Introduction
Brain metastasis occurs frequently in women with
advanced breast cancer. However, early breast cancer patients
developing metastasis to the brain as the first site of recurrence
are occasionally encountered. Certain studies suggest that only
10–16% of patients with metastatic breast cancer have clinically
apparent brain metastases, and autopsy series have revealed an
incidence of brain metastasis of 30% in breast cancer patients
(1). However, it is a matter of
great concern that few effective treatments are available for the
treatment of clinically apparent brain metastases. It is well known
that almost none of the anticancer agents are effective against
brain metastases due to the presence of the blood-brain barrier.
Therefore, the main treatment method for brain metastases is either
radiotherapy or surgical therapy. However, even after these
treatments, the mean 1-year survival rate is estimated to be less
than 20% (2,3). Therefore, more effective treatments
for brain metastases are required.
Some clinicians have suggested that the incidence of
brain metastasis in breast cancer patients has increased following
the introduction of trastuzumab, a humanized recombinant monoclonal
antibody against human epidermal growth factor receptor
(HER)-2/neu, although the cause/effect relationship remains unclear
and controversial. A number of possibilities have, however, been
proposed: i) HER-2-positive breast tumors are more likely to
metastasize to the brain; ii) trastuzumab may promote the
development of brain metastasis; iii) prolongation of the survival
period owing to the efficacy of trastuzumab against visceral
metastases results in an increase in the incidence of brain
metastasis. If trastuzumab was not effective against only brain
metastases, the blood-brain barrier could play an important role in
the development of brain metastases. However, we think that changes
in the biological features of the tumors also contribute to the
development of brain metastasis.
The HER-2 expression status is a predictive factor
for the efficacy of trastuzumab and also a prognostic factor in
breast cancer patients. In general, it is well known that breast
cancer patients with HER-2/neu overexpression have a poor
prognosis. The estrogen receptor (ER) and progesterone receptor
(PgR) are the main hormone receptors expressed in breast cancers,
and the expression status of these receptors is the best predictive
factor for hormonal therapy. Moreover, ER- and PgR-negative breast
cancers show poor prognosis and are more likely associated with
brain metastasis (4–8). p53 and bcl-2 are apoptosis-related
factors, and Ki-67 is a proliferation-related factor. Evaluating
the differences in the expression of these factors between primary
breast tumors and brain metastases is very important in order to
elucidate the mechanism of development of brain metastasis.
In the present study, we comparatively evaluated the
expression of these factors by immunohistochemistry in primary
breast tumors and metachronous brain metastases. Moreover, the
relationships between the expression of these factors and prognosis
were also examined.
Materials and methods
Patients and tissue samples
This study was conducted on 21 patients who were
diagnosed as having breast cancer and who underwent breast surgery
at the Saitama Cancer Center, Japan, between 1984 and 2004. All 21
patients developed metachronous brain metastasis and underwent
craniotomy and tumor resection, followed by whole-brain
radiotherapy. None of the patients had received trastuzumab before
the onset of the brain metastasis. For all of the 21 patients,
specimens of the primary breast and metastatic brain tumors were
available for the study. All of the resected tissues were fixed in
10% formalin solution, embedded in paraffin and stained with
H&E for routine histopathologic examination. The expression of
ER, PgR and HER-2 in the primary breast cancers was compared to
that in usual invasive breast cancers. These usual breast cancers
consisted of 439 (for ER and PgR) or 586 (for HER-2) consecutive
tumor samples collected at the Saitama Cancer Center between 2003
and 2004.
Immunohistochemistry
Immunohistochemical staining was performed on the
resected tissues. Sections were deparaffinized with xylene and
dehydrated through a graded series of ethanol. To enhance the
antigenicity, the sections were immersed in 10 mmol/l citrate
buffer (pH 6.0) and autoclaved. Endogenous peroxidase activity was
blocked with 0.3% hydrogen peroxide in methanol. Sections were then
incubated for 1 h with monoclonal antibodies against ER (1:50), PgR
(1:50), p53 (1:100), bcl-2 (1:40), Ki-67 (1:50) or polyclonal
antibody against HER-2/neu (1:1,600) (all from Dako, Glostrup,
Denmark). Incubation with a secondary antibody (peroxidase-labeled
Envision polymer reagent; Dako) was performed for 45 min at room
temperature. After visualization of the reaction complexes with
0.05% 3,3′-diaminobenzidine tetrahydrochloride and 0.03% hydrogen
peroxide in 50 mmol/l Tris-HCL buffer (pH 7.6), the sections were
examined under a light microscope.
