Introduction
Breast cancer is the most common malignancy and the
principal cause of cancer-related death among women globally
(1). Statistics show that each
year there are over 1.1 million women newly diagnosed with breast
cancer worldwide. Each year 410,000 women die from the disease
(2). The total cost of illness for
breast cancer has been estimated at $3.8 billion, of which $1.8
billion represents medical care due to side effects during
treatment. Among women who received chemotherapy this equated to
more than $1,200 in additional health care expenditures related to
chemotherapy and more than $17,000 in additional costs for
ambulatory care as compared to women who did not receive
chemotherapy (3).
Today, it is well known that anticancer treatment by
surgery, radiotherapy or chemotherapy has improved the prognosis of
the disease and has increased survival. In breast cancer,
antineoplastic chemotherapy has improved the overall clinical
response. The administration of taxane has increased the response
rate from 50 to 68%; with the combination of epirubicin and
paclitaxel the overall response rate is 66% (4).
However, various side effects have been associated
with chemotherapy and radiotherapy. These side effects, not only
affect the tumor, but also target bone marrow activity and divide
lymphocytes causing lymphocytopenia (5) which may induce subsequent clinical
immunodeficiency (6).
Chemotherapeutic drugs produce T-cell depletion, which is more
severe in CD4+ than in CD8+ T lymphocytes, a
decrease in the dendritic cell function and an alteration in the
production of pro-inflammatory and anti-inflammatory cytokines.
Antineoplastic chemotherapy also induces side
effects such as fatigue (7,8),
skeletal muscle wasting and atrophy (9), as well as elevated levels of tumor
necrosis factor, inactivity and weight loss. In 1948, Karnofsky
developed a performance status scale as a multi-measure assessment
of the quality of life for cancer patients during medical treatment
(10,11). Such investigations revealed that
chemotherapy, not only generates medical benefits during the
disease, but unfortunately also worsens the quality of life during
treatment (12–15).
An improved immune response helps to prevent
chemotherapy-induced side effects. An immunotherapy agent increases
the populations of T-cells, dendritic and natural killer (NK) cells
that are the most potent effectors in the host antitumor response.
Immunotherapy agents are an alternative therapy used to boost
antitumor immunity and to improve the clinical response to cancer
chemotherapeutic treatment.
An immunological agent that has been considered in
the context of cancer immunotherapy is the dialyzable leukocyte
extract (DLE) or transfer factor, which has no reported side
effects or toxicity. DLE was first described in 1955 by Lawrence
and Borkowsky (16). In 1970,
Kirkpatrick found that antigen-specific DLE therapy results in the
induction of cell-mediated immunity and successful response to the
corresponding antigen (17).
Currently, DLE is defined as a dialyzed heterogeneous mixture of
low molecular weight (<10 kDa) substances released from
disintegrated blood or tissue leukocytes. DLE is believed to
transfer the ability to express delayed-type hypersensitivity and
cell-mediated immunity from an immune donor to a non-immune
recipient (18). DLE has been used
as a therapeutic agent in the treatment of autoimmune diseases
(19), bacterial diseases
(20), asthma and allergies
(19) (Luna-Baca GA, Linares M,
Santacruz-Valdes C, et al: Immunological study of patients
with herpetic stromal keratitis treated with dialyzable leukocyte
extracts. 13th International Congress of Immunology, 2007). Such
treatment has consistently led to improved prognosis.
Therefore, DLE represents an attractive alternative
to complement chemotherapy, which can be used to enhance the immune
system after disturbances resulting from the side effects of
chemotherapy. DLE in vitro is effective in improving
cellular immunity (18) and in
regulating the production of different cytokines involved in tumor
progression (21–25).
In breast cancer cell line assays, bovine DLE (bDLE)
induced cytotoxic effects despite suppressing the expression of p53
mRNA, bab-1, c-myc, bax, bcl-2 and bad mRNA (26,27).
In clinical trials, patients with advanced breast cancer were
treated with pooled dialyzable transfer factor from healthy adult
donors (non-specific) without chemotherapy or radiotherapy, after
which the disease progressed (21,28).
In other reports, the administration of DLE directly to the tumor
was found to reduce tumor size and increase CD2+,
CD4+, CD8+ and NK cell counts in rats with
glioblastoma multiforme (29). DLE
as an adjuvant of chemotherapy has been associated with tumor
regression and temporary stabilization in several types of cancer
(30), such as breast cancer,
nasopharyngeal carcinoma (31),
metastatic renal carcinoma (32),
prostate cancer (33) and others
(34).
Previously, we reported the use of bDLE as an
adjuvant therapy to complement bevacizumab (Avastin), cetuximab
(Erbitux), cytokines and cisplatin in transarterial
chemoembolization (TACE). bDLE was shown to reduce tumor size in a
lung cancer (stage III) patient and led to complete remission in 3
patients with primary pancreatic cancer (moderately
differentiated). Furthermore, cellular immunity parameters were
maintained within reference ranges after chemotherapy
(Rodriguez-Padilla C, García de la Fuente A, Díaz R, et al:
Intra-arterial chemo-inmuno target therapy plus conformal XRT in
brain tumors. 16th International Congress on Anti-Cancer Treatment
Paris, France, 2005) (Rodriguez-Padilla C, Ixtepan L, García de la
Fuente A, et al: Transarterial chemoembolization (TACE) with
bevacizumab (avastin), cetuximab (erbitux) and immunomodulators and
image-guided radiation therapy (IGRT) in patients with lung cancer.
19th International Congress on Anti-Cancer Treatment Paris, France,
2008). The quality of life, as measured by the Karnofsky
performance scale, increased.
