Introduction
FOLFOX4 therapy [folinic acid (FOL), fluorouracil
(F) plus oxaliplatin (OX)] (1) and
FOLFIRI therapy (FOL, F, plus irinotecan (IRI)] (2) are international standard treatments
for advanced colorectal cancer (3). In recent years, it has also been
recommended that molecular targeting drugs such as bevacizumab (BV)
(4,5) or cetuximab (Cet) (6,7) be
combined with FOLFOX4 or FOLFIRI.
The median patient survival time (MST) was reported
to be significantly longer for FOLFOX4 + BV therapy (12.9 months)
than for FOLFOX4 alone (10.8 months), confirming that the addition
of BV increased the efficacy of the treament (4). The MST was also reported to be
significantly longer for FOLFIRI + Cet (8.9 months) than for
FOLFIRI alone (8.0 months) (8).
These observations suggest that chemotherapy combined with
molecular targeting drugs is more effective for treatment of
advanced colorectal cancer. Thus, the addition of such molecular
targeting drugs to chemotherapy has been recommended (Saltz LB, et
al: Proc ASCO 170: abs. 4028, 2007).
Anemia is one of the most common adverse effects of
chemotherapy. However, Follézou et al (9) reported an increase in the serum iron
level after the administration of various anticancer drugs,
including 5-FU, actinomycin D, adriacin and cyclophosphamide. Yet,
there have been no reports concerning the effect of FOLFOX4 and
FOLFIRI on serum iron levels. Therefore, in the present study, we
evaluated the effect of FOLFOX4 and FOLFIRI therapies on changes in
serum levels of iron as well as transferrin and ferritin.
Materials and methods
Subjects
Fifty-eight subjects (92 cases) were enrolled in
this study. They were admitted to Tobu Chiiki Hospital (Tokyo
Metropolitan Health and Medical Treatment Corporation, Tokyo,
Japan) and received FOLFOX4 or FOLFIRI therapy alone or in
combination with BV between April 2005 and September 2008. Prior to
the enrollment, informed consent was obtained from all the
subjects. The patient characteristics are presented in Table I.
 | Table I.Characteristics of the subjects. |
Table I.
Characteristics of the subjects.
| No. of subjects | 58 |
| Gender
(male/female) | 35/23 |
| Mean age (years) | 69.4±7.7 |
| Dukes’ stage
(A/B/C/D) | 1/9/28/20 |
| Colon/rectum | 37/21 |
| Recurrence | |
| Liver | 23 |
| Lung | 9 |
| Peritoneum | 7 |
| Lymph node | 5 |
| Local
recurrence | 5 |
| Bone | 1 |
| Brain | 1 |
| Other | 3 |
| Unresectable | 4 |
Measurement of the serum iron level
Serum iron was measured by the hospital laboratory
before and 48 h after treatment in the 44 patients receiving
FOLFOX4 therapy. The normal range of serum iron was 60–210 μg/dl
for men and 50–170 μg/dl for women. The serum iron level was also
measured before and after treatment in the 11 patients receiving
FOLFOX4 + BV. Furthermore, serum iron levels were compared before
and after the introduction of BV in the 10 patients who received
FOLFOX4 + BV after FOLFOX4 alone.
Serum iron was measured before and after treatment
in the 31 patients who received FOLFIRI therapy, and in the 6
patients who received FOLFIRI + BV. The serum iron level was also
compared before and after the introduction of BV in the 5 patients
who received FOLFIRI + BV after FOLFIRI alone.
Measurement of transferrin and
ferritin
Transferrin and ferritin levels were measured before
and after treatment at SRL, Inc. (Tokyo, Japan) in the 15 and 14
patients who received FOLFOX4 and FOLFIRI therapy, respectively.
The normal range of transferrin was 190–300 mg/dl for men and
200–340 mg/dl for women, while the normal range of ferritin was
39.4–340 ng/ml for men and 3.6–114 ng/ml for women.
Measurement of urinary iron
Urinary iron was measured at the hospital laboratory
on the day of treatment and on the next day in 5 and 7 patients who
received FOLFOX4 and FOLFIRI therapy, respectively.
