Introduction
Among the tumors that affect the brain, gliomas of
astrocytic, oligodendroglial and ependymal origin are responsible
for more than 70% of tumor occurrences (1). Of these, astrocytomas are the most
frequent histological type. They are classified according to their
malignant potential and graded on the basis of histological
criteria recommended by the World Health Organization (WHO)
(2). Over the last few years,
knowledge of the mechanisms of tumor progression leading to
astrocytomas has increased. This has led to the supplementation of
histological grade with new potential prognostic markers. Among
these, there has been increasing interest concerning the role of
the quantification of vessels in astrocytomas, with ongoing
investigations being conducted (3).
In 1991, Weidner et al were the first to
report on the prognostic value of measurements of angiogenesis
using a model of primary breast tumors (4). Many investigators have supported the
notion of an inverse correlation between microvascular density
(MVD) and prognosis (5–7), while others have refuted this
(8). MVD has also been shown to be
a prognostic indicator of postoperative survival among patients
with astrocytomas (3,9,10).
However, the quantification of the vascularization of brain tumors
has been limited due to the cell heterogeneity found in these
tumors (9). Other authors have
demonstrated significant differences in MVD between tumors in
children and adults (3).
For brain tumors, MIBI (2-methoxyisobutyl
isonitrile) has been shown to be accurate in identifying recurrence
following treatment for high-grade gliomas, thereby making it
possible to differentiate them from radionecrosis (11), although there are divergent
opinions on this subject (12).
Furthermore, MIBI aids in differentiating between the findings from
neoplastic and non-neoplastic lesions (11,13),
allows tumor grade to be evaluated (11), and assists in predicting
therapeutic response (11,14,15).
The intensity of uptake is related not only to breaking the
blood-brain barrier, but also to tumor metabolic activity (11,16,17).
Thus, MIBI may be used to evaluate the biological characteristics
of brain tumors and helps to determine their proliferative
potential and prognosis (11,18,19).
Because of its molecular characteristics, MIBI is preferentially
absent in mitochondria (20,21).
Since the mitochondrial activity in tumors is usually high, this
generates an increase in the transmembrane gradient, which favors
its accumulation in tumor tissue significantly.
Vascularization is important as a prognostic factor
in many types of neoplasias (3–5,22,23),
but such evaluations are only possible today through direct
analysis of slides using several methods (4,22)
that all have limitations due to the heterogeneity of these tumors
(3). Various imaging techniques
have been proposed as valuable tools for evaluating vascularization
in brain tumors, among them SPECT-MIBI (16,17).
This method has revealed a significant correlation between MIBI
uptake rates and length of survival, according to the
aggressiveness of the recurrent malignant gliomas (24). However, the amount of robust
information on its relationship with MVD remains insufficient, thus
allowing for new perspectives regarding this approach.
The aim of this study was to evaluate MVD in
low-grade astrocytomas (LGAs), anaplastic astrocytomas (AAs) and
multiform glioblastomas (GBMs) using immunohistochemistry with
anti-CD34 monoclonal antibodies to determine the relationship
between immunohistochemical data and the parameters obtained from
SPECT-MIBI.
Materials and methods
Study population and protocol
A study of cross-sectional type was conducted,
within which demographic and KPS (Karnofsky performance scale) data
were gathered retrospectively from 48 patients (29 men and 19
women; mean age 48.8 years, SD 15.9, range 20–73 years) who had
been admitted to the Hospital de Câncer de Barretos - Fundação Pio
XII. None of these patients had undergone any previous surgical or
therapeutic procedures. Only cases of supratentorial gliomas that
had been diagnosed in accordance with WHO criteria (2) as LGAs (of which only diffuse
astrocytomas were selected), AAs or GBMs were included. All
patients underwent brain SPECT with MIBI before any procedures.
Tumor tissue specimens of an adequate quantity were available in
paraffin blocks for all patients. The study was approved by the
ethics committee of the Hospital de Câncer - Fundação Pio XII.