Based on our previous experience with bDLE, the main
objective of the present study was to assess the clinical and
immune responses with regard to quality of life in breast cancer
patients who were undergoing standard chemotherapy and who also
received adjuvant therapy (bDLE).
Patients and methods
Patients
A total of 43 women with confirmed histological
diagnoses of breast cancer were included in the study. Female
patients over 18 years of age were seronegative for human
immunodeficiency virus, human T-cell leukemia virus type 1,
hepatitis B and hepatitis C. Patients who were randomly selected
for the treatment group had a Karnofsky performance status of ≥60%.
None of the patients received cell proliferation stimulants during
chemotherapy [Neupogen or granulocyte colony-stimulating factor
(G-CSF)], drugs to stimulate appetite or corticosteroids. The
Institutional Review Board and Ethics Committee of the Universidad
Autonoma de Nuevo Leon, Mexico approved the trial, and all patients
gave their written informed consent.
The chemotherapy commonly employed for local disease
includes doxorubicin and cyclophosphamide (AC), AC followed by
paclitaxel, cyclophosphamide, doxorubicin and fluorouracil (FAC),
cyclophosphamide, methotrexate, fluorouracil (CMF), docetaxel,
doxorubicin and cyclophosphamide (TAC). For metastatic disease the
regimens may also include epirubicin, Navelvine, Aromasin or
Xeloda.
Adjuvant therapy
The bDLE used in our study as an adjuvant therapy in
patients who received chemotherapy was produced by the Laboratory
of Immunology and Virology at the Universidad Autonoma de Nuevo
Leon, Mexico, following a modified protocol described by Lawrence
and Borkowsky (16). bDLE is a
mixture of low molecular weight molecules acquired from the
dialyzation of disintegrated bovine spleens. The bDLE was
lyophilized, tested for endogenous pyrogens using the Limulus
amoebocyte lysate assay (MP Biomedicals Inc.) and determined to be
free of bacterial contamination by culturing in different media as
well as by in vivo mouse inoculations.
Study assessment
The design of the study included 43 breast cancer
patients divided as follows: 25 breast cancer patients monitored
for clinical and immunological responses during chemotherapy
treatment with bDLE as adjuvant therapy and a control group that
included 18 breast cancer patients receiving chemotherapy without
bDLE as adjuvant. The administration of bDLE lasted 9 months,
starting with 1-week administration of bDLE alone prior to
chemotherapy, with continued administration during the chemotherapy
cycle (3–6 months) up to 1 month after the completion of
chemotherapy. The dose administered to each patient was defined
according to the patient's immunologic status. For the first 15
days, the daily administration of bDLE was as follows: i) 1–3% of B
lymphocytes, 5 oral units; ii) 4–6% of B lymphocytes, 4 units (2
oral/2 i.m.); iii) >6% of B lymphocytes, 1 unit alternating oral
and i.m. daily. All patients began the bDLE treatment before
chemotherapy and continued with the daily treatment during all
chemotherapy cycles and several months after the completion of the
chemotherapy. If patients achieved a complete remission before 3
months with bDLE and chemotherapy, treatment was limited to bDLE
until the follow-up appointment, at which point patients were
evaluated immunologically based on their lymphocyte profiles.
Evaluation of the immunologic
response
The immunologic parameters of the patients were
monitored during chemotherapy in both groups. In addition, in the
group that received bDLE as adjuvant the cellular immune response
before receiving bDLE was evaluated also 1 month after finishing
chemotherapy (description of the protocol design in Fig. 1). Monitoring involved obtaining
complete and differential blood counts, as well as flow cytometric
analysis of peripheral mononuclear cells. Flow cytometry was used
to count NK cells, B lymphocytes and T lymphocytes. Flow cytometry
was performed on a Beckman Coulter Altra No. AE47042. Data were
obtained and analyzed using Software Expo 32 version 1.2.
bDLE stimulates an immune response mediated by
cytokines that indirectly stimulate the proliferation of
hematopoietic progenitor cells in bone marrow, as reported used
pig-DLE in rats after radiotherapy (18). We evaluated several concentrations
of IL-3 and IL-7 in serum with and without bDLE as an adjuvant
during chemotherapy using an ELISA assay according to the protocol
by Peprotech Company.
Evaluation of the clinical response
A total of 43 patients were evaluated for the
clinical response to cancer chemotherapy treatment with or without
bDLE as adjuvant, as determined by standard radiographic studies or
PET-CT scan imaging. Clinical tumor response was compared to the
control group (without bDLE) according to the International Union
Against Cancer Criteria. A complete response (CR) was defined as
the disappearance of all clinical evidence of disease. A partial
response (PR) was defined as a ≥50% decrease in the sum of the
products of perpendicular diameters of all measurable lesions for
at least 1 month with no increase in any lesion and no appearance
of new lesions. Patients with mixed or minor responses or
progressive disease were considered nonresponders (NR) (38).
Quality of life
Quality of life was measured in the group with and
without bDLE using the Karnofsky performance scores before
treatment with bDLE and after 1 month following the end of the
chemotherapy regimen.
Statistical analysis
A t-test was used to compare lymphocyte cell
populations and Karnofsky performance scores obtained before and
after bDLE treatment. Statistical significance was established as
P<0.05. Individual values given in the figures represent the
mean of 25 patients ± SEM (for those who received adjuvant therapy)
or 18 patients ± SEM (for those who did not receive adjuvant
therapy).
