymocyte
globulin was used mainly in sensitized patients (defined as having
CDC >10% or testing positive for panel-reactive antibodies), or
in patients with a high number of HLA mismatches. Sulfamethoxazole
and ganciclovir were used as antimicrobial prophylaxis drugs and
the majority of the patients routinely underwent pre-operative
hemodialysis or peritoneal dialysis to remove toxins from the body.
Prior to transplantation, ABO-incompatible (ABOi) recipients
received immunosuppressive preconditioning for 1-4 weeks, with
different preconditioning regimens selected according to the
recipient's initial anti-A/-B antibody titer. All ABOi renal
transplant recipients were administered rituximab at a dosage of
between 200-500 mg ~2 weeks prior to surgery. Recipients with an
initial anti-A/-B antibody titer ≤1:4 were administered rituximab
only, whereas those with an initial anti-A/-B antibody titer ≥1:8
were additionally treated with plasmapheresis and/or
double-filtration plasmapheresis, as appropriate, to remove
existing anti-A/-B antibodies. ABOi-associated living kidney
transplantation could be performed after the anti-A/-B antibody
titer was reduced to ≤1:8.
Data collection and outcomes
Clinical and laboratory examination data were
collected from medical records and kidney transplant program
databases at pre-transplantation, 1, 2, 3 and 7-day, 1, 3 and
6-month post-transplantation stages. Laboratory examination data
included the following: The concentrations of serum creatinine
(Scr) and urea, estimated glomerular filtration rate (eGFR), the
levels of blood glucose (GLU) and hemoglobin (Hb), percentages of
reticulocytes (RET%), absolute reticulocyte number (RET#),
reticulocyte hemoglobin content (RET-He), low-fluorescence
intensity reticulocytes (LFRs), medium-fluorescence intensity
reticulocytes (MFRs), high-fluorescence intensity reticulocytes
(HFRs), white blood cell count (WBC), the percentage of neutrophils
(N%) and the absolute number of neutrophils (N#) and the percentage
of lymphocytes (L%) and the absolute number of lymphocytes (L#)
were measured. Renal function indicators were measured using a
Roche automated biochemistry analyzer (Roche Corporation). Blood
routine indexes were tested using a SYSMEX™ XE-2100
automatic five-category blood cell analyzer. The occurrence of
post-transplant adverse events was associated with DGF and DGF was
defined either as having received dialysis within 1 week of
transplantation or having an Scr >400 µmol/l within 7 days
post-operatively despite not requiring hemodialysis.
Statistical analysis
Continuous variables are presented as the mean ±
standard deviation or as the median and the interquartile range and
categorical data are expressed as the number (%). Categorical data,
including demographic, clinical and immunological characteristics,
were analyzed using Pearson's χ2 test (or χ2
for trend testing when three categories of variables were present)
or Fisher's exact test, whereas continuous variables were analyzed
using the t-test (or one-way ANOVA for three categories of
variables) or the Mann-Whitney U test (Kruskal-Wallis test for
three categories of variables), as appropriate. Post hoc analyses
were conducted using the Bonferroni method. Odds ratios (ORs) and
P-values for clinically relevant covariates were obtained by
applying binary logistic regression in univariate analyses, taking
into account variables such as HLA mismatches, the age and sex of
the patients, CDC results, blood group ABOi, donor type and
pre-transplant HLA antibodies. Variables with P<0.05 in the
univariate analysis were introduced into the multivariate model and
significant predictors of the occurrence of adverse events were
identified by multivariate logistic regression analysis.
Statistical analyses were performed using the SPSS 25.0 software
package (IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Demographic and clinical
characteristics of the patients
A total of 356 patients underwent kidney
transplantation between June 2022 and June 2023. Patients who did
not meet the age requirement, were not first-time transplant
recipients, or had missing antibody screening data (see the
Patients and methods section) were excluded from the present
study, resulting in the inclusion of a total of 112 patients in the
analysis. Among them, 84 (75%) were male and 28 (25%) were female,
with an average age of 38.17±9.08 years. The baseline and clinical
characteristics of the patients included in the present study are
shown in Table I.
 | Table IDemographic and clinical
characteristics of the study cohort. |
Table I
Demographic and clinical
characteristics of the study cohort.
| Characteristic | CDC>10%
n=16 | CDC≤10% n=96 | P-value | HLA 0-2 MM
n=20 | HLA 3-6 MM
n=92 | P-value |
|---|
| Age | | | | | | |
|
18-34 n
(%) | 7 (43.8) | 39 (40.6) | 1.000 | 9 (45.0) | 37 (40.2) | 0.502 |
|
35-49 n
(%) | 7 (43.8) | 43 (44.8) | | 10 (50.0) | 40 (43.5) | |
|
50-60 n
(%) | 2 (12.5) | 14 (14.6) | | 1 (5.0) | 15 (16.93) | |
|
Mean | 38.17±9.08 | | | 38.17±9.08 | | |
| Sex (%) | | | | | | |
|
Male | 16(100) | 68 (70.8) | 0.029 | 12 (60.0) | 72 (7 8.3) | 0.087 |
|
Female | 0 (0) | 28 (29.2) | | 8 (40.0) | 20 (21.7) | |
| Blood type
compatibility, n (%) | | | | | | |
|
ABO-compatible | 14 (87.5) | 92 (95.8) | 0.441 | 18 (90.0) | 88 (95.7) | 0.291 |
|
ABO-incompatible | 2 (12.5) | 4 (4.2) | | 2 (10.0) | 4 (4.3) | |
| Donor category, n
(%) | | | | | | |
|
LD | 6 (37.5) | 53 (55.2) | 0.189 | 20(100) | 39 (42.4) | 0.000 |
|
DCD | 10 (62.5) | 43 (44.8) | | 0 (0) | 53 (57.6) | |
| HLA-A mismatches
(%) | | | | | | |
|
0 | 0 (0) | 12 (12.5) | 0.219 | 10(50) | 2 (2.2) | 0.000 |
|
1 | 10 (62.5) | 62 (64.6) | | 10(50) | 62 (67.4) | |
|
2 | 6 (37.5) | 22 (22.9) | | 0 (0) | 28 (30.4) | |
| HLA-B mismatches, n
(%) | | | | | | |
|
0 | 1 (6.3) | 6 (6.3) | 0.626 | 6(30) | 1 (1.1) | 0.000 |
|
1 | 6 (37.5) | 47 (49.0) | | 14(70) | 39 (42.4) | |
|
2 | 9 (56.3) | 43 (44.8) | | 0 (0) | 52 (56.5) | |
| HLA-C mismatches, n
(%) | | | | | | |
|
0 | 1 (6.3) | 11 (11.5) | 0.718 | 7(35) | 5 (5.4) | 0.000 |
|
1 | 9 (56.3) | 58 (60.4) | | 13(65) | 54 (58.7) | |
|
2 | 6 (37.5) | 27 (28.1) | | 0 (0) | 33 (35.9) | |
| HLA-DR mismatches,
n (%) | | | | | | |
|
0 | 0 (0) | 11 (11.5) | 0.018 | 11(55) | 0 (0) | 0.000 |
|
1 | 6 (37.5) | 59 (61.5) | | 9(45) | 56 (60.9) | |
|
2 | 10 (62.5) | 26 (27.1) | | 0 (0) | 36 (39.1) | |
| HLA-DQ mismatches,
n (%) | | | | | | |
|
0 | 0 (0) | 10 (10.4) | 0.017 | 7(35) | 3 (3.3) | 0.000 |
|
1 | 8(50) | 69 (71.9) | | 13(65) | 64 (69.6) | |
|
2 | 8(50) | 17 (17.7) | | 0 (0) | 25 (27.2) | |
| CDC, n (%) | | | - | | | |
| CDC>10% | - | - | | 0 (0) | 16 (17.4) | 0.071 |
| CDC≤10% | - | - | | 20(100) | 76 (82.6) | |
| Pre-transplant
serum antibodies, n (%) | | | | | | |
|
HLA+MICA+ | 1 (6.3) | 5 (5.2) | 0.219 | 1 (5.0) | 5 (5.4) | 0.321 |
|
HLA-MICA+ | 0 (0) | 7 (7.3) | | 3 (15.0) | 4 (4.3) | |
|
HLA+MICA- | 0 (0) | 15 (15.6) | | 2 (10.0) | 13 (14.1) | |
|
MICA-HLA- | 15 (93.8) | 69 (71.9) | | 14 (70.0) | 70 (76.1) | |
| Pre-transplant HLA
antibodies, n (%) | | | | | | |
|
HLA class
I+HLA class II+ | 0 (0) | 3 (3.1) | 0.789 | 0 (0) | 3 (3.3) | 1.000 |
