Introduction
Gastric cancer is the fourth most common malignancy
and the second leading cause of cancer-related death in the world
(1). Incidence rates vary across
continents, being higher in Asia, Central and South America and
Europe (1). At least in developed
counties, both incidence and death rates have been constantly
decreasing in the last 50 years. Furthermore, according to the
Surveillance, Epidemiology and End Results database, the 5-year
survival for all gastric cancer patients treated in the US
increased by 50% from 1975 to 2003 (from 16 to 24%). However, in
2008 21,500 new diagnoses of gastric cancer were estimated in the
US (2) and 12,600 in Italy
(http://www.tumori.net/stime).
As expected, patients with localized disease have a
higher 5-year survival rate (82%) compared to patients with
regional (24%) or distant metastases (3%) (2). In Western counties, only 25–40% of
the patients have localized disease at diagnosis, whereas such a
subgroup is much larger in Japan and in countries that adopt
aggressive screening programs.
Surgical resection of the primary tumor and regional
lymph nodes is the fundamental treatment for gastric cancer.
Although extensive lymphadenectomy (D2 resection) is the standard
procedure in Japan since the 1980’s (3), four prospective randomized trials
that assessed the role of D1 (peri-gastric lymph nodes along the
lesser and the greater curvature) vs. D2 (including lymph nodes
along the left gastric artery, the common hepatic artery, the
celicac trunk, the splenic hilum and the splenic artery) resection
in the management of gastric cancer failed to demonstrate any
advantage in terms of survival in favor of extended surgery
(4–7).
In Western countries, more than 50% of the radically
resected patients experience local or distant recurrence, thus
prompting the evaluation of the best surgical procedure and the
best complementary strategy to surgery. The role of chemotherapy as
adjuvant treatment has been controversial for a long time. Several
randomized trials run in Western countries during the ‘80s and ‘90s
failed to demonstrate a clear survival benefit for post-operative
chemotherapy as compared to surgery alone, mostly because they were
underpowered and had a small sample size (8–21).
A great number of meta-analyses has been performed,
including published trials comparing adjuvant chemotherapy to
surgery alone (22–33). Most of them have suggested a small
survival advantage for adjuvant treatment, but such data were
considered unconvincing due to the different selection of the
considered trials, the inconsistent surgical and medical treatment
and, in some cases, the questionable methodology. Moreover,
evidence from large, well-designed, randomized trials is more
conclusive and easier to translate into clinical practice than
suggestions from meta-analyses of small, under-powered trials.
Thus, in the 1990’s the effort of researchers worldwide was focused
on large-sized, sufficiently powered trials designed to