Results
Patient characteristics
To establish a general screening assessment of the
effect of bDLE as an adjuvant during chemotherapy, 25 patients with
a diagnosis of breast cancer were selected randomly. The study also
included 18 breast cancer patients who did not receive adjuvant
treatment with bDLE during chemotherapy. In both groups, the
patients who had disseminated metastasis were affected in a median
of three organs/tissues, principal bones, liver and lung. Among
patients who received bDLE during chemotherapy, 84% were positive
for tumor markers. In the control group, 88% were positive for
tumor markers. According to Karnofsky performance scale
classification, 0 patients were able to work and 1 patient was not.
All patients received a tailored oncology treatment scheme
depending on disease stage (Table
I).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | With bDLE (%) | Without bDLE (%) |
|---|
| Total patients,
43 | 25 (60) | 18 (40) |
| Pathological
stage | | |
| I | 2 (8) | 2 (11) |
| II | 13 (52) | 5 (28) |
| III | 4 (16) | 5 (28) |
| IV | 6 (24) | 6 (33) |
| Total | 25 (100) | 18 (100) |
| Tumor markers | | |
| RE | 13 (52) | 10 (56) |
| RP | 8 (32) | 7 (39) |
| Her2 | 7 (28) | 6 (33) |
| Total | 25 (100) | 18 (100) |
| Performance
status | | |
| 0 | 1 (4) | 2 (12) |
| 1 | 24 (96) | 16 (88) |
| Clinical
treatment |
| Surgery | 13 (52) | 18 (100) |
| Chemotherapy | 25 (100) | 18 (100) |
| Radiotherapy | 1 (4) | 7 (39) |
| Hormonal | 8 (32) | 8 (44) |
Immunologic response
The median total white blood cell count before
chemotherapy was 5,928±339/mm3. During chemotherapy,
this measure was slightly reduced in the group that received bDLE
as adjuvant to 5,554±374/mm3 and in the control group to
4,779±435/mm3. The percentages of monocytes, basophils,
eosinophils and neutrophils were always reported to be in the
reference range values for our laboratory, even during chemotherapy
treatment.
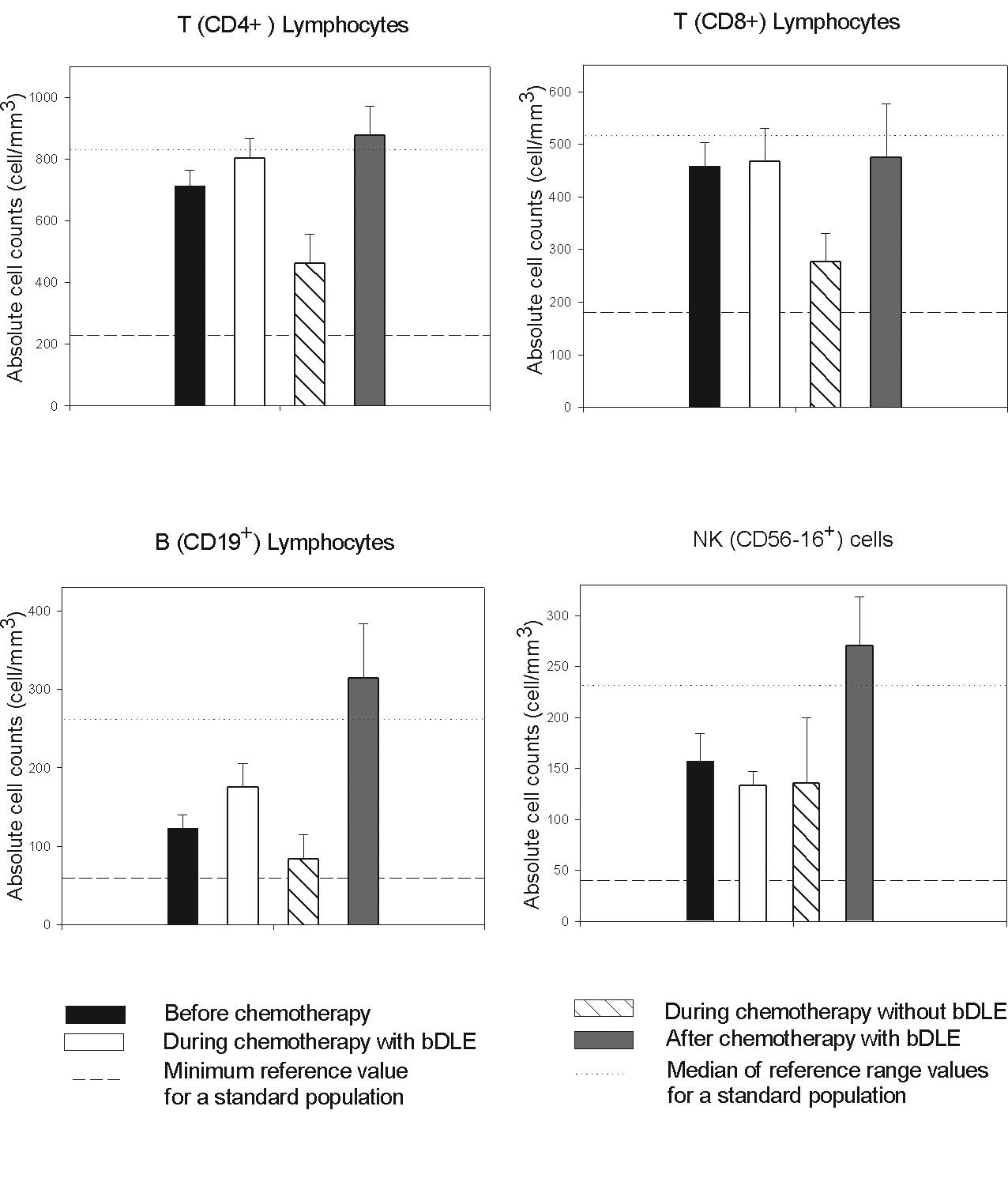
No myelosuppression in lymphoid populations was
observed in patients receiving the bDLE treatment, while in
patients undergoing chemotherapy without bDLE the absolute numbers
of CD4+, CD8+ and B lymphocytes were reduced
compared to the reference range values as shown in Fig. 2. Interestingly, a significant
increase in the numbers of NK cells (P<0.05) and B lymphocytes
(P<0.05) was observed 1 month after the completion of
chemotherapy in patients receiving bDLE as an adjuvant (Table II). The proportion of lymphocytes
was maintained at reference values during treatment with bDLE as
adjuvant. Levels in the control group were below reference values
as reported by Mackall et al for patients undergoing
chemotherapy (5).
 | Table II.Effect of bDLE treatment on the
cellular immune response. |
Table II.