Acquisition of brain SPECT images with
MIBI
Examinations were performed 15 min after intravenous
administration of 720 MBq of MIBI labeled with Tc99m. Tomographic
images were captured using a GE Millenium VG gamma camera equipped
with two high resolution detectors, with a 128x128 matrix and 360°
rotation, thus making it possible to obtain 120 two-dimensional
frames of the brain, 25 sec/frame. The images obtained were
reconstructed in a 128x128 matrix using Butterworth-filtered
back-projection, with a cutoff of 0.25 and order of five (25), in accordance with a semi-automated
protocol furnished by the manufacturer. The examinations were
interpreted by an experienced nuclear medicine physician in the
Department of Nuclear Medicine of Hospital de Câncer - Fundação Pio
XII.
Interpretations were initially performed by visual
analysis and classified as normal or abnormal. The latter were then
subdivided into abnormal images of mild intensity (when uptake
could be discerned, but less than in the scalp), moderate intensity
(when the uptake was as intense as the scalp) and marked intensity
(when the uptake was equivalent to the salivary glands).
Semiquantitative analysis was performed to investigate the mean
count in the tumor area and in a mirror area in the contralateral
hemisphere (14,16,24,26).
This made it possible to obtain an index (tumor/contralateral side
index, T/CL) as the ratio between these measurements.
Histopathological classification
Slides stained with H&E were produced from the
paraffin blocks in order to review the diagnoses and the
histopathological grade. These reviews were performed by two
experienced pathologists following WHO criteria (2).
Immunohistochemical reactions
Sections (3 μm) were cut from the paraffin blocks
from each case. These were mounted on slides and silanized
(3-aminopropyltriethoxilane, A-3648, Sigma, USA) and deparaffinized
in a heated chamber at 60°C for 12 h for immunohistochemical
reactions.
The streptavidin-biotin-peroxidase technique was
used with anti-CD34 class II monoclonal antibodies (clone QBEnd-10,
code m7165, titration 1:800; Dako Cytomation, Glostrup, Denmark).
Antigen recovery was carried out on a Pascal pan, using citrate at
pH 6.0. Amplification was performed using the Advance™ HRP System
(Dako Cytomation).
Microscopic analysis of
immunohistochemical reactions
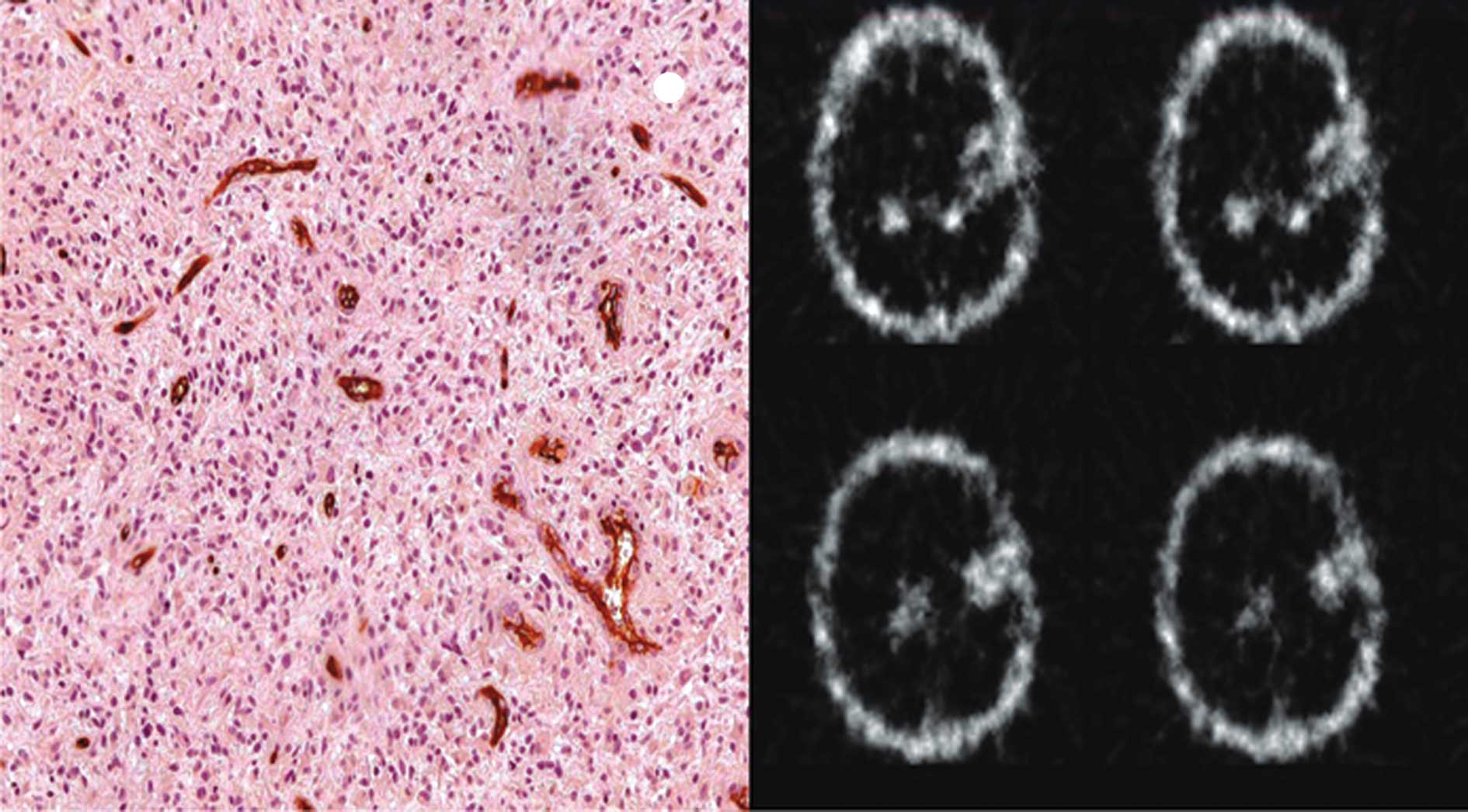
MVD was analyzed as described by Weidner et
al, with some modifications (4). Two observers without knowledge of the
case diagnoses viewed the slides under a dual-view optical
microscope (Olympus BX 41) at a magnification of x40, searching for
the areas of greatest concentration of immunolabeled vessels (hot
spots). At each hot spot, a magnification of x200 was used to
photograph the optical field. A total of three fields were
photographed, one in each hot spot of each tumor. Adobe®
Photoshop 7.0 software was used to open and amplify the images. The
