Introduction
Coscinium fenestratum (Gaertn.) Colebr.,
generally known as Hamm in the Thai language, is a medicinal plant
belonging to the family of Menispermaceae (1). Decoction and tincture of the stem
have been used in ethnomedicine, especially in northeastern
Thailand, for the treatment of cancer, diabetes mellitus and
arthritis. In addition, this plant has been used in the traditional
Ayurvedic and Siddha systems of medicine in India and Sri Lanka for
treating diabetes mellitus (2).
However, the molecular targets of this plant in anti-cancer
activity have not been elucidated.
The non-steroidal anti-inflammatory drug
(NSAID)-activated gene (NAG-1) was identified in COX-negative cells
by PCR-based subtractive hybridization from an NSAID-induced
library as a divergent member of the TGF-β superfamily (3). The over-expression of NAG-1 in cancer
cells results in growth arrest and an increase in apoptosis,
suggesting that NAG-1 has anti-tumorigenic activity. NAG-1
expression is also up-regulated by a number of dietary compounds
and anticancer drugs (4–8). Similarly, ATF3 is a pro-apoptotic
protein, and many anti-tumorigenic compounds, including
phytochemicals, induce this protein at the transcriptional level
(9). ATF3 has been postulated to
be a tumor suppressor gene since it coordinates the expression of
genes that may be linked to cancer (10). In contrast, the cyclin D1
proto-oncogene is an important regulator of G1 to S phase
progression in many different cell types (11). Overexpression of cyclin D1 has been
described in several forms of human cancer (12,13).
A number of therapeutic agents have been observed to suppress
cyclin D1 expression in vitro (14–16),
indicating that the suppression of cyclin D1 may offer a useful
avenue for therapeutic intervention.
Peroxisome proliferator-activated receptor γ (PPARγ)
is a ligand-activated transcription factor that regulates numerous
biological processes, including energy and lipid metabolism, and
cell proliferation (17). The
PPARγ agonists can also affect cell proliferation, differentiation
and apoptosis in a PPARγ-dependent and/or -independent manner, and
thereby represent a potentially important therapeutic family of
compounds for cancer treatment. Many studies describe the
beneficial effects of PPARγ agonists for the treatment of lung
(18), ovarian (19), breast (20) and colorectal cancer (21,22).
Subsequent study from our laboratory also suggested that PPARγ
ligands control many genes that are involved in cellular and
physiological pathways in a PPARγ-dependent manner (23). In contrast, we have also
demonstrated that the PPARγ ligand MCC-555 induces NAG-1 and
apoptosis in HCT-116 cells (24)
in a PPARγ-independent manner. In the present study, we
investigated the anti-proliferative effects and binding activity
for PPARγ of 80% ethanolic stem extract of C. fenestratum
(80ET) and its two additional fractions separated by its polarity,
dichloromethane (DCM) and aqueous fractions (WF) of the stem of
C. fenestratum, as well as berberine, a major constituent of
C. fenestratum. Our data suggest that the stem extracts
extract and its fractions show anti-proliferative activity as
assessed by several measurements described here. The
anti-proliferative activity of the stem extract probably results
from berberine; however, the DCM fraction may contain a unique
compound that induces PPARγ activity.
Materials and methods
Plant materials
The dried stems of C. fenestratum were
obtained from Udonthani province, Thailand, in January 2004. The
powdered sample was pharmacognostically identified by us
(macroscopic, microscopic and TLC characteristics), as previously
reported (25). The voucher
specimen was deposited at the Department of Pharmacognosy, Faculty
of Pharmacy, Mahidol University, Bangkok, Thailand (WCs04).
Extract preparation
The powdered plant material (100 g) was extracted
ten times with 80% ethanol (300 ml) at room temperature in cycles
of 48 h each on an orbital shaker. The combined extract was
evaporated to dryness on a boiling water-bath to yield a dried
80ET. The 80ET was then dissolved in dichloromethane (DCM, 7:300
w/v). The mixture was placed in a separator funnel, and 300 ml of
distilled water was added. After shaking, the mixture was
thoroughly drained off, allowing the DCM layer to separate. This
extraction was repeated three times. The combined DCM and aqueous
(WF) layers were collected and evaporated to yield DCM and WF
fractions, respectively.
TLC analysis
The content of berberine in the extracts was
determined by validated TLC-densitometry as previously described
(26). Briefly, 10 mg of the
extract was transferred into a 10-ml volumetric flask containing 5
ml methanol, sonicated for 10 min and diluted to 10 ml with
methanol. Filtered on Whatman no. 1 filter paper, a 5-μl aliquot of
sample was applied on the precoated silica gel GF254 plate. The
plate was then developed with butanol:glacial acetic acid:water
(14:3:4), and the analysis was repeated three times. Chromatograms
were evaluated via peak area after scanning in absorbance mode at
415 nm. For TLC fingerprint, the extracts extract of C.
fenestratum and its fractions were dissolved in methanol, and a
5-μl sample was applied to the plate, corresponding to ∼100 μg for
each dry weight. A butanol:glacial acetic acid:water (70:15:20)
solvent system was used for TLC. The plate was sprayed with
Dragendroff’s spraying reagent.