Effect of bDLE treatment on the
cellular immune response.
| Treatment | Leukocytes |
CD4+ |
CD8+ |
CD19+ |
CD56−16+ |
|---|
| Before bDLE | 5,928±339 | 713±50 | 458±46 | 123±17 | 157±27 |
| After bDLE | 5,554±374 | 877±95 | 475±102 | 314±69 | 271±48 |
| P-value | 0.05 | 0.05 | 0.05 | <0.05 | <0.05 |
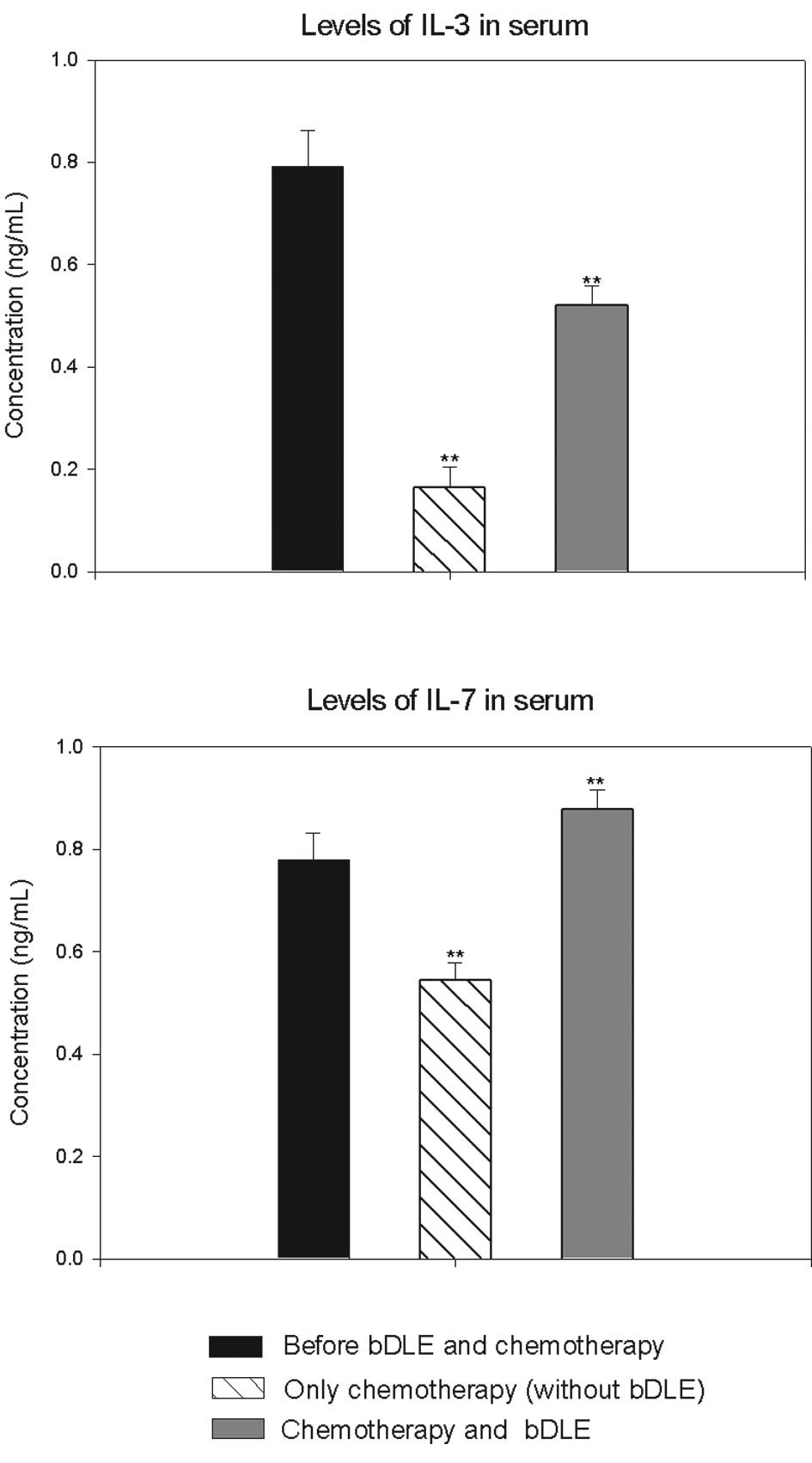
In addition, IL-3 levels were reduced by 80% in the
group that received chemotherapy without bDLE. In the group with
bDLE as adjuvant during chemotherapy we observed that levels were
reduced by only 34%. However, IL-7 levels were increased in the
group that received bDLE as adjuvant (11%) prior to chemotherapy in
combination with bDLE. This measure was reduced by 30% in the
control group during chemotherapy as compared to before
chemotherapy (Fig. 3).
Clinical response
Clinical response was evaluated using standard
radiographic studies or PET-CT scan imaging.
