Contents
Introduction
Effects of acetogenins on mammalian DNA polymerases
α, β and λ
Effects of acetogenins on human DNA topoisomerases I
and II
Effects of acetogenins on human cancer cell line,
HL-60
Three-dimensional structures of acetogenins
Effects of compounds 5 on DNA metabolic enzymes
Effects of compound 5 on cultured human cancer
cells
Effects of the influence on HL-60 cells by compound
5
Discussion
Conclusion
Introduction
Acetogenins are a class of potent bioactive
compounds in various plant species in the Annonaceae family
(1). These acetogenins are a
relatively new class of fatty acid-derived natural products that
have a wide range of biological activities, such as cytotoxic,
antitumor and immunosuppressive effects (1–3).
They are characterized by the presence of one to three
tetrahydrofuran (THF) rings in the center of a long alkyl chain
with a butenolide moiety at the end. Besides such classical types,
acetogenins with a tetrahydropyran (THP) ring in the long chain,
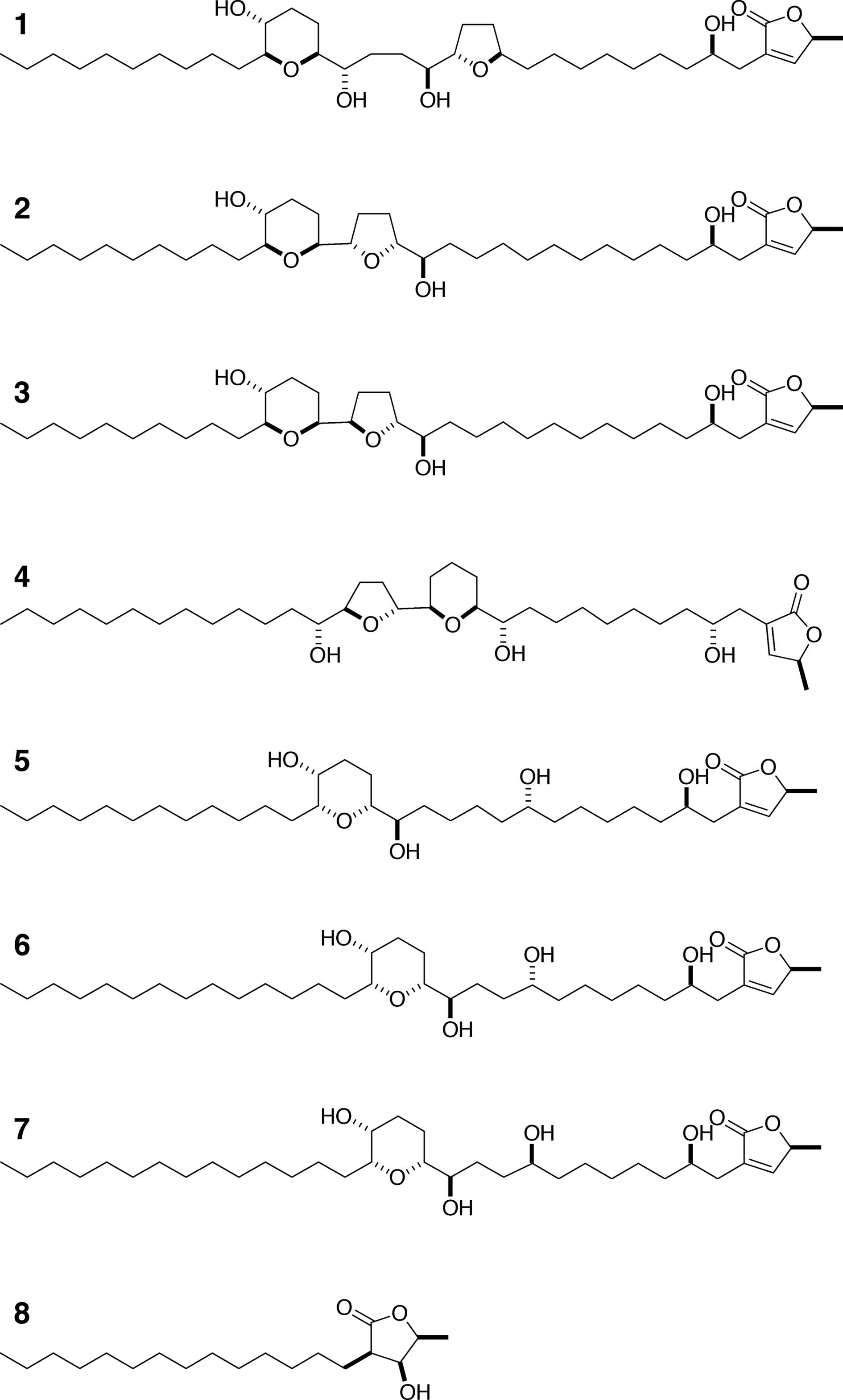
such as mucocin (compound 1), jimenezin (compound 2), muconin
(compound 4), pyranicin (compound 5) and pyragonicin (compound 6)
have also been discovered (Fig. 1)
(4–7).
DNA polymerase (pol) catalyzes the addition of
deoxyribonucleotides to the 3′-hydroxyl terminus of primed
double-stranded DNA molecules (8).
The human genome encodes at least 15 pols to conduct cellular DNA
synthesis (9,10). Eukaryotic cells contain three
replicative pols (α, δ and ε), mitochondrial pol γ and at least
twelve non-replicative pols [β, ζ, η, θ, ι, κ, λ, µ, ν, terminal
deoxynucleotidyl transferase (TdT) and REV1] (9–11).
DNA topoisomerases (topos) are key enzymes that
control the topological state of DNA. Type I enzymes act by
transiently nicking one of the two DNA strands. Type II enzymes
nick both DNA strands which are ATP-dependent and are involved in
many vital cellular processes that influence DNA replication,
transcription, recombination, integration and chromosomal
segregation (12).
DNA metabolic enzymes, such as pols and topos, are
not only essential for DNA replication, repair and recombination,
but are also involved in cell division. Selective inhibitors of
these enzymes are considered as a group of potentially useful
anti-cancer and anti-parasitic agents, because some inhibitors
suppress human cancer cell proliferation and have cytotoxicity
(13–16).
