Introduction
Since hybrid capture II (HC-II) was approved by the
US Food and Drug Administration (FDA) in 1999, it has been utilized
in China for nearly 10 years. At present, HC-II is the most
advanced viral DNA detection technique, and also the only technique
used in clinical human papillomavirus (HPV) DNA detection.
Epidemiologic studies have shown that cervical cancer is an
infectious disease. Persistent infection of high-risk (HR)-HPV is a
necessary condition of cervical cancer and precancerous lesions –
cervical intraepithelial neoplasia (CIN) (1). HPV DNA detection by use of HC-II has
played an important role in the screening of cervical cancer and
CINs (2). The application of HC-II
has reached a consensus in some clinical fields: i) combined use
with liquid-based cytology (LBC) in cervical cancer screening to
improve sensitivity and specificity; ii) use in a triage of
patients with atypical squamous cells of unknown significance or
more severe cytological lessions; iii) use in the follow-up of the
patients with CIN II or more severe histological lessions. However,
there is controversy as to how to analyze and assess the viral load
of HR-HPV by use of HC-II and the relation between viral load and
cervical lesions. At present, there are three viewpoints. i) Viral
loads of HR-HPV DNA increase with the severity of cervical
neoplasias (3,4). ii) Both cervical cancer and CINs are
highly influenced by HR-HPV viral loads (5). iii) From cervical inflammation to
CINs, the titer of the HC-II test does not demonstrate a clear
line, but the higher the titer, the greater possibility of
high-grade squamous intraepithelial lesions (6). In this study, we analyzed the results
of a sequential screening of outpatients at the Department of
Obstetrics and Gynecology of the China-Japan Friendship Hospital,
and we aimed to explore the relationship between HR-HPV viral load
and the severity of cervical lesions, and to clarify the clinical
significance of the titer of HR-HPV DNA determined by HC-II.
Materials and methods
Study population
From September 2006 to March 2009, 2,761 women who
visited the Department of Obstetrics and Gynecology of the
China-Japan Friendship Hospital consented to cervical cancer
screening using HC-II and LBC. Written informed consent was
obtained from all patients. All of the cases met the following
criteria: defined sexual behavior, no current pregnancy, no
previous hysterectomy, and no previous ablative or excisional
therapy of the cervix. The mean age of the 2,761 women was
39.25±10.08 years of age (range, 17–80 years).
Cytology
A sample of drop epidermic cells from the ectocervix
and endocervix was obtained by performing complete rotations with a
cervical cytobrush and immediately immersed in fixative solution.
Thin-layer cytology specimens were obtained using the Surepath
liquid-based cytology and Thinprep liquid-based cytology tests.
Cytologic diagnosis was made according to the cervical cytology
criteria of the 2001 Bethesda System (7).
HPV DNA testing using hybrid capture
II
HR-HPV DNA detection was performed using the
automated HC-II test system (Qiagen, Gaithersburg, MD, USA). The
samples were analyzed for the 13 most common HR-HPV DNA types: 16,
18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68. Specimens were
considered positive for HPV infection when the relative lighting
unit (RLU) value was equal to or greater than the positive control
(PC), which corresponds to 1 ng/l of HPV DNA (∼4700 copies of the
HPV genome/ml assay).
Colposcopy and biopsy
Colposcopic examination of the cervix was performed
in women with either HR-HPV-positive results or abnormalities in
cytology, or both. Negative results in both cytology and HR-HPV
testing predicted a low risk of cervical neoplasia. A punch biopsy
was taken from key sites of acetowhite epithelium, mosaic,
punctation, leukoplakia and atypical vessels that were represented
after daubing with 5% acetum. If no abnormal lesion was found, a
punch biopsy was taken from positions 2, 4, 8 and 10 o’clock of the
cervix according to routine biopsy methods. Endocervical canal
curettage was performed when colposcopic examination was
unsatisfactory. The patients with high-grade lesions accepted loop
electrosurgical excision procedure (LEEP), or cold knife conization
(CKC), or even hysterectomy for further diagnosis and treatment.
The most severe diagnosis was determined as final histopathological
diagnosis. The diagnostic criteria of high-grade lesions were
defined as equal to or more severe than CIN II.