Statistical analysis
The t-test was used to compare the two groups, and
p<0.05 was considered to be significant. Data are expressed as
the mean ± standard deviation (SD).
Results
Changes in serum iron levels during
FOLFOX4 therapy
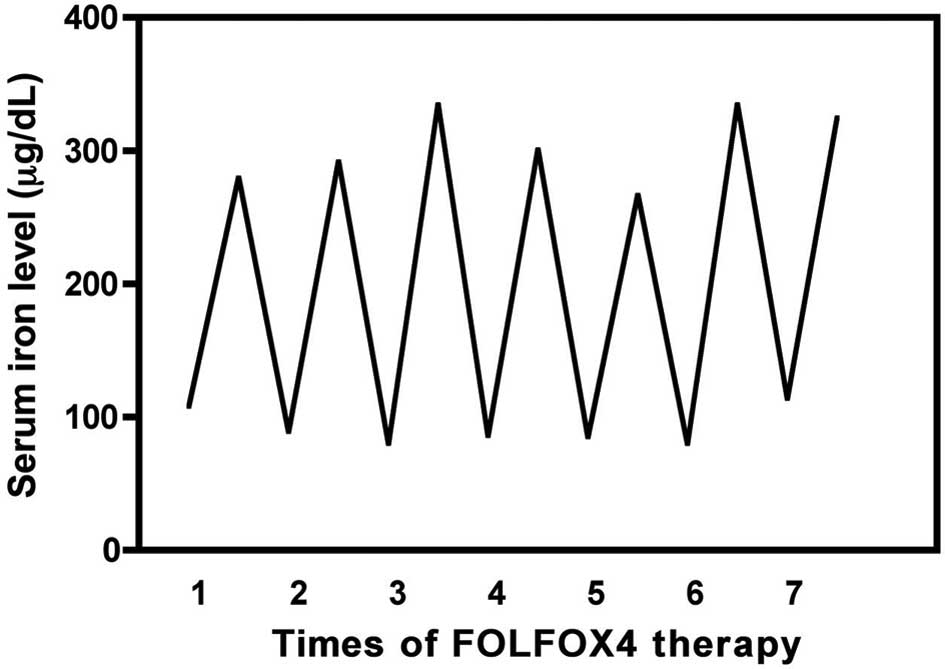
A typical pattern of the changes in the serum iron
levels before and after FOLFOX4 therapy is shown in Fig. 1. The serum iron level transiently
increased after treatment (48 h) and then returned to baseline
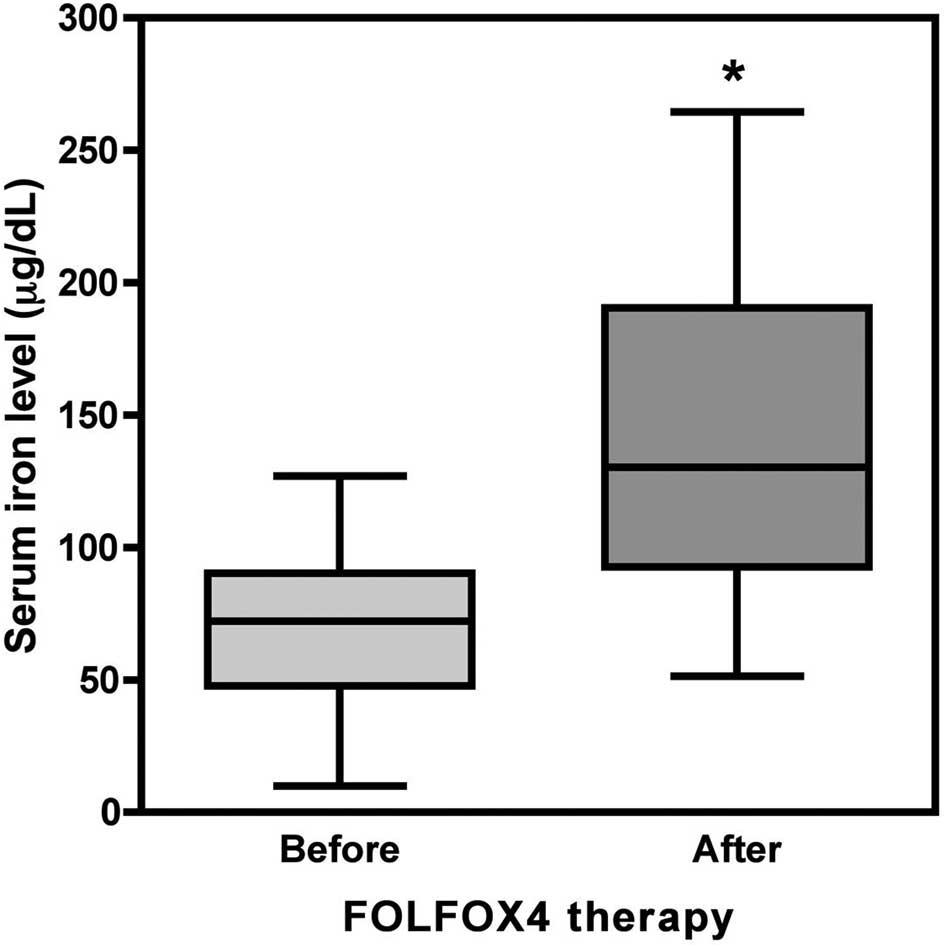
within 2 weeks. In the FOLFOX4 group (44 patients and 272 blood
samples), the serum iron level was 68.24±25.20 μg/dl before
treatment and increased significantly to 143.34±62.18 μg/dl
afterwards (p<0.001, Fig. 2),
showing an increase of 238.54±127.17%. In the FOLFOX4 + BV group,
the serum iron level also increased transiently after treatment (48
h), and then returned to baseline within 2 weeks (data not shown).