Specimen analysis
Two investigators performed the microscopic
examinations independently. Immunoreactivity was quantified by
evaluating a minimum of 1,000 carcinoma cells in the histologic
sections examined in randomly selected fields under a high-powered
objective. Strong to moderate immunostaining was considered as
positive staining and faint to no staining was considered as
negative staining. The results for ER, PgR and p53 were considered
positive when >10% of the nuclei of the carcinoma cells showed
positive staining for the respective markers. As for bcl-2, the
expression status was considered positive when >25% of the
cytoplasm of the carcinoma cells showed positive staining. The
Ki-67 labeling index (LI) was calculated as the percentage of
carcinoma cells with Ki-67-positive nuclei. A Ki-67 LI ≥20% was
considered to be a high value of the index. For the evaluation of
HER-2/neu expression, semi-quantitative analysis was performed
according to the scoring guidelines laid down in the
HercepTestTM (Dako) instruction guide. In brief, the
specimens were scored as follows: 0, no staining or membrane
staining in <10% of the tumor cells; 1+, faint or barely
perceptible membrane staining in >10% of the tumor cells; 2+,
weak to moderate complete membrane staining in >10% of the tumor
cells; 3+, strong complete membrane staining in >30% of the
tumor cells. Scores of 0 and 1+ represented a negative result for
HER-2/neu overexpression, whereas scores of 2+ and 3+ were
considered to represent HER-2/neu overexpression. Only the membrane
staining intensity and patterns were evaluated as laid down in the
guideline.
Statistical analysis
The χ2 analysis was used for univariate
categorical variable analysis. In relation to ER, PgR, HER-2, bcl-2
and p53 expression, survival curves were calculated using the
Kaplan-Meier method and compared by the log-rank test. The Ki-67 LI
in the primary tumors was compared to that in the brain metastases
using a paired t-test. A P-value <0.05 was considered as
denoting statistical significance.
Results
Clinical and pathological
characteristics
All 21 patients were female, with a median age of 47
years (range 33–69) at the time of diagnosis of the primary breast
cancer. Of the 21 patients, 1 had received pre-operative
chemotherapy prior to the breast surgery. Thirteen patients had
received at least one form of postoperative hormonal therapy, 16
had received at least one form of postoperative chemotherapy and 10
had received both after the breast surgery. Only 1 patient had
received trastuzumab after the brain surgery. The average interval
from primary diagnosis to death was 56 months, whereas the average
interval from the time of development of brain metastasis to death
was 12 months. Death was ascribed to brain metastasis in 11 of the
17 patients and to non-central nervous system causes in 6 of the 17
patients (Table I).
 | Table I.Demographic data. |
Table I.
Demographic data.
| Characteristics | n=21 |
|---|
| Age (years) | |
| Median at
10 Dx | 47 |
| Range at
10 Dx | 33–69 |
| Average TTD from
10 Dx (months) | 56.0 |
| Average TTD from Met
(months) | 12.0 |
| Average TTM from
10 Dx (months) | 44.5 |
| Cause of death
(n=17) | |
| CNS/non-CNS | 11/6 |
| No. of Met | |
|
Single/multiple | 8/13 |
Characteristics of the primary
tumors
The primary tumors were positive for ER, PgR, HER-2,
p53 and bcl-2 expression in 43% (9/21), 29% (6/21), 33% (7/21), 33%
(7/21) and 57% (12/21) of the cases, respectively. The Ki-67 LI was
high in 67% (14/21) of the primary tumors and not high in the
remaining 33% cases (7/21), with a mean ± SD percentage of
immunoreactive cells of 25.6±14.6%. The percentages of ER- and/or
PgR-positive cases in the primary tumors were significantly lower
than the corresponding percentages in the usual invasive breast
cancers (ER, P=0.0002; PgR, P=0.00018). However, there was no
significant difference in the percentage of HER-2-positive
specimens between the primary tumors and usual invasive breast
cancers (P=0.64).
Characteristics of the brain
metastases
The brain metastases were positive for ER, PgR,
HER-2, p53 and bcl-2 expression in 43% (9/21), 19% (4/21), 43%
(9/21), 48% (10/21) and 43% (9/21) of the cases, respectively. The
Ki-67 LI was high in 95% (20/21) of the brain metastases and not
high in the remaining 5% cases (1/21), with a mean ± SD percentage
of immunoreactive cells of 44.3±19.8%.