Non-classical THP acetogenins have become
interesting compounds because of their powerful antitumor activity;
thus, total synthesis of the natural and non-natural acetogenins
(compounds 1–8 of Fig. 1) was
achieved (17–25). Since McLaughlin et al and
Mata et al reported that some acetogenins have cytotoxicity
against human cancer cell lines (4–7), the
purpose of this review is to investigate the biochemical action of
the compounds against DNA metabolic enzymes such as pols and topos,
and to use the compound as an antineoplastic agent.
Therefore, we describe the inhibitory activities of
chemically synthesized acetogenins against pols, topos and other
DNA metabolic enzymes, as well as cellular proliferation processes
such as DNA replication of human cancer cells. The analysis of the
relationship between the essential molecular structure and
bioactive function of acetogenins shows that acetogenins are an
ideal model for the development of new anti-cancer drugs.
Effects of acetogenins on mammalian DNA
polymerases α, β and λ
The structures of the acetogenins (compounds 1–8),
which were chemically synthesized, are shown in Fig. 1. The inhibitory activity of
mammalian pols, such as calf pol α, rat pol β and human pol λ,
against 10 µM of each compound was investigated. For pols,
poly(dA)/oligo(dT)12–18 (A/T = 2/1) and
2′-deoxythymidine 5′-triphosphate (dTTP) were used as the DNA
template-primer and nucleotide (i.e., 2′-deoxyribonucleotide
5′-triphosphates, dNTP) substrate, respectively. One unit of pol
activity was defined as the amount of enzyme that catalyzed the
incorporation of 1 nmol dNTP (i.e., dTTP) into synthetic DNA
template-primers in 60 min at 37°C under the normal reaction
conditions for each enzyme (26,27).
Pol α and pols β and λ were used as representative replicative and
repair/recombination-related pols, respectively (8–10).
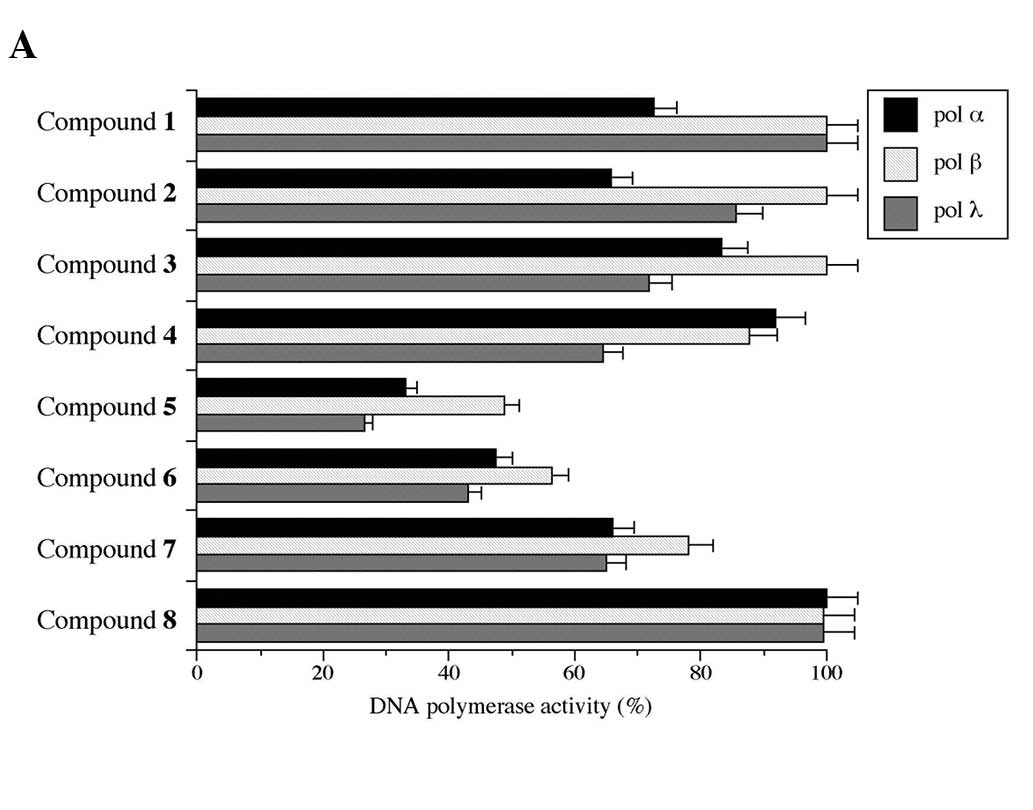
As shown in Fig.
2A, compounds 5 and 6 significantly inhibited the activities of
these pols, while compound 8 had no effect. Compound 5 showed the
strongest inhibition of pol α, β and λ activities in the tested
compounds, and 50% inhibition was observed at doses of 5.3, 9.6 and
2.3 µM, respectively. When activated DNA (i.e., DNA digested by
bovine deoxyribonuclease I) was used as the DNA template-primer
instead of poly(dA)/oligo(dT)12–18 (A/T = 2/1), the mode
of inhibition of these compounds did not change (data not
shown).
Effects of acetogenins on human DNA
topoisomerases I and II
Topo inhibitory activity of acetogenins was then
investigated. The relaxation activity of topos I and II from humans
was determined by detecting the conversion of supercoiled plasmid
DNA to its relaxed form (28).
Compounds 5–7 (10 μM), respectively, inhibited the activities of
topos dose-dependently, while the other compounds did not (Fig. 2B). In human topos I and II,
compound 5 showed the strongest inhibition of the tested compounds.
Topo I and II inhibitors, camptothecin and etoposide, inhibited the
relaxation activities of topos I and II with IC50 values
of 85 and 70 μM, respectively (28). Therefore, compound 5 was a stronger
inhibitor of topos I and II than camptothecin and etoposide,
respectively.
These results suggested that the inhibitory activity
of acetogenins between mammalian pols and human topos had the same
tendency, and the inhibitory effect on pols was almost as strong as
that on topos. Thus, the mechanism of the inhibitory effect of
acetogenins, including compound 5, on cultured human cancer cells
was investigated.