Cell cultures, reagents and plasmids
Cell lines were purchased from ATCC (Rockville, MD,
USA). The human colorectal carcinoma cell lines HCT-116 and SW480
were maintained in McCoy’s 5A and RPMI-1640 medium, respectively,
supplemented with 10% fetal bovine serum (Cellgro, VA, USA),
penicillin and streptomycin (10 mg/ml). 3T3-L1 mouse embryonic
fibroblast cells were grown in DMEM. Berberine chloride was
purchased from Sigma (St. Louis, MO, USA). Four copies of a Gal4
binding site (MH100×4-TK-LUC) and chimeric receptors
(pCMX-Gal-mPPARγ-LBD) were previously reported (21). The NAG-1 antibody was previously
described (3). ATF3, actin and
cyclin D1 antibodies were purchased from Santa Cruz Biotechnology
(Santa Cruz, CA, USA). The secondary antibodies linked to HRP were
purchased from Cell Signaling Technology (Beverly, MA, USA). All
chemicals were purchased from Fisher Scientific, unless otherwise
specified.
Cell proliferation analysis
The effects of berberine, 80ET, DCM and WF on cell
proliferation in HCT-116 human colorectal cancer cells were
investigated using the CellTiter 96 Aqueous One Solution Cell
Proliferation Assay (Promega, WI, USA). The cells were seeded at a
concentration of 1,000 cells/well in six replicates and maintained
overnight. The cells were then treated with various concentrations
of berberine, 80ET, DCM and WF dissolved in dimethylsulfoxide
(DMSO) in the presence of serum. At 0, 1, 2 and 4 days after
treatment, 20 μl of CellTiter96 Aqueous One solution was added to
each well and the plate was incubated for 1 h at 37°C. An
absorbance at 490 nm was recorded in an enzyme-linked immunosorbent
assay (ELISA) plate reader (Bio-Tek Instruments, Winooski, VT,
USA).
Western blot analysis
HCT-116 cells were grown to 60–80% confluence in
6-cm plates followed by a 24-h treatment with various
concentrations of berberine, 80ET, DCM and WF, or with DMSO as
vehicle control in the absence of serum. Total cell lysates were
then isolated using RIPA buffer (1× PBS, 1% NP-40, 0.5% sodium
deoxycholate, 0.1% SDS) supplemented with protease inhibitors (1 mM
PMSF, 5 μg/ml aprotinin and 5 μg/ml leupeptin) and phosphatase
inhibitors (1 mM Na3VO4 and 1 mM NaF).
Protein concentration was determined by the BCA protein assay
(Pierce, Rockford, IL, USA), using BSA as the standard. Protein (30
μg) was separated by SDS-PAGE and transferred for 1 h onto a
nitrocellulose membrane (Schleicher & Schuell, NH, USA). The
blots were blocked for 1 h with 5% skim milk in TBS/Tween 0.05%
(TBS-T) and probed with a specific primary antiserum in TBS-T and
5% non-fat dry milk at 4°C overnight. After washing with TBS-T, the
blots were treated with horseradish peroxidase-conjugated secondary
antibody for 1 h and washed several times. Proteins were detected
by the enhanced chemiluminescence system.
Transient transfections
Transient transfections were performed using
Lipofectamine (Invitrogen, CA, USA) according to the manufacturer’s
instructions. HCT-116 cells were plated in 12-well plates at a
concentration of 1×105 cells/well. After growth for 18
h, plasmid mixtures containing MH100× 4-Tk-LUC plasmid (0.25 µg)
and pCMX-GalmPPARγ-LBD (0.25 µg) were co-transfected with pRL-null
vector (0.05 µg) for 5 h. The transfected cells were cultured in
the absence or presence of various concentrations of berberine,
80ET, DCM and WF. Rosiglitazone (RGZ) (Cayman Chemicals, MI, USA;
100% purity) was used as a positive control. Cells were harvested
in 1× luciferase lysis buffer, and luciferase activity was
normalized to the pRL-null luciferase activity using a dual
luciferase assay kit (Promega, WI, USA).
Caspase activity
Caspase activity was measured using the Apo-ONE
homogeneous Caspase-Glo 3/7 Assay kit (Promega) according to the
manufacturer’s protocol. Cell lysates (50 μg protein) were obtained
using RIPA buffer containing protease inhibitors and incubated with
50 μl of Caspase-Glo 3/7 reagent in 96-well plates for 1 h.
Luminescence was measured using a plate-reading luminometer
(FLX800; BioTek).