In the group with bDLE as adjuvant therapy, 15 (60%)
patients experienced a CR to treatment, 8 (32%) experienced a PR
and 2 (8%) were NRs (Table III).
Among stage I patients in this group, 2 (100%) patients experienced
a complete response (CR); among stage II patients, 9 (70%)
experienced a CR and 4 (30%) patients experienced a PR; for stage
III patients, 2 (50%) experienced a CR and 2 (50%) patients
experienced a PR; among stage IV patients, 2 (33%) experienced a
CR, 2 (33%) experienced a PR and 2 (33%) patients experienced
NR.
 | Table III.Clinical responses to treatment. |
Table III.
Clinical responses to treatment.
| Stage | Treatment | Complete response
(%) | Partial response
(%) | No response
(%) | Overall response
for stage (%) |
|---|
| I | With bDLE | 2 (100) | 0 | 0 | 100 |
| Without bDLE | 2 (100) | 0 | 0 | |
| II | With bDLE | 9 (70) | 4 (30) | 0 | 72 |
| Without bDLE | 4 (80) | 1 (20) | 0 | |
| III | With bDLE | 2 (50) | 2 (50) | 0 | 33 |
| Without bDLE | 1 (20) | 3 (60) | 1 (20) | |
| IV | With bDLE | 2 (33) | 2 (33) | 2 (33) | 17 |
| Without bDLE | 0 | 5 (83) | 1 (17) | |
In the control group (without adjuvant treatment
with bDLE during chemotherapy), 7 (39%) patients experienced a CR
to treatment (chemotherapy or radiotherapy), 9 (50%) experienced a
PR and 2 (11%) were NRs (Table
III). Among stage I patients in the control group, 2 (100%)
patients experienced a CR; for stage II, 4 (80%) patients
experienced a CR and 1 (20%) patient experienced a PR; among stage
III patients, 1 (20%) experienced a CR, 3 (60%) experienced a PR
and 1 (20%) patient exhibited NR; among stage IV patients, none
experienced a CR, 5 (83%) patients experienced a PR (17%) and 1
patient experienced NR.
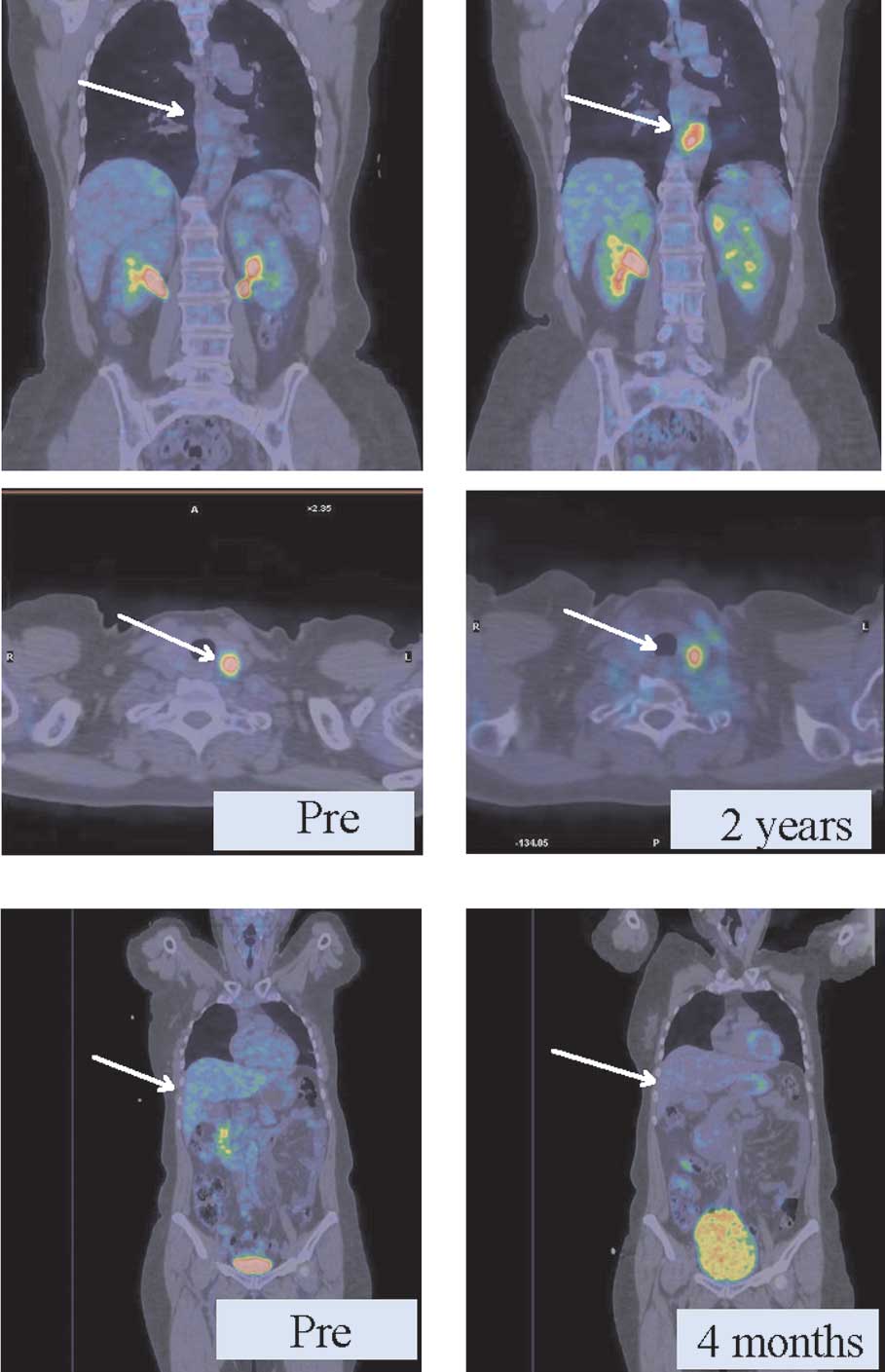
PET-CT imaging
We used PET-CT to evaluate both groups and observed
that in the group with bDLE as adjuvant the regression of
metastatic lesions in diverse anatomic locations was obtained in
less time than in the control group (without bDLE). As shown in
Fig. 4A, patients with metastatic
breast cancer without adjuvant therapy had persistent thyroid
lesions and a new lesion around the aorta (2 cm) after 2 years of
chemotherapy. In another case (Fig.