vessels were counted on the computer screen. Individual
microvessels (arterioles and venules) were counted in each of these
areas, and the MVD was determined as the ratio of the total number
of microvessels counted divided by the three fields examined
(17).
Any CD34-positive endothelial cells or groupings of
endothelial cells (clusters) that were clearly separated from the
tumor cells and other glial elements were considered to be a single
countable microvessel. Those that appeared to be derived from the
same vessel, if separate, were also counted. Each fixed lumen was
counted as a single countable microvessel. If there was no lumen,
but only a single CD34-positive cell visible, this cell was also
interpreted as a single microvessel (3,4,17).
Statistical analysis
A descriptive analysis was performed on the data in
terms of frequencies and percentages, central trend and dispersion
measurements, means, medians, standard deviations, minimums and
maximums. The Kolmogorov-Smirnov test was applied to investigate
the adherence of the quantitative variables to a normal
distribution curve. For the dependent variable of MVD, this test
was applied in accordance with the subdivisions of each
demographic, clinical and interventional category (27). For associations between qualitative
variables relating to anatomopathology and visual analysis, the
Fisher’s exact test was applied.
Since some variables did not present a normal
distribution, it was decided to use non-parametric tests. To
compare the means between the independent variables and MVD, the
Mann-Whitney test was applied when the independent variable had two
categories, and the Kruskal-Wallis test was used when the
independent variable had more than two categories. To investigate
correlations between the dependent variable (MVD) and the
tumor/contralateral side (T/CL) index, Spearman’s correlation was
used. Statistical significance was set as p<0.05. Data input,
consistency and analysis were performed using SPSS software for
Windows, version 16.0.
Results
As shown in Table
I, the single forms of clinical presentation most commonly
found were headache (37.5%), motor deficit (25%) and convulsions
(14.6%). Most of these patients (81.3%) presented KPS of ≥70%.
Among the paraffin-block specimens, 75% were obtained from surgical
procedures with full or partial macroscopic resection of the tumor,
and the remainder came from biopsies. GBMs accounted for 24 cases
(50%) of the sample, while the LGA and AA groups represented 16
(33.3%) and 8 cases (16.7%), respectively. Visual analysis of the
SPECT-MIBI examinations did not find any abnormality in 13
examinations (27.1%), while the remaining 35 examinations (72.9%)
presented mild, moderate or marked abnormalities.
 | Table I.Description of the clinical, surgical
and anatomopathological data and visual analysis of the SPECT-MIBI
results. |
Table I.