Effects of acetogenins on human cancer cell
line, HL-60
Pols and topos have recently emerged as important
cellular targets for chemical intervention in the development of
anti-cancer agents. Acetogenins therefore are useful in
chemotherapy, and the cytotoxic effect of eight compounds against
the human promyelocytic leukemia cell line HL-60, derived from a
cancer patient, was investigated. The survival rate of cultured
human cancer cells was determined by MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide)
assay (29).
As shown in Fig.
2C, 10 μM of compound 5 had the strongest growth inhibitory
effect on HL-60, while compounds 6 and 7 had the second and third
strongest, respectively. At a concentration of 100 μM, compounds
1–7 strongly suppressed cancer cell growth, while compound 8 had no
influence (i.e., in order of cytotoxicity observed at a
concentration of 10 μM: compound 5 > compound 6 > compound 7
> compound 1 = compound 2 = compound 3 = compound 4 >
compound 8). The suppression of cell growth had almost the same
tendency as the inhibition of mammalian pols and human topos among
the 8 compounds (Fig. 2A and B,
respectively), suggesting that the cause of cancer cell influence
involves the activities of pols, including replicative and
repair/recombination pols, as well as topos.
Three-dimensional structures of
acetogenins
To obtain more information about the molecular basis
for differential inhibition spectra exhibited by the acetogenins
prepared, computational analyses of compounds 1–8 were performed
(Fig. 3). Acetogenin models were
simulated with force-field parameters based on the Consistent
Valence Force Field. Temperature was set at 298 K. Calculations
based on simulation images were carried out using Insight II
(Accelrys Inc., San Diego, CA, USA). Electrostatic potentials on
the surface of compounds were analyzed by WebLab ViewerLite
software (version 3.2; Accelrys Inc.).
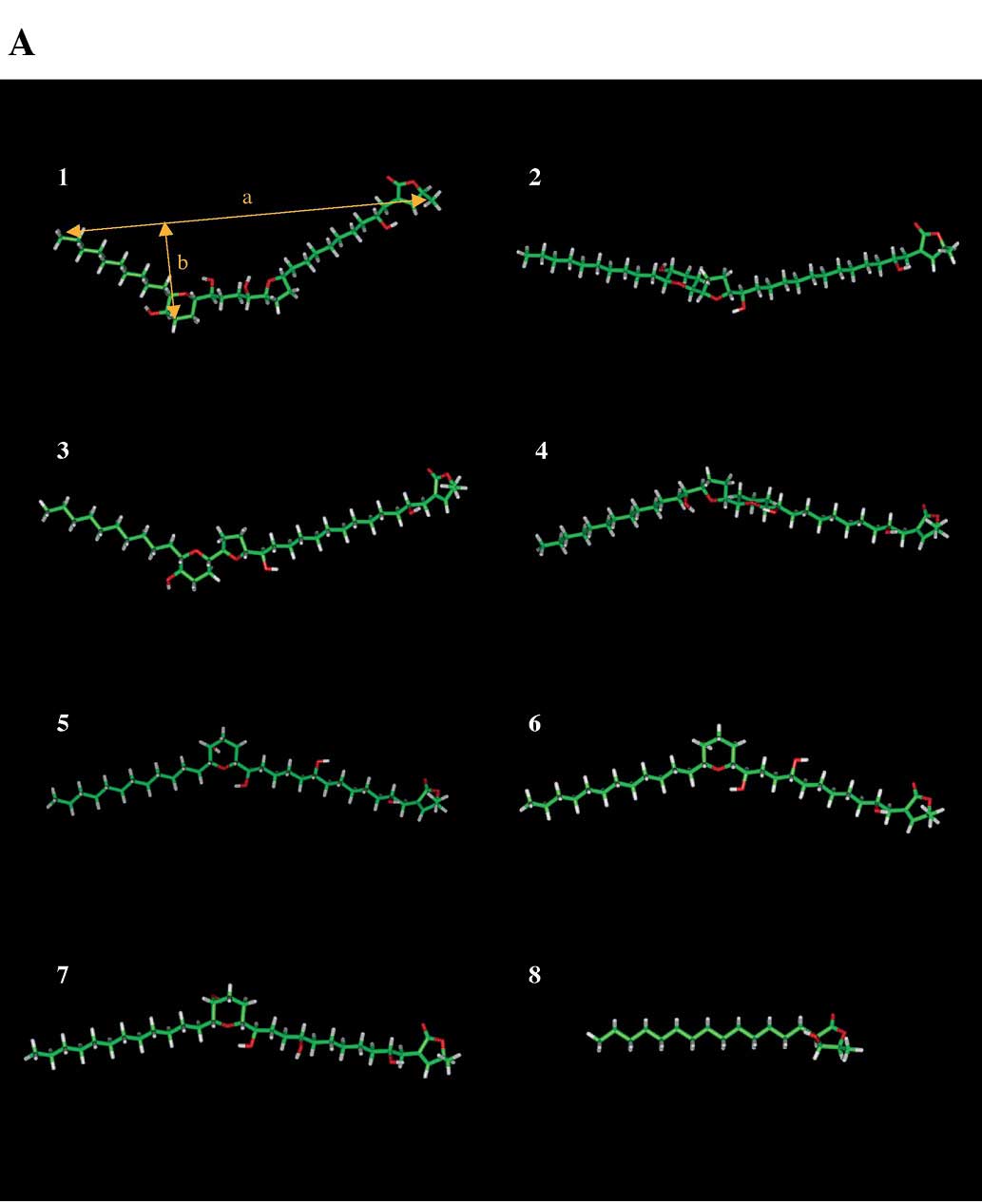
Fig. 3A shows the
front view of the three-dimensional structures, from which were
calculated energy-minimized compounds. The molecular length ‘a’ and
width ‘b’ of the three-dimensional structure of the compounds based
on Fig. 3A are indicated in
Table I. Compounds tested as
acetogenins have a butenolide or γ-lactone moiety at the end.