4B), retroperitoneal retrohepatic metastases exhibited a PR,
with the same metabolic activity (6 SUV) after only 4 months of
receiving bDLE treatment and 5 cycles of chemotherapy.
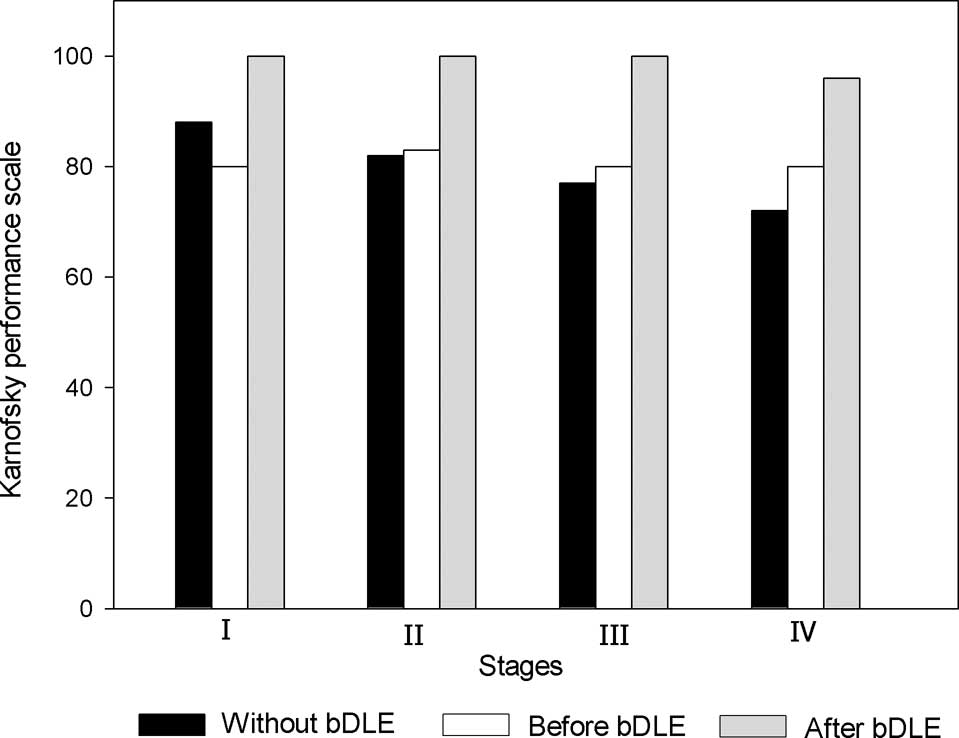
Quality of life
Quality of life was measured using the Karnofsky
performance scores. In the patients who received bDLE adjuvant
therapy during chemotherapy, average Karnofsky scores increased
from 70 to 90, which reflected an overall clinical improvement in
the health status of the patients (Fig. 5). Of the patients who received
chemotherapy treatment without bDLE adjuvant therapy, the average
final score was 80.
Toxicity
The administration of bDLE therapy was safe and well
tolerated. None of the patients died during the reported
period.
Discussion
DLE, commonly known as transfer factor, is an
immunotherapy agent that has been reported to improve the
immunological response in cancer patients (Rodriguez-Padilla C,
García de la Fuente A, Díaz R, et al: Intra-arterial chemo
inmuno target therapy plus conformal XRT in brain tumors. 16th
International Congress on Anti-Cancer Treatment Paris, France,
2005) (36). Various reports have
used different clinical assays to investigate DLE as an adjuvant
therapy. These studies have consistently reported improvement in
the clinical response to treatment, but there is a lack of
information about the clinical parameters that are improved in
those patients (37–39). In this clinical study, we randomly
sampled breast cancer patients to explore the immunological and
clinical response to bDLE treatment as an adjuvant to chemotherapy.
In particular, we focused on the clinical effects of bDLE as an
adjuvant therapy during chemotherapy.
Myelosuppression is a common side effect of
chemotherapy that is accompanied by lymphopenia, neutropenia and
thrombocytopenia. (6). In this
study, our results showed a protective effect of bDLE on
CD4+ T lymphocytes, CD8+ T lymphocytes,
CD19+ B lymphocytes and NK cells (Fig. 2). The absolute numbers of these
lymphocytes in the bDLE-treated patients during chemotherapy
(Fig. 2) were always higher than
expected as compared to our control group and as reported by
Mackall et al for patients undergoing chemotherapy (5). In addition, we observed that the
levels of IL-3 and IL-7 were higher in the group that received bDLE
as an adjuvant during chemotherapy as compared to the control
group.
These factors likely underline the immunological
protection afforded by bDLE during chemotherapy as reported by
Vacek et al (18) using
pig-DLE.