In the FOLFOX4 + BV group (11 patients and 46 blood samples), the
serum iron level was 65.59±15.87 μg/dl before treatment and
increased significantly to 147.55±44.55 μg/dl after treatment
(p<0.001, Fig. 3), showing an
increase of 247.16±60.70%.
Changes in serum iron levels during
FOLFIRI therapy
A typical pattern of the changes in the serum iron
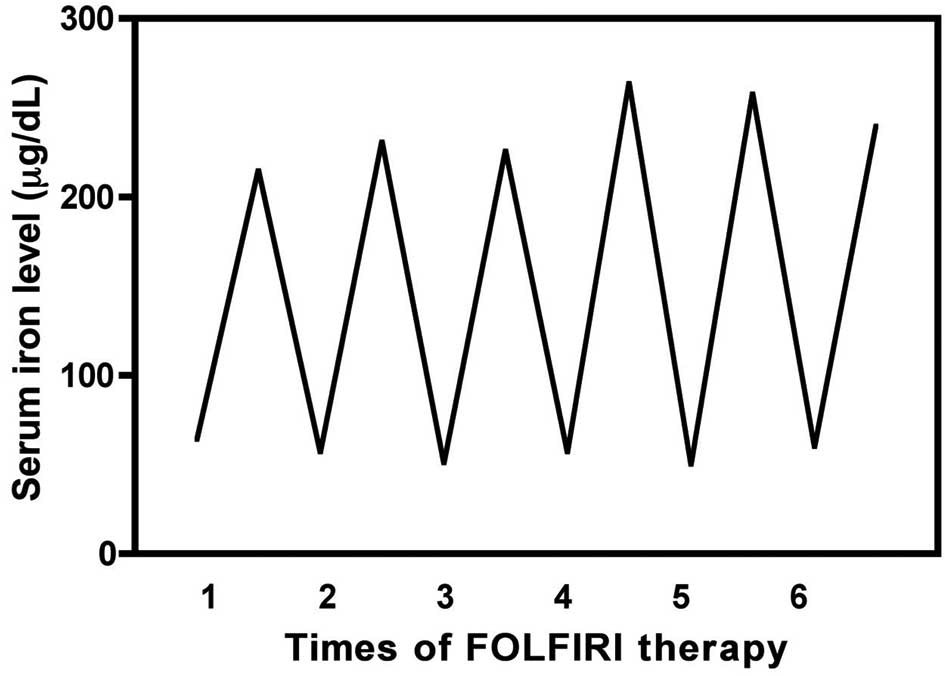
levels before and after FOLFIRI therapy is shown in Fig. 4. The serum iron level transiently
increased after treatment (48 h) and then returned to baseline
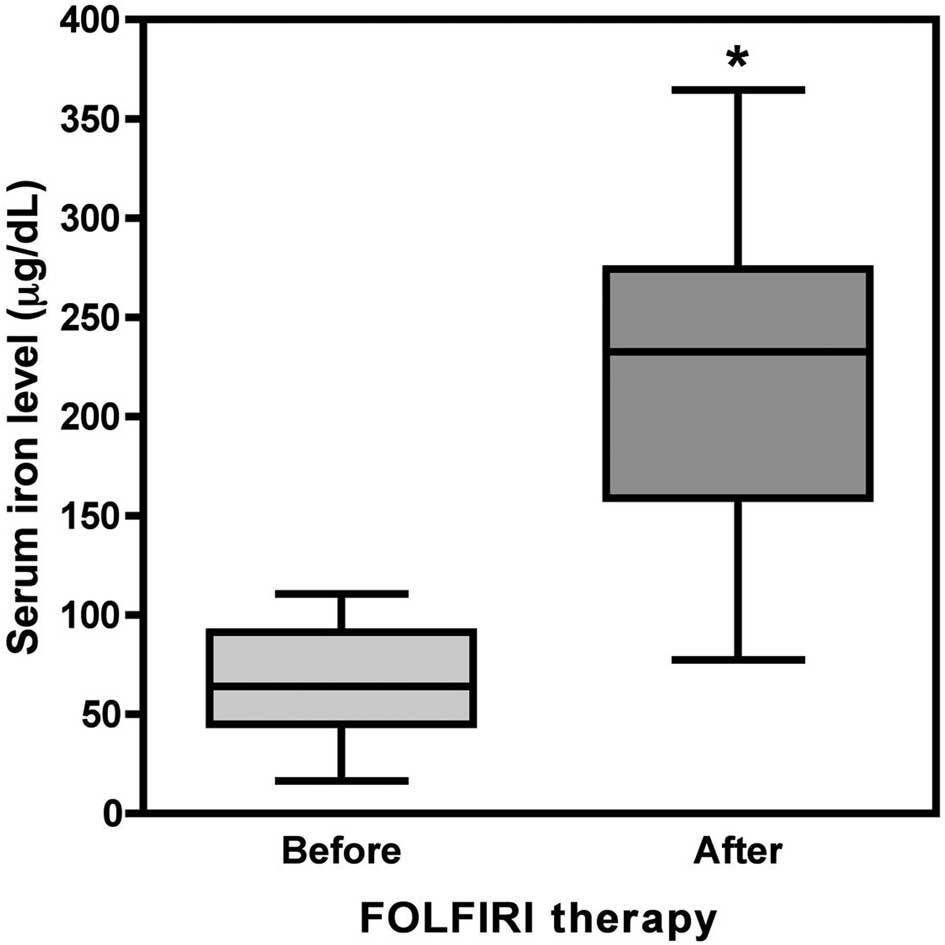
within 2 weeks. In the FOLFIRI group (31 patients and 231 blood
samples), the serum iron level was 66.01±27.47 μg/dl before
treatment and increased significantly to 221.69±78.51 μg/dl
afterwards (p<0.001, Fig. 5),
showing an increase of 399.94±6.25%. In the FOLFIRI + BV group, the