3T3-L1 cell differentiation and oil-red o
staining
3T3-L1 cells were grown to confluence in 6
cm-plates. For adipocyte differentiation, differentiation medium
(DM) containing 1 μg/ml insulin, 1 μM dexamethasone and 0.5 mM
isobutylmethyl xanthine was added to the culture. DM was changed
every 2 days until day 4. Thereafter, DMEM containing 10% FBS and
insulin only was subsequently replaced every 2 days. DCM (50 μg/ml)
was added 24 h before and during differentiation, and DMSO was used
as a vehicle for untreated cells. On day 9, the cells were washed
with PBS, fixed with 10% formalin at room temperature, and stained
with oil-red O for 10 min. Pictures were captured using a
microscope (Nikon Eclipse E 600).
Statistical analysis
Statistical analysis was performed with the
Student’s unpaired t test, with statistical significance set at
*P<0.05, **P<0.01 and ***P<0.001.
Results
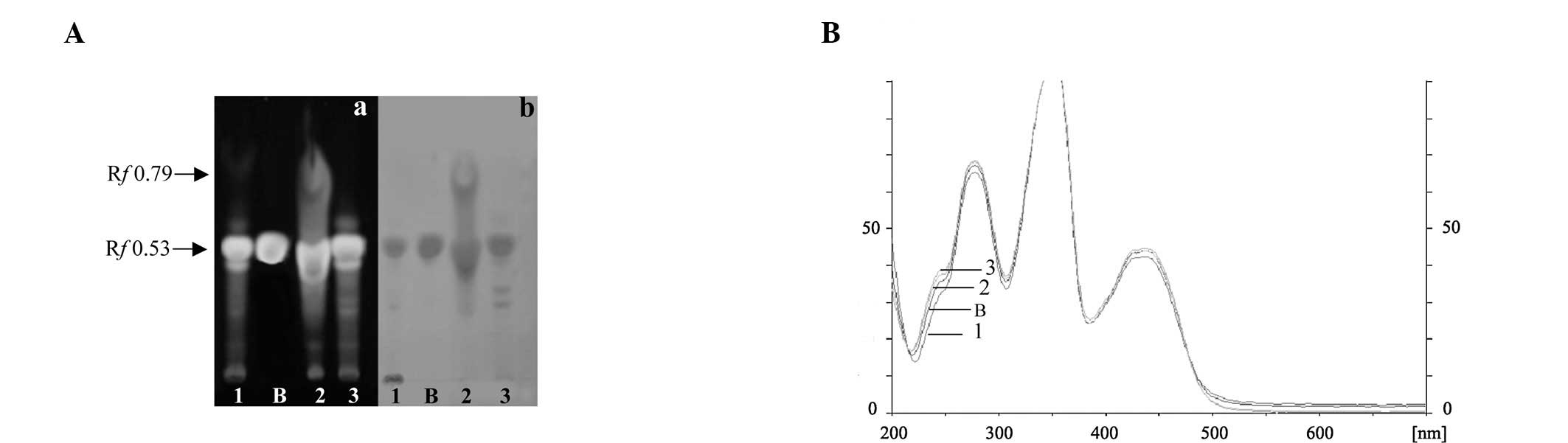
TLC analysis was performed to investigate the
phytochemical profiles of the extracts. Berberine was found to be a
major constituent seen in all the extracts as a yellow fluorescence
spot detected under UV 366 nm and an orange spot detected with
Dragendroff’s spraying reagent (Fig.
1A). The spot for berberine in the sample was confirmed by
comparing the Rf value (0.53) and the spectrum of the spot with
that of the standard. Peak purity of the sample was fully in
conformity with the standard (Fig.
1B). The berberine contents of 80ET, DCM and WF expressed as
the mean ± SD were 18.45±1.39, 18.02±1.03, and 17.95±0.97 (% w/w),
respectively. In the DCM fraction, other compounds, which may be
alkaloid compounds, were observed as a blue fluorescent zone under
uV 366 nm and an orange zone with Dragendroff’s spraying reagent at
Rf 0.79 (Fig. 1A).