|
HLA class
I-HLA class II+ | 0 (0) | 3 (3.1) | | 0 (0) | 3 (3.3) | |
|
HLA class
I+HLA class II- | 1 (6.3) | 14 (14.6) | | 3 (15.0) | 12 (13.0) | |
|
HLA class
I-HLA class II- | 15 (93.8) | 76 (79.2) | | 17 (85.0) | 74 (80.4) | |
| Post-transplant
serum antibodies, n (%) | | | | | | |
|
HLA+MICA+ | 1 (6.3) | 5 (5.2) | 1.000 | 2 (10.0) | 4 (4.3) | 0.567 |
|
HLA-MICA+ | 0 (0) | 4 (4.2) | | 1 (5.0) | 3 (3.3) | |
|
HLA+MICA- | 4 (25.0) | 26 (27.1) | | 5 (25.0) | 25 (27.2) | |
|
MICA-HLA- | 11 (68.8) | 61 (63.5) | | 12 (60.0) | 60 (65.2) | |
| Post-transplant HLA
antibodies (%) | | | | | | |
|
HLA class
I+HLA class II+ | 1 (6.3) | 6 (6.3) | 1.000 | 0 (0) | 7 (7.6) | 0.498 |
|
HLA class
I-HLA class II+ | 1 (6.3) | 5 (5.2) | | 1 (5.0) | 5 (5.4) | |
|
HLA class
I+HLA class II- | 3 (18.8) | 20 (20.8) | | 6 (30.0) | 17 (18.5) | |
|
HLA class
I-HLA class II- | 11 (68.8) | 65 (67.7) | | 13 (65.0) | 63 (68.5) | |
| Pre-transplant
antibody MFI median (IQR) | | | | | | |
|
Anti-HLA
class I | 0 (0-0) | 0 (0-0) | 0.234 | 0 (0-0) | 0 (0-0) | 0.957 |
|
Anti-HLA
class II | 0 (0-0) | 0 (0-0) | 0.306 | 0 (0-0) | 0 (0-0) | 0.243 |
|
Anti-MICA | 0 (0-0) | 0 (0-0) | 0.530 | 0 (0-0) | 0 (0-0) | 0.199 |
| Post-transplant
antibody MFI median (IQR) | | | | | | |
|
Anti-HLA
class I | 0 (0-92.48) | 0 (0-223.60) | 0.281 | 0 (0-0) | 0 (0-0) | 0.073 |
|
Anti-HLA
class II | 0 (0-0) | 0 (0-0) | 0.715 | 0 (0-0) | 0 (0-0) | 0.076 |
|
Anti-MICA | 0 (0-0) | 0 (0-0) | 0.141 | 0 (0-0) | 0 (0-0) | 0.147 |
| Post-transplant de
novo antibody, n (%) | | | | | | |
|
De
novo HLA antibody | 5 (31.3) | 21 (21.9)75 | 0.522 | 4 (20.0) | 22 (23.9) | 0.924 |
|
De
novo MICA antibody | 0 (0) | 4 (4.2) | 1.000 | 0 (0) | 4 (4.3) | 1.000 |
| Adverse event
(within six months), n (%) | | | | | | |
| DGF | 2 (12.5) | 17 (17.7) | 0.877 | 0 (0) | 19 (20.7) | 0.022 |
When patients were stratified according to CDC-XM
levels, the CDC >10% group consisted of all male patients,
mainly with HLA-DR and -DQ MM and the majority of them had two
alleles mismatched. Only one patient was found to have anti-HLA and
anti-MICA antibodies prior to transplantation, of which the HLA
antibody was HLA class I-positive and class II-negative and the
remaining 15 patients were not found to have anti-HLA or anti-MICA
antibodies. Furthermore, none of the patients in this group were
found to have class II anti-HLA antibodies prior to
transplantation. Alternatively, when patients were stratified
according to HLA matching, the donors in the HLA 0-2 MM group were
all from living donors (LDs) and the patients in this group had a
CDC ≤10%, whereas all patients with CDC >10% were in the HLA 3-6
MM group. Moreover, all patients in the HLA 0-2 MM group were found
not to have class II anti-HLA antibodies prior to transplantation,
whereas six patients in the HLA 3-6 MM group were found to have
class II anti-HLA antibodies prior to transplantation, of which two
patients had antibodies against HLA-DQ and the other four patients
had antibodies against HLA-DR. No DGF was identified in the HLA 0-2
MM group, which was significantly different compared with that in
the HLA 3-6 MM group (P=0.022). In the CDC ≤10% and HLA 3-6 MM
groups, almost half of the donor kidneys came from LDs (55.2 and
42.4%, respectively) whereas half of them came from donation after
cardiac death (DCD) (44.8 and 57.6%, respectively).
Of all patients, the vast majority were
ABO-compatible (ABOc), with only six (5.3%) patients being ABOi. A
total of twenty-eight (25%) of the patients had anti-HLA and/or
anti-MICA antibodies prior to transplantation, of which 15 (13.4%)
patients were only anti-HLA antibody-positive, seven (6.3%)
patients were only anti-MICA antibody-positive and six (5.3%)
patients were positive for both antibodies. Moreover, 15 (13.4%)
patients were only anti-HLA class I antibody-positive, three (2.7%)
patients were only class II anti-HLA antibody-positive and three
(2.7%) patients were positive for both antibodies. Finally, a total
of 40 (35.7%) patients had anti-HLA and/or anti-MICA antibodies
following transplantation, which represented an increase compared
with the numbers of patients prior to transplantation.
Association between CDC-XM and
laboratory results following kidney transplantation
Subsequently, the renal function of the two groups
of patients with different CDC-XM levels were analyzed, which
revealed that the levels of Scr decreased with post-operative time,
leveling off after 7 days (Fig.
2A). The urea levels were found to decrease with time following
surgery, leveling off after 1 month and a significant difference in
the urea levels was observed between the two groups at 3 days
post-operatively (P<0.05; Fig.
2B). By contrast, eGFR levels increased with post-operative
time, leveling off after 7 days (Fig.
2C). The Scr levels of the CDC >10% group were found to be
significantly higher compared with those of the CDC ≤10% group at 1
day (P=0.008), 2 days (P=0.006), 3 days (P=0.003) and 7 days
(P=0.038) post-surgery (Table
SIA). As for the eGFR levels, significantly higher eGFR levels
were observed in the CDC ≤10% group at 1 day (P=0.038), 2 days
(P=0.018) and 3 days (P=0.010) post-operatively (Fig. 2C and Table SIC).
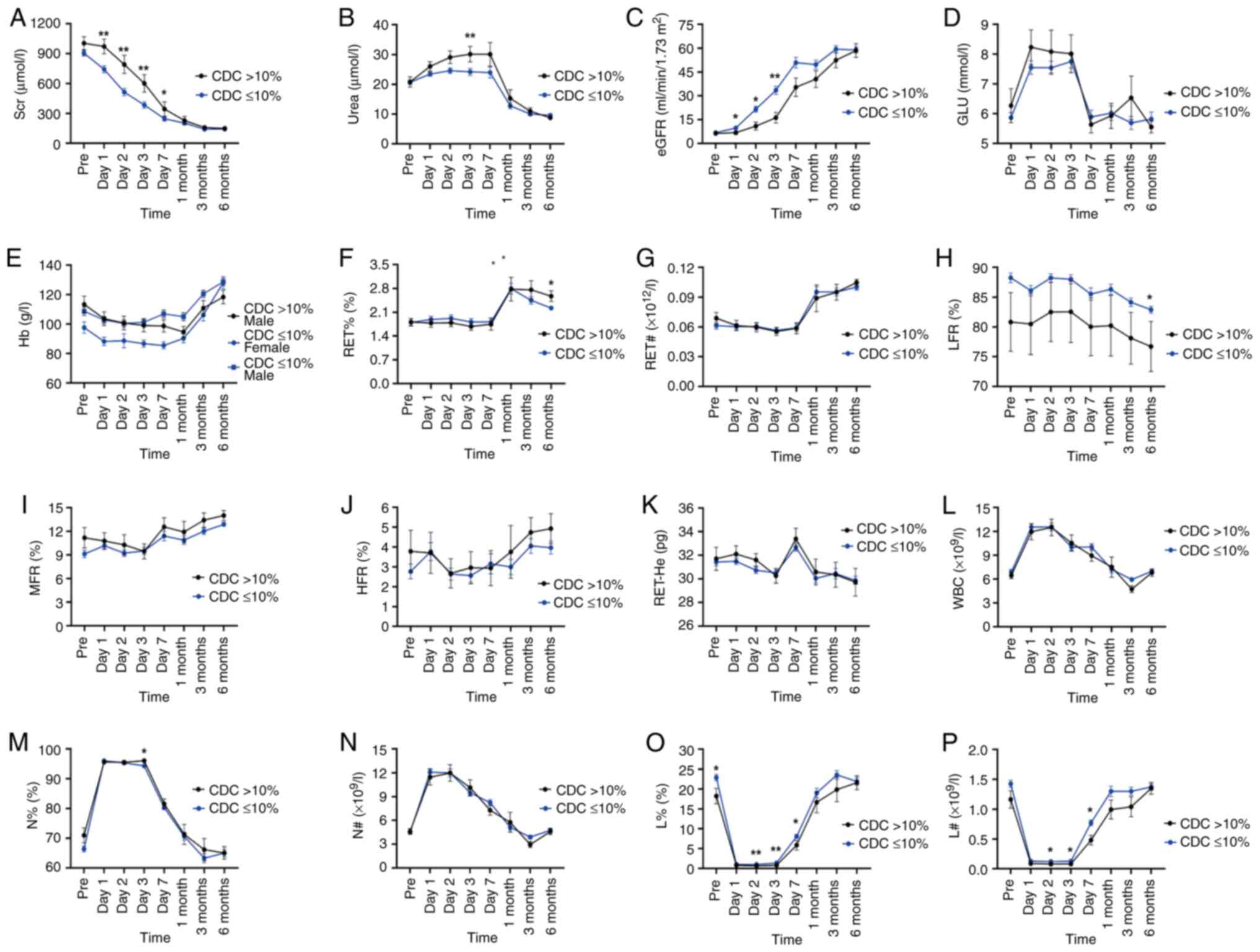 | Figure 2Laboratory indicator level of renal
transplant recipients according to the CDC-XM levels, which
represented in a line graph. (A) Dynamics of Scr at each follow-up
time point. (B) Dynamics of urea at each follow-up time point. (C)
Dynamics of eGFR at each follow-up time point. (D) Dynamics of GLU
at each follow-up time point. (E) Dynamics of Hb at each follow-up
time point. (F) Dynamics of RET% at each follow-up time point. (G)
Dynamics of RET# at each follow-up time point. (H) Dynamics of LFR
at each follow-up time point. (I) Dynamics of MFR at each follow-up
time point. (J) Dynamics of HFR at each follow-up time point. (K)
Dynamics of RET-He at each follow-up time point. (L) Dynamics of
WBC at each follow-up time point. (M) Dynamics of N% at each
follow-up time point. (N) Dynamics of N# at each follow-up time
point. (O) Dynamics of L% at each follow-up time point. (P)
Dynamics of L# at each follow-up time point. *P≤0.05 and
**P≤0.01. RTR Scr, serum creatinine; eGFR, estimated
glomerular filtration rate; GLU, blood glucose; Hb, hemoglobin;
RET%, percentages of reticulocytes; RET#, absolute reticulocyte
number; RET-He, reticulocyte hemoglobin content; LFR,
low-fluorescence intensity reticulocytes; MFR, medium-fluorescence
intensity reticulocytes; HFR, high-fluorescence intensity
reticulocytes; WBC, white blood cell count; N%, the percentage of
neutrophils; N#, the absolute number of neutrophils; L%, the
percentage of lymphocytes; L#, the absolute number of lymphocytes;
SEM, standard error of mean. |
The blood GLU levels in the two groups with
different CDC-XM levels were maintained at high levels during the
first 3 post-operative days, which was probably due to the
influence of post-operative infusion. However, the blood GLU levels
did not differ markedly between the two groups at all time points
observed (Fig. 2D).