Comparison of the expression in the
primary tumors and brain metastases
The concordance rate between the primary tumors and
brain metastases was high for p53 [86% (18/21)], ER, PgR and HER-2
[81% (17/21) for all three]. The negative-conversion rates for ER
and PgR were 22% (2/9) and 50% (3/6), respectively (Table II); all of these patients had
received postoperative hormonal therapy after breast surgery. The
Ki-67 LI was significantly higher in the brain metastases than that
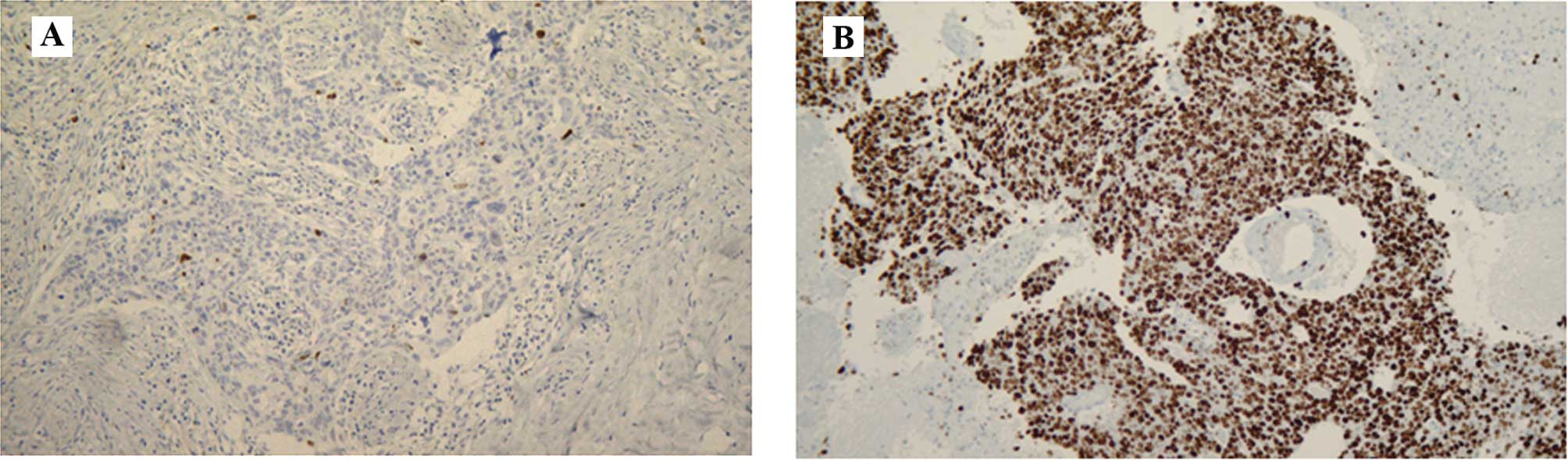
in the primary tumors (P=0.003) (Figs.
1 and 2).
 | Table II.Positive expression rate and
conversion of expression. |
Table II.
Positive expression rate and
conversion of expression.
| Positive expression
rate
| | | | |
|---|
| Primary | Brain | PCC | NCC | CR | NCR |
|---|
| ER | 43% (9/21) | 43% (9/21) | 2 | 2 | 81% (17/21) | 22% (2/9) |
| PgR | 29% (6/21) | 19% (4/21) | 1 | 3 | 81% (17/21) | 50% (3/6) |
| HER-2 | 33% (7/21) | 43% (9/21) | 3 | 1 | 81% (17/21) | 14% (1/7) |
| p53 | 33% (7/21) | 48% (10/21) | 3 | 0 | 86% (18/21) | 0% (0/7) |