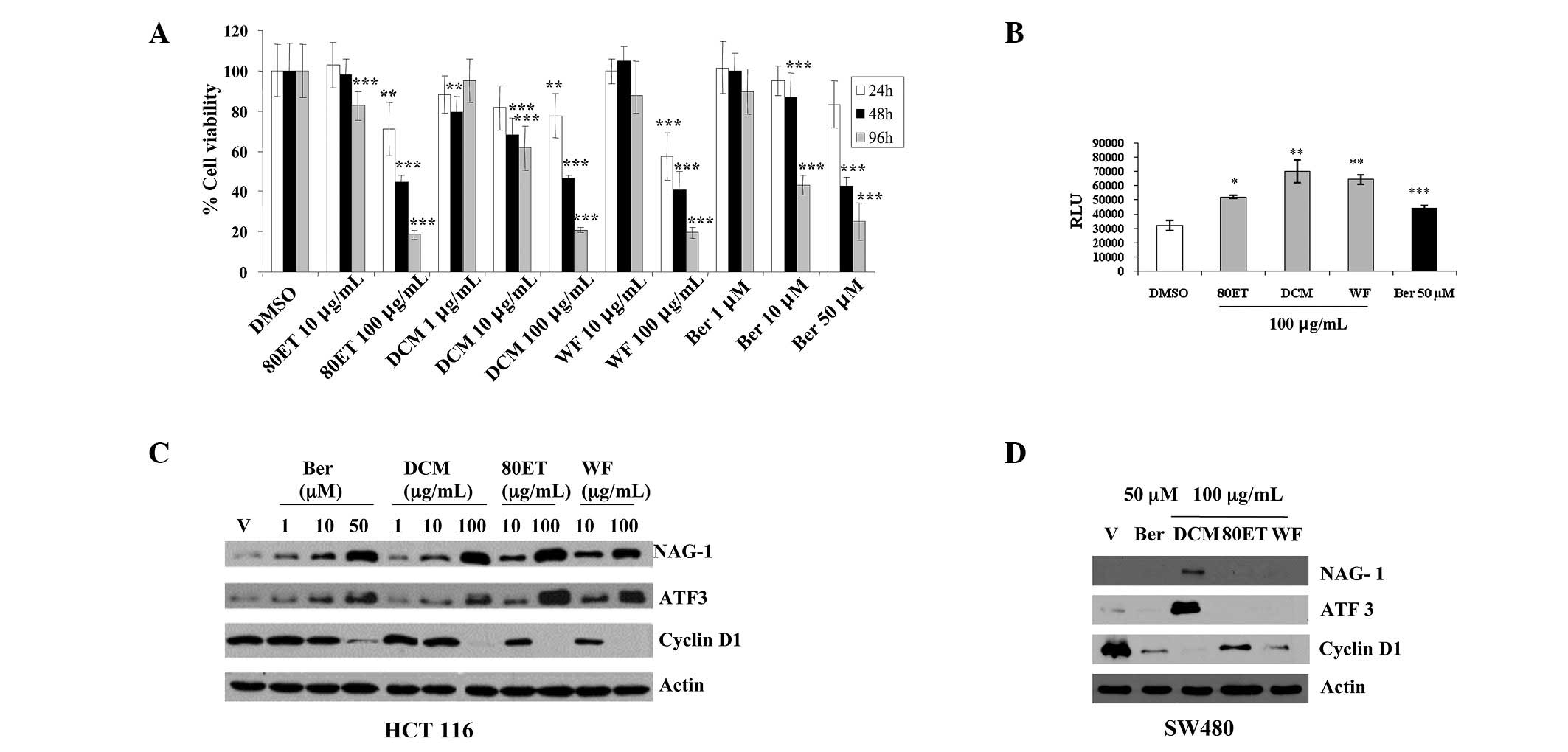
To investigate the effects of the stem extract
(80ET), its two fractions (WF and DCM) and its major constituent
berberine on cell viability on the human colorectal carcinoma
HCT-116 cell line, the cells were treated with 10–100 μg/ml of 80ET
and WF, 1–100 μg/ml of DCM, and 1–50 μM of berberine for 24, 48 and
96 h. The reduction in cell proliferation in a dose- and
time-dependent manner in response to the treatments is shown in
Fig. 2A. At 24 h, a marked
reduction in viability was detected with the concentration of 100
μg/ml of 80ET (26.55%) and WF (37.27%). After 96 h, all the higher
dose treatments significantly inhibited cell growth (P<0.001).
It is evident that all the treatments had a cytotoxic effect on the
HCT-116 cell line, and that cell growth arrest was induced, in
part, by apoptosis as assessed by caspase activity (Fig. 2B). As shown in Fig. 2C, the 24-h treatment with the
extract, its fractions and berberine increased NAG-1 and ATF3
expression and strongly suppressed cyclin D1 expression in a
dose-dependent manner. Protein expression was further examined in
another colorectal cancer cell line, SW480. Cyclin D1 suppression
by all the treatments was similar to that seen in HCT-116 cells,
whereas ATF3 and NAG-1 induction was seen only in DCM-treated
samples (Fig. 2D).