Subsequently, various indicators of erythrocytes in
the two groups of patients with different CDC-XM levels were
analyzed and the results showed that there was a statistically
significant difference in the RET% and LFR values comparing between
the two groups at 6 months (P<0.05; Fig. 2F and H), whereas no statistically significant
differences in the Hb level or the RET#, MFR and HFR values, or the
RET-He values were observed at all observed time points (P>0.05;
Fig. 2 and Table SII).
Among the leukocyte indices, significant differences
in the N%, L% and L# values were noted between the two groups at
different CDC-XM levels (P<0.05; Fig. 2M, O and P),
although no significant differences in the WBC and N# values were
observed between the two groups (P>0.05; Fig. 2L and N). The N% values of the two groups were
found to be significantly different at 3 days post-operatively
(P=0.027; Table SIL). Similarly,
significant differences in the L% values between the two groups
were noted at pre-transplant (P=0.031), 2 days (P=0.007), 3 days
(P=0.002) and 7 days (P=0.040) post-operative kidney
transplantation (Table SIN).
Moreover, a significant difference in L# values was observed
between the two groups at 2 days (P=0.020), 3 days (P=0.013) and 7
days (P=0.015) post-operatively (Table SIO). Furthermore, the values of L%
and L# decreased sharply at 1 day post-operatively and remained at
extremely low levels for the first 3 days following
transplantation, before beginning to gradually increase, eventually
returning to pre-operative levels and leveling off at 1 month
(Fig. 2O and P).
Association between HLA compatibility
and laboratory results following kidney transplantation
Subsequently, patients were grouped according to the
degree of HLA matching and the curve changes of the renal function
indicators Scr, urea and eGFR were found to be similar to those in
the CDC groups. Kidney transplant recipients in the HLA 0-2 MM
group had significantly lower Scr levels compared with those in the
HLA 3-6 MM group at 1 day (P<0.001), 2 days (P<0.001), 3 days
(P<0.001), 7 days (P<0.001) and 1 month (P=0.041)
post-operatively. Although a slight trend was observed at the
pre-transplant stage, this did not reach the level of significance
(P=0.096; Table SIIIA). Regarding
the urea levels, significantly higher values were observed in the
HLA 3-6 MM group at 2 days (P<0.001), 3 days (P<0.001) and 7
days (P=0.001) (Fig. 3B and
Table SIIIB) compared with the
HLA 0-2 MM group. At 1 day (P=0.001), 2 days (P<0.001), 3 days
(P<0.001) and 7 days (P<0.001) following renal
transplantation, the eGFR levels were significantly higher in the
HLA 0-2 MM group (Fig. 3C;
Table SIIIC).
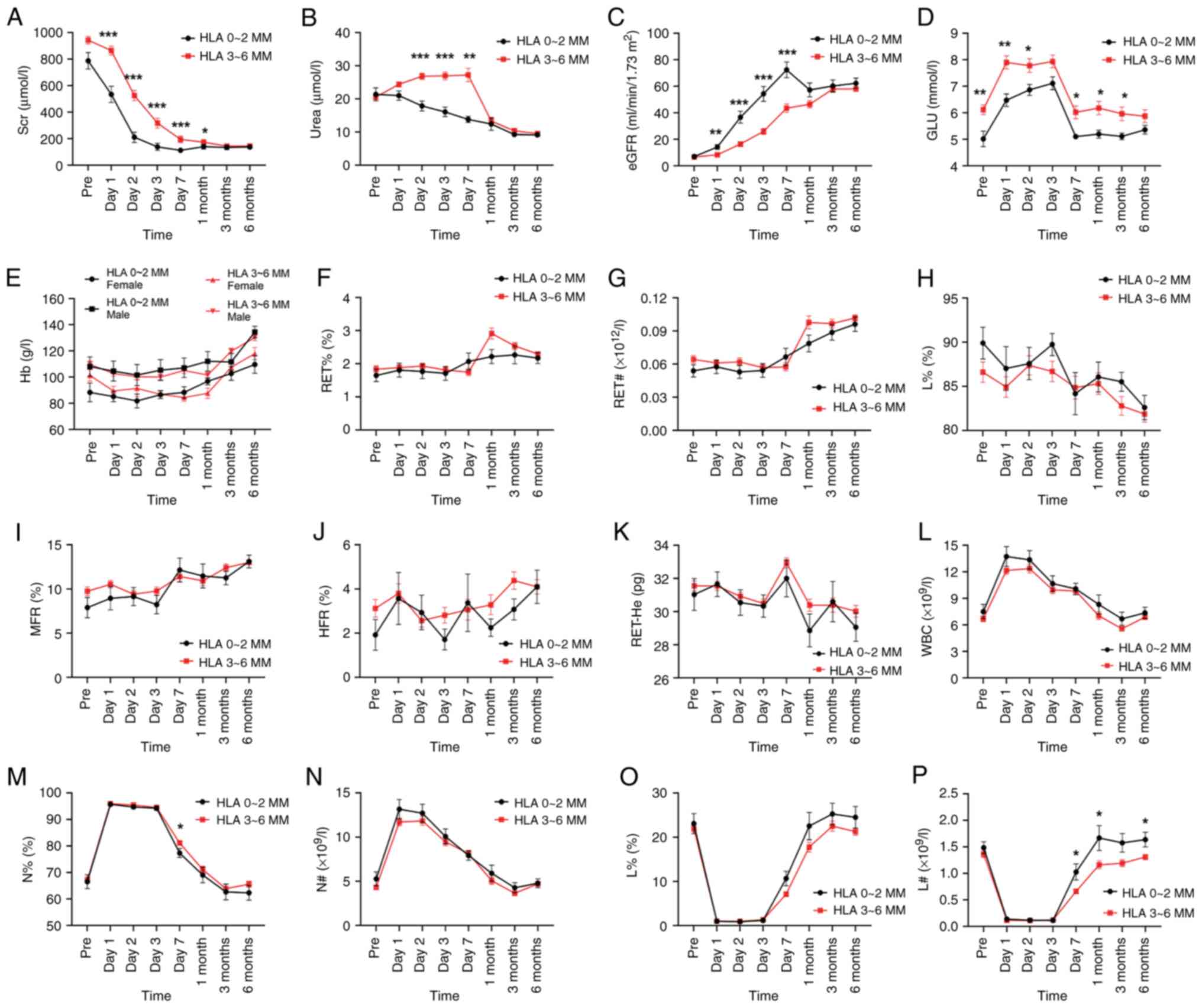 | Figure 3Laboratory indicator level of renal
transplant recipients according to the HLA matching, which
represented in a line graph. (A) Dynamics of Scr at each follow-up
time point. (B) Dynamics of urea at each follow-up time point. (C)
Dynamics of eGFR at each follow-up time point. (D) Dynamics of GLU
at each follow-up time point. (E) Dynamics of Hb at each follow-up
time point. (F) Dynamics of RET% at each follow-up time point. (G)
Dynamics of RET# at each follow-up time point. (H) Dynamics of LFR
at each follow-up time point. (I) Dynamics of MFR at each follow-up
time point. (J) Dynamics of HFR at each follow-up time point. (K)
Dynamics of RET-He at each follow-up time point. (L) Dynamics of
WBC at each follow-up time point. (M) Dynamics of N% at each
follow-up time point. (N) Dynamics of N# at each follow-up time
point. (O) Dynamics of L% at each follow-up time point. (P)
Dynamics of L# at each follow-up time point. Values are represented
as the mean ± SEM. Values of P≤0.05 were considered statistically
significant. *P≤0.05; **P≤0.01;
***P≤0.001. Scr, serum creatinine; eGFR, estimated
glomerular filtration rate; GLU, blood glucose; Hb, hemoglobin;
RET%, percentages of reticulocytes; RET#, absolute reticulocyte
number; RET-He, reticulocyte hemoglobin content; LFR,
low-fluorescence intensity reticulocytes; MFR, medium-fluorescence
intensity reticulocytes; HFR, high-fluorescence intensity
reticulocytes; WBC, white blood cell count; N%, the percentage of
neutrophils; N#, the absolute number of neutrophils; L%, the
percentage of lymphocytes; L#, the absolute number of
lymphocytes. |
Blood GLU level in the HLA 0-2 MM group was
significantly lower compared with that in the HLA 3-6 MM group at
the pre-transplant (P=0.002), 1-day (P=0.002), 2-day (P=0.021),
7-day (P=0.019), 1-month (P=0.038) and 3-month (P=0.030) stages. In
addition, the HLA 0-2 MM group was found to have lower blood GLU
levels compared with the HLA 3-6 MM group during the observation
period (Fig. 3D and Table SIIID).
Regarding the various indicators of erythrocytes
that were monitored in the present study, including Hb levels,
RET%, RET#, LFR, MFR and HFR values and the RET-He, no significant
differences were identified between the two groups at all
pre-operative and post-operative time points (P>0.05; Tables SIV and SIIIE-J).
Subsequently, the various leukocyte indices between
the HLA 0-2 MM and HLA 3-6 MM groups were compared, which revealed
that the differences in WBC, N# and L% values were not
statistically significant (P>0.05; Fig. 3L, N and O),
although the differences in the N% and L# values between the two
groups were statistically significant (P<0.05; Fig. 3M and P). The N% values were significantly lower
in the HLA 0-2 MM group compared with the HLA 3-6 MM group at 7
days (P=0.017; Table SIIIL)
post-operatively. Regarding the L# levels, significantly higher L#
values were observed in the HLA 0-2 MM group at 7 days (P=0.030), 1
month (P=0.041) and 6 months (P=0.012) post-operatively (Fig. 3P and Table SIIIO). Finally, the curves of the
L% and L# values were found to be similar to those of the CDC
groups (Fig. 3O and P).