| bcl-2 | 57% (12/21) | 43% (9/21) | 1 | 4 | 76% (16/21) | 33% (4/12) |
Survival data
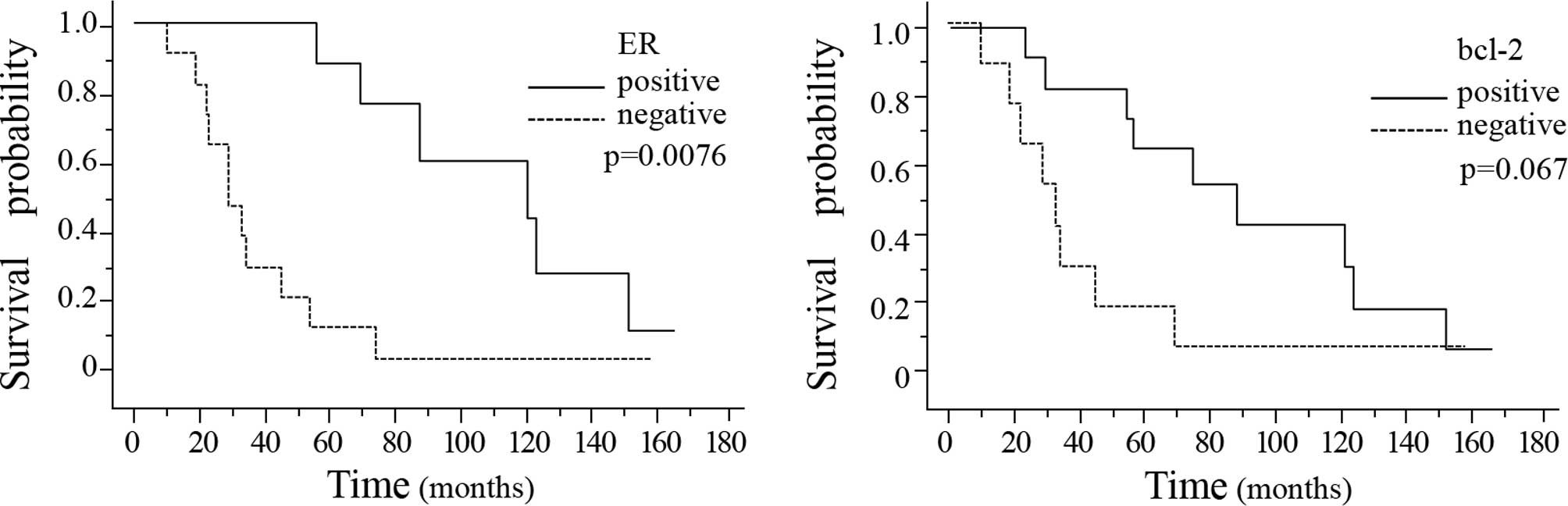
The time from primary diagnosis to death was
significantly longer in the breast cancer patients showing positive
ER expression in the primary breast cancer tissue (P=0.0076)
(Fig. 3A). Moreover, the interval
from primary diagnosis to death also tended to be longer in the
patients showing positive bcl-2 expression in the primary breast
cancer tissue as compared to that in the patients showing negative
bcl-2 expression in the primary breast tumor (P=0.067) (Fig. 3B). There were no significant
differences in the interval from the diagnosis of brain metastasis
to death associated with the presence/absence of ER, PgR, HER-2,
p53 or bcl-2 in either the primary breast tumors or the brain
metastases (Table III). The
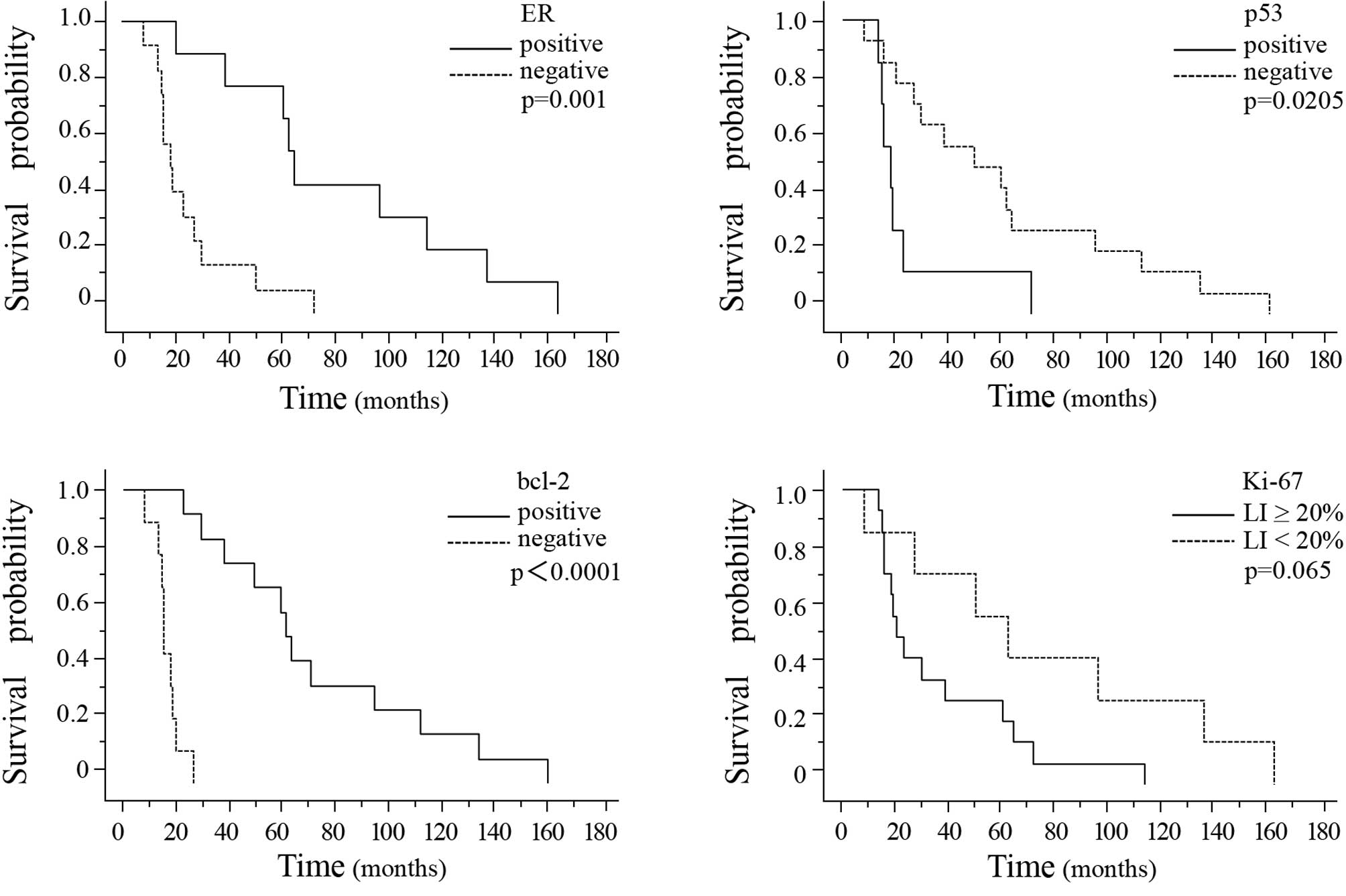
interval from primary diagnosis to the development of brain
metastasis was significantly longer in the breast cancer patients
showing ER-positivity, p53-negativity or bcl-2-positivity in the
primary breast cancer tissue (Fig.