Statistical analysis
Based on the criteria of histopathology, the
sensitivity, specificity, positive-predictive value and
negative-predictive value were calculated. The difference of mean
data was estimated by the t-test, and HPV test results and final
histologic diagnoses were analyzed by the χ2 test. Based
on the quartiles of the logarithms of RLU/PC, the positive cases of
HR-HPV DNA were divided into 3 groups: low-degree loads
(log10RLU/PC =0–1.73), moderate-degree loads
(log10RLU/PC =1.74–2.78) and high-degree loads
(log10RLU/PC =2.79–3.73). The patients with negative HPV
(log10RLU/PC <0) were considered as the normal
control group. Statistical analysis software SPSS 13.0 was used.
P<0.05 was considered statistically significant.
Results
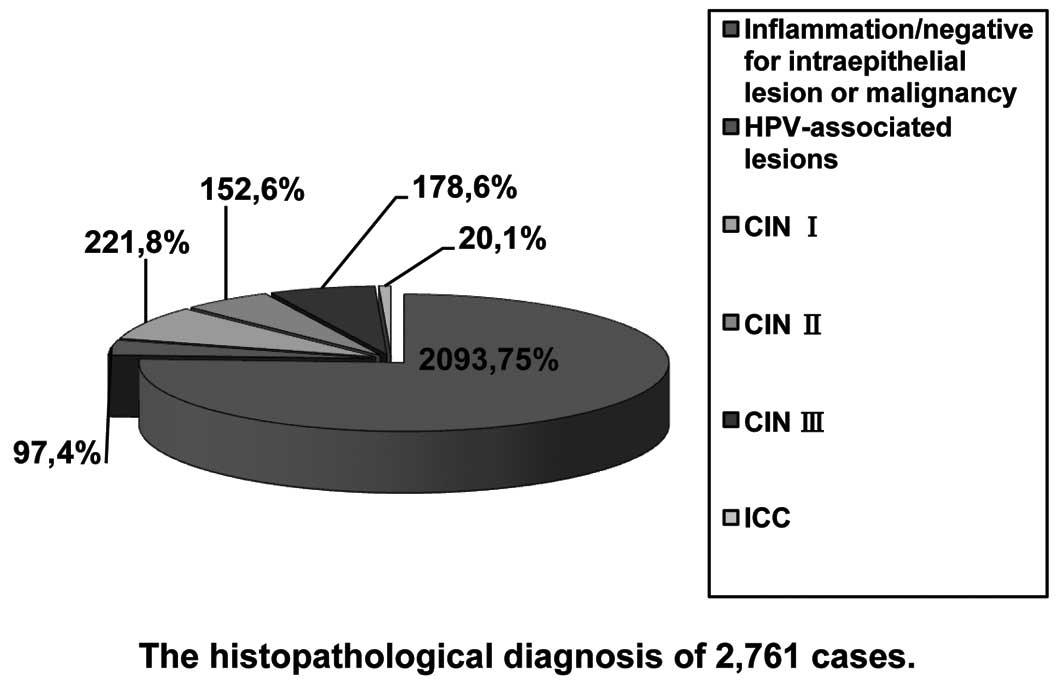
Histopathological results
Cervical biopsies were obtained upon consent in
1,051 women by colposcopy. HPV-associated lesions implied transient
HPV infection in the uterine cervix. Final pathological diagnoses
included HPV-associated lesions in 97 cases, CIN I in 221 cases,
CIN II in 152 cases, CIN III in 178 cases, invasive cervical cancer
(ICC) in 20 cases and inflammation in 383 cases, respectively. A
total of 1,710 women both LBC- and HR-HPV-negative had an extremely
low-risk to develop high-grade CINs during the next several years
(8). We considered them to be
negative for intraepithelial lesions or malignancy (Fig. 1).
Age analysis
The distribution of patient age with different
histopathological diagnoses is shown in Table I.
 | Table I.Age and cervical lesions. |
Table I.