Effects of CDC-XM and HLA
compatibility on renal function
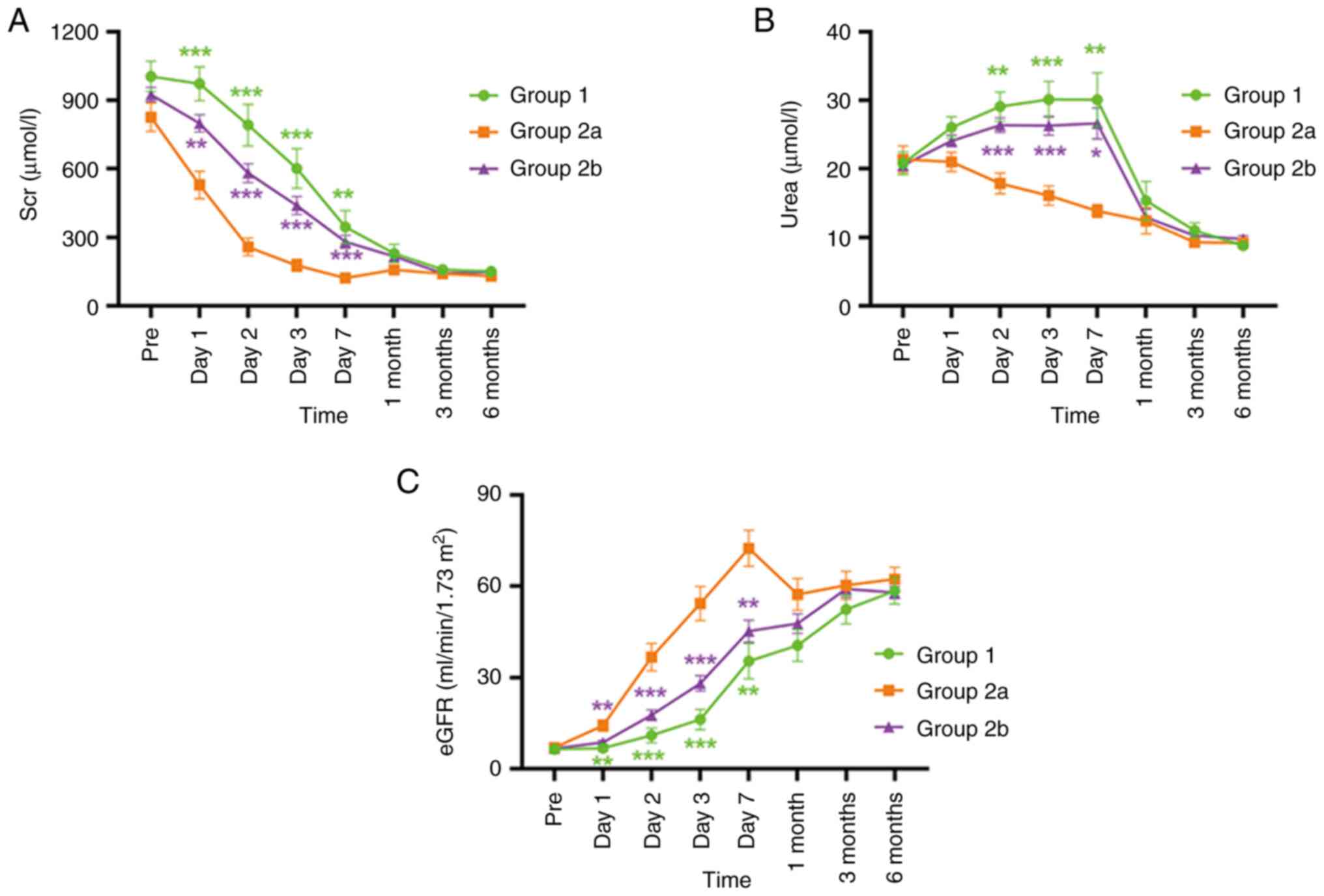
When the CDC levels and HLA matching were assessed
together, all patients were divided into three groups: Group 1 (CDC
>10% and HLA 3-6 MM), group 2a (CDC ≤10% and HLA 0-2 MM) and
group 2b (CDC ≤10% and HLA 3-6 MM). This analysis revealed that
patients in group 2a (that is, who met both CDC ≤10% and HLA 0-2
MM) had improved results compared with both groups 1 and 2b in
terms of their Scr, urea and eGFR data at all follow-up time points
following surgery and also markedly faster and improved recovery
levels of renal function within one week following surgery
(Fig. 4 and Table SV). Urinary protein (qualitative)
data in routine urinalysis, which showed significant differences
only in the HLA subgroups at the second postoperative month
(Fig. S1; Tables SIX and SX.).
Effects of HLA-A, -B and -DR
mismatches on renal function
Subsequently, the impact of mismatches at each locus
of HLA-A, HLA-B and HLA-DR on post-operative renal function was
evaluated and this analysis revealed that patients who received
0-allele MM or 1-allele MM kidney transplants had an improved renal
function compared with those who received a 2-allele MM transplant;
moreover, the matching effect was very significant at the A locus
comparing among the three loci and these patients experienced a
greater impact on kidney function compared with the B and DR loci
(Tables SVI, SVII and SVIII). In addition, the Scr, urea and
eGFR values were found to be markedly improved in patients who
received 0-allele MM or 1-allele MM kidney transplants compared
with those who received a 2-allele MM kidney transplant at each
follow-up time point one week after transplantation (Table SVI). However, in the patients
with MM at the B and DR loci, the differences in renal function
were mainly observed comparing between the 1-allele and 2-allele MM
groups. Although renal function was optimal in patients with
0-allele MM in the three locus MM groups, no significant
differences were observed at each follow-up time point following
transplantation comparing between 0-allele MM and 1-allele MM
transplanted patients and this was probably due to the small number
of patients studied in the 0-allele MM group. There was no
significant difference in renal function between ABO-compatible and
-incompatible recipients (Fig. S2
and Table SXI). However, in the
early postoperative period, recipients receiving LD kidney
transplantation had better renal function than recipients receiving
DCD (Fig. S3 and Table SXII) .
Incidence of adverse events and
multivariate logistic regression analysis
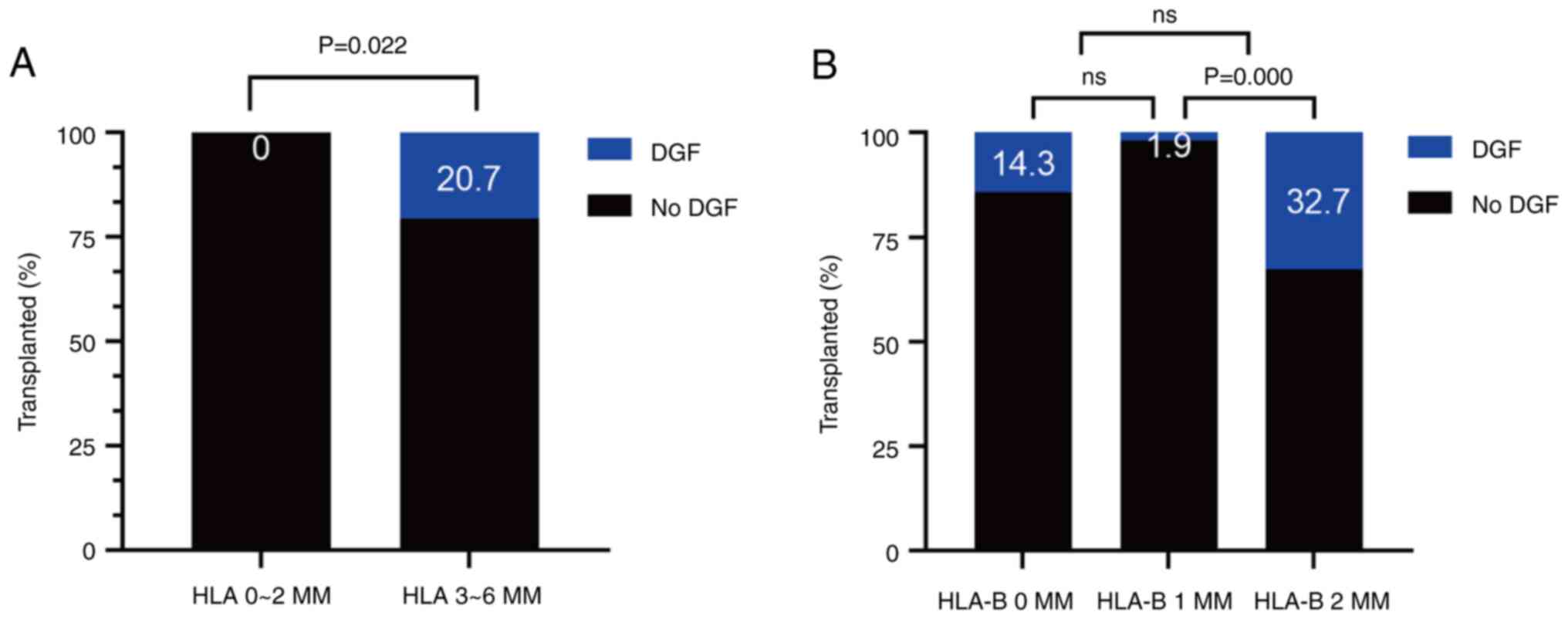
The incidence of DGF (20.7%) in the HLA 3-6 MM group
was found to be higher compared with that in the HLA 0-2 MM group
and this difference was statistically significant (P=0.022;
Fig. 5A). However, this difference
was not significant in the CDC-XM group (Fig. S4E). Subsequently, the proportion
of patients with adverse events was analyzed in the MM patient
groups with different HLA loci. The results obtained demonstrated
that the highest proportion of patients with DGF was observed in
the 2-B MM group (32.7%) and this difference was found to be
statistically significant (Fig.