Description of the clinical, surgical
and anatomopathological data and visual analysis of the SPECT-MIBI
results.
| Variable | n | % |
|---|
| Symptoms or signs
of presentation | | |
| Headache | 18 | 37.5 |
| Convulsions | 7 | 14.6 |
| Motor
deficit | 12 | 25.0 |
| Associated and
other symptoms | 10 | 21.0 |
| Unknown | 1 | 2.1 |
| KPS (%) | | |
| ≤40 | 1 | 2.1 |
| 50–60 | 8 | 16.7 |
| 70–80 | 19 | 39.6 |
| 90–100 | 9 | 18.8 |
| Unknown | 11 | 22.9 |
| Intervention | | |
| Biopsy | 12 | 25.0 |
| Surgery | 36 | 75.0 |
|
Anatomopathology | | |
| Glioblastoma
multiforme | 24 | 50.0 |
| Anaplastic
astrocytoma | 8 | 16.7 |
| Low-grade
astrocytoma | 16 | 33.3 |
| Visual
analysis | | |
| Normal | 13 | 27.1 |
| Mildly
abnormal | 10 | 20.8 |
| Moderately
abnormal | 18 | 37.5 |
| Markedly
abnormal | 7 | 14.6 |
| Total | 48 | 100.0 |
Among these 35 abnormal examinations, the mean count
in the tumor was 1226.1, and in the mirror image it was 285.2. The
mean T/CL index was 5.8. The mean number of vessels found in the 48
slides was 71 vessels/field as shown in Table II.
 | Table II.Description of the mean count
parameters and MVD. |
Table II.
Description of the mean count
parameters and MVD.
| Variable | No. of
examinations | Mean (SD) | Median |
|---|
| MCT | 35 | 1226.1
(1132.3) | 817.0 |
| MCMI | 35 | 285.2 (291.1) | 185.0 |
| T/CL index | 35 | 5.8 (5.4) | 3.9 |
| MVD | 48 | 71.1 (33.9) | 64.9 |
In analyzing the differences in mean MVD in relation
to gender and the interventions that gave rise to the specimens,
there was no significance except in relation to the
anatomopathological variable (p=0.013). However, the MVD for the
GBMs was lower, with a mean of 58.8 vessels/field, while for the AA
and LGA cases it was 107.4 and 71.4 vessels/field, respectively, as
indicated in Table III.
 | Table III.Difference in the mean MVD between
the categories. |
Table III.
Difference in the mean MVD between
the categories.
| Variable | No. | Mean (SD) | Median | p-value |
|---|
| Gender | | | | |
| Male | 29 | 70.7 (33.9) | 71.3 | 0.983a |
| Female | 19 | 71.8 (34.9) | 63.3 | |
| Intervention | | | | |
| Biopsy | 12 | 69.4 (41.1) | 51.1 | 0.668a |
| Surgery | 36 | 71.7 (31.7) | 68.9 | |
|
Anatomopathology | | | | |
| Glioblastoma
multiforme | 24 | 58.8 (20.0) | 55.9 | 0.013b |
| Anaplastic
astrocytoma | 8 | 107.4 (42.4) | 114.1 | |
| Low-grade
astrocytoma | 16 | 71.4 (34.8) | 76.0 | |
The difference in mean MVD between the AAs and LGAs
was significant (p=0.040). However, there was no significant
difference in mean MVD between normal and abnormal examinations
(p=0.057), as shown in Table IV. A
similar analysis for the GBM group did not reach significance
(p=0.295) (result not shown in the Table). Correlation between the
T/CL index and MVD was also non-significant, as shown in Table V.
 | Table IV.Difference in the mean MVD between
anaplastic astrocytomas and low-grade astrocytomas, and between
examinations with normal and abnormal uptake. |
Table IV.
Difference in the mean MVD between
anaplastic astrocytomas and low-grade astrocytomas, and between
examinations with normal and abnormal uptake.
| Variable | No. of
examinations | Mean (SD) | p-value |
|---|
|
Anatomopathology | | | |
| Anaplastic
astrocytoma | 8 | 107.4 (42.40) | 0.040a |
| Low-grade
astrocytoma | 16 | 71.4 (34.82) | |
| Uptake | | | |
| Normal | 10 | 64.9 (34.84) | 0.057a |
| Abnormal | 14 | 96.6 (40.13) | |
 | Table V.Correlations of quantitative
variables with MVD. |
Table V.