4A–C). Of the three markers, bcl-2 was the most sensitive
prognostic marker (P<0.0001). The interval from primary
diagnosis to the detection of brain metastasis tended to be longer
in patients showing a Ki-67 LI of <20% in the breast cancer
tissue (Fig. 4D).
 | Table III.Survival data (mean time to death in
months). |
Table III.
Survival data (mean time to death in
months).
| ER
| PgR
| HER-2
| p53
| bcl-2
|
|---|
| + | − | + | − | + | − | + | − | + | − |
|---|
| Staining, breast | | | | | | | | | | |
| Survival from
diagnosis of primary tumor (n=21) | 112.2 | 37.2 | 92.3 | 63.3 | 43.7 | 79.4 | 39.1 | 84.4 | 92.8 | 36.7 |
| P-value | 0.0076 | 0.18 | 0.38 | 0.24 | 0.067 |
| Survival from
diagnosis of brain metastasis (n=21) | 37.3 | 11.3 | 13.5 | 24.0 | 26.5 | 21.6 | 11.0 | 27.4 | 26.6 | 21.1 |
| P-value | 0.13 | 0.88 | 0.69 | 0.60 | 0.98 |
| Staining,
brain | | | | | | | | | | |
| Survival from
diagnosis of brain metastasis (n=21) | 28.0 | 19.5 | 36.5 | 19.1 | 17.6 | 28.4 | 10.2 | 30.2 | 26.3 | 21.0 |
| P-value | 0.78 | 0.53 | 0.30 | 0.35 | 0.97 |
Discussion
In the present study, we demonstrated a striking
increase in the Ki-67 LI in the metachronous brain metastases as
compared to that in the corresponding primary breast tumors. We
clarified the differences in the characteristics between the
primary breast cancer and the metachronous brain metastases in this
study. The Ki-67 nuclear antigen is known to be present during the
G1, S, G2 and M, but not the G0, phases of the cell cycle in
continuously proliferating cells. Therefore, immunohistochemical
staining with an antibody against Ki-67 serves as a useful means to
determine the cell proliferative activity (9). It has been reported that breast
cancer patients with a high Ki-67 LI in the primary tumor are
likely to have a poor prognosis (10–15).
Some investigators have demonstrated that a high Ki-67 LI is a
predictive marker of a better response to chemotherapy in breast
cancer patients (16–18), although the precise relationship
between the Ki-67 LI and the efficacy of chemotherapy is still
controversial (19). This may be
partially attributed to the heterogeneity of Ki-67 expression.
Shabani et al, using a small sample size, also showed
significantly higher values of the Ki-67 LI in brain metastases
than in primary cancers (the origins of various origins) (20). Moreover, some investigators have
reported that the Ki-67 LI was significantly higher in metastatic
axillary lymph nodes than in the corresponding primary breast
tumors (21,22). The differences in the Ki-67 LI
between primary breast tumors and metastatic tumors have not yet
been clarified in relation to other organs. However, it could be
reasonably presumed that the Ki-67 LI would be higher in metastatic
tissues than that in the primary tumors. In the present study, we
confirmed that the Ki-67 LI was significantly higher in the brain
metastases than in the primary breast cancers, despite the
existence of heterogeneity.
As compared to the case in usual invasive breast
cancer, the percentage of ER- and/or PgR-positive cases was
significantly lower in the patients with metachronous brain
metastases, which was consistent with previous reports (4–8).
However, the percentage of HER-2-positive cases was not
significantly different between these two groups. It has been
reported that 42–48% of breast tumors associated with metachronous
brain metastases show positive HER-2 expression (23,24).
Thus, our result of 33% seems to be relatively low; we speculated
that this may simply be due to the small sample size in our
study.