Age and cervical lesions.
| Histopathological
diagnosis | Mean ± SD | Age range | No. | P-value |
|---|
| Inflammation/negative
for intraepithelial lesion or malignancy | 39.63±10.36 | 17–80 | 2093 | |
| HPV-associated
lesions | 37.68±9.03 | 23–74 | 97 | 0.041a |
| CIN I | 36.61±9.38 | 20–59 | 221 | |
| CIN II | 38.45±9.01 | 19–65 | 152 | |
| CIN III | 39.13±8.52 | 20–71 | 178 | |
| ICC | 41.24±12.41 | 29–60 | 20 | 0.043b |
Morphological analysis
Pathological change with kiolocytosis was found in
76.92% (170/221) of CIN I, 65.13% (99/152) of CIN II and 38.20%
(68/178) of CIN III cases, respectively (χ2=63.621,
P<0.01). Gland involvement in the cervix was found in 3.17%
(7/221) of CIN I, 23.03% (35/152) of CIN II and 74.72% (133/178) of
CIN III cases, respectively (χ2=240.281, P<0.01).
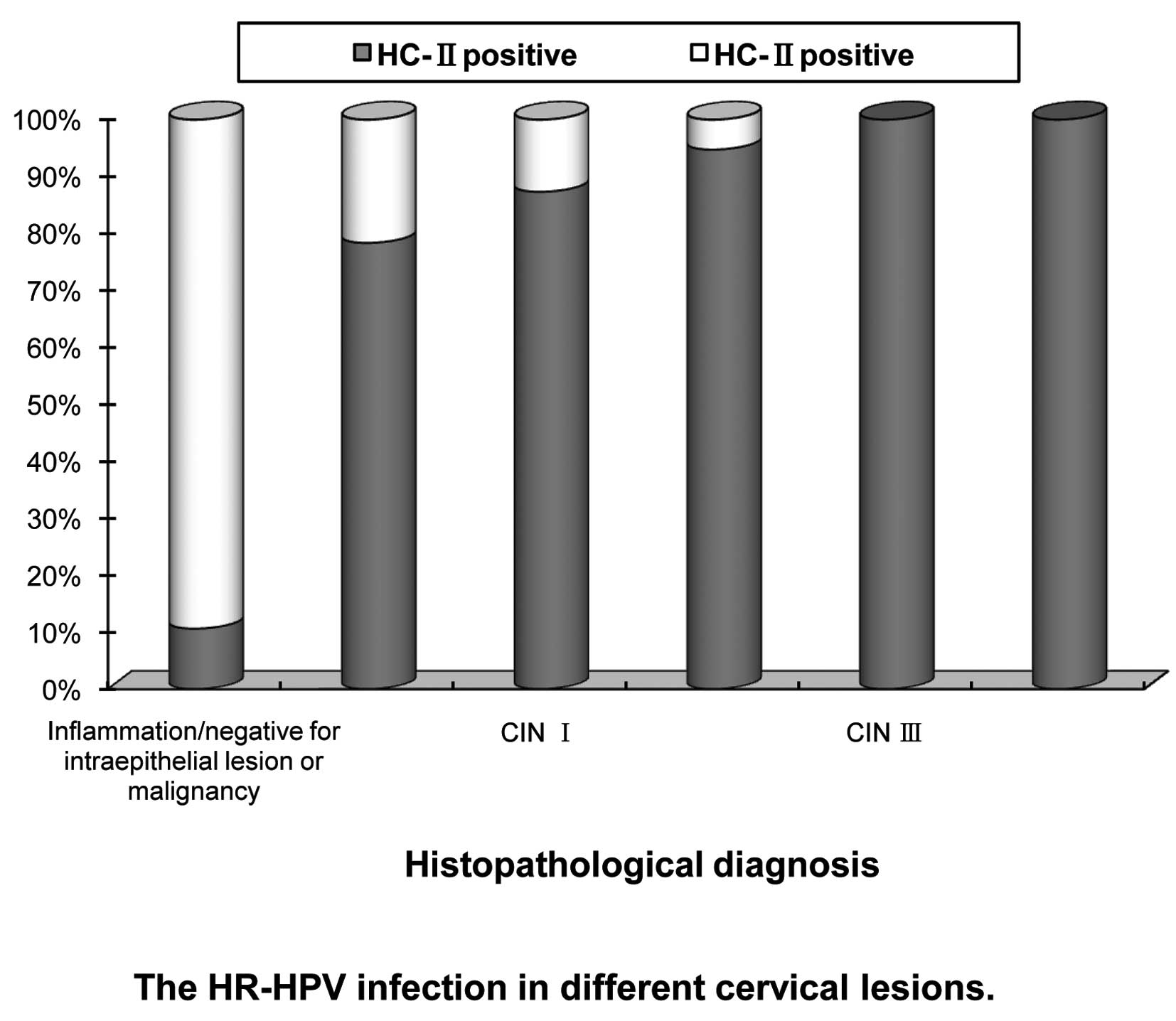
Results of hybrid capture II test
Positive HR-HPV DNA was found in 30.17% (833/2761)
of all cases. The positive rates were 78.35% (76/97) in
HPV-associated lesions, 87.33% (193/221) in CIN I, 94.74% (144/152)
in CIN II, 100% (178/178) in CIN III and 100% (20/20) in ICC cases,
respectively (χ2=46.781, P<0.01), while it was only
10.61% (222/2093) in inflammation/negative for intraepithelial
lesion or malignancy cases (Fig.