The administration of bDLE in this study resulted in
an increased clinical response. The difference was principally
observed in stage III and IV patients. The median survival reported
after the appearance of metastases is approximately 20–25 months,
hence the importance of obtaining a clinical response as rapidly as
possible. We observed that those metastatic patients receiving bDLE
exhibited improved clinical responses in 6–12 months, as compared
to the group that did not receive adjuvant therapy with bDLE. In
the latter group, the clinical response was as expected at
approximately 2–3 years (data not shown).
Therefore, in future studies with bDLE as adjuvant
chemotherapy, it will be necessary to focus specifically on the
group that improved (patients with metastatic disease). To further
verify enhancement due to bDLE treatment, we recommend a study with
a larger population.
bDLE treatment in combination with chemotherapy
resulted in an increase in the Karnofsky performance scores after
several chemotherapy cycles; patients reached a 90 on the Karnofsky
performance scores, which implies minor symptoms and the ability to
work (10). By contrast, the
average score for the control group (without bDLE) was 80. During
the interviews, we observed that patients improved in their general
health and state of mind even 1 month after chemotherapy.
Therefore, adjuvant therapy with bDLE reduces economic losses as
well as the physical incapacitation suffered by cancer
patients.
In conclusion, our results pertaining to the
administration of bDLE as an adjuvant therapy during breast cancer
chemotherapy can be used for clinical decision-making and for
improving the quality of life during treatment. We propose the use
of bDLE as an adjuvant to complement conventional chemotherapies in
cancer. bDLE would be particularly useful to improve immunological
response, symptomatology and general patient prognosis.
Acknowledgements
This is the first publication to
report a clinical approach to define the anticancer effects of bDLE
in breast cancer patients. The following funding source supported
the data collection process: the Programa de Apoyo a la
Investigacion en Ciencia y Tecnologia (PAICyT) from the Universidad
Autonoma de Nuevo Leon, Mexico and the Consejo Nacional de Ciencia
y Tecnologia (CONACyT), Mexico.
References
|
1.
|
Bray F, McCarron P and Parkin DM: The
changing global patterns of female breast cancer incidence and
mortality. Breast Cancer Res. 6:229–239. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Stewart BW and Coates AS: Cancer
prevention: a global perspective. J Clin Oncol. 23:392–403. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Smith MD and McGhan WF: Financial facts
about treating breast cancer. Bus Health. 14:67–68.
70:1996.PubMed/NCBI
|
|
4.
|
Spitler LE: Clinical uses of transfer
factor. Calif Med. 118:471973.PubMed/NCBI
|
|
5.
|
Mackall CL, Fleisher TA, Brown MR, Magrath
IT, Shad AT, Horowitz ME, Wexler LH, Adde MA, McClure LL and Gress
RE: Lymphocyte depletion during treatment with intensive
chemotherapy for cancer. Blood. 84:2221–2228. 1994.PubMed/NCBI
|
|
6.
|
Van der Most RG, Currie AJ, Robinson BW
and Lake RA: Decoding dangerous death: how cytotoxic chemotherapy
invokes inflammation, immunity or nothing at all. Cell Death
Differ. 15:13–20. 2008.PubMed/NCBI
|
|
7.
|
Piper BF, Borneman T, Sun VC, Koczywas M,
Uman G, Ferrell B and James RL: Cancer-related fatigue: role of
oncology nurses in translating National Comprehensive Cancer
Network Assessment Guidelines into practice. Clin J Oncol Nurs.
12:37–47. 2008. View Article : Google Scholar
|
|
8.
|
Berger AM: Patterns of fatigue and
activity and rest during adjuvant breast cancer chemotherapy. Oncol
Nurs Forum. 25:51–62. 1998.PubMed/NCBI
|
|
9.
|
Goodman MN: Tumor necrosis factor induces
skeletal muscle protein breakdown in rats. Am J Physiol.
260:E727–E730. 1991.PubMed/NCBI
|
|
10.
|
Karnofsky DA, Abelmann WH and Craver LF:
The use of the nitrogen mustards in palliative treatment of
carcinoma. Cancer. 1:634–656. 1948. View Article : Google Scholar
|
|
11.
|
Priestman TJ and Baum M: Evaluation of
quality of life in patients receiving treatment for advanced breast
cancer. Lancet. 1:899–900. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Kasper CE: Sarcolemmal disruption in
reloaded atrophic skeletal muscle. J Appl Physiol. 79:607–614.
1995.PubMed/NCBI
|
|
13.
|
Kasper CE: Recovery of plantaris muscle
from impaired physical mobility. Biol Res Nurs. 1:4–11. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kasper CE and Sarna LP: Influence of
adjuvant chemotherapy on skeletal muscle and fatigue in women with
breast cancer. Biol Res Nurs. 2:133–139. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
St Pierre BA, Kasper CE and Lindsey AM:
Fatigue mechanisms in patients with cancer: effects of tumor
necrosis factor and exercise on skeletal muscle. Oncol Nurs Forum.
19:419–425. 1992.PubMed/NCBI
|
|
16.
|
Lawrence HS and Borkowsky W: Transfer
factor – current status and future prospects. Biotherapy. 9:1–5.
1996.
|
|
17.
|
Kirkpatrick CH: Transfer of cellular
immunity with transfer factor. J Allergy Clin Immunol. 63:71–73.
1979. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Vacek A, Hofer M, Schneiderova H and
Svoboda J: Ultrafiltered pig leukocyte extract (UPLE, IMUNOR)
potentiates hematopoiesis-stimulating effects of G-CSF in vitro and
improves the outcome of treatment of hematopoietic radiation damage
in mice with G-CSF. Immunopharmacol Immunotoxicol. 27:647–659.