In the present study, the concordance rates between
the primary breast tumors and the brain metastases were high (more
than 76%) for ER, PgR, HER-2, p53 and bcl-2 expression. Of the 21
primary breast tumors, 9 cases were positive for ER and 6 for PgR.
The negative conversion rate was 22% (2/9) for ER and 50% (3/6) for
PgR; all patients that showed negative ER and/or PgR conversions
had received hormonal therapy after the breast surgery. These
results raise the possibility of a relationship between negative
conversion for hormone receptor expression and the efficacy of
hormonal therapy. However, this phenomenon (negative conversion)
does not always occur after hormonal therapy. On the other hand,
negative ER and/or PgR conversions have also been found to be
frequently induced by pre-operative hormonal therapy (25). Although the mechanisms underlying
these phenomena have yet to be fully elucidated, negative
conversion of hormone receptor expression appears to be an
important factor in the resistance to hormonal therapies.
The percentage of p53-positive cases was higher by
15% in the brain metastases than in the primary breast tumors; that
is, 3 of the 14 p53-negative primary breast tumors showed positive
conversion in the brain metastases. Some investigators have
suggested that there is a considerable difference in the rate of
p53 abnormalities between immunohistochemical and mutational
analysis (26–28). Immunohistochemical analysis detects
mutated p53 protein, but not wild-type p53. This is thought to be
due to the longer half-life of mutated p53 protein. Failure of
apoptosis is attributed to the p53 mutation. Thus, the detection of
p53 protein is considered to be associated with worsening tumor
characteristics. On the other hand, although bcl-2 is generally
considered to be an anti-apoptotic protein, most investigators
agree that bcl-2-positive breast cancers show good responses to
hormonal therapy. Moreover, bcl-2 expression has been reported to
be associated with favorable histopathological features and
positive clinical outcomes (29,30).
This may seem paradoxical, but we consider that the presence of the
bcl-2 protein sustains the estrogen-dependent proliferative
organization of the tumors. The percentage of bcl-2-positive cases
was lower by 14% in the brain metastases than in the primary breast
tumors; that is, 4 of the 12 bcl-2-positive primary breast tumors
showed negative conversion in the brain metastases, and 1 of the 9
bcl-2-negative primary breast tumors showed positive conversion in
the brain metastases. Positive hormone receptor expression is a
favorable prognostic factor in breast cancer patients; therefore,
the decrease in the percentage of bcl-2-positive cases in the brain
metastases is not likely to reflect a favorable prognosis. In this
study, the increase in the number of p53-positive cases and
Ki-67-positive cells, and the decrease in the number of
bcl-2-positive cases in the brain metastases suggest a worsening of
the tumor characteristics in brain metastases.
In our study, the interval from primary diagnosis to
death was significantly longer in the breast cancer patients who
showed positive ER expression in the primary breast cancer tissue.
This tendency was also observed for bcl-2 expression. Furthermore,
the interval from primary diagnosis to the detection of brain
metastasis was significantly longer in the breast cancer patients
showing ER-positivity, p53-negativity or bcl2-positivity in the
primary breast cancer tissue. In relation to ER and bcl-2, the
prolongation of the interval from primary diagnosis to the
detection of brain metastasis contributed to the prolongation of
the interval from primary diagnosis to death. From this point of
view, ER and bcl-2 are important prognostic factors in these
patients.
We also examined the correlation between the Ki-67
LI and prognosis. There was no relationship between the Ki-67 LI in
breast cancer tissue and the interval from primary diagnosis to
death. Moreover, there was also no relationship between the Ki-67
LI in the brain metastases and the interval from the detection of
brain metastasis to death. Caly et al reported the Ki-67 LI
as a prognostic factor in terms of both disease-free survival and
overall survival (OS) based on a study of 257 breast cancer
patients (15). In our study, the
absence of a relationship between the Ki-67 LI in the breast cancer
tissue and the OS could be attributable to the small sample size.
However, the interval from primary diagnosis to the detection of
brain metastasis tended to be longer in breast cancer patients
showing a Ki-67 LI of less than 20% in the breast cancer
tissue.
In conclusion, we comparatively evaluated the
immunohistochemical expression profiles between primary breast
tumors and brain metastases in this study. There were no
significant differences in the expression of ER, PgR, HER-2, p53 or
bcl-2 between the two tissues. However, the Ki-67 LI was
significantly higher in the brain metastases than in the primary
breast tumors. Furthermore, a high Ki-67 LI in the primary breast
tumor was associated with a shorter interval from the primary
diagnosis to the development of brain metastasis. These results
suggest that the tumor characteristics are worse in the brain
metastases. In addition, ER and/or bcl-2 expression was shown to be
an important prognostic factor in breast cancer patients with
metachronous brain metastases. Further studies in larger samples
are required to understand the relationship between the
immunohistochemical expression profiles of primary breast tumor and
brain metastases.
