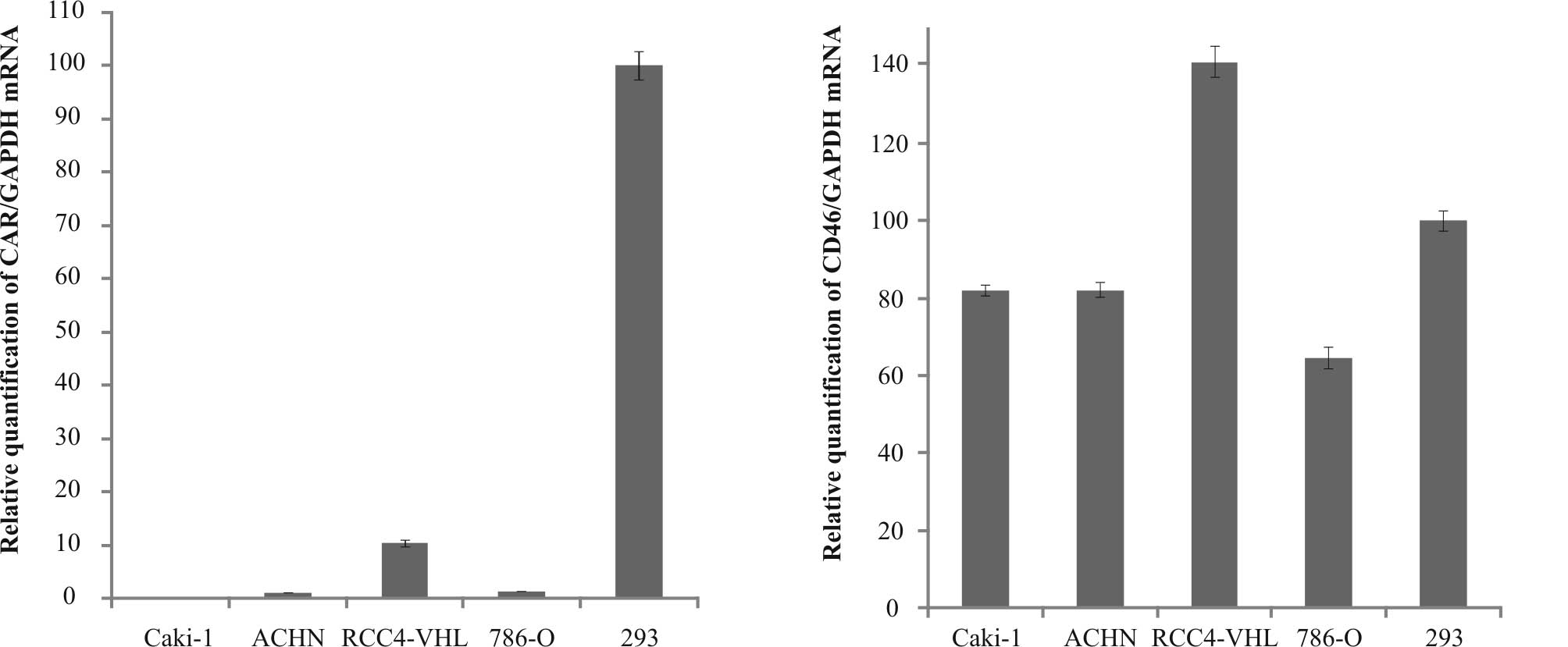
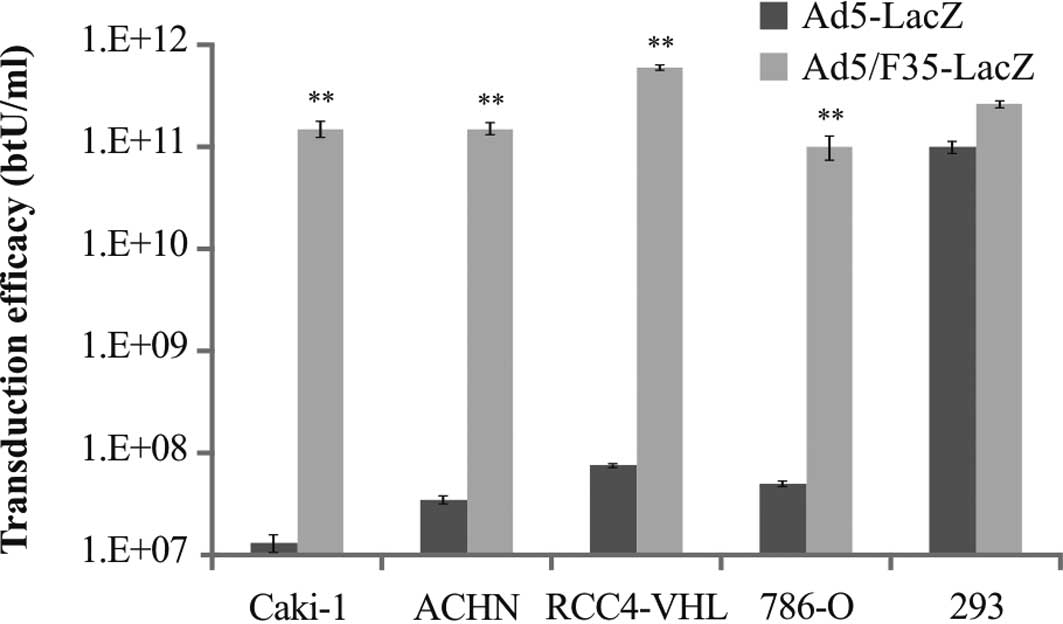
|
1.
|
American Cancer Society: Cancer Facts and
Figures 2009. American Cancer Society; Atlanta: 2009
|
|
2.
|
Coppin C, Porzsolt F, Awa A, Kumpf J,
Coldman A and Wilt T: Immunotherapy for advanced renal cell cancer.
Cochrane Database Syst Rev. 1:CD0014252005.
|
|
3.
|
Castellano D, Del Muro XG, Pérez-Gracia
JL, González-Larriba JL, Abrio MV, Ruiz MA, Pardo A, Guzmán C,
Cerezo SD and Grande E: Patient-reported outcomes in a phase III,
randomized study of sunitinib versus interferon-α as first-line
systemic therapy for patients with metastatic renal cell carcinoma
in a European population. Ann Oncol. 20:1803–1812. 2009.PubMed/NCBI
|
|
4.
|
Rini BI, Halabi S, Taylor J, Small EJ and
Schilsky RL; Cancer and Leukemia Group B: Cancer and Leukemia Group
B 90206: a randomized phase III trial of interferon-alpha or
interferon-alpha plus anti-vascular endothelial growth factor
antibody (bevacizumab) in metastatic renal cell carcinoma. Clin
Cancer Res. 10:2584–2586. 2004. View Article : Google Scholar
|
|
5.
|
Yang JC, Haworth L, Sherry RM, Hwu P,
Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX and
Rosenberg SA: A randomized trial of bevacizumab, an anti-vascular
endothelial growth factor antibody, for metastatic renal cancer. N
Engl J Med. 349:427–434. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Hamilton MM, Byrnes GA, Gall JG, Brough
DE, King CR and Wei LL: Alternate serotype adenovector provides
long-term therapeutic gene expression in the eye. Molecular Vision.
14:2535–2546. 2008.PubMed/NCBI
|
|
7.
|
Bergelson JM, Cunningham JA, Droguett G,
Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowel RL and
Finberg RW: Isolation of a common receptor for Coxsackie B viruses
and adenoviruses 2 and 5. Science. 275:1320–1323. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Okegawa T, Sayne JR, Nutahara K, Pong RC,
Saboorian H, Kabbani W, Higashihara E and Hsieh JT: A histone
deacetylase inhibitor enhances adenoviral infection of renal cancer
cells. J Urol. 177:1148–1156. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Mizuguchi H and Hayakawa T: Targeted
adenovirus vectors. Hum Gene Ther. 15:1034–1044. 2004. View Article : Google Scholar
|
|
10.
|
Gaggar A, Shayakhmetov DM and Lieber A:
CD46 is a cellular receptor for group B adenoviruses. Nat Med.