2005. View Article : Google Scholar
|
|
19.
|
Pizzo PA, Henderson ES and Leventhal BG:
Acute myelogenous leukemia in children: a preliminary report of
combination chemotherapy. J Pediatr. 88:125–130. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Franco-Molina MA, Mendoza-Gamboa E,
Castillo-Leon L, Tamez-Guerra RS and Rodriguez-Padilla C: Bovine
dialyzable leukocyte extract protects against LPS-induced, murine
endotoxic shock. Int Immunopharmacol. 4:1577–1586. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Atzpodien J, Kuchler T, Wandert T and
Reitz M: Rapid deterioration in quality of life during
interleukin-2- and alpha-interferon-based home therapy of renal
cell carcinoma is associated with a good outcome. Br J Cancer.
89:50–54. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Ojeda MO, van't Veer C, Fernandez Ortega
CB, Arana Rosainz MJ and Buurman WA: Dialyzable leukocyte extract
differentially regulates the production of TNFalpha, IL-6 and IL-8
in bacterial component-activated leukocytes and endothelial cells.
Inflamm Res. 54:74–81. 2005. View Article : Google Scholar
|
|
23.
|
Kirkpatrick CH, Rozzo SJ, Mascali JJ and
Merryman CF: Murine transfer factor. II. Transfer of delayed
hypersensitivity to synthetic antigens. J Immunol. 134:1723–1727.
1985.PubMed/NCBI
|
|
24.
|
Kirkpatrick CH: Therapeutic potential of
transfer factor. N Engl J Med. 303:390–391. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Kirkpatrick CH: Transfer of delayed
cutaneous hypersensitivity with transfer factor. Cell Immunol.
41:62–71. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Franco-Molina MA, Mendoza-Gamboa E,
Miranda-Hernandez D, Zapata-Benavides P, Castillo-Leon L,
Isaza-Brando C, Tamez-Guerra RS and Rodriguez-Padilla C: In vitro
effects of bovine dialyzable leukocyte extract (BDLE) in cancer
cells. Cytotherapy. 8:408–414. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Mendoza-Gamboa E, Franco-Molina MA,
Zapata-Benavides P, Castillo-Tello P, Vera-Garcia ME, Tamez-Guerra
RS and Rodriguez-Padilla C: Bovine dialyzable leukocyte extract
modulates AP-1 DNA-binding activity and nuclear transcription
factor expression in MCF-7 breast cancer cells. Cytotherapy.
10:212–219. 2008. View Article : Google Scholar
|
|
28.
|
Oettgen HF, Old LJ, Farrow JH, Valentine
FT, Lawrence HS and Thomas L: Effects of dialyzable transfer factor
in patients with breast cancer. Proc Natl Acad Sci USA.
71:2319–2323. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Pineda B, Estrada-Parra S, Pedraza-Medina
B, Rodriguez-Ropon A, Perez R and Arrieta O: Interstitial transfer
factor as adjuvant immunotherapy for experimental glioma. J Exp
Clin Cancer Res. 24:575–583. 2005.PubMed/NCBI
|
|
30.
|
Meier CR and LoBuglio AF: Transfer factor:
a potential agent for immunotherapy of cancer. World J Surg.
1:617–623. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Goldenberg GJ and Brandes LJ: In vivo and
in vitro studies of immunotherapy of nasopharyngeal carcinoma with
transfer factor. Cancer Res. 36:720–723. 1976.PubMed/NCBI
|
|
32.
|
Moss RW: Cancer and complementary and
alternative medicine in Italy: personal observations and historical
considerations. Integr Cancer Ther. 3:173–188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Pizza G, De VC, Cuzzocrea D, Menniti D,
Aiello E, Maver P, Corrado G, Romagnoli P, Dragoni E and LoConte G:
A preliminary report on the use of transfer factor for treating
stage D3 hormone-unresponsive metastatic prostate cancer.
Biotherapy. 9:123–132. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Rodriguez-Flores A, Castillo-Juarez P,
Vazquez D, Gonzalez-Guzman R, Rico-Martinez G and Estrada-Parra S:
Immunological evaluation of patients with osteosarcoma received
adjuvant treatment with specific transfer factor. J Immunol.
182:632009.
|
|
35.
|
Dudley ME, Wunderlich JR, Yang JC, Sherry
RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE and
Mavroukakis SA: Adoptive cell transfer therapy following
non-myeloablative but lymphodepleting chemotherapy for the
treatment of patients with refractory metastatic melanoma. J Clin
Oncol. 23:2346–2357. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Franco-Molina MA, Mendoza-Gamboa E,
Zapata-Benavides P, Vera-Garcia ME, Castillo-Tello P, Garcia dlF,
Mendoza RD, Garza RG, Tamez-Guerra RS and Rodriguez-Padilla C:
IMMUNEPOTENT CRP (bovine dialyzable leukocyte extract) adjuvant
immunotherapy: a phase I study in non-small cell lung cancer
patients. Cytotherapy. 10:490–496. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Fujisawa T: Transfer factor immunotherapy
as an adjunct to surgery in lung cancer. Nihon Kyobu Shikkan Gakkai
Zasshi. 23:68–73. 1985.PubMed/NCBI
|
|
38.
|
Krown SE, Pinsky CM, Hirshaut Y, Hansen JA
and Oettgen HF: Effects of transfer factor in patients with
advanced cancer. Isr J Med Sci. 14:1026–1038. 1978.PubMed/NCBI
|
|
39.
|
Vacek A, Hofer M, Hola J, Weiterova L,
Streitova D and Svoboda J: The role of G-CSF and IL-6 in the
granulopoiesis-stimulating activity of murine blood serum induced
by perorally administered ultrafiltered pig leukocyte extract,
IMUNOR. Int Immunopharmacol. 7:656–661. 2007. View Article : Google Scholar
|