Correlations of quantitative
variables with MVD.
| Variable | No. | r-value | p-value |
|---|
| T/CL index in LGA
and AA | 14 | 0.238 | 0.413a |
| T/CL index in
GBM | 21 | −0.084 | 0.716a |
On the other hand, Table VI indicates that there was a
greater likelihood that the GBM group would present abnormal
examination results in relation to the AA and LGA cases (87.5 vs.
58.3%; p=0.049).
 | Table VI.Likelihood of abnormal examination
result in relation to histological type. |
Table VI.
Likelihood of abnormal examination
result in relation to histological type.
| Visual
analysis | Anatomopathology
| |
|---|
| LGA/AA | GBM | |
|---|
|
| |
|---|
| No. (%) | No. (%) | p-valuea |
|---|
| Normal | 10 (41.7) | 3 (12.5) | 0.049 |
| Abnormal | 14 (58.3) | 21 (87.5) | |
| Total | 24 (100.0) | 24 (100.0) | |
Discussion
In the present study, data on 48 adult patients with
supratentorial astrocytomas were evaluated in an attempt to
establish a relationship between MVD in these tumors and the
results of analysis using SPECT-MIBI. Gender did not influence MVD,
which has not been previously discussed in the literature. GBMs
accounted for 50% of the sample evaluated as shown in Table I, although there was no
randomization. Despite the lower diagnostic accuracy provided by
biopsies (28), which suggests
that studies with such samples should be regarded separately
(9), in our study there was no
significant difference in mean MVD between the samples obtained
through biopsy and those obtained through surgery, as shown in
Table III.
There have been discussions on the relationship
between MVD and tumor prognosis, not only outside the central
nervous system (5,7,23),
but also within (3,9,10).
Moreover, the presence of vascular proliferation determined
non-quantitatively distinguishes high- and low-grade astrocytomas
(2). For this reason, it would be
expected that a greater MVD would be obtained with increasing tumor
grade. Such a relationship was found between the LGAs and AAs, but
not for the GBMs, as indicated in Table III. In 2005, Yao et al found
a significant difference only between GBMs and LGAs (10), while Leon et al, considering
only AA and GBM cases in 1996, showed that there was a significant
association between microvascular grade and histology (9). Furthermore, the latter author showed
that microvascular grade and MVD were significant predictors of
postoperative survival, and that the vascular grade was more
intense, which was explained by the existence of glomeruloid
vascular structures. In fact, these structures are clusters of
vessels that influence the choice of hotspots, given that the
microvascular grade is higher in these areas. Nonetheless, they are
counted as single vessels at a higher magnification, thereby
probably resulting in an underestimation of the count of individual
microvessels (9). Extensive areas
of necrosis also confer additional explanations, although, in the
present study, there was constant attention to avoiding areas that
were close to necrosis. The existence of necrosis makes
immunohistochemical analyses difficult (29), while MVD has failed to show any
correlation with histological grade in tumors presenting necrosis
(30). Tumor necrosis, and in
particular large-scale ischemic necrosis, is significantly more
frequent in primary GBMs (31,32).
Therefore, the presence of glomeruloid formations and necrosis,
which were elements observed in the present study, may explain why
there was an underestimation of the microvessel counts in the GBMs.
It was understood that the identification of hot spots, from the
manner in which this was carried out, might not faithfully
represent the appropriate area for counting the vessels inside the
tumor. This would particularly be the case in GBMs, in which the
heterogeneous characteristic of brain tumors appeared to be more
evident.
This heterogeneity has limited the use of
pathological analysis techniques (9), thus explaining why no relationship
between tumor grade and MVD was found by some authors (33). Moreover, different methods have
been used to quantify MVD (17,34),
which leads to a lack of standardization and additional
difficulties in establishing the real role of vessel quantification
as a prognostic indicator.
It is known that 95% of GBMs start out as GBMs,
while only 5% originate from LGAs or AAs. Thus, GBMs may be primary
or secondary. These constitute distinct entities that affect
patients at different ages and develop through different genetic
routes. LGA and AA represent a continuum within the disease, with
similar genetic characteristics, and thus differ from GBM, which
has separate genetic characteristics (35,36).