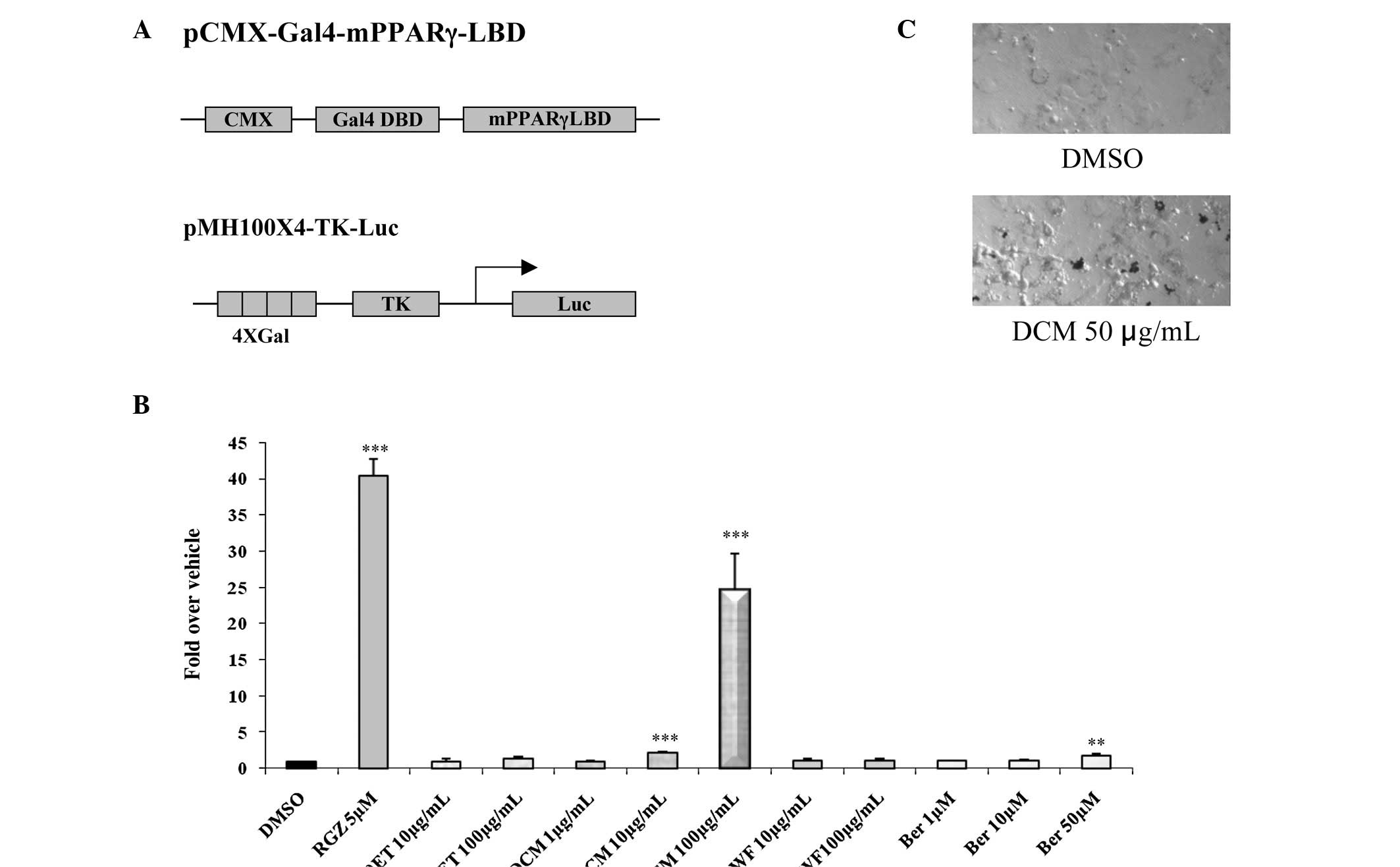
We also examined whether compounds in 80ET, DCM, WF
and berberine bind to PPARγ as a ligand. PPARγ ligands are capable
of binding to the PPARγ transcription factor, which then forms a
heterodimeric complex with retinoid X receptor that functions as a
central regulator of differentiation and a modulator of cell
growth. Using a reporter system, HCT-116 cells were plated in
12-well plates and transfected with four copies of a Gal4 binding
site (pMH100×4-TK-Luc) and chimeric receptors
(pCMX-Gal4-mPPARγ-LBD) (Fig. 3A).
After the cells were treated with 10–100 μg/ml of 80ET and WF,
1–100 μg/ml of DCM and 1–50 μM of berberine for 24 h, luciferase
activity was measured to assess transactivation for the PPARγ
receptor. As shown in Fig. 3B, RGZ
5 µg/ml, a positive control, demonstrated a 40-fold increased
induction, while DCM 10 and 100 showed 2.2- and 24.4-fold
inductions of PPARγ ligand binding activity, respectively. However,
80ET, WF and low doses of berberine treatments did not show
dramatic induction in activity. Therefore, the DCM fraction may
exclusively contain a compound that facilitates PPARγ activity of
this plant. Finally, we examined the effect of DCM fraction on
3T3-L1 cell differentiation and found an elevated fat accumulation
as assessed by oil-red O staining (Fig. 3C), indicating that DCM treatment
increases PPARγ activation during adipocyte differentiation.
Discussion
Natural products have played an important role in
drug discovery and development. Specifically, C. fenestratum
is a widely used medicinal plant in Southeast Asia (27). The major constituents of the stem
are protoberberine alkaloids such as berberine, palmatine and
jatrorrhizine (26,28–30).