serum iron level also increased transiently after treatment (48 h)
and then returned to baseline within 2 weeks (data not shown). In
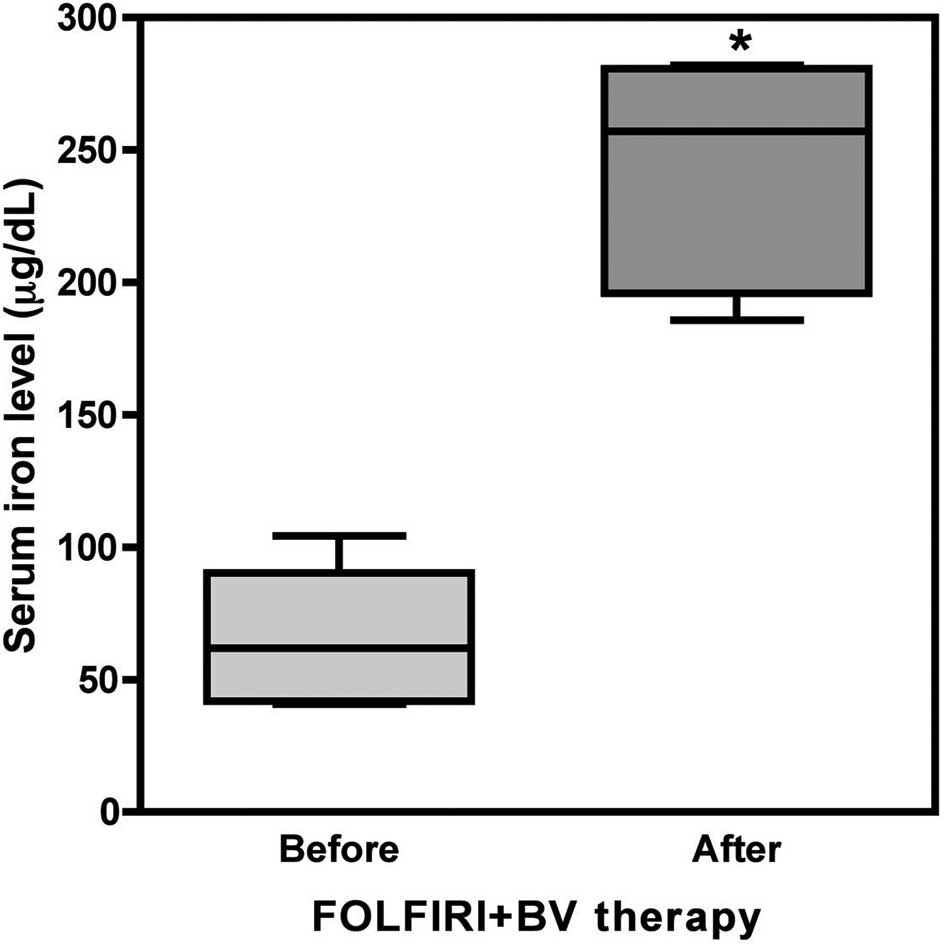
the FOLFIRI + BV group (6 patients and 26 blood samples), the serum
iron level was 64.68±23.60 μg/dl before treatment and increased
significantly to 244.55±40.54 μg/dl after treatment (p<0.001,
Fig. 6), showing an increase of
440.33±156.22%.
Since there was little difference in the changes in
serum iron between FOLFOX4 and FOLFIRI therapy when these regimens
were combined with BV (Figs. 2,
3, 5 and 6),
BV was considered to impart no influence on the changes in iron
levels. To confirm this, changes in serum iron were examined in
patients who underwent FOLFOX4 + BV after FOLFOX4 alone. No
difference was noted in the serum iron levels after treatment
between FOLFOX4 alone (80.84±53.94 μg/dl, n=10) and FOLFOX4 + BV
(76.03±34.84 μg/dl). Furthermore, the serum iron levels were also
measured in patients who underwent FOLFIRI + BV after FOLFIRI
alone. Similarly, there was no difference in the serum iron levels
after the treatment between FOLFIRI alone (205.09±139.37 μg/dl,
n=5) and FOLFIRI + BV (257.45±151.63 μg/dl).
Changes in transferrin and ferritin
levels during FOLFOX4 and FOLFIRI therapies
The influence of chemotherapy on transferrin (an
iron-transporting protein) (10)
and ferritin (an iron storage protein) (11,12)
was also investigated. In the 15 patients of the FOLFOX4 group,
transferrin levels were not different before (256.79±80.13 mg/dl)
and after (233.53±80.70 mg/dl) the treatment (p=0.14). In the 14
patients of the FOLFOLI group, the transferrin level was
236.15±54.31 mg/dl before the treatment and decreased slightly to
196.50±36.85 mg/dl after the treatment (p<0.001), but these
changes were within the normal range of serum transferrin.