In the present study, out of the 48 specimens evaluated, there were
24 GBMs, which were studied separately. It is important to note
that, due to the presence of extensive areas of necrosis, it was
not possible to establish a correlation between MVD and the
GBMs.
Anti-CD34 monoclonal antibodies were chosen for the
present study. Although they are non-specific for endothelial
cells, they present good sensitivity for vascular endothelium
(37) and are an efficient
vascular marker for glial tumors (33). Several other markers have been
described, but none have been indicated as the most recommended or
most efficient. Comparative studies on different antibodies have
shown divergent results, particularly with regard to prognosis
(7,23,33).
The main criticism that could be directed towards anti-CD34 is that
it not only marks the neoformed vessels, but also the normal
vessels existing inside the tumor (5,7).
Furthermore, it has been demonstrated that not all neoformed
vessels have evident circulation (17). It should be emphasized that this
peculiar characteristic of anti-CD34 was regarded as a point of
interest by the present authors, since they judged that the
SPECT-MIBI images would be dependent on the vascular supply of the
tumor.
The mechanisms for MIBI uptake are still not well
defined, although some authors have reported that MIBI uptake does
not depend on MVD and only reflects the rupturing of the
blood-brain barrier (16,17). Nonetheless, the existence of
methodological inconsistencies in quantifying MVD means that its
true value in relation to MIBI uptake cannot be assessed with
certainty. MIBI has been widely used to differentiate radionecrosis
from tumor recurrence (11), but
there were three cases of GBM in the present study in which no
abnormal areas of uptake were viewed (Table VI). A similar finding was described
previously (12,17), which indicates the need for caution
in interpreting these examinations.
There was a significant difference in mean MVD
between LGA and AA cases, but a significant difference (p=0.057)
was not noted between uptake and MVD (Table IV). However, we believe that the
sample size may have influenced this result, and that evaluation of
a larger sample would lead to the finding of significance in this
tumor group. In 2004, Staudenherz et al stated that MVD is
not crucial for the scintigraphic viewing of brain tumors using
99mTc-MIBI (17). In their sample,
there were four cases, of which three were astrocytomas and one was
an oligodendroglioma. There were no associations between
histological grade and MVD, and only a descriptive analysis was
made.
The T/CL indices obtained in the present study did
not present any significant correlation and, in addition, the index
was negative for the GBMs (Table V
and Fig. 1). In addition, there
was no significant difference between the means of the examinations
with normal and abnormal uptake among the GBM cases. There have
been many studies on MVD or SPECT-MIBI in brain tumors (3,9,10,12,14,16-19,24-26,33,34,38),
but this is the first that sought to establish a relationship
between MVD and a numerical indicator obtained from
semi-quantitative analysis on SPECT-MIBI images.
We observed that there was a greater likelihood that
GBM cases would present abnormal SPECT-MIBI results in relation to
tumors of lower grade (Table VI).
Histologically, GBMs are characterized by intense vascular
proliferation (2), but no
association with such data could be confirmed by current
immunohistochemical methods due to the limitations cited above.
The results reported in this study must be
interpreted with caution due to a number of methodological
shortcomings that could limit the internal validity of our study,
such as GBM extensive necrosis, MVD quantification and the small
number of specimens analyzed. Therefore, it was not possible to
fully ascertain whether the use of the SPECT-MIBI approach may
serve as an adjunctive resource to the clinical armamentarium.
In conclusion, the results from the present study
indicate that the use of MVD, determined immunohistochemically, had
a significant relationship with histological grade in the LGAs and
AAs, but not in relation to the GBMs. On the other hand, the
association between SPECT-MIBI and the MVD of the LGA and AA cases
was not significant, nor was the association between SPECT-MIBI and
the MVD of the GBM cases.
Acknowledgements
Special thanks to Dr Edmundo Carvalho
Mauad for the encouragement and dedication to academic
training.
References
|
1.
|
Kleihues P, Louis DN, Scheithauer BW,
Rorke LB, Reifenberger G, Burger PC and Cavanee WK: The WHO
classification of tumors of the nervous system. J Neuropathol Exp
Neurol. 6:215–225. 2002.
|
|
2.
|
Lois DN, Ohgaki H, Wiestler OD and Cavanee
WK: WHO Classification of Tumors of the Central Nervous System.