2).
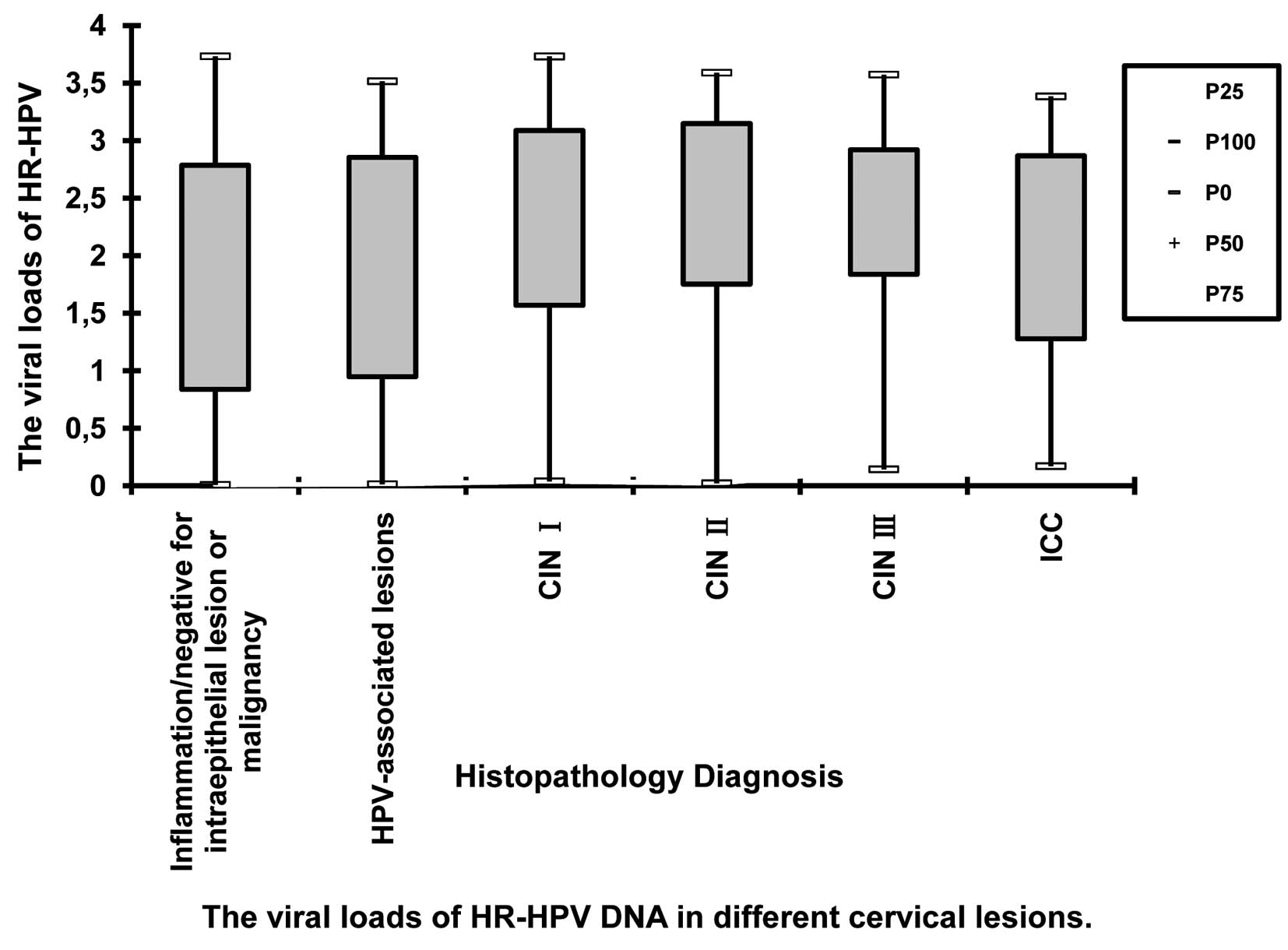
Analysis of viral loads
The viral loads of HR-HPV DNA tested by HC-II are
listed in Table II. The median
log10RLU/PC valuewas 1.77 in inflammation/negative for
intraepithelial lesion or malignancy cases, 1.83 in HPV-associated
lesions, 2.44 in CIN I, 2.65 in CIN II, 2.44 in CIN III and 2.13 in
ICC cases, respectively. The distribution of viral loads of HR-HPV
DNA in different cervical lesions is shown in Fig. 3.
 | Table II.Range of RLU/PC in different cervical
lesions. |
Table II.
Range of RLU/PC in different cervical
lesions.
| Histopathological
diagnosis | RLU/PC (mean ±
SD) | RLU/PC range | Median RLU/PC | No. |
|---|
| Inflammation/negative
for intraepithelial lesion or malignancy | 424.26±721.19 | 1.02–5399.48 | 59.46 | 222 |
| HPV-associated
lesions | 512.15±764.19 | 1.03–3268.43 | 67.78 | 76 |
| CIN I | 753.95±978.27 | 1.09–5387.61 | 276.37 | 193 |
| CIN II | 871.08±1003.52 | 1.05–3884.58 | 448.52 | 144 |
| CIN III | 603.40±740.25 | 1.39–3743.53 | 272.86 | 178 |
| ICC | 466.44±673.05 | 1.48–2414.92 | 135.08 | 20 |
The positive cases were divided into 3 groups:
low-degree loads, moderate-degree loads and high-degree loads,
respectively. The distributions of viral loads in different
cervical lesions are shown in Table