In the 15 patients of the FOLFOX4 group, the
ferritin levels were not different before (192.32±224.88 ng/dl) and
after (210.15±210.16 ng/dl) the treatment (p=0.67). Similarly, in
the 14 patients of the FOLFIRI group, the ferritin levels were not
different before (211.48±181.83 ng/dl) and after (220.15±182.97
ng/dl) the treatment (p=0.83). These changes were all within the
normal range of serum ferritin.
Urinary iron excretion during FOLFOX4 and
FOLFIRI therapies
To determine whether the changes in serum iron
during chemotherapy were related to the urinary iron excretion,
urine samples were collected on the day of treatment and on the
next day to measure the urinary iron level in 5 and 7 patients of
the FOLFOX4 and FOLFIRI groups, respectively. Although urinary iron
excretion was 0.09 mg/day on the day of treatment in 1 subject
receiving FOLFIRI, it was within the normal range (<0.2 mg/day).
Moreover, urinary iron excretion was below the detection limit
(0.03 mg/day) in all of the other subjects.
Discussion
Recently, a powerful and effective combination
chemotherapy has become available due to the development of
antitumor chemotherapeutical agents and molecular targeting drugs.
However, the incidence of serious adverse reactions has also
increased. Almost all anticancer agents have the potential to
induce myelosuppression by eliciting the apoptosis/necrosis of
immature myelopoietic cells. In particular, severe leukopenia,
thrombocytopenia and erythropenia are serious adverse events that
lead to the termination of treatment. FOLFOX4 and FOLFIRI therapies
are standard treatments for advanced colorectal cancer; however,
they cause characteristic adverse reactions, such as peripheral
neuropathy and severe diarrhea as well as conventional reactions
like myelosuppression (13,14).
During our preliminary studies on the adverse events
caused by FOLFOX4 or FOLFIRI therapy, an increase in the serum iron
level was sometimes observed, while the red blood cell count
remained unchanged. Focusing on this finding, the present study was
carried out.
In regard to the chemotherapy-induced changes in the
serum iron level, Follézou et al reported that serum iron
levels transiently increased during chemotherapy (9). However, their study differed from the
present investigation in the following respects. Their patients
received relatively older anticancer drugs, such as 5-FU, adriacin,
and cyclophosphamide, and they did not measure the levels of
transferrin or ferritin. Furthermore, they did not evaluate the
effect of tumor cell death and hepatic damage on the increase in
serum iron levels.
In the present study, we measured serum iron as well
as transferrin and ferritin levels in patients who received FOLFOX4
or FOLFIRI therapy alone or in combination with BV. The serum iron
level showed a transient increase in patients receiving FOLFOX4 or
FOLFIRI therapy alone. In most of the patients, serum iron
increased above the normal range (60–210 μg/dl for men and 50–170
μg/dl for women) and sometimes reached 400 μg/dl. We confirmed that
the serum iron level similarly increased regardless of the
administration of BV, suggesting that the transient increase in
serum iron was not due to BV, but was presumably caused by FOLFOX4
or FOLFIRI therapy alone. In contrast, transferrin levels were not
essentially changed during chemotherapy with FOLFOX4 or FOLFIRI.
Moreover, ferritin levels were not basically changed by FOLFOX4 and
FOLFIRI therapies.
Levels of aspartate aminotransferase, alanine
aminotransferase and hemoglobin did not change during chemotherapy
(data not shown). In addition, urinary excretion of iron was not
increased by the chemotherapy. These observations suggest that the
transient increase in serum iron was not due to the destruction of
hepatocytes or erythrocytes. However, it is possible that iron was
transiently released from tumor cells into the blood by
chemotherapy. To examine this possibility, FOLFIRI therapy was
administered to a patient after tumor resection (1 patient and 6
blood samples), and serum iron levels were measured (Fig. 7). As a result, it was revealed that
the serum iron level increased transiently after FOLFIRI therapy
even in the patient who had undergone tumor resection. Since a
transient increase in serum iron level was also observed after
tumor resection (56.50±5.32 μg/dl before versus 239.0±18.95 μg/dl
after the chemotherapy; an increase of 427.25±61.62%), the
increased iron was unlikely derived from tumor cells but was likely
derived from normal cells. Thus, it is reasonable to speculate that
the increased iron in sera was mainly derived from normal cells,
since the number of normal cells was much higher than that of the
tumor cells in the body. However, it cannot be ruled out that iron
is partially released from tumor cells into the blood during
chemotherapy in cancer-bearing patients.