In folkloric medicine, people favor the use of the stems and roots
of C. fenestratum rather than the leaves due to their
greater alkaloid content. Previous study has shown that the stem of
C. fenestratum exerts an anti-proliferative effect against
lung carcinoma and/or the lung metastatic cell lines A549, LLC and
B16-BL6 via the induction of morphological change and DNA
fragmentation (31). Our study
showed that the extracts of this plant, including 80ET, DCM, WF and
berberine, inhibit cell proliferation and further provide potential
targets in colorectal cancer. NAG-1 and ATF3 have pro-apoptotic and
anti-tumorigenic activities in colorectal cancer cells and other
cell lines (3,8,9,32).
Of note, both ATF3 and NAG-1 are induced by the green tea catechin
ECG and by indole-3-carbinol treatment, supporting the concept that
ATF3 and NAG-1 play a role in phytochemical-induced apoptosis and
are important molecular target proteins of chemopreventive
compounds. In addition, all three extracts and berberine strongly
reduced cyclin D1 expression in HCT-116 and SW480 cells. Cyclin D1
plays a role in the regulation of cell growth (33), and plant-derived compounds
effectively block cell cycle progression by inhibiting the
expression of cyclin D1 (32,34,35).
Since all the extracts showed a similar effect on
anti-proliferation, berberine may be one of the active constituents
causing cyclin D1 suppression. However, only DCM increases both
NAG-1 and ATF3 expression in SW480 cells (Fig. 2D). The difference between SW480 and
HCT-116 cells is p53 expression. Wild-type p53 gene is expressed in
HCT-116 cells, whereas SW480 cells produce a mutant p53 gene. Thus,
80ET and WF increase NAG-1 and ATF3 expression via p53 tumor
suppressor proteins, whereas DCM may have the activity to increase
NAG-1 and ATF3 expression in a p53-independent manner. Indeed, both
NAG-1 and ATF3 are known to be regulated by p53 tumor suppressor
protein at the transcription level (36,37).
In this study, we demonstrated that the DCM fraction
exhibited considerable PPARγ-binding activity and the induction of
pro-apoptotic genes. Although Yin et al reported that
berberine is capable of exerting a glucose-lowering effect in
hepatocytes that is insulin independent, similar to that of
metformin, and can stimulate apoptosis in several cancer cells
(38), our data strongly suggest
that berberine is a weak PPARγ ligand and NAG-1/ATF3 inducer,
compared to the DCM fraction (Figs.
2 and 3B). This suggests that
components in the DCM fraction play a role in anti-proliferative
activity through PPARγ binding ability. Further studies are
required to clarify the molecular mechanisms of individual
compounds from the DCM fraction.
In conclusion, this study provides information on
the anti-proliferative effect of C. fenestratum. Berberine
may be an active constituent of the plant, possessing
anti-proliferative activity. However, the DCM fraction shows
improved anti-proliferation activity in colorectal cancer cells.
The DCM fraction of the C. fenestratum stem extract appears
to be a most promising fraction for consideration as an
anti-tumorigenic agent in future cancer management.
Abbreviations:
|
NSAID
|
non-steroidal anti-inflammatory
drug;
|
|
NAG-1
|
NSAID-activated gene-1;
|
|
ATF3
|
activating transcription factor 3;
|
|
PPARγ
|
peroxisome proliferator-activated
receptor γ;
|
|
DCM
|
dichloromethane;
|
|
ET
|
ethanolic extract;
|
|
WF
|
aqueous fraction;
|
|
DMSO
|
dimethyl sulfoxide
|
Acknowledgements
We thank Misty R. Bailey (University
of Tennessee) for her critical reading of the manuscript. This work
was supported by the Center of Excellence in Livestock Diseases and
Human Health from the University of Tennessee. Financial support
for P. Rojsanga was provided by the Royal Golden Jubilee Ph.D.
Program (PHD/0235/2544), Thailand.
References
|
1.
|
Wattanathorn J, Uabundit N, Itarat W,
Mucimapura S, Laopatarakasem P and Sripanidkulchai B: Neurotoxicity
of Coscinium fenestratum stem, a medicinal plant used in
traditional medicine. Food Chem Toxicol. 44:1327–1333. 2006.
|
|
2.
|
Punitha IS, Rajendran K and Shirwaikar A
and Shirwaikar A: Alcoholic stem extract of Coscinium
fenestratum regulates carbohydrate metabolism and improves
antioxidant status in streptozotocin-nicotinamide induced diabetic
rats. Evid Based Complement Alternat Med. 2:375–381. 2005.
|
|
3.
|
Baek SJ, Kim KS, Nixon JB, Wilson LC and
Eling TE: Cyclooxygenase inhibitors regulate the expression of a
TGF-beta superfamily member that has proapoptotic and
antitumorigenic activities. Mol Pharmacol. 59:901–908.
2001.PubMed/NCBI
|
|
4.
|
Martinez JM, Sali T, Okazaki R, Anna C,
Hollingshead M, Hose C, Monks A, Walker NJ, Baek SJ and Eling TE:
Drug-induced expression of nonsteroidal anti-inflammatory
drug-activated gene/macrophage inhibitory
cytokine-1/prostate-derived factor, a putative tumor suppressor,
inhibits tumor growth. J Pharmacol Exp Ther. 318:899–906. 2006.