Compounds 1–7 also consist of one or two rings, such as THF and/or
THP, in the center, but compound 8 has no other rings. Since
compound 8 did not influence the activities of pols, topos and
HL-60 cell growth, the moiety of THF and/or THP rings of compounds
1–7 may be important for the inhibition of mammalian pols, human
topos and human cancer cell growth. The molecular length of
compounds 1–7 was ∼1.9-fold longer than that of compound 8 (i.e.,
33.63–37.76 Å and 19.80 Å, respectively). Moreover, the molecular
length (33–38 Å) of the compound is essential for these inhibitory
activities. Compounds 5–7 contain a THP ring in the center of the
structure, and the moiety has a slightly V-type shape. Compounds
1–4 also have this V-type shape. The width of compounds 5–7 is less
than that of compounds 1, 3 and 4, but is larger than that of
compound 2 (Table I). These
results suggested that the width of the three-dimensional structure
of compounds 5–7 (i.e., 5.43–5.94 Å) is important for pols, topos
and cancer cell growth inhibition.
 | Table I.Molecular length and width of
three-dimensional structure of compounds 1–8. |
Table I.
Molecular length and width of
three-dimensional structure of compounds 1–8.
| Compound | Length (Å) | Width (Å) |
|---|
| 1 | 33.63 | 8.65 |
| 2 | 37.76 | 5.11 |
| 3 | 36.96 | 7.49 |
| 4 | 37.13 | 6.24 |
| 5 | 37.03 | 5.94 |
| 6 | 34.40 | 5.55 |
| 7 | 34.80 | 5.43 |
| 8 | 19.80 | 5.43 |
Fig. 3B indicates
that a comparison of the electrostatic potential surfaces of the
acetogenins revealed a difference in their overall disposition and
affinity. The electrostatic potential at each point on a constant
electronic density surface (approximating the van der Waals surface
for each arrangement) is represented graphically, with red
corresponding to regions where the electrostatic potential is most
negative, and blue corresponding to the most positive regions. As
shown in Fig. 3B, compounds 5–7
have four hydroxyl groups, and compound 5 has the same
three-dimensional position of these groups as compound 6,
supporting the enhancement of the negative and positive
electrostatic potential on O and H atoms of the -OH group,
respectively. The other compounds are markedly different from
compounds 5 and 6. These results suggested that the
three-dimensional position of hydrophobicity, such as the hydroxyl
group, was essential for the inhibitory activities of pols, topos
and cancer cell growth.
Effects of compounds 5 on DNA metabolic
enzymes
Since compound 5 had the strongest bioactivity of
the acetogenins investigated, this review focuses on compound 5 in
the latter part. As shown in Table
II, this compound inhibited the activities of the mammalian
pols tested with IC50 values of 2.3–15.8 µM. The pol λ
inhibitory effect of compound 5 was the strongest in mammalian
pols. Furthermore, this compound inhibited animal pols from fish
(cherry salmon) pol δ, and insect (fruit fly) pols α, δ and ε at
almost the same concentration as the inhibition of mammalian pols.
On the other hand, compound 5 did not significantly influence the
activities of pols I (α-like pol) and II (β-like pol) from plants
(cauliflower) and prokaryotes such as the Klenow fragment of E.
coli pol I, Taq pol and T4 pol.
 | Table II.IC50 values of compound 5
on the activities of various DNA polymerases and other DNA
metabolic enzymes. |
Table II.
IC50 values of compound 5
on the activities of various DNA polymerases and other DNA
metabolic enzymes.
| Enzyme | IC50
value (μM) |
|---|
| Mammalian DNA
polymerases | |
| Calf DNA
polymerase α | 5.3±0.4 |
| Rat DNA
polymerase β | 9.6±0.8 |
| Human DNA
polymerase γ | 5.9±0.4 |
| Human DNA
polymerase δ | 8.4±0.7 |
| Human DNA
polymerase ε | 15.8±1.4 |
| Human DNA
polymerase η | 10.2±0.9 |
| Human DNA
polymerase ι | 13.0±1.2 |
| Human DNA
polymerase κ | 11.1±1.0 |
| Human DNA
polymerase λ | 2.3±0.2 |
| Calf terminal
deoxynucleotidyl transferase | 6.3±0.5 |
| Fish DNA
polymerases | |
| Cherry salmon DNA
polymerase δ | 8.8±0.7 |
| Insect DNA
polymerases | |
| Fruit fly DNA
polymerase α | 6.5±0.5 |
| Fruit fly DNA
polymerase δ | 8.9±0.8 |
| Fruit fly DNA
polymerase ε | 14.0±1.3 |
| Plant DNA
polymerases | |
| Cauliflower DNA
polymerase I (α-like) | >200 |
| Cauliflower DNA
polymerase II (β-like) | >200 |
| Prokaryotic DNA
polymerases | |
| E. coli
DNA polymerase I (Klenow fragment) | >200 |
| Taq DNA
polymerase | >200 |
| T4 DNA
polymerase | >200 |
| Other DNA metabolic
enzymes | |
| Calf primase of
DNA polymerase α | >200 |
| T7 RNA
polymerase | >200 |
| Human DNA
topoisomerase I | 5.0±1.5 |
| Human DNA
topoisomerase II | 7.5±2.0 |
| T4 polynucleotide
kinase | >200 |
| Bovine
deoxyribonuclease I | >200 |
In the DNA metabolic enzymes tested, compound 5 also
inhibited the activities of human topos I and II with
IC50 values of 5.0 and 7.5 μM, respectively (Table II). This compound did not inhibit
the activities of the other DNA metabolic enzymes tested, including
calf primase of pol α, T7 RNA polymerase, T4 polynucleotide kinase
and bovine deoxyribonuclease I.
To determine whether the inhibitor resulted in
binding to DNA or the enzyme, the interaction of compound 5 with
double-stranded DNA (dsDNA) was investigated based on the thermal
transition of dsDNA with or without the compound. The melting
temperature of dsDNA with an excess amount of compound 5 (100 μM)
was measured using a spectrophotometer equipped with a
thermoelectric cell holder. In the concentration range used, no
thermal transition of the melting temperature was observed. In
contrast, when ethidium bromide, a typical intercalating compound,
was used as a positive control a clear thermal transition was
produced. These observations indicated that compound 5 did not
intercalate to DNA as a template-primer; thus, the compound
directly binds to the enzyme and inhibits its activity.