Acknowledgements
The authors acknowledge financial
support by a grant from the ‘Brain Metastasis of Breast Cancer’
Research Group (Director, Dr Michihide Mitsumori) of the Japanese
Breast Cancer Society.
References
|
1.
|
Lin NU, Bellon JR and Winer EP: CNS
metastases in breast cancer. J Clin Oncol. 22:3608–3617. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Shaffrey ME, Mut M, Asher AL, Burri SH,
Chahlavi A, Chang SM, Farace E, Fiveash JB, Lang FF, Lopes MB,
Markert JM, Schiff D, Siomin V, Tatter SB and Voqelbaum MA: Brain
metastasis. Curr Probl Surg. 41:665–741. 2004. View Article : Google Scholar
|
|
3.
|
Engel J, Eckel R, Aydemir U, Aydemir S,
Kerr J, Schlesinger-Raab A, Dirschedl P and Holzel D: Determinants
and prognoses of locoregional and distant progression in breast
cancer. Int J Radiat Oncol Biol Phys. 55:1186–1195. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Hicks DG, Short SM, Prescott NL, Tarr SM,
Coleman KA, Yoder BJ, Crowe JP, Choueiri TK, Dawson AE, Butt GT,
Tubbs RR, Casey G and Weil RJ: Breast cancers with brain metastases
are more likely to be estrogen receptor negative, express the basal
cytokeratin CK5/6, and overexpress HER2 or EGFR. Am J Surg Pathol.
30:1097–1103. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Ryberg M, Nielsen D, Osterlind K, Andersen
PK, Skovsgaard T and Dombernowsky P: Predictors of central nervous
system metastasis in patients with metastatic breast cancer: A
competing risk analysis of 579 patients treated with
epirubicin-based chemotherapy. Breast Cancer Res Treat. 91:217–225.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Evans AJ, James JJ, Cornford EJ, Chan SY,
Burrell HC, Pinder SE, Gutteridge E, Robertson JF, Hornbuckle J and
Cheung KL: Brain metastasis from breast cancer: identification of a
high-risk group. Clin Oncol. 16:345–349. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Slimane K, Andre F, Delaloge S, Dunant A,
Perez A, Grenier J, Massard C and Spielmann M: Risk factors for
brain relapse in patients with metastatic breast cancer. Ann Oncol.
15:1640–1644. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Maki D and Grossman RI: Patterns of
disease spread in meta-static breast carcinoma: influence of
estrogen and progesterone receptor status. AJNR Am J Neuroradiol.
21:1064–1066. 2000.PubMed/NCBI
|
|
9.
|
Gerdes J, Lemke H, Baisch H, Wacker HH,
Schwab U and Stein H: Cell cycle analysis of a cell
proliferation-associated human nuclear antigen defined by the
monoclonal antibody Ki-67. J Immunol. 133:1710–1715.
1984.PubMed/NCBI
|
|
10.
|
Molino A, Micciolo R, Turazza M, Bonetti
F, Piubello Q, Bonetti A, Nortilli R, Pelosi G and Cetto GL: Ki-67
immunostaining in 322 primary breast cancers: associations with
clinical and pathological variables and prognosis. Int J Cancer.
74:433–437. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Railo M, Lundin J, Haglund C, von Smitten
K, von Boguslawsky K and Nordling S: Ki-67, p53, Er-receptors,
ploidy and S-phase as prognostic factors in T1 node negative breast
cancer. Acta Oncol. 36:369–374. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Pierga JY, Leroyer A, Viehl P, Mosseri V,
Chvillard S and Magdelenat H: Long term prognostic value of growth
fraction determination by Ki-67 immunostaining in primary operable
breast cancer. Breast Cancer Res Treat. 37:57–64. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Railo M, Nordling S, von Boguslawsky K,
Leivonen M, Kyllonen L and von Smitten K: Prognostic value of Ki-67
immunolabelling in primary operable breast cancer. Br J Cancer.
68:579–583. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Veronese SM, Gambacorta M, Gottardi O,
Scanzi F, Ferrari M and Lampertico P: Proliferation index as a
prognostic marker in breast cancer. Cancer. 71:3926–3931. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Caly M, Genin P, Al Ghuzlan A, Elie C,
Freneaux P, Klijanienko J, Rosty C, Sigal-Zafrani B,
Vincent-Salomoni A, Douggaz A, Zidane M and Sastre-Garau X:
Analysis of correlation between mitotic index, MIB1 score and
S-phase fraction as proliferation markers in invasive breast
carcinoma. Methodological aspects and prognostic value in a series
of 257 cases. Anticancer Res. 24:3283–3288. 2004.
|
|
16.
|
Petit T, Wilt M, Velten M, Millon R,
Rodier JF, Borel C, Mors R, Haegele P, Eber M and Ghnassia JP:
Comparative value of tumour grade hormonal receptors, Ki-67, HER-2
and topoisomerase II alpha status as predictive markers in breast
cancer patients treated with neoadjuvant anthracycline-based
chemotherapy. Eur J Cancer. 40:205–211. 2004. View Article : Google Scholar
|
|
17.
|
Bonetti A, Zaninelli M, Rodella S, Molino
A, Sperotto L, Piubello Q, Bonetti F, Nortilli R, Turazza M and
Cetto GL: Tumor proliferative activity and response to first-line
chemotherapy in advanced breast carcinoma. Breast Cancer Res Treat.