5B). No clear HLA matching effects of the HLA-C, -DR and -DQ
loci were identified in the occurrence of adverse events; however,
all of them were associated with an increase in the number of
allelic mismatches and a corresponding increase in the incidence of
adverse event rates, accordingly. Notably, no patients in the HLA
0-2 MM or the HLA-DR 0 MM groups developed DGF (Figs. 5A and S1C). In addition, the incidence of DFG
was not significant in ABO-compatible compared with incompatible
renal transplant recipients, and none of the six ABOi patients
developed DGF (Fig. S5).
Of the 112 adult kidney transplant cases, 19 (17.0%)
developed DGF (Table II).
Patients who develop DGF were more likely to be male (47.4 vs.
20.4%; P=0.029) and older (45.3 vs. 36.7 years; P<0.001).
Furthermore, they tended to have a higher BMI (24.06 vs. 22.4%;
P=0.042), a poorer HLA match condition (3-6 HLA-A+B+DR mismatches:
100 vs. 78.5%; P=0.022) and diabetes mellitus as the underlying
cause of ESRD (21.1 vs. 3.2%; P=0.016). Moreover, the ABOi (21.1
vs. 2.2; P=0.006) and DCD (94.7 vs. 38.7; P=0.000) values were also
higher in patients with DGF compared with those without DGF. In the
multivariable logistic regression analysis, recipients whose age
was ≥40 years and who had a BMI ≥25, had the strongest predictors
of DGF (ORs=1.097 and 1.555 and P=0.030 and 0.005,
respectively; Table III).
Finally, a reduced risk of DGF was identified in LD transplantation
cases (OR=0.026; P=0.006), or in cases with a good HLA match
(OR=0.378; P=0.029).
 | Table IIDemographics of study patients. |
Table II
Demographics of study patients.
| Characteristic | No DGF n=93 | DGF n=19 | P-value |
|---|
| Mean age,
years | 36.7±8.4 | 45.3±8.8 | 0.000 |
| Sex, n (%) | | | 0.029 |
|
Male | 74 (79.6) | 10 (52.6) | |
|
Female | 19 (20.4) | 9 (47.4) | |
| Mean BMI
(kg/m2) | 22.4±3.2 | 24.06±2.6 | 0.042 |
| Diabetes, n
(%) | 3 (3.2) | 4 (21.1) | 0.016 |
| CDC, n (%) | | | 0.704 |
|
CDC>10% | 14 (17.2) | 2 (10.5) | |
|
CDC≤10% | 79 (82.8) | 17 (89.5) | |
| Donor category, n
(%) | | | 0.000 |
|
LD | 57 (61.3) | 1 (5.3) | |
|
DCD | 36 (38.7) | 18 (94.7) | |
| Pre-transplant HLA
antibodies, n (%) | | | 0.720 |
|
HLA class
I+HLA class II+ | 2 (2.2) | 0 (0) | |
|
HLA class
I-HLA class II+ | 2 (2.2) | 1 (5.3) | |
|
HLA class
I+HLA class II- | 12 (12.9) | 3 (15.8) | |
|
HLA class
I-HLA class II- | 77 (82.7) | 15 (78.9) | |
| Mean HLA-A+B+DR
mismatches | | | 0.022 |
|
0-2 | 20 (21.5) | 0 (0) | |
|
3-6 | 73 (78.5) | 19(100) | |
| Types of dialysis,
n (%) | | | 0.516 |
|
HD | 58 (62.3) | 15 (78.9) | |
|
PD | 23 (24.7) | 4 (21.1) | |
| Mean time on
dialysis, years | 2.9±4.1 | 3.5±4.0 | 0.074 |
| ABO
incompatibility, n (%) | 2 (2.2) | 4 (21.1) | 0.006 |
 | Table IIIMultivariate regression analysis of
DGF. |
Table III
Multivariate regression analysis of
DGF.
| Variable | OR | 95%CI | P-value |
|---|
| Recipient age
≥40 | 1.097 | 1.009-1.194 | 0.030 |
| Recipient BMI
≥25 | 1.555 | 1.139-2.124 | 0.005 |
| Recipient
diabetes | 0.147 | 0.010-2.117 | 0.159 |
| Recipient sex | 0.529 | 0.134-2.082 | 0.362 |
| Donor category | 0.026 | 0.002-0.346 | 0.006 |
| Time on dialysis
≥5 | 0.883 | 0.762-1.023 | 0.097 |
| ABO
incompatibility | 2.328 | 0.201-26.945 | 0.499 |
| HLA-A+B+DR
mismatches ≥3 | 0.378 | 0.158-0.903 | 0.029 |
Discussion
It is well known that CDC-XM and HLA typing are two
important methods to assess donor-recipient compatibility and for
predicting the occurrence of transplant rejection. In the present
study, the differences in laboratory results and clinical outcomes
of kidney transplant recipients were evaluated from the
pre-operative stage to 6 months post-operatively, comparing between
groups based on pre-transplantation CDC-XM levels and HLA-A+B+DR
matching stratification, respectively. CDC-XM was the first method
that was developed to assess donor-recipient compatibility and its
implementation allowed transplants to be performed safely by
mitigating the risk of hyper-ARs. However, the method has the
disadvantages of lacking specificity and sensitivity. As a result,
several modifications have been made to the standard CDC-XM assay
over the years to improve its sensitivity, including the
introduction of a washing step, extended incubation times and the
addition of anti-human globulin reagents (6). In addition, treatment of the serum
with dithiothreitol was introduced to distinguish genuine positive
XM reactions from those triggered by clinically irrelevant IgM
antibodies (7). More sensitive and
specific HLA antibody detection techniques have been developed over
the most recent three decades, including flow cytometry crossmatch
(FCXM) methods and Luminex®-based single antigen beads
(SAB) assay. Given the expansive development of these
histocompatibility-testing methods that has occurred compared with
CDC-XM, which showed poor sensitivity and high false-positive
reactivity, FCXM and SAB have become more favored as tests for the
pre-transplantation screening of organs. However, in numerous
countries in Asia, CDC-XM is still retained due to its
cost-effectiveness. By contrast, a vast majority of transplant
centers in North America and also in several European countries
such as the United Kingdom, have replaced CDC-XM with more
sensitive FCXM techniques. To improve the accuracy of immunization
risk assessment, laboratories have also seen the introduction of
FCXM and SAB techniques. In clinical practice, patients with a CDC
result of 11-20% may be eligible for kidney transplantation
following a comprehensive evaluation. It should be noted that the
success of kidney transplantation is also affected by numerous
other factors, including other immune compatibility indicators of
the donor and recipient, immunosuppressive therapy following
transplantation, among other factors. Since the majority of studies
to date have excluded patients with CDC >10% and relatively few
studies have been published on the prognosis of kidney transplant
patients with CDC >10%, these patients were included in the
analysis in the present study and a comparative analysis was also
performed with patients with CDC ≤10%.
As the number of HLA-A+B+DR mismatches increases, so
does the risk of rejection after kidney transplantation and the
likelihood of graft loss. In a comparative analysis of over 130,000
kidney transplant survival cases, a statistically strong and
significant HLA matching effect was shown when HLA-A+B+DR
mismatching was used as a reference (8). Therefore, HLA-A+B+DR locus matching
is considered to be the central cornerstone of renal allocation.
When HLA-A+B+DR mismatching involves 0, 1 or 2 alleles, a high
degree of donor-recipient matching may still be achieved, although
the number of HLA antigens, the number of antigen combinations and
their unequal distribution across racial and ethnic populations
makes it very difficult to find well-matched donors, especially in
areas with a diverse population (9). Of the 112 patients in the present
study, only 20 (17.9%) patients were well-matched; that is, less
than one-fifth of the total study population. In the past, the
effect of HLA-DQ matching has been underemphasized and DQ antigens
have not yet been part of kidney allocation algorithms due to the
high association of DR antigens with certain DQ antigens, a
phenomenon known as ‘linkage disequilibrium’. However, there is
growing evidence that HLA-DQ mismatching can act as a predictor of
AR and that it is independent of multiple relevant clinical
covariates (10,11). In the present study, the incidence
of DGF in the 2-DQ MM group (32%) was only 0.7% lower compared with
that in the group with the highest incidence of DGF (namely, the
2-B MM group; 32.7%) and the incidence was higher compared with
that in the 2-A MM (17%) and 2-DR MM (25%) groups. Therefore,
additional studies are required to further evaluate whether it is
necessary to additionally quantify the effect of HLA-DQ matching
compared with the HLA-A, -B and -DR loci. The value of HLA matching
in kidney transplantation and its role in prolonging graft
survival, have been widely recognized. As a result, most
organ-sharing organizations give preference to well-matched kidney
recipients for transplantation. At present, HLA-typing techniques
have evolved from simple serological methods to advanced molecular
techniques that increase the importance of HLA matching by
extending it to more precise matching at the allele (or even the
epitope) level, which would minimize the risk of sensitization and
thereby improve the success rate of transplantation.
The recently introduced solid-phase assay,
Luminex®, provides a reliable and sensitive method for
identifying panel-reactive antibodies and distinguishing between
HLA class I and II antibodies. A study by Süsal et al
(12) found that kidney transplant
recipients who were negative for both HLA class I and II antibodies
exhibited a higher 2-year graft survival rate compared with those
who were positive for both HLA class I and II antibodies and this
difference was found to be statistically significant (P<0.001).
Similarly, Michielsen et al (13) reported a similar observation by
analyzing the clinical correlation between pre-transplant DSA and
non-donor-specific anti-HLA antibodies (nDSA). In the present
study, no patients in the CDC >10% group were identified as
being positive for both HLA-class I and II antibodies prior to
transplantation. Also note that the sensitization degree of renal
recipients with CDC >10% was higher compared with that of renal
recipients with CDC ≤10%. Therefore, in the comprehensive
evaluation of kidney transplantation for those patients with CDC
>10%, attempts should be made to try to avoid selecting patients
with positive pre-transplant HLA class I and II antibodies. Solgi
et al (14) observed that
pre-transplant sensitivity to HLA class II antigens was strongly
associated with a higher incidence of AR during the first
post-operative year (P=0.004). In the present study, the proportion
of patients with post-operative adverse events decreased with
increasing CDC levels, although the difference was not found to be
significant. All patients in the CDC >10% group were negative
for pre-transplant HLA class II antibodies, suggesting that
negative pre-transplant HLA class II antibodies may be associated
with improved clinical outcomes.