These results suggested that compound 5 is a potent
and selective inhibitor of animal pols and human topos.
Consequently, the mechanism of the inhibitory effect of compound 5
on human cancer cells was investigated.
Effects of compound 5 on cultured human
cancer cells
The growth suppression specificity of human cancer
cell species by compound 5 was investigated (Table III). This compound inhibited the
growth of the cancer cell lines tested, and the range of
IC50 values was 9.4–16.1 μM. The inhibitory effect on
non-adherent cell lines, such as BALL-1 and HL-60, was
approximately 1.5-fold stronger than that on other adhering cell
lines. The inhibitory effect of the 48-h culture was as strong as
that of the 24-h incubation. The LD50 values of compound
5 for cancer cell growth were almost the same as the
IC50 values for pols and topos. Therefore, this compound
may penetrate cancer cells and reach the nucleus, inhibiting the
activities of pols and topos. Moreover, the inhibition of enzyme
activities by compound 5 may lead to cell growth suppression. Since
compound 5 was the strongest cell growth inhibitor of HL-60 in the
human cancer cell lines tested, this cell line was examined in the
latter part of this review.
 | Table III.LD50 values of compound 5
on the growth of human cancer cell lines. |
Table III.
LD50 values of compound 5
on the growth of human cancer cell lines.
| Species of human
cells | Type of cancer | LD50
values (μM) |
|---|
| A549 | Lung cancer | 15.2±1.3 |
| BALL-1 | B cell acute
lymphoblastoid leukemia | 9.9±0.9 |
| HCT116 | Colon carcinoma
cancer | 14.8±1.2 |
| HeLa | Cervix cancer | 16.1±1.4 |
| HL-60 | Promyelocytic
leukemia | 9.4±0.8 |
| NUGC-3 | Stomach cancer | 15.6±1.3 |
Effects of the influence on HL-60 cells by
compound 5
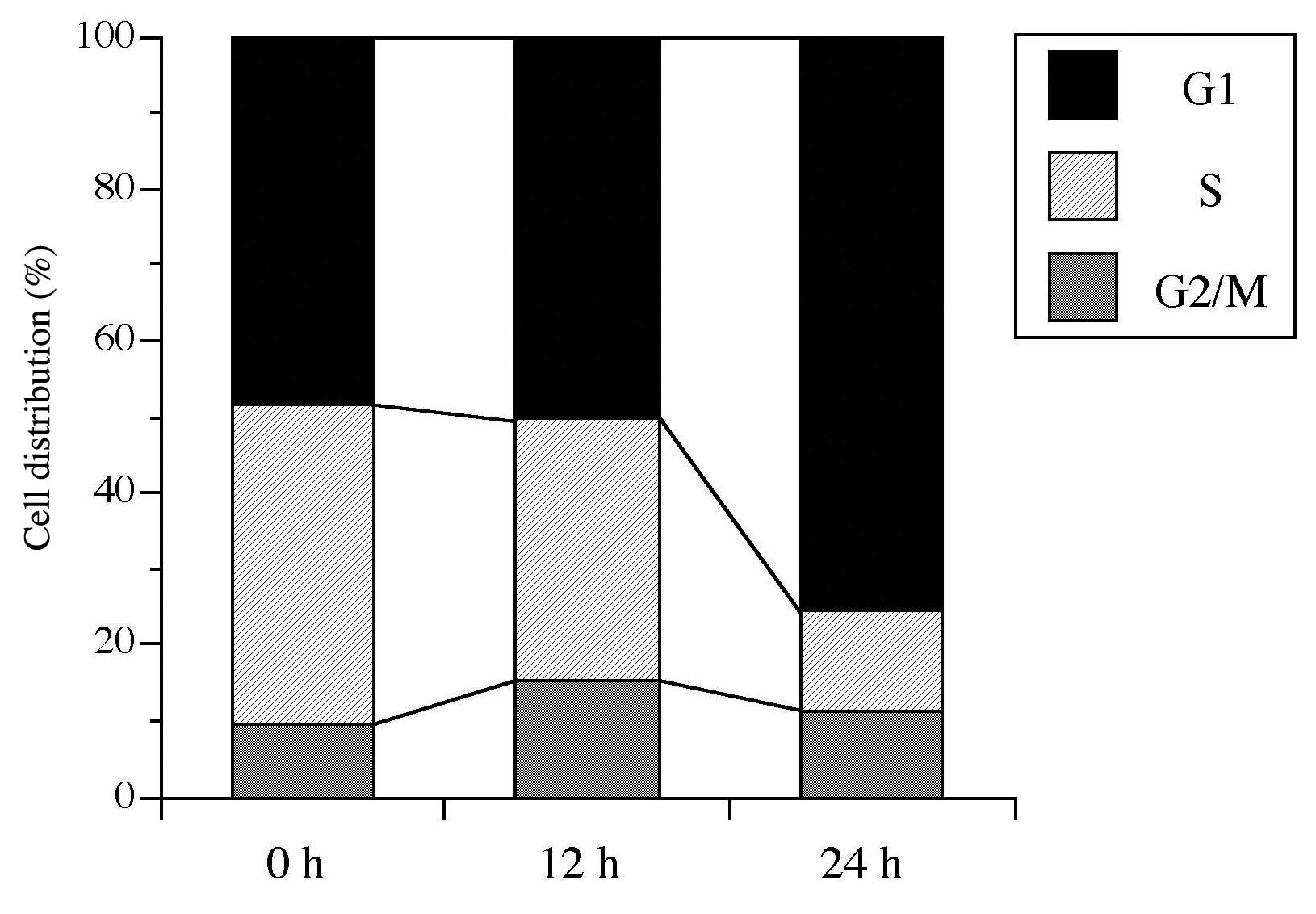
The cell cycle distribution of compound 5-treated
HL-60 cells was investigated. As shown in Fig. 4, the cell cycle fraction was
recorded after 12 and 24 h of treatment with the LD50
value of the compound (i.e., 9.4 μM). Consequently, among cells
treated with compound 5 for 12 h, the population of cells in the
G2/M phase increased (9.6–15.2%), the percentage of cells in the S
phase decreased from 42.1 to 34.5%, and the G1 phase was not
affected. These results suggested that the actions of this compound
blocked the G2/M phase in HL-60 cells. Following 24 h treatment of
compound 5, the cell population in the G1 phase significantly
increased from 48.3 to 75.6%, but the S phase decreased
(42.1-13.1%). Dehydroaltenusin, a specific pol α inhibitor,
inhibited the cell cycle in the G1 phase, including the early S
phase (30), while classical topo
inhibitors, such as etoposide, arrested the cell cycle at the G2/M
phase (31). Therefore, compound 5
is more effective in the inhibition of pols than topos in the 24-h
cell incubation, although compound 5 inhibited the activities of
mammalian pols and human topos, and the inhibitory effect on topos
(IC50 values 5.0–7.5 μM) was almost the same as that for
pols (IC50 values 2.3–15.8 μM) in vitro (Table II).