IARC; Lyon: 2007
|
|
3.
|
Birlik B, Canda S and Ozer E: Tumour
vascularity is of prognostic significance in adult, but not
paediatric astrocytomas. Neuropathol Appl Neurobiol. 32:532–538.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis – correlation in invasive
breast carcinoma. N Eng J Med. 324:1–8. 1991.
|
|
5.
|
Tanaka F, Otake Y, Yanagihara K, et al:
Evaluation of angiogenesis in non-small cell lung cancer:
Comparison between anti-CD34 antibody and anti-CD105 antibody. Clin
Cancer Res. 7:3410–3415. 2001.PubMed/NCBI
|
|
6.
|
Offersen B, Pfeiffer P, Hamilton-Dutoit S
and Overgaad J: Patterns of angiogenesis in non-small cell lung
carcinoma. Cancer. 91:1500–1509. 2001. View Article : Google Scholar
|
|
7.
|
Ding S, Li C, Lin S, et al: Comparative
evaluation of microvessel density determined by CD34 or CD105 in
benign and malignant gastric lesions. Hum Pathol. 37:861–866. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Amar A, Giovanini AF, Rosa MP, Yamassaki
HO, De Carvalho MB and Rapoport A: Densidade microvascular no
carcinoma de língua. Rev Assoc Med Bras. 48:204–208. 2002.
|
|
9.
|
Leon SP, Folkerth RD and Black PM:
Microvessel density is a prognostic indicator for patients with
astroglial brain tumors. Cancer. 77:362–372. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Yao Y, Kubota T, Takeuchi H and Sato K:
Prognostic significance of microvessel density determined by an
anti-CD105/endoglin monoclonal antibody in astrocytic tumors:
comparison with an anti-CD31 monoclonal antibody. Neuropathology.
25:201–206. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Baldari S, Pecorella R, Cosentino S and
Minutoli F: Investigation of brain tumours with 99mTc-MIBI SPECT. Q
J Nucl Med. 46:336–345. 2002.PubMed/NCBI
|
|
12.
|
Goethals I, De Winter O, Dierckk R,
Annovazzi A, Signore A and van de Wiele C: False-negative Tc-99m
scintigraphy in histopathologically proved recurrent high-grade
oligodendroglioma. Clin Nucl Med. 28:299–301. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Minutoli F, Angileri FF, Conti A, et al:
Timing of examination affects reliability of
99mTc-methoxyisobutylisonitrile SPECT in distinguishing neoplastic
from nonneoplastic brain hematomas. J Nucl Med. 46:574–579.
2005.
|
|
14.
|
Andrews DW, Das R, Kim S, Zhang J and
Curtis M: Technetium-MIBI as a glioma imaging agent for the
assessment of multidrug resistance. Neurosurgery. 40:1323–1332.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Sasajima T, Shimada N, Naitoh Y, Takahashi
M, Hu Y, Satohh T and Mizoi K: 99mTc-MIBI imaging for prediction of
therapeutic effects of second-generation MDR1 inhibitors in
malignant brain tumors. Int J Cancer. 121:2637–2645. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Staudenherz A, Fazeny B, Marosi C, et al:
Does 99mTc-sestamibi in high-grade malignant brain tumors reflect
blood-brain barrier damage only? Neuroimage. 12:109–111. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Staudenherz A, Wolfsberg S, Killer M, et
al: Microvessel density is not crucial for scintigraphic
visualization of brain tumors using 99mTc-MIBI. Microvasc Res.
67:218–222. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Nagamachi S, Jinnouchi S, Nabeshima K, et
al: The correlation between 99mTc-MIBI uptake and MIB-1 as a
nuclear proliferation marker in glioma – a comparative study with
201TL. Neuroradiology. 43:1023–1030. 2001.PubMed/NCBI
|
|
19.
|
Ak I, Gulbas Z, Altinel F and Vardareli E:
TC-99m MIBI uptake and its relation to the proliferative potential
of brain tumors. Clin Nucl Med. 28:29–33. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Carvalho PA, Chiu ML, Kronauge JF,
Kawamura M, Jones AG, Holman BL and Piwnica-Worms D: Subcellular
distribution and analysis of technetium-99-MIBI in isolated
perfused rat hearts. J Nucl Med. 33:1516–1522. 1992.PubMed/NCBI
|
|
21.