To address the insufficient numbers of kidney donors
to meet clinical needs, efforts are being made worldwide to expand
the donor pool, including performing kidney transplantations under
CDC-positive and ABOi conditions. CDC-positivity is highly
associated with hyper-AR and immediate graft loss and two major
clinical desensitization methods, high-dose intravenous
immunoglobulin (IVIG) and plasma exchange combined with low-dose
IVIG, have been used to decrease the circulating anti-HLA antibody
load in patients, thereby increasing the chance of transplantation
for sensitized patients. ABOi has also long been considered a
contraindication to successful transplantation. Increasingly
critical organ supply shortages has forced the development of
strategies to overcome the ABO antibody barrier. Desensitization is
usually achieved using therapeutic apheresis and B-cell depletion
therapies, which are accompanied by strong immunosuppression.
Despite the use of stronger immunosuppressive therapies for ABOi
kidney transplantation, no increases in the incidence of rejection,
infectious complications or malignancy have been reported (15). In the present study, there were a
total of six (5.3%) patients with ABOi, who received pre-operative
desensitization until the anti-A/B antibody titer was below the
target titer prior to transplantation. No significant difference in
incidence of post-operative adverse events was observed between the
ABOi and ABOc groups and the six patients with ABOi did not develop
DGF post-operatively. ABOi living donor kidney transplant (LDKT)
patients have significantly improved survival rates compared with
patients who either remain on the waiting list (P=0.001) or who
receive an ABOc deceased donor kidney transplant (DDKT; P=0.048)
and that the higher the titer of anti-A/-B antibody in ABOi kidney
transplant patients, the lower is the survival rate of the patients
(16). In their study, Massie
et al (17) also show that
patients treated with ABOi-LDKT have higher cumulative survival
rates at 5 and 10 years (90.0 and 75.4%, respectively) compared
with similar patients who remained on the waiting list or received
an ABOc-LDKT/ABOc-DDKT (81.9 and 68.4%, respectively). In the
present study, levels of Scr, urea and eGFR did not differ markedly
between the ABOi and ABOc groups at each follow-up time point
during the observation period, which may have been associated with
the intensive immunosuppressive therapy received by ABOi patients.
Regarding the impact of long-term prognosis, in a study by others
(18), similar renal function was
found between these two types of patients; furthermore, no
significant differences were identified in terms of the renal
function comparing the two patient groups. In recent years, ABOi
kidney transplantation has become a routine procedure. With this
approach, ~30% of previously rejected LDs are now able to donate
their kidneys, thereby markedly expanding the LD pool. However,
transplantation in the presence of severe ABOi puts the patient at
a higher risk of early rejection, infection and
infection-associated mortality. Therefore, when possible, ABOc
donors should be preferred for kidney transplantation.
Renal function monitoring may be used to assess the
functional status of the transplanted kidney to determine whether
the excretory function of the transplanted kidney is normal and
whether there are problems such as AR or chronic rejection. In the
present study, statistically significant differences were
identified in terms of the Scr, urea and eGFR values in both
groupings. During the observation period, especially within the
first month after surgery, the renal function of the CDC ≤10% and
HLA 0-2 MM groups was markedly improved compared with that of the
CDC >10% and HLA 3-6 MM groups and the improved status of the
performance of renal function was even more obvious when patients
met the conditions of CDC ≤10% and HLA 0-2MM at the same time. This
suggested that, in the early stage following kidney
transplantation, CDC-negativity and improved HLA matching are more
helpful in terms of facilitating the rapid and high-quality
recovery of renal function in the short-term period after surgery.
In the clinical setting, the measurement of Scr and urea nitrogen
levels is of great value in terms of helping to determine renal
function, as these fulfill a key role in maintaining metabolic
homeostasis and eliminating metabolic wastes from the body: When
both parameters are elevated, it is usually indicative of impaired
renal function (19). Parameters
such as urinary albumin and the urinary protein-to-creatinine
ratio, which are used as markers of early renal impairment, may
also be used to assess the extent of renal damage more accurately
(20,21). However, the present study was
unable to include such more sensitive indicators in the analysis
due to the lack of quantitative tests for proteinuria and
creatinine in their post-operative follow-up examinations. Although
it did collect the results of qualitative tests for urinary
proteins in the routine urinalysis, the present study found that
the number of patients with positive urinary protein decreased over
time during the 6 months following surgery and the proportions of
patients with positive urinary protein in the CDC ≤10% and HLA 0-2
MM groups were smaller compared with those in the CDC >10% and
HLA 3-6 MM groups at the same time point. However, clinical
decision-making in renal disease depends heavily on eGFR levels, as
this is the primary surrogate marker for clinical monitoring of
allograft function and long-term graft survival (22,23).
It has been reported previously that patients who receive kidney
transplants with high eGFR levels that remain stable for one year
tend to have transplanted organs from younger donors (23). These patients have a relatively low
immunological risk and do not develop persistent allograft injury,
as well as fewer histological lesions associated with allograft
injury. The majority of the donors in the HLA 0-2 MM group in the
present study were from LDs of similar age to the recipients and
therefore all patients in this group had higher eGFR levels
compared with those in the HLA 3-6 MM group post-operatively. A
previous study of 325 DCD and 409 LD kidney transplantations
revealed that eGFR was markedly lower in DCD renal recipients
compared with LD renal recipients on post-operative days 7 and
30(24). Similar results were
found in the present study, where it was shown that kidney
transplant recipients who received a transplant from an LD had
markedly enhanced renal function, including the Scr, urea and eGFR
levels, compared with kidney transplant recipients who received DCD
at pre-transplant and each of the subsequent follow-up time points
within one month post-operatively. Renal function was also markedly
improved in the LD group compared with the DCD group at the
pre-transplant stage due to the overall enhanced physical states of
the LD group recipients compared with the DCD group. Furthermore,
in addition to differences in renal function in the short-term
post-operative period, other clinical outcomes, such as the graft
survival rate, also differed between the donor types. Chen et
al (25) analyzed 6,719 renal
transplant recipients from the Chinese Renal Transplantation
Scientific Registry and found that, compared with the transplants
from DCD, improved outcomes were observed in the LD group,
including the 3-year graft survival rate (LD=95.8% vs. DCD=91.3%),
DGF (LD=2.4% vs. DCD=17.7%), infection (LD=10.7% vs. DCD=20.7%),
graft loss (LD=2.3% vs. DCD=6.3%) and death (LD=1.3% vs.
DCD=3.2%).
The erythrocyte system has been shown to be
especially important for endocrine metabolism after renal
transplantation. Erythropoietin (EPO) is a glycoprotein hormone
produced mainly by the kidneys and its main effect is to stimulate
erythropoiesis in the bone marrow, thereby increasing the
concentration of Hb (26). It has
been shown that testosterone induces an increase in Hb and
hematocrit, an effect that has been associated with the stimulation
of EPO expression and a decrease in the concentrations of ferritin
and hepcidin (27). In the present
study, the Hb levels of the same group of male patients were found
to be higher compared with those of the female patients during the
same time period, which may be due to the fact that the level of
testosterone in males is higher compared with that of females and
therefore the secretion of EPO is relatively higher, which
consequently promotes erythropoiesis and Hb synthesis.
Reticulocytes can effectively reflect the hematopoietic status of
the body's erythrocyte system. In the present study, the majority
of patients were mildly anemic prior to surgery, whereas in the
middle and late post-operative periods, as the transplanted renal
function gradually recovered, the secretion of EPO was observed to
increase, the reticulocytes reached a higher level and the Hb level
gradually increased. Compared with the pre-operative and early
post-operative periods, the numbers of reticulocytes markedly
increased, which led to a significant recovery of the patients'
anemia. Iron deficiency (ID) is highly prevalent in kidney
transplant recipients and has been shown to be independently
associated with an excess mortality risk in this population
(28). Moreover, the parameter
RET-He is affected only by the amount of iron intake, but it
accurately reflects iron deficiency in patients (29). RET-He of patients fluctuated
consistently within the normal range in each group of patients,
suggesting that iron deficiency was not present in these patients
within the first 6 months following renal transplantation and that
iron deficiency correction was not required. A previously published
longitudinal study showed that patients with pre-transplant ID
remained iron-deficient following transplantation and that ferritin
levels tended to decrease in the first few months after
transplantation (30). Therefore,
the presence or absence of iron deficiency after transplantation
depends on a variety of factors, including the patient's overall
health, recovery of transplanted kidney function, dietary habits
and the presence of chronic blood loss. In the time frame selected
for the present study, in the CDC group, the RET% and LFR values
were significantly different only at the 6-month post-operative
stage (P<0.05). In the HLA group, the differences in Hb level,
RET%, RET#, LFRs, MFRs, HFRs and RET-He were found not to be
statistically significant (P>0.05) comparing between the two
groups, suggesting that the pre-operative CDC level and HLA
matching may not have a significant effect on post-operative
erythropoiesis and metabolism, although this observation still
needs to be confirmed by a retrospective or prospective study with
a larger sample size.
Leukocytes form an important part of the human
immune system and their primary functions include immune defense
and participation in immune regulation. In particular, neutrophils
and lymphocytes have critically important roles for transplant
patients. As kidney transplantation is an invasive treatment
modality, which in turn causes a stress response, the immune system
exhibits a reactive inflammatory response, with the numbers of WBCs
and neutrophils increasing rapidly from the first post-operative
day to a level that is far above the normal range. In the present
study, the patients' lymphocyte parameters were at extremely low
levels within one week post-operatively and both the L# and L%
values were markedly decreased compared with those of the
pre-operative period. This may have been due to the use of
immunosuppression (including lymphocyte depletion), as a direct
reduction in the number of lymphocytes post-operatively has been
noted in previous studies (31,32),
or alternatively, the cause may have been a blockade of lymphocyte
activation, conductance and expression (33). When the recovery in the number of
lymphocytes started on the third post-operative day, the lymphocyte
parameters L# and L% were consistently lower in the HLA 3-6 MM
group compared with the HLA 0-2 MM group due to the persistence of
stronger immunosuppression. Studies have also suggested that the
neutrophil/lymphocyte ratio (NLR) and lymphocyte count may be risk
factors for graft and patient prognosis. NLR is a simple tumor
marker that predicts the prognosis of certain solid malignancies,
including renal cell carcinoma, bladder uroepithelial carcinoma and
prostate carcinoma (34,35). Another study shows that, when a
patient's lymphocyte count falls below 750/mm3 at a
given follow-up time, the risk of both graft failure and death is
increased compared with similar patients who were without
lymphopenia at the same time point (36). Therefore, neutrophil and lymphocyte
parameters should be closely monitored during long-term follow-up
following renal transplantation, as they are inexpensive to
monitor, easily accessible and widely available parameters for
assessing the prognostic status of renal transplantation. The
present study did have certain limitations in that the examination
of lymphocyte subsets was lacking in the routine follow-up of renal
transplant patients in our hospital. However, over the past two
decades, there has been increasing evidence to show that monitoring
peripheral blood lymphocyte subsets in renal transplant patients is
important for improving the success rate of renal transplantation,
for protecting the function of the transplanted kidney and for
improving the quality of life of patients (37-39).