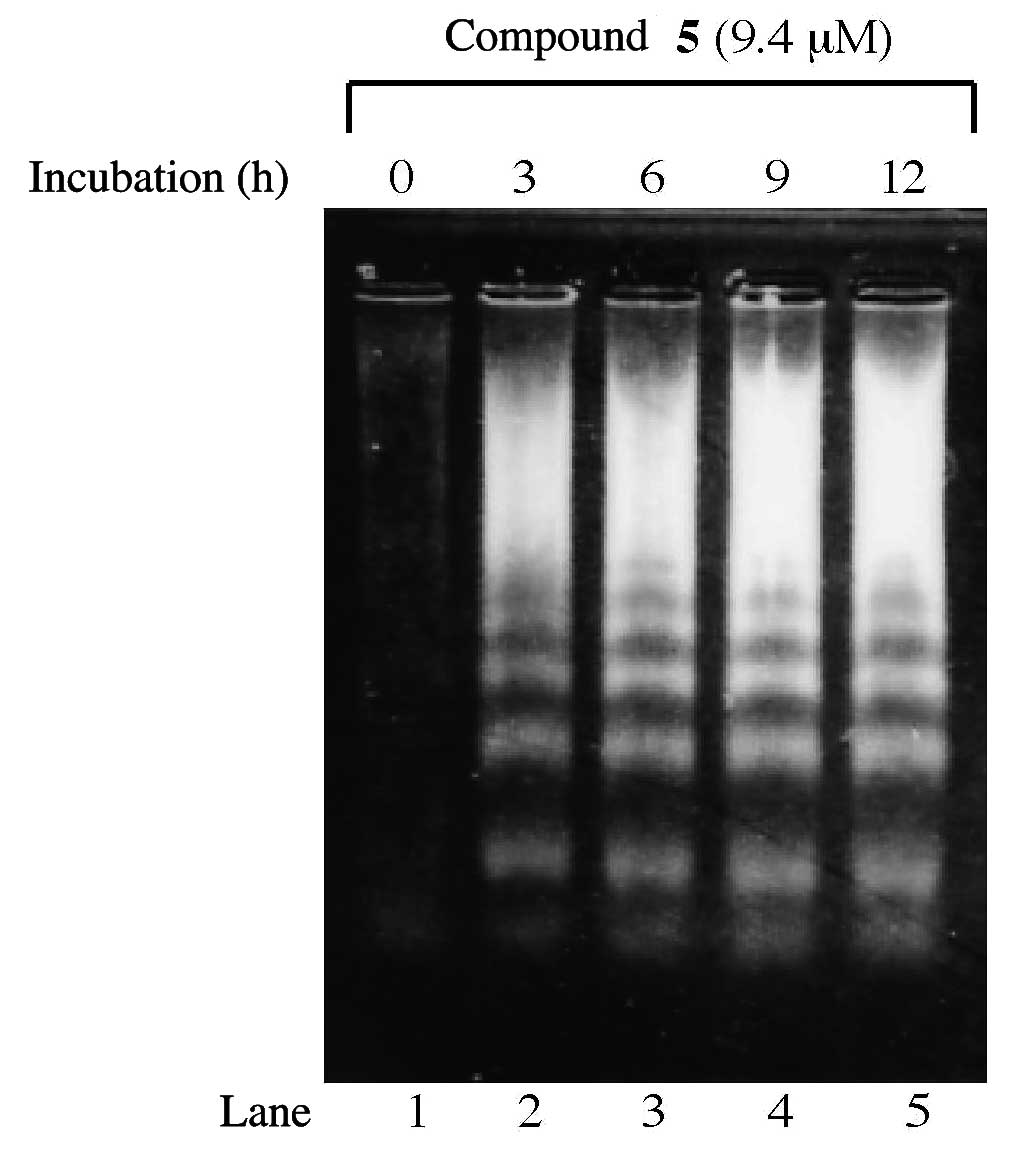
To examine whether the decrease in cell numbers
caused by compound 5 was due to apoptosis, DNA fragmentation was
analyzed by electrophoresis. DNA ladder formation was
dose-dependently observed in HL-60 cells treated with the
LD50 value of the compound (i.e., 9.4 μM), and ladders
were apparent at 3 h (Fig. 5).
These results suggested that apoptotic effects were evident in the
cells, and the effect of the compound must involve a combination of
growth arrest and cell death.
Discussion
Most acetogenins exhibit potent and selective in
vitro anti-tumor activities. For example, compound 1 (mucocin)
reportedly shows significant inhibitory activities against A-549
(lung cancer) and PACA-2 (pancreatic cancer) solid tumor lines
(32). As the main mode of action,
blockage of the mitochondrial NADH-ubiquinone oxidoreductase in
complex I, which is a membrane-bound and essential enzyme for ATP
production, is discussed (32).
Furthermore, these natural products were shown to inhibit a
ubiquinone-linked NADH oxidase found in the plasma membrane of
specific tumor cell lines, including some which show multidrug
resistance (33). However,
research on the inhibition of acetogenins against pol and topo
activities is limited, probably due to the small quantities of
natural products. Since the total synthesis of bioactive
acetogenins, such as compound 5, is possible (17–25),
these compounds should be provided and studied in pharmaceutical
research throughout the world.