III.
 | Table III.The distributions of viral loads of
HR-HPV in different cervical lesions. |
Table III.
The distributions of viral loads of
HR-HPV in different cervical lesions.
|
log10RLU/PC
|
|---|
| 0.00–1.73 | 1.74–2.78 | 2.79–3.73 | Total |
|---|
| Inflammation/negative
for intraepithelial lesionor malignancy (%) | 107 (48.20) | 59 (26.58) | 56 (25.22) | 222 |
| HPV-associated
lesions (%) | 37 (48.69) | 16 (21.05) | 23 (30.26) | 76 |
| CIN I (%) | 54 (27.98) | 67 (34.71) | 72 (37.31) | 193 |
| CIN II (%) | 35 (24.31) | 47 (32.64) | 62 (43.05) | 144 |
| CIN III (%) | 37 (20.78) | 81 (45.51) | 60 (33.71) | 178 |
| ICC (%) | 7 (35) | 7 (35) | 6 (30) | 20 |
The differences in HR-HPV viral load distribution in
different cervical lesions were statistically significant
(χ2=57.957, P<0.01). There was no statistical
significance between inflammation/negative for intraepithelial
lesion or malignancy cases and HPV-associated lesions
(χ2=1.231, P=0.540). Also, there was no statistical
significance between CINs and ICC (CIN I vs. CIN II,
χ2=1.212, P=0.545; CIN II vs. CIN III,
χ2=5.592, P=0.061; CIN III vs. ICC, χ2=2.155,
P=0.340). However, the difference between HPV-associated lesions
and CIN I was statistically significant (χ2=10.974,
P=0.004).