DGF is a manifestation of post-transplant acute
renal failure and is an important complication of kidney
transplantation. In clinical series, the most frequently reported
donor and recipient factors associated with DGF have been shown to
be BMI, previous transplantation and diabetes in male recipients
and increased age, donor type and BMI in female donors (40). Additional factors frequently
reported are warm ischemia time, cold ischemia time, prior
sensitization and HLA mismatches (41). The age and BMI of the recipient, as
well as HLA mismatches, were confirmed in the present study as
being important factors for the development of DGF. In the present
study, the incidence of DGF was 1.7% in cases of LD kidney
transplantation and 32.1% in cases of DCD kidney transplantation
and these findings were found to be consistent with those of
previous studies (42-44).
None of the patients in the HLA 0-2 MM group developed DGF,
probably since the donors in this group were all from LDs and had
improved HLA matching. In addition to the non-immunological factors
aforementioned, immune factors also have a role in the development
of DGF. A serum study from a multicenter collaborative
transplantation study shows that the presence of class I and II HLA
antibodies prior to transplantation is a strong predictor that
almost doubles the risk of DGF, whereas the presence of only class
I HLA antibodies or only class II antibodies show no significant
effect (45). Moreover, in another
study, the risk of DGF was almost doubled in patients with
pre-formed DSA compared with those either without HLA antibodies or
with HLA antibodies against third-party antigens, despite the
results of CDC-XM being negative (46). The aforementioned study also found
that patients who developed antibodies directed against donor
HLA-DRB1 had the highest incidence of DGF (69%), which may explain
the phenomenon that no patients in the HLA-DR 0 MM group developed
DGF in the present study. Due to the small sample size of the
present study, however, which included 21 patients who were
positive for HLA antibodies prior to transplantation and featured
only three patients who were positive for both HLA class I and II
antibodies, it was not possible to derive conclusions similar to
those reported in other studies. At the same time, because few of
the patients included in the present study had positive DSA, no
large-sample size DSA data were available for analysis. In
conclusion, it may be said that DGF has a significant negative
impact on graft survival; therefore, it is crucial to take
appropriate measures to prevent the development of DGF in renal
transplant patients.
The present study did, however, also have a number
of limitations. First, retrospective studies are limited by the
availability of data and so it was not possible to include all the
variables and indicators associated with the prognosis of renal
transplant patients. Some of the examination indicators that are
more relevant to disease and more specific are not available to us.
For example, lymphocyte subsets, urinary albumin, or the ratio of
urinary albumin to creatinine. Secondly, small amounts of data were
missing and to compensate for this the present study employed
appropriate algorithms to replace the missing data values, which
may have led to biased results. Furthermore, since the follow-up
period of the present study was from the pre-operative period to
six months post-operatively, it was not possible to analyze the
long-term outcomes of the patients. Additionally, the small sample
size of the collected data and insufficient sample size of HLA
0-2MM group calculated by PASS software in HLA grouping may limit
the general usability and reproducibility of the results of the
present study. Therefore, prospective, multicenter trials with
longer follow-up periods are needed to address the aforementioned
issues, with a view to obtaining more comprehensive and reliable
findings.
In conclusion, the present study has demonstrated
that renal transplant recipients with CDC ≤10% and HLA 0-2 MM
tended to have improved recovery of renal function in the early
post-operative period compared with transplant recipients with CDC
>10% and HLA 3-6 MM. When the conditions of CDC ≤10% and HLA 0-2
MM were both met, kidney transplant recipients had an improved
post-operative prognosis, including faster recovery of renal
function and lower incidence of DGF. In a multifactorial analysis
of adverse events, recipient age, recipient BMI, donor type and HLA
mismatch were significant risk factors for DGF. In addition,
pre-operative CDC-XM levels and HLA matching did not markedly
affect post-operative erythropoiesis or metabolism. Differences in
laboratory findings between groups were mainly concentrated in the
first week after the operation, whereas recovery between 1 and 6
months was essentially comparable, suggesting that the long-term
recovery of kidney transplant recipients may be associated with a
wider range of factors and that other factors including the source
of the donor kidney, nDSA and DSA may also have an effect on the
transplant results. Therefore, in kidney transplantation, doctors
need to comprehensively consider various factors to formulate the
optimal transplantation plan for patients.
Supplementary Material
Comparison of proteinuria at each
follow-up time after surgery. (A) Comparison of proteinuria with
different CDC levels at each follow-up time; (B) Comparison of
proteinuria with different degrees of HLA matching at each
follow-up time.
(A) Serum creatinine, (B) urea and (C)
estimated glomerular filtration levels of RTRs according to the
ABO-compatibility, which represented in a line graph. Values are
represented as the mean±SEM. Values of P≤0.05 were considered
statistically significant. Scr: serum creatinine; eGFR: estimated
glomerular filtration rate; ABOi: ABO-incompatible; ABOc:
ABO-compatible; SEM: standard error of mean.
Serum creatinine (A), urea (B) and
estimated glomerular filtration (C) levels of RTRs according to the
ABO-compatibility. *P≤0.05; **P≤0.01;
***P≤0.001. Scr, serum creatinine; eGFR, estimated
glomerular filtration rate; ABOi, ABO-incompatible; ABOc,
ABO-compatible; SEM, standard error of mean.
Comparison of the incidence of adverse
events. Comparison of the incidence of DGF with different degrees
of (A) HLA-A, (B) -C, (C) -DR and (D) -DQ locus matching.
Comparison of the incidence of DGF with different (E) CDC levels
and (F) presence of HLA antibodies before transplantation. Values
of P≤0.05 were considered statistically significant.ns: P>0.05;
DGF: delayed graft function.
Comparison of the incidence of adverse
events. Comparison of the incidence of DGF with different ABO
compatibility. ns: P>0.05; ABOi: ABO-incompatible; ABOc:
ABO-compatible; DGF: delayed graft function.
Markers in patients categorized by the
CDC-XM levels.
Hb in patients categorized by the
CDC-XM levels.
Markers in patients categorized by HLA
matching.
Statistical description of Hb in
patients categorized by the HLA matching.
Markers in patients categorized by
CDC-XM level and HLA matching.
Markers in patients categorized in
patients categorized by HLA-A locus mismatch.
Markers in patients in patients
categorized by HLA-B locus mismatch.
Markers in patients categorized by
HLA-DR locus mismatch.
Markers in patients categorized by ABO
compatibility.
Statistical description of Proteinuria
(qualitative) in patients categorized by the CDC-XM levels.
Statistical description of Proteinuria
(qualitative) in patients categorized by the HLA matching.
Markers in patients categorized by
donor category.
Acknowledgements
Not applicable.
Funding
Funding: The present study received funding from Major Project
of Humanities and Social Sciences Research in Anhui (grant no.
SK2021ZD0032) and Anhui Medical University Clinical and Early
Discipline Co-construction Project and Key Project of Natural
Science Research of Higher Education Institutions in Anhui Province
(grant no. 2024AH050739).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
MZ and WX conceived the present study. MZ, SX and CX
participated in the design of the present study. SX, CX, ZZ, CQ, XS
and XW participated in data collection. CX and ZZ analyzed and
interpreted the data. CQ, XS and XW drafted the manuscript. SX, CX,
ZZ, CQ, XS and XW revised and edited the manuscript. MZ, WX and SX
confirmed the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The authors obtained appropriate institutional
review board approval. Ethical approval was granted by The Ethics
Committee of the First Affiliated Hospital of Anhui Medical
University (approval no. PJ2024-05-30). The data and samples
utilized in the present study were obtained from the hospital's
electronic medical record system and did not involve any privacy or
information of the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pan GH, Chen Z, Xu L, Zhu JH, Xiang P, Ma
JJ, Peng YW, Li GH, Chen XY, Fang JL, et al: Low-dose tacrolimus
combined with donor-derived mesenchymal stem cells after renal
transplantation: A prospective, non-randomized study. Oncotarget.
7:12089–12101. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Strohmaier S, Wallisch C, Kammer M,
Geroldinger A, Heinze G, Oberbauer R and Haller MC: Survival
benefit of first single-organ deceased donor kidney transplantation
compared with long-term dialysis across ages in transplant-eligible
patients with kidney failure. JAMA Netw Open.
5(e2234971)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li J, Luo Y, Wang X and Feng G: Regulatory
B cells and advances in transplantation. J Leukoc Biol.
105:657–668. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sellarés J, de Freitas DG, Mengel M, Reeve
J, Einecke G, Sis B, Hidalgo LG, Famulski K, Matas A and Halloran
PF: Understanding the causes of kidney transplant failure: The
dominant role of antibody-mediated rejection and nonadherence. Am J
Transplant. 12:388–399. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lim WH, Wong G, Heidt S and Claas FHJ:
Novel aspects of epitope matching and practical application in
kidney transplantation. Kidney Int. 93:314–324. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gunawansa N, Rathore R, Sharma A and
Halawa A: Crossmatch strategies in renal transplantation: A
practical guide for the practicing clinician. J Transplant Surg.