|
Piwinca-Worms D, Kronauge JF and Chiu ML:
Enhancement by tetraphenylborate of technetium-99m-MIBI uptake
kinetics and accumulation in cultured chick myocardial cells. J
Nucl Med. 32:1992–1999. 1991.PubMed/NCBI
|
|
22.
|
Offersen B, Sorensen F, Yilmaz M, Knoop A
and Overgaard J: Chalkley estimates of angiogenesis in early breast
cancer. Acta Oncol. 41:695–703. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Mineo TC, Ambrogi V, Baldi A, Rabitti C,
Bollero P, Vicenzi B and Tonini G: Prognostic impact of VEGF, CD31,
CD34 and CD105 expression and tumor vessel invasion after radical
surgery for IB-IIA non-small cell lung cancer. J Clin Pathol.
57:591–597. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Beauchesne P and Soler C: Correlation of
99m Tc-MIBI brain spect (Functional index ratios) and survival
after treatment failure in malignant glioma patients. Anticancer
Res. 22:3081–3085. 2002.PubMed/NCBI
|
|
25.
|
Soler C, Beauchesne P, Maatougui K, et al:
Technetium-99msestamibi brain single-photon emission tomography for
detection of recurrent gliomas after radiation therapy. Eur J Nucl
Med. 25:1649–1657. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
San Pedro EC, Yilmaz M, Liu HG, Rosenfeld
SS and Mountz JM: A new semiquantitative method for comparing brain
tumor uptake of Tc-99m sestamibi and TL-201. Clin Nucl Med.
24:868–873. 1999.PubMed/NCBI
|
|
27.
|
Field A: Discovering Statistic Using SPSS.
2nd edition. SAGE; California: 2005
|
|
28.
|
Glantz MJ, Burger PC, Herdon JE II,
Friedman AH, Caircross JG, Vick NA and Shold SC Jr: Influence of
the type of surgery on the histologic diagnosis in patients with
anaplastic gliomas. Neurology. 41:1741–1744. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Aronsson DE and Muhr C: Quantification of
sensitivity of endothelial cell markers for the astrocytoma and
oligodendroglioma tumors. Anticancer Res. 22:343–346.
2002.PubMed/NCBI
|
|
30.
|
Shivakumar S, Prabhakar BT, Jayashree K,
Rajan MG and Salimath BP: Evaluation of serum vascular endothelial
growth factor (VEGF) and microvessel density (MVD) as prognostic
indicators in carcinoma breast. J Cancer Res Clin Oncol.
135:627–636. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Tohama Y, Gratas C, van Meir EG, et al:
Necrogenesis and Fas/APO-1 (CD95) expression in primary (de novo)
and secondary glioblastomas. J Neuropathol Exp Neurol. 57:239–245.
1998.PubMed/NCBI
|
|
32.
|
Homma T, Fukushima T, Vaccarella S, et al:
Correlation among pathology, genotype and patient outcomes in
glioblastoma. J Neuropathol Exp Neurol. 65:846–854. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Netto GC, Bleil CB, Hilbig A and Coutinho
LM: Immunohistochemical evaluation of the microvascular density
through the expression of TGF-β (CD 105/endoglin) and CD 34
receptors and expression of the vascular endothelial growth factor
(VEGF) in oligodendrogliomas. Neuropathology. 28:17–23.
2008.PubMed/NCBI
|
|
34.
|
Vaquero J, Zurita M, Coca S, Oya S and
Morales C: Prognostic significance of clinical and
angiogenesis-related factors in low-grade oligodendrogliomas. Surg
Neurol. 54:229–234. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Ohgaki H and Kleihues P: Population-based
studies on incidence, survival rates and genetic alterations in
astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol.
64:479–489. 2005.PubMed/NCBI
|
|
36.
|
Ohgaki H and Kleihues P: Genetic pathways
to primary and secondary glioblastoma. Am J Pathol. 170:1445–1453.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Folkerth RD: Descriptive analysis and
quantification of angiogenesis in human brain tumors. J Neurooncol.
50:165–172. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Traweek ST, Kandalaft PL, Metha P and
Battifora H: The human hematopoietic progenitor cell antigen (CD34)
in vascular neoplasia. Am J Clin Pathol. 96:25–31. 1991.PubMed/NCBI
|