The range of RLU/PC values tested by HC-II was
widely distributed from one to thousands in different cervical
lesions. When the data were transformed to logarithms, there was no
statistical significance between CINs and ICC. This implied that
the viral loads of HR-HPV DNA had no correlation with the severity
of the cervical lesions. In inflammation/negative for
intraepithelial lesion or malignancy cases and HPV-associated
lesions, the viral loads of HR-HPV were low-degree loads when
analyzed by the median RLU/PC, or analyzed by the Chi-square test
among different groups. But there was statistically significant
difference between moderate-degree loads in CINs and high-degree
loads in ICC.
The data showed that the positive HR-HPV rate in
women >40 year of age was the highest (43.46%). But there was no
statistical significance between the viral loads of HR-HPV and
patient age (χ2=3.968, P=0.410) (Table IV).
 | Table IV.Age and viral loads of HR-HPV DNA. |
Table IV.
Age and viral loads of HR-HPV DNA.
| Age
| |
|---|
|
log10RLU/PC | <30 (%) | 30–40 (%) | >40 (%) | Total (%) |
|---|
| 0.00–1.73 | 54 (19.49) | 92 (33.22) | 131 (47.29) | 277 (33.25) |
| 1.74–2.78 | 53 (19.13) | 103 (37.18) | 121 (43.69) | 277 (33.25) |
| 2.79–3.73 | 63 (22.58) | 106 (37.99) | 110 (39.43) | 279 (33.50) |
| Total | 170 (20.41) | 301 (36.13) | 362 (43.46) | 833 |
Discussion
HPV infection and cervical
intraepithelial neoplasias
Based on their association with cervical cancer and
precursor lesions, HPV may be divided into high-risk (HR)-HPV and
low-risk (LR)-HPV types. LR-HPV infection often results in
condyloma acuminatum of the reproductive organs and low-grade
lesions of the cervix. It often has obvious features of morphology.
The classical characteristic is appearance of kiolocytosis in
malpighian epithelium. It is characterized by karyomegaly, nuclear
enlargement with binucleation, irregularities in the nuclear
membrane and hyperchromasia. Perinucleus cavity may be found, and
kiolocytosis has diagnostic significance. But HR-HPV infection may
be short of morphological evidence. If HR-HPV infection continues
for 8–10 years, high-grade CIN (CIN II and III) may occur, and
invasive cervical cancer may progress within 5–10 years. Some
studies revealed that HPV infection exists in 99.80% of cervical
cancer cases (9).
In this study, the morphological concomitance of
kiolocytosis was 76.92% in CIN I, 65.13% in CIN II and 38.20% in
CIN III cases, respectively. This implied that LR-HPV infection
decreases significantly as the CIN grade progresses. However, in
our study positive HR-HPV tested by HC-II was 87.33% in CIN I,
94.74% in CIN II and 100% in CIN III cases, respectively. This
implied that the infection of HR-HPV increases as the lesion
progresses. These results prove that LR-HPV infection is correlated
with low-grade lesions (CIN I), while HR-HPV infection is necessary
for high-grade lesions (CINs II and III) and cervical cancer.
HR-HPV infection played a major role in the occurrence and
progression of cervical cancer. Morphological study showed that
with the progression of CINs, cervical gland involvement
predominantly increased. This demonstrated that invasion of
high-grade lesions might increase, and these lesions should be
treated more actively.
Relationship between the viral loads and
cervical intra-epithelial neoplasias and invasive cervical
cancer
The range of RLU/PC values was widely distributed in
this study from one to thousands including the normal cervix,
inflammation of the cervix, CINs and ICC. Thus, the viral loads
could not be used to define whether the lesion was benign or
malignant or its severity. To investigate the relation between
viral loads and lesions, RLU/PC values were transformed into
logarithms and categorized into four groups. It was found that
there was no statistical significance between inflammation/negative
for intraepithelial lesion or malignancy cases and HPV-associated
lesions, and the viral loads of these groups were at a low level.