38:289–297. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
MacGrogan G, Mauriac L, Durand M, Bonichon
F, Trojani M, De Mascarel I and Coindre JM: Primary chemotherapy in
breast invasive carcinoma: predictive value of the
immunohistochemical detection of hormonal receptors, p53, c-erbB-2,
MiB1, pS2 and GST pi. Br J Cancer. 74:1458–1465. 1996. View Article : Google Scholar
|
|
19.
|
Aas T, Geisler S, Eide GE, Haugen DF,
Varhaug JE, Bassoe AM, Thorsen T, Berntsen H, Borresen-Dale AL,
Akslen LA and Lonning PE: Predictive value of tumor cell
proliferation in locally advanced breast cancer treated with
neoadjuvant chemotherapy. Eur J Cancer. 39:438–446. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Shabani HK, Kitange G, Tsunoda K, Anda T,
Tokunaga Y, Shibata S, Kaminogo M, Hayashi T, Ayabe H and Iseki M:
Immunohistochemical expression of E-cadherin in metastatic brain
tumors. Brain Tumor Pathol. 20:7–12. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Buxant F, Anaf V, Simon P, Favt I and Noel
JC: Ki-67 immunostaining activity is higher in positive axillary
lymph nodes than in the primary breast tumor. Breast Cancer Res
Treat. 75:1–3. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Cabibi D, Mustacchio V, Martorana A,
Tripodo C, Campione M, Calascibetta A, Sanguedolce R and Aragona F:
Lymph node metastases displaying lower Ki-67 immunostaining
activity than the primary breast cancer. Anticancer Res.
26:4357–4360. 2006.PubMed/NCBI
|
|
23.
|
Lear-Kaul KC, Yoon HR,
Kleinschmidt-DeMasters BK, McGavran L and Singh M: HER-2/neu status
in breast cancer metastases to central nervous system. Arch Pathol
Lab Med. 127:1451–1457. 2003.PubMed/NCBI
|
|
24.
|
Fuchs IB, Loebbecke M, Buhler H,
Stoltenburg-Didinger G, Heine B, Lichtenegger W and Schaller G:
HER2 in brain metastases: issues of concordance, survival, and
treatment. J Clin Oncol. 20:4130–4133. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Ellis MJ, Coop A, Singh B, Tao Y,
Llombart-Cussac A, Janicke F, Mauriac L, Quebe-Fehling E,
Chaudri-Ross HA, Evans DB and Miller WR: Letrozole inhibits tumor
proliferation more effectively than tamoxifen independent of HER1/2
expression status. Cancer Res. 63:6523–6531. 2003.PubMed/NCBI
|
|
26.
|
Dunn JM, Hastrich DJ, Newcomb P, Webb JCJ,
Maitland NJ and Farndon JR: Correlation between p53 mutations and
antibody staining in breast carcinoma. Br J Surg. 80:1410–1412.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Thompson AM, Anderson TJ, Condie A,
Prosser J, Chetty U, Carter DC, Evans HJ and Steel CM: p53 allele
losses, mutations and expression in breast cancer and their
relationship to clinicopathological parameters. Int J Cancer.
50:528–532. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Borrensen AL, Hovig E, Smith-Sorensen B,
Malkin D, Lystad S, Andersen TI, Nesland JM, Isselbacher KJ and
Friend SH: Constant denaturation gel electrophoresis (CDGE) as a
rapid screening technique for p53 mutations. Proc Natl Acad Sci
USA. 88:8405–8409. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Binder C, Marx D, Overhoff R, Binder L,
Schauer A and Hiddemann W: Bcl-2 protein expression in breast
cancer in relation to established prognostic factors and other
clinicopathological variables. Ann Oncol. 6:1005–1010.
1995.PubMed/NCBI
|
|
30.
|
Gee JM, Robertson JF, Ellis IO, Willsher
P, McClelland RA, Hoyle HB, Kyme SR, Finlay P, Blamey RW and
Nicholson RI: Immunocytochemical localization of bcl-2 protein in
human breast cancers and its relationship to a series of prognostic
markers and response to endocrine therapy. Int J Cancer.
59:619–628. 1994. View Article : Google Scholar
|