View Article : Google Scholar
|
|
5.
|
Baek SJ, Kim JS, Jackson FR, Eling TE,
McEntee MF and Lee SH: Epicatechin gallate-induced expression of
NAG-1 is associated with growth inhibition and apoptosis in colon
cancer cells. Carcinogenesis. 25:2425–2432. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Baek SJ, Wilson LC and Eling TE:
Resveratrol enhances the expression of non-steroidal
anti-inflammatory drug-activated gene (NAG-1) by increasing the
expression of p53. Carcinogenesis. 23:425–434. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Piyanuch R, Sukhthankar M, Wandee G and
Baek SJ: Berberine, a natural isoquinoline alkaloid, induces NAG-1
and ATF3 expression in human colorectal cancer cells. Cancer Lett.
258:230–240. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Lee SH, Cekanova M and Baek SJ: Multiple
mechanisms are involved in 6-gingerol-induced cell growth arrest
and apoptosis in human colorectal cancer cells. Mol Carcinog.
47:197–208. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Cho KN, Sukhthankar M, Lee SH, Yoon JH and
Baek SJ: Green tea catechin (-)-epicatechin gallate induces tumour
suppressor protein ATF3 via eGR-1 activation. Eur J Cancer.
43:2404–2412. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Lu D, Wolfgang CD and Hai T: Activating
transcription factor 3, a stress-inducible gene, suppresses
Ras-stimulated tumorigenesis. J Biol Chem. 281:10473–10481. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Alao JP: The regulation of cyclin D1
degradation: roles in cancer development and the potential for
therapeutic invention. Mol Cancer. 6:242007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Arber N, Sutter T, Miyake M, Kahn SM,
Venkatraj VS, Sobrino A, Warburton D, Holt PR and Weinstein IB:
Increased expression of cyclin D1 and the Rb tumor suppressor gene
in c-K-ras transformed rat enterocytes. Oncogene. 12:1903–1908.
1996.PubMed/NCBI
|
|
13.
|
Ratschiller D, Heighway J, Gugger M,
Kappeler A, Pirnia F, Schmid RA, Borner MM and Betticher DC: Cyclin
D1 overexpression in bronchial epithelia of patients with lung
cancer is associated with smoking and predicts survival. J Clin
Oncol. 21:2085–2093. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Huang JW, Shiau CW, Yang YT, Kulp SK, Chen
KF, Brueggemeier RW, Shapiro CL and Chen CS: Peroxisome
proliferator-activated receptor gamma-independent ablation of
cyclin D1 by thiazolidinediones and their derivatives in breast
cancer cells. Mol Pharmacol. 67:1342–1348. 2005. View Article : Google Scholar
|
|
15.
|
Deep G, Singh RP, Agarwal C, Kroll DJ and
Agarwal R: Silymarin and silibinin cause G1 and G2-M cell cycle
arrest via distinct circuitries in human prostate cancer PC3 cells:
a comparison of flavanone silibinin with flavanolignan mixture
silymarin. Oncogene. 25:1053–1069. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Stepulak A, Sifringer M, Rzeski W,
Endesfelder S, Gratopp A, Pohl EE, Bittigau P, Felderhoff-Mueser U,
Kaindl AM, Buhrer C, Hansen HH, Stryjecka-Zimmer M, Turski L and
Ikonomidou C: NMDA antagonist inhibits the extracellular
signal-regulated kinase pathway and suppresses cancer growth. Proc
Natl Acad Sci USA. 102:15605–15610. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Barak Y, Nelson MC, Ong ES, Jones YZ,
Ruiz-Lozano P, Chien KR, Koder A and Evans RM: PPAR gamma is
required for placental, cardiac, and adipose tissue development.
Mol Cell. 4:585–595. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Bren-Mattison Y, van Putten V, Chan D,
Winn R, Geraci MW and Nemenoff RA: Peroxisome
proliferator-activated receptor-gamma [PPAR(gamma)] inhibits
tumorigenesis by reversing the undifferentiated phenotype of
metastatic non-small cell lung cancer cells (NSCLC). Oncogene.
24:1412–1422. 2005.
|
|
19.
|
Vignati S, Albertini V, Rinaldi A, Kwee I,
Riva C, Oldrini R, Capella C, Bertoni F, Carbone GM and Catapano
CV: Cellular and molecular consequences of peroxisome
proliferator-activated receptor-gamma activation in ovarian cancer
cells. Neoplasia. 8:851–861. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Elstner E, Muller C, Koshizuka K,
Williamson EA, Park D, Asou H, Shintaku P, Said JW, Heber D and
Koeffler HP: Ligands for peroxisome proliferator-activated
receptorgamma and retinoic acid receptor inhibit growth and induce
apoptosis of human breast cancer cells in vitro and in BNX mice.