If the transient increase in serum iron observed in
the present study can estimate the outcome of FOLFOX4 or FOLFIRI
therapy, it could be used as one of the potential biomarkers for
monitoring antitumor chemotherapy. In fact, our preliminary studies
revealed that the efficacy of FOLFOX4 or FOLFIRI therapy is
correlated with the response of serum iron. We are now planning to
investigate the relationship between the changes in serum iron and
the outcome of chemotherapy in a larger polulation of patients.
Acknowledgements
We would like to thank the surgeons
and the other medical staff at Tobu Chiiki Hospital for their
cooperation.
References
|
1.
|
De Gramont A, Figer A, Seymour M, et al:
Leucovorin and fluorouracil with or without oxaliplatin as
first-line treatment in advanced colorectal cancer. J Clin Oncol.
18:2938–2947. 2000.
|
|
2.
|
Rougier P, Cutsem EV, Bajetta E, et al:
Randomised trial of irinotecan versus fluorouracil by continuous
infusion after fluorouracil failure in patients with metastatic
colorectal cancer. Lancet. 352:1407–1412. 1998. View Article : Google Scholar
|
|
3.
|
Grothey A and Sargent D: Overall survival
of patients with advanced colorectal cancer correlates with
availability of fluorouracil, irinotecan, and oxaliplatin
regardless of whether doublet or single-agent therapy is used first
line. J Clin Oncol. 23:9441–9442. 2005. View Article : Google Scholar
|
|
4.
|
Giantonio BJ, Catalano PJ, Meropol NJ, et
al: Bevacizumab in combination with oxaliplatin, fluorouracil, and
leucovorin (FOLFOX4) for previously treated metastatic colorectal
cancer: Results from the Eastern Cooperative Oncology Group study
E3200. J Clin Oncol. 25:1539–1544. 2007. View Article : Google Scholar
|
|
5.
|
Hurwitz H, Fehrenbacher L, Novotny W, et
al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for
metastatic colorectal cancer. N Engl J Med. 350:2335–2342. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Cunningham D, Humblet Y, Siena S, et al:
Cetuximab monotherapy and cetuximab plus irinotecan in
irinotecan-refractory metastatic colorectal cancer. N Engl J Med.
351:337–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Saltz LB, Lenz H-J, Kindler HL, et al:
Randomized Phase II trial of cetuximab, bevacizumab, and irinotecan
compared with cetuximab and bevacizumab alone in
irinotecan-refractory colorectal cancer: The BOND-2 study. J Clin
Oncol. 25:4557–4561. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Cutsem EV, Kohne C-H, Hitre E, et al:
Cetuximab and chemotherapy as initial treatment for metastatic
colorectal cancer. N Engl J Med. 360:1408–1417. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Follézou JY and Bizon M: Cancer
chemotherapy induces a transient increase of serum-iron level.
Neoplasma. 33:225–231. 1986.PubMed/NCBI
|
|
10.
|
Hentze MW, Muckenthaler MU, Andrews NC, et
al: Molecular control of mammalian iron metabolism. Cell.
117:285–297. 2004.PubMed/NCBI
|
|
11.
|
Iwai K: An ubiquitin ligase recognizing a
protein oxidized by iron: implications for the turnover of
oxidatively damaged proteins. J Biochem. 134:175–182. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Rouault TA: The role of iron regulatory
proteins in mammalian iron homeostasis and disease. Nat Chem Biol.
2:406–414. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Goldberg RM, Sargent DJ, Morton RF, et al:
A randomized controlled trial of fluorouracil plus leucovorin,
irinotecan, and oxaliplatin combinations in patients with
previously untreated metastatic colorectal cancer. J Clin Oncol.
22:23–30. 2004. View Article : Google Scholar
|
|
14.
|
Douillard JY, Cunningham D, Roth AD, et
al: Irinotecan combined with fluorouracil compared with
fluorouracil alone as first-line treatment for metastatic
colorectal cancer: a multicentre randomised trial. Lancet.
355:1041–1047. 2000. View Article : Google Scholar
|