The log10RLU/PC values were 1.77 and 1.83, and this
level was lower than those of CINs and ICC. The viral loads of CINs
and ICC were at a moderate or high level. There was no relationship
between the viral loads and the severity of cervical lesions. In
this study log10RLU/PC values of CINs were higher than
that of ICC. This might have been partly due to the relatively
fewer cases of ICC. However, the median log10RLU/PC
value for CIN I was the same as that for CIN III; both were 2.44
and lower than that in CIN II (2.65). This showed that the
log10RLU/PC value does not reflect the severity of
cervical lesions. On the other hand, due to the limitations of
histopathology, the actual lesion sample was at times not acquired
by biopsy and might induce positive results for HC-II with negative
results for histopathology in some cases.
Clinical significance of HR-HPV DNA
testing using hybrid capture II
The threshold value of RLU/PC is defined by 1.0. But
RLU/PC <1.0 does not mean absence of HPV infection. RLU/PC
<1.0 means 4700 HPV copies/ml. According to this threshold
value, we could optimally evaluate the sensitivity and specificity
for the risk of high-grade cervical lesions. Some studies showed
(8,10) that better sensitivity and
specificity could not be obtained by increasing the threshold value
of RLU/PC. Therefore, HR-HPV DNA tested by HC-II had clinical
significance at RLU/PC ≥1. Based on the histopathology in this
study, the sensitivity of HR-HPV DNA tested by HC-II for detecting
high-grade cervical lesions was 97.71%, the specificity was 79.64%,
the positive-predictive value was 41.06%, and the
negative-predictive value was 99.59%, respectively. A vast amount
of data in China and other countries revealed that the main
advantage of HR-HPV DNA detection is its extremely high
negative-predictive value (11–15).
It is possible to infer that individuals with negative HR-HPV have
an extremely low risk of cervical cancer. In this study, the
sensitivity and negative-predictive value were very high. Thus, as
HC-II is a simple accessible method, we can apply it independently
for the screening of cervical cancer and precancerous lesions. In
our study, patients in the CIN I group were the youngest in age,
and the range of RLU/PC values was widely distributed. This implied
that the HPV virus might be in a free state in infected young
women. The virus might be duplicated temporarily in a large amount,
but might be eliminated by the host through the autoimmunity
mechanism. High-grade cervical lesions would occur only when the
viral DNA integrated with the host DNA. Studies have shown that
persistent HR-HPV infection is a necessary condition of CINs and
cervical cancer (16). LEEP and
CKC have become routine treatment for CINs, but lesions may recur
after clinical treatment. How to monitor cases with persistent
HR-HPV infection and follow up cases after treatment is still
unclear. Yet, HC-II testing offers a reliable method for the
management and follow-up after treatment of cervical precancerous
lesions.
In conclusion, the viral loads of HR-HPV DNA tested
by HC-II had no correlation with the severity of cervical lesions.
The viral load of inflammatory cervical lesions was markedly lower
than those of CINs and ICC. The positive rate of HR-HPV increased
significantly with the progression of cervical lesions. HC-II
testing has important value both in the screening of cervical
lesions and in the management and follow-up after treatment of
cervical precancerous lesions.
References
|
1.
|
Bosch FX, Manos MM, Munoz N, Sherman M,
Jansen AM, Peto J, Schiffman MH, Moreno V, Kurman R and Shah KV:
Prevalence of human papillomavirus in cervical cancer: a worldwide
perspective. International Biological Study on Cervical Cancer
(IBSCC) Study Group. J Natl Cancer Inst. 87:796–802. 1995.
View Article : Google Scholar
|
|
2.
|
Ying J and Lingya P: Role of high-risk
human papillomavirus testing in the screening and management of
cervical cancer precursors. Acta Acade Med Sinic. 29:691–696.
2007.PubMed/NCBI
|
|
3.
|
Zhihong H, Deying Q, Ding W, Danhua H,
Minjian C and Yanhong SH: Study of the relationship between loads
of human papilloma virus in cervical carcinoma and cervical
intraepithelial neoplasia. Matern Child Health Care Chin.