Proc Natl Acad Sci USA. 95:8806–8811. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Baek SJ, Wilson LC, Hsi LC and Eling TE:
Troglitazone, a peroxisome proliferator-activated receptor gamma
(PPAR gamma) ligand, selectively induces the early growth
response-1 gene independently of PPAR gamma. A novel mechanism for
its anti-tumorigenic activity. J Biol Chem. 278:5845–5853. 2003.
View Article : Google Scholar
|
|
22.
|
Sarraf P, Mueller E, Smith WM, Wright HM,
Kum JB, Aaltonen LA, De la Chapelle A, Spiegelman BM and Eng C:
Loss-of-function mutations in PPAR gamma associated with human
colon cancer. Mol Cell. 3:799–804. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Cekanova M, Yuan JS, Li X, Kim K and Baek
SJ: Gene alterations by peroxisome proliferator-activated receptor
gamma agonists in human colorectal cancer cells. Int J Oncol.
32:809–819. 2008.PubMed/NCBI
|
|
24.
|
Yamaguchi K, Lee SH, Eling TE and Baek SJ:
A novel peroxisome proliferator-activated receptor gamma ligand,
MCC-555, induces apoptosis via posttranscriptional regulation of
NAG-1 in colorectal cancer cells. Mol Cancer Ther. 5:1352–1361.
2006. View Article : Google Scholar
|
|
25.
|
Rungsimakan S: Pharmacognostic Properties
of Khamin Khruea. Department of Pharmacognosy, Chulalongkorn
University; Bangkok: pp. 1882001
|
|
26.
|
Rojsanga P, Gritsanapan W and Suntornsuk
L: Determination of berberine content in the stem extracts of
Coscinium fenestratum by TLC densitometry. Med Princ Pract.
15:373–378. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Wongcome T, Panthong A, Jesadanont S,
Kanjanapothi D, Taesotikul T and Lertprasertsuke N: Hypotensive
effect and toxicology of the extract from Coscinium
fenestratum (Gaertn.) Colebr. J Ethnopharmacol. 111:468–475.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Malhotra S, Taneja SC and Dhar KL: Minor
alkaloids from Coscinium fenestatum. Phytochemistry.
28:1998–1999. 1989. View Article : Google Scholar
|
|
29.
|
Pinho PMM, Pinto MMM, Kijjoa A, Pharadai
K, Diaz JG and HerZ W: Protoberberine alkaloids from Coscinium
fenestratum. Phytochemistry. 31:1403–1407. 1992. View Article : Google Scholar
|
|
30.
|
Siwon J, Verpoorte R, van Essen GFA and
Baerheim Svendsen A: Studies on Indonesian medicinal plants III.
The alkaloids of Coscinium fenestratum. Planta Med. 38:24–32. 1980.
View Article : Google Scholar
|
|
31.
|
Ueda JY, Tezuka Y, Banskota AH, Le Tran Q,
Tran QK, Harimaya Y, Saiki I and Kadota S: Antiproliferative
activity of Vietnamese medicinal plants. Biol Pharm Bull.
25:753–760. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Baek SJ, Okazaki R, Lee SH, Martinez J,
Kim JS, Yamaguchi K, Mishina Y, Martin DW, Shoieb A, McEntee MF and
Eling TE: Nonsteroidal anti-inflammatory drug-activated gene-1 over
expression in transgenic mice suppresses intestinal neoplasia.
Gastroenterology. 131:1553–1560. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Morgan DO: Principles of CDK regulation.
Nature. 374:131–134. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Shukla S and Gupta S: Apigenin-induced
cell cycle arrest is mediated by modulation of MAPK, PI3K-Akt and
loss of cyclin D1 associated retinoblastoma dephosphorylation in
human prostate cancer cells. Cell Cycle. 6:1102–1114. 2007.
View Article : Google Scholar
|
|
35.
|
Lim YC, Lee SH, Song MH, Yamaguchi K, Yoon
JH, Choi EC and Baek SJ: Growth inhibition and apoptosis by
(-)-epicatechin gallate are mediated by cyclin D1 suppression in
head and neck squamous carcinoma cells. Eur J Cancer. 42:3260–3266.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Baek SJ, Wilson LC and Eling TE:
Resveratrol enhances the expression of non-steroidal
anti-inflammatory drug-activated gene (NAG-1) by increasing the
expression of p53. Carcinogenesis. 23:425–432. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Yan C, Jamaluddin MS, Aggarwal B, Myers J
and Boyd DD: Gene expression profiling identifies activating
transcription factor 3 as a novel contributor to the proapoptotic
effect of curcumin. Mol Cancer Ther. 4:233–241. 2005.PubMed/NCBI
|
|
38.
|
Yin J, Hu R, Chen M, Tang J, Li F, Yang Y
and Chen J: Effects of berberine on glucose metabolism in vitro.
Metabolism. 51:1439–1443. 2002. View Article : Google Scholar : PubMed/NCBI
|