1:8–15. 2017.
|
|
7
|
Tellis VA, Matas AJ, Senitzer D, Louis P,
Glicklich D, Soberman R and Veith FJ: Successful transplantation
after conversion of a positive crossmatch to negative by
dissociation of IgM antibody. Transplantation. 47:127–129.
1989.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Opelz G and Döhler B: Effect of human
leukocyte antigen compatibility on kidney graft survival:
Comparative analysis of two decades. Transplantation. 84:137–143.
2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cecka JM: HLA matching for renal
transplantation: The last word? Transplantation. 100:975–976.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lim WH, Chapman JR, Coates PT, Lewis JR,
Russ GR, Watson N, Holdsworth R and Wong G: HLA-DQ mismatches and
rejection in kidney transplant recipients. Clin J Am Soc Nephrol.
11:875–883. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Leeaphorn N, Pena JRA, Thamcharoen N,
Khankin EV, Pavlakis M and Cardarelli F: HLA-DQ mismatching and
kidney transplant outcomes. Clin J Am Soc Nephrol. 13:763–771.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Süsal C, Döhler B and Opelz G:
Presensitized kidney graft recipients with HLA class I and II
antibodies are at increased risk for graft failure: A collaborative
transplant study report. Hum Immunol. 70:569–573. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Michielsen LA, Wisse BW, Kamburova EG,
Verhaar MC, Joosten I, Allebes WA, van der Meer A, Hilbrands LB,
Baas MC, Spierings E, et al: A paired kidney analysis on the impact
of pre-transplant anti-HLA antibodies on graft survival. Nephrol
Dial Transplant. 34:1056–1063. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Solgi G, Furst D, Mytilineos J, Pourmand G
and Amirzargar AA: Clinical relevance of pre and post-transplant
immune markers in kidney allograft recipients: Anti-HLA and MICA
antibodies and serum levels of sCD30 and sMICA. Transpl Immunol.
26:81–87. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zschiedrich S, Jänigen B, Dimova D,
Neumann A, Seidl M, Hils S, Geyer M, Emmerich F, Kirste G, Drognitz
O, et al: One hundred ABO-incompatible kidney transplantations
between 2004 and 2014: A single-centre experience. Nephrol Dial
Transplant. 31:663–671. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Koo TY, Lee J, Lee Y, Kim HW, Kim BS, Huh
KH and Yang J: Outcomes of ABO-incompatible living donor kidney
transplantation compared to waiting or deceased donor kidney
transplantation. Am J Nephrol. 55:235–244. 2024.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Massie AB, Orandi BJ, Waldram MM, Luo X,
Nguyen AQ, Montgomery RA, Lentine KL and Segev DL: Impact of
ABO-incompatible living donor kidney transplantation on patient
survival. Am J Kidney Dis. 76:616–623. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chow KV, Flint SM, Shen A, Landgren A,
Finlay M, Murugasu A, Masterson R, Hughes P and Cohney SJ:
Histological and extended clinical outcomes after ABO-incompatible
renal transplantation without splenectomy or rituximab.
Transplantation. 101:1433–1440. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lyman JL: Blood urea nitrogen and
creatinine. Emerg Med Clin North Am. 4:223–233. 1986.PubMed/NCBI
|
|
20
|
Ruilope LM, Ortiz A, Lucia A, Miranda B,
Alvarez-Llamas G, Barderas MG, Volpe M, Ruiz-Hurtado G and Pitt B:
Prevention of cardiorenal damage: Importance of albuminuria. Eur
Heart J. 44:1112–1123. 2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Levey AS, Atkins R, Coresh J, Cohen EP,
Collins AJ, Eckardt KU, Nahas ME, Jaber BL, Jadoul M, Levin A, et
al: Chronic kidney disease as a global public health problem:
approaches and initiatives-a position statement from kidney disease
improving global outcomes. Kidney Int. 72:247–259. 2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Clayton PA, Lim WH, Wong G and Chadban SJ:
Relationship between eGFR decline and hard outcomes after kidney
transplants. J Am Soc Nephrol. 27:3440–3446. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Raynaud M, Aubert O, Reese PP, Bouatou Y,
Naesens M, Kamar N, Bailly É, Giral M, Ladrière M, Le Quintrec M,
et al: Trajectories of glomerular filtration rate and progression
to end stage kidney disease after kidney transplantation. Kidney
Int. 99:186–197. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang X, Lyu J, Yu X, Wang L, Peng W, Chen
J and Wu J: Comparison of graft outcome between donation after
circulatory death and living-donor kidney transplantation.
Transplant Proc. 52:111–118. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen L, Bai H, Jin H, Zhang T, Shi B, Cai
M and Wang Y: Outcomes in kidney transplantation with mycophenolate
mofetil-based maintenance immunosuppression in China: A
large-sample retrospective analysis of a national database. Transpl
Int. 33:718–728. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cantarelli C, Angeletti A and Cravedi P:
Erythropoietin, a multifaceted protein with innate and adaptive
immune modulatory activity. Am J Transplant. 19:2407–2414.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bachman E, Travison TG, Basaria S, Davda
MN, Guo W, Li M, Connor Westfall J, Bae H, Gordeuk V and Bhasin S:
Testosterone induces erythrocytosis via increased erythropoietin
and suppressed hepcidin: evidence for a new
erythropoietin/hemoglobin set point. J Gerontol A Biol Sci Med Sci.
69:725–735. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Winkelmayer WC, Lorenz M, Kramar R, Hörl
WH and Sunder-Plassmann G: Percentage of hypochromic red blood
cells is an independent risk factor for mortality in kidney
transplant recipients. Am J Transplant. 4:2075–2081.
2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ogawa C, Tsuchiya K and Maeda K:
Reticulocyte hemoglobin content. Clin Chim Acta. 504:138–145.
2002.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tornero F, Prats D, Alvarez-Sala JL,
Coronel F, Sanchez A and Barrientos A: Iron deficiency anemia after
successful renal transplantation. J Urol. 149:1398–1400.
1993.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Bonnefoy-Berard N, Verrier B, Vincent C
and Revillard JP: Inhibition of CD25 (IL-2R alpha) expression and
T-cell proliferation by polyclonal anti-thymocyte globulins.
Immunology. 77:61–67. 1992.PubMed/NCBI
|
|
32
|
Alamartine E, Bellakoul R and Berthoux F:
Randomized prospective study comparing OKT3 and antithymocyte
globulins for treatment of the first acute cellular rejection of
kidney allografts. Transplant Proc. 26:273–274. 1994.PubMed/NCBI
|
|
33
|
Soulillou JP, Cantarovich D, Le Mauff B,
Giral M, Robillard N, Hourmant M, Hirn M and Jacques Y: Randomized
controlled trial of a monoclonal antibody against the interleukin-2
receptor (33B3.1) as compared with rabbit antithymocyte globulin
for prophylaxis against rejection of renal allografts. N Engl J
Med. 322:1175–1182. 1990.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kawahara T, Fukui S, Sakamaki K, Ito Y,
Ito H, Kobayashi N, Izumi K, Yokomizo Y, Miyoshi Y, Makiyama K, et
al: Neutrophil-to-lymphocyte ratio predicts prostatic carcinoma in
men undergoing needle biopsy. Oncotarget. 6:32169–32176.
2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kawahara T, Furuya K, Nakamura M, Sakamaki
K, Osaka K, Ito H, Ito Y, Izumi K, Ohtake S, Miyoshi Y, et al:
Neutrophil-to-lymphocyte ratio is a prognostic marker in bladder
cancer patients after radical cystectomy. BMC Cancer.
16(185)2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Dujardin A, Lorent M, Foucher Y, Legendre
C, Kerleau C, Brouard S and Giral M: DIVAT Consortium.
Time-dependent lymphocyte count after transplantation is associated
with higher risk of graft failure and death. Kidney Int.
99:1189–1201. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bloom RD and Augustine JJ: Beyond the
biopsy: Monitoring immune status in kidney recipients. Clin J Am
Soc Nephrol. 16:1413–1422. 2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Long W, Zhang H, Yuan W, Lan G, Lin Z,
Peng L and Dai H: The role of regulatory B cells in kidney
diseases. Front Immunol. 12(683926)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Huang DL, He YR, Liu YJ, He HY, Gu ZY, Liu
YM, Liu WJ, Luo Z and Ju MJ: The immunomodulation role of Th17 and
Treg in renal transplantation. Front Immunol.
14(1113560)2023.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Nashan B, Abbud-Filho M and Citterio F:
Prediction, prevention, and management of delayed graft function:
where are we now? Clin Transplant. 30:1198–1208. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Redfield RR, Scalea JR, Zens TJ, Muth B,
Kaufman DB, Djamali A, Astor BC and Mohamed M: Predictors and
outcomes of delayed graft function after living-donor kidney
transplantation. Transpl Int. 29:81–87. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ojo AO, Wolfe RA, Held PJ, Port FK and
Schmouder RL: Delayed graft function: Risk factors and implications
for renal allograft survival. Transplantation. 63:968–974.
1997.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Sellers MT, Gallichio MH, Hudson SL, Young
CJ, Bynon JS, Eckhoff DE, Deierhoi MH, Diethelm AG and Thompson JA:
Improved outcomes in cadaveric renal allografts with pulsatile
preservation. Clin Transplant. 14:543–549. 2000.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Molnar MZ, Kovesdy CP, Rosivall L,
Bunnapradist S, Hoshino J, Streja E, Krishnan M and Kalantar-Zadeh
K: Associations of pre-transplant anemia management with
post-transplant delayed graft function in kidney transplant
recipients. Clin Transplant. 26:782–791. 2012.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Morath C, Döhler B, Kälble F, Pego da
Silva L, Echterdiek F, Schwenger V, Živčić-Ćosić S, Katalinić N,
Kuypers D, Benöhr P, et al: Pre-transplant HLA antibodies and
delayed graft function in the current era of kidney
transplantation. Front Immunol. 11(1886)2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Peräsaari JP, Kyllönen LE, Salmela KT and
Merenmies JM: Pre-transplant donor-specific anti-human leukocyte
antigen antibodies are associated with high risk of delayed graft
function after renal transplantation. Nephrol Dial Transplant.
31:672–678. 2016.PubMed/NCBI View Article : Google Scholar
|