Compound 5 directly inhibited animal pol and human
topo activities (Table II), but
did not bind to DNA. These observations suggested some structural
similarity between the enzymes at the compound 5 binding site,
although the characteristics of pols and topos, including their
modes of action, amino acid sequences and three-dimensional
structures, are markedly different. We previously reported that
several inhibitors, long-chain fatty acids (26,27)
and triterpenoids (34,35) of mammalian pol β also inhibited
topo II activity. The two enzymes had a structural homology at the
DNA-binding site (36–38). Moreover, the DNA-binding site of
long-chain fatty acids on the pol was the same domain (i.e.,
N-terminal 8-kDa domain of pol β) as that of triterpenoids
(36,39); therefore, compound 5 was expected
to have similar characteristics. Pols and topos have recently
emerged as important cellular targets for chemical intervention in
the development of anti-cancer agents. Therefore, information
concerning the structural characteristics of these inhibitors may
provide valuable insight for the design of new anti-cancer
agents.
In acetogenins, the molecular length and width, as
well as the surface area of the neighboring negative and positive
charges are regarded as important for mammalian pol inhibition,
human topo inhibition and human cancer cell cytotoxicity.
Continuing computer simulation analyses, however, will result in
more effective pol and topo inhibitors than compound 5.
Conclusion
Chemically synthesized acetogenins inhibited the
activities of mammalian pols, human topos and human cancer cell
growth. In particular, compound 5 (pyranicin) showed the strongest
inhibition of the tested acetogenins, and it was revealed that the
inhibition of pol and topo activities by compound 5 influenced not
only cell proliferation but also the cell cycle and apoptosis
induction. Therefore, compound 5 is the lead compound of
potentially useful cancer chemotherapy agents.
Abbreviations:
|
pol
|
DNA-directed DNA polymerase (EC
2.7.7.7);
|
|
topo
|
DNA topoisomerase;
|
|
THF
|
tetrahydrofuran;
|
|
THP
|
tetrahydropyran;
|
|
TdT
|
terminal deoxynucleotidyl
transferase;
|
|
IC50
|
50% inhibitory concentration;
|
|
dTTP
|
2′-deoxythymidine 5′-triphosphate;
|
|
dNTP
|
2′-deoxyribonucleotide
5′-triphosphate;
|
|
LD50
|
50% lethal dose
|
Acknowledgements
This work was supported in part by a
Grant-in-Aid for Kobe-Gakuin University Joint Research (A), and the
‘Academic Frontier’ Project for Private Universities: matching fund
subsidy from the Ministry of Education, Science, Sports, and
Culture of Japan (MEXT), 2006–2010, (H.Y. and Y.M.). Y.M.
acknowledges a Grant-in-Aid for Young Scientists (A) (No. 19680031)
from MEXT, Grants-in-Aid from the Nakashima Foundation (Japan),
Foundation of Oil and Fat Industry Kaikan (Japan), The Salt Science
Research Foundation, No. 08S3 (Japan), and a Grant from the
Industrial Technology Research Program from NEDO (Japan).
References
|
1.
|
Rupprecht JK, Hui YH and McLaughlin JL:
Annonaceous acetogenins: a review. J Nat Prod. 53:237–278. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Alali FQ, Liu XX and McLaughlin JL:
Annonaceous acetogenins: recent progress. J Nat Prod. 62:504–540.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Berrnejo A, Figadere B, Zafra-Polo MC,
Barrachina I, Estornell E and Cortes D: Acetogenins from
Annonaceae: recent progress in isolation, synthesis and mechanisms
of action. Nat Prod Rep. 22:269–303. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Alali FQ, Rogers L, Zhang Y and McLaughlin
JL: Unusual bioactive annonaceous acetogenins from Goniothalamus
giganteus. Tetrahedron. 54:5833–5844. 1998. View Article : Google Scholar
|
|
5.
|
Chavez D, Acevedo LA and Mata R:
Jimenezin, a novel annonaceous acetogenin from the seeds of
Rollinia mucosa containing adjacent
tetrahydrofuran-tetrahydropyran ring system. J Nat Prod.
61:419–421. 1998. View Article : Google Scholar
|
|
6.
|
Shi G, Alfonso D, Fatope MO, Zeng L, Gu
ZM, Zhao GX, He K, MacDougal JM and McLaughlin JL: Mucocin: a new
annonaceous acetogenin bearing a tetrahydropyran ring. J Am Chem
Soc. 117:10409–10410. 1995. View Article : Google Scholar
|
|
7.
|
Shi G, Kozlowski JF, Schwedler JT, Wood
KV, MacDougal JM and McLaughlin JL: Muconin and mucoxin: additional
nonclassical bioactive acetogenins from Rollinia mucosa. J
Org Chem. 61:7988–7989. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kornberg A and Baker TA: Eukaryotic DNA
polymerase. DNA replication (2nd edition). Freeman WH and Co. (New
York). 197–225. 1992.
|
|
9.
|
Hubscher U, Maga G and Spadari S:
Eukaryotic DNA polymerases. Annu Rev Biochem. 71:133–163. 2002.
View Article : Google Scholar
|
|
10.
|
Bebenek K and Kunkel TA: DNA repair and
replication. Advances in Protein Chemistry. 69:Yang W: Elsevier.
(San Diego). 137–165. 2004.
|
|
11.
|
Takata K, Shimizu T, Iwai S and Wood RD:
Human DNA polymerase N (POLN) is a low fidelity enzyme capable of
error-free bypass of 5S-thymine glycol. J Biol Chem.