9:1408–1412. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Segerman A, Atkinson JP, Marttila M,
Dennerquist V, Wadell G and Arnberg N: Adenovirus type 11 uses CD46
as a cellular receptor. J Virol. 77:9183–9191. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Marttila M, Persson D, Gustafsson D,
Liszewski MK, Atkinson JP, Wadell G and Arnberg N: CD46 is a
cellular receptor for all species B adenoviruses except types 3 and
7. J Virol. 79:14429–14436. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Toyoda E, Doi R, Kami K, Mori T, Ito D,
Koizumi M, Kida A, Nagai K, Ito T, Masui T, Wada M, Tagawa M and
Uemoto S: Adenovirus vectors with chimeric type 5 and 35 fiber
proteins exhibit enhanced transfection of human pancreatic cancer
cells. Int J Oncol. 33:1141–1147. 2008.PubMed/NCBI
|
|
14.
|
Yu L, Shimozato O, Li Q, Kawamura K, Ma G,
Namba M, Ogawa T, Kaiho I and Tagawa M: Adenovirus type 5
substituted with type 11 or 35 fiber structure increases its
infectivity to human cells enabling dual gene transfer in
CD46-dependent and independent manners. Anticancer Res.
27:2311–2316. 2007.PubMed/NCBI
|
|
15.
|
Mizuguchi H and Hayakawa T: Adenovirus
vectors containing chimeric type 5 and type 35 fiber proteins
exhibit altered and expanded tropism and increase the size limit of
foreign genes. Gene. 20:69–77. 2002. View Article : Google Scholar
|
|
16.
|
Ni S, Gaggar A, Di Paolo N, Li ZY, Liu Y,
Strauss R, Sova P, Morihara J, Feng Q, Kiviat N, Touré P, Sow PS
and Lieber A: Evaluation of adenovirus vectors containing serotype
35 fibers for tumor targeting. Cancer Gene Ther. 13:1072–1081.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Yu L, Takenobu H, Shimozato O, Kawamura K,
Nimura Y, Seki N, Uzawa K, Tanzawa H, Shimada H, Ochiai T and
Tagawa M: Increased infectivity of adenovirus type 5 bearing type
11 or type 35 fibers to human esophageal and oral carcinoma cells.
Oncol Rep. 14:831–835. 2005.PubMed/NCBI
|
|
18.
|
Terao S, Acharya B, Suzuki T, Aoi T, Naoe
M, Hamada K, Mizuguchi H and Gotoh A: Improved gene transfer into
renal carcinoma cells using adenovirus vector containing RGD motif.
Anticancer Res. 29:2997–3002. 2009.PubMed/NCBI
|
|
19.
|
Koizumi N, Mizuguchi H, Kondoh M, Fujii M,
Nakanishi T, Utoguchi N and Watanabe Y: Efficient gene transfer
into differentiated human trophoblast cells with adenovirus vector
containing RGD motif in the fiber protein. Biol Pharm Bull.
29:1297–1299. 2006. View Article : Google Scholar
|
|
20.
|
Wechsel HW, Petri E, Feil G, Nelde HJ,
Bichler KH and Loesr W: Renal cell carcinoma: immunohistological
investigation of expression of the integrin alpha v beta 3.
Anticancer Res. 19:1529–1532. 1999.PubMed/NCBI
|
|
21.
|
Chen L, Chen D, Gong M, Na M, Li L, Wu H,
Jiang L, Qian Y, Fang G and Xue X: Concomitant use of Ad5/35
chimeric oncolytic adenovirus with TRAIL gene and taxol produces
synergistic cytotoxicity in gastric cancer cells. Cancer Lett.
284:141–148. 2009. View Article : Google Scholar
|
|
22.
|
Toivonen R, Suominen E, Grenman R and
Savontaus M: Retargeting improves the efficacy of a
telomerase-dependent oncolytic adenovirus for head and neck cancer.
Oncol Rep. 21:165–171. 2009.PubMed/NCBI
|