21:1557–1559. 2006.
|
|
4.
|
Shumin L, Wenhua ZH, Lingying W, Fanghui
ZH, Manni H, Nan L and Feng CH: Preliminary study on the
relationship between loads of human papillomavirus in cervical
carcinoma and cervical intraepithelial neoplasia. Chin J Obstet
Gynecol. 39:400–402. 2004.
|
|
5.
|
Fanghui ZH, Junfei M, Youlin Q, Shoude R,
Ling L and Wenhua ZH: Association between high-risk human
papillomavirus DNA load and cervical intraepithelial lesion. Chin J
Epidemiol. 25:921–924. 2004.PubMed/NCBI
|
|
6.
|
Li J, Jinghe L, Youfang W and Xuemei CH:
Relationship of HR-HPV DNA loads with the stages of CIN. Reprod
Contrac. 26:422–425. 2006.
|
|
7.
|
Apgar BS, Zoschnick L and Wright TC Jr:
The 2001 Bethesda System terminology. Am Fam Physician.
68:1992–1998. 2003.
|
|
8.
|
Syrjanen S, Shabalova IP, Petrovichev N,
Kozachenko VP, Zakharova T, Paianidi J, Podistov JI, Chemeris G,
Sozaeva LG, Lipova EV, Tsidaeva I, Ivanchenko OG, Pshepurko AA,
Zakharenko S, Nerovina R, Kljukina LB, Erokhina OA, Branovskaja MF,
Nikitina M, Grunberga V, Grunberg A, Juschenko A, Tosi P, Cintorino
M, Santopietro R and Syrjanen KJ: Human papillomavirus testing and
conventional Pap smear cytology as optional screening tools of
women at different risks for cervical cancer in countries of the
former Soviet Union. J Low Genit Tract Dis. 6:97–110. 2002.
View Article : Google Scholar
|
|
9.
|
Tarkanen J, Auvinen E, Nieminen P, Malmi
R, Vartianen J, Timonen T, Laurila P, Raisanen I, Unnerus HA, Sakki
A, Mattila P, van Den, Brule AV and Tapper AM: HPV DNA testing as
an adjunct in the management of patients with low-grade cytological
lesions in Finland. Acta Obstet Gynecol Scand. 86:367–372. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Ordi J, Alonso I, Torne A, Esteve R,
Sierra E, Campo E and Puiq-Tintore LM: Human papillomavirus load in
Hybrid Capture II assay: does increasing the cutoff improve the
test? Gynecol Oncol. 99:313–319. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Yufei C and Guangze ZH: The roles of
high-risk human papillomavirus detection in cervical cancer
screening. Matern Child Health Care Chin. 21:3434–3436. 2006.
|
|
12.
|
Sherman ME, Lorincz AT, Scott DR,
Wacholder S, Castle PE, Glass AG, Mielzynska-Lohnas I, Rush BB and
Schiffman M: Baseline cytology, human papillomavirus testing, and
risk for cervical neoplasia: a 10-year cohort analysis. J Natl
Cancer Inst. 95:46–52. 2003.PubMed/NCBI
|
|
13.
|
Cuzick J, Szarewski A, Cubie H, Hulman G,
Kitchener H, Luesley D, McGoogan E, Menon U, Terry G, Edwards R,
Brooks C, Desai M, Gie C, Ho L, Jacobs I, Pickles C and Sasieni P:
Management of women who test positive for high-risk types of human
papillomavirus: the HART study. Lancet. 362:1871–1876. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Clavel C, Cucherousset J, Lorenzato M,
Caudroy S, Nou JM, Nazeyrollas P, Polette M, Bory JP, Gabriel R,
Ouereux C and Birembaut P: Negative human papillomavirus testing in
normal smears selects a population at low risk for developing
high-grade cervical lesions. Br J Cancer. 90:1803–1808.
2004.PubMed/NCBI
|
|
15.
|
Deying Q, Jianmin C, Ding W, Renhai Z,
Aihua L, Yanhong SH, Danhua H and Zhihong H: Combining high-risk
human papillomavirus DNA test and cytological test to detect early
cervical dysplasia. Chin J Obstet Gynecol. 41:34–37.
2006.PubMed/NCBI
|
|
16.
|
Munoz N, Bosch FX, Sanjose SDE, Herrero R,
Castellsaque X, Shah KV, Snijder PJ and Meijer CJ; the
International Agency for Research on Cancer Multicenter Cervical
Cancer Study Group: Epidemiologic classification of human
papillomavirus types associated with cervical cancer. N Engl J Med.
348:518–527. 2003. View Article : Google Scholar : PubMed/NCBI
|