281:23445–23455. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Wang JC: DNA topoisomerases. Annu Rev
Biochem. 65:635–692. 1996. View Article : Google Scholar
|
|
13.
|
Chakraborty AK and Majumder HK: Mode of
action of pentavalent antimonials: specific inhibition of type I
DNA topoisomerase of Leishmania donovani. Biochem Biophys
Res Commun. 152:605–611. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Liu LF: DNA topoisomerase poisons as
antitumor drugs. Annu Rev Biochem. 58:351–375. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Ray S, Hazra B, Mittra B, Das A and
Majumder HK: Diospyrin, a bisnaphthoquinone: a novel inhibitor of
type I DNA topoisomerase of Leishmania donovani. Mol
Pharmacol. 54:994–999. 1998.PubMed/NCBI
|
|
16.
|
Sakaguchi K, Sugawara F and Mizushina Y:
Inhibitors of eukaryotic DNA polymerases. Seikagaku. 74:244–251.
2002.PubMed/NCBI
|
|
17.
|
Pupo MT, Vieira PC, Fernandes JB and da
Silva MFGF: γ-Lactones from Trichilia claussenii.
Phytochemistry. 48:307–310. 1998.
|
|
18.
|
Takahashi S and Nakata T: Total synthesis
of an antitumor agent, mucocin, based on the ‘chiron approach’. J
Org Chem. 67:5739–5752. 2000.PubMed/NCBI
|
|
19.
|
Takahashi S, Kubota A and Nakata T:
Stereoselective total synthesis of mucocin, an antitumor agent.
Angew Chem Int Ed Engl. 41:4751–4754. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Takahashi S, Maeda K, Hirota S and Nakata
T: Total synthesis of a new cytotoxic acetogenin, jimenezin, and
the revised structure. Org Lett. 1:2025–2028. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Takahashi S, Kubota A and Nakata T:
Stereoselective total synthesis of muconin. Tetrahedron.
59:1627–1638. 2003. View Article : Google Scholar
|
|
22.
|
Takahashi S, Kubota A and Nakata T: Total
synthesis of a cytotoxic acetogenin, pyranicin. Org Lett.
5:1353–1356. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Takahashi S, Ogawa N, Koshino H and Nakata
T: Total synthesis of the proposed structure for pyragonicin. Org
Lett. 7:2783–2786. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Takahashi S, Hongo H, Ogawa N, Koshino H
and Nakata T: Convergent synthesis of pyragonicin. J Org Chem.
71:6305–6308. 2006. View Article : Google Scholar
|
|
25.
|
Takahashi S, Ogawa N, Sakairi N and Nakata
T: Stereoselective synthesis of
(2R,3S,4S)-3-hydroxy-4-methyl-2-tetradecyl-4-butanolide starting
from 2,5-anhydro-D-mannitol. Tetrahedron. 61:6540–6545. 2005.
View Article : Google Scholar
|
|
26.
|
Mizushina Y, Tanaka N, Yagi H, Kurosawa T,
Onoue M, Seto H, Horie T, Aoyagi N, Yamaoka M, Matsukage A, Yoshida
S and Sakaguchi K: Fatty acids selectively inhibit eukaryotic DNA
polymerase activities in vitro. Biochim Biophys Acta. 1308:256–262.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Mizushina Y, Yoshida S, Matsukage A and
Sakaguchi K: The inhibitory action of fatty acids on DNA polymerase
β. Biochim Biophys Acta. 1336:509–521. 1997.
|
|
28.
|
Ishimaru C, Yonezawa Y, Kuriyama I,
Nishida M, Yoshida H and Mizushina Y: Inhibitory effects of
cholesterol derivatives on DNA polymerase and topoisomerase
activities, and human cancer cell growth. Lipids. 43:373–382. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Murakami-Nakai C, Maeda N, Yonezawa Y,
Kuriyama I, Kamisuki S, Takahashi S, Sugawara F, Yoshida H,
Sakaguchi K and Mizushina Y: The effects of dehydroaltenusin, a
novel mammalian DNA polymerase α inhibitor, on cell proliferation
and cell cycle progression. Biochim Biophys Acta. 1674:193–199.
2004.
|
|
31.
|
Nishida K, Seto M and Ishida R: Different
susceptibilities of postmitotic checkpoint-proficient and
-deficient Balb/3T3 cells to ICRF-193, a catalytic inhibitor of DNA
topoisomerase II. Jpn J Cancer Res. 92:193–202. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Degli Esposti M: Inhibitors of
NADH-ubiquinone reductase: an overview. Biochim Biophys Acta.
1364:222–235. 1998.PubMed/NCBI
|
|
33.
|
Oberlies NH, Chang CJ and McLaughlin JL:
Structure-activity relationships of diverse Annonaceous acetogenins
against multidrug resistant human mammary adenocarcinoma
(MCF-7/Adr) cells. J Med Chem. 40:2102–2106. 1997. View Article : Google Scholar
|
|
34.
|
Mizushina Y, Tanaka N, Kitamura A, Tamai
K, Ikeda M, Takemura M, Sugawara F, Arai T, Matsukage A, Yoshida S
and Sakaguchi K: The inhibitory effect of novel triterpenoid
compounds, fomitellic acids, on DNA polymerase β. Biochem J.
330:1325–1332. 1998.PubMed/NCBI
|
|
35.
|
Tanaka N, Kitamura A, Mizushina Y,
Sugawara F and Sakaguchi K: Fomitellic acids, triterpenoid
inhibitors of eukaryotic DNA polymerases from a basidiomycete,
Fomitella fraxinea. J Nat Prod. 61:193–197. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Mizushina Y, Ohkubo T, Date T, Yamaguchi
T, Saneyoshi M, Sugawara F and Sakaguchi K: Mode analysis of a
fatty acid molecule binding to the N-terminal 8-kDa domain of DNA
polymerase β. J Biol Chem. 274:25599–25607. 1999.
|
|
37.
|
Mizushina Y, Sugawara F, Iida A and
Sakaguchi K: Structural homology between DNA binding sites of DNA
polymerase β and DNA topoisomerase II. J Mol Biol. 304:385–395.
2000.
|
|
38.
|
Mizushina Y, Iida A, Ohta K, Sugawara F
and Sakaguchi K: Novel triterpenoids inhibit both DNA polymerase
and DNA topoisomerase. Biochem J. 350:757–763. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Mizushina Y, Ohkubo T, Sugawara F and
Sakaguchi K: Structure of lithocholic acid binding to the
N-terminal 8-kDa domain of DNA polymerase β. Biochemistry.
39:12606–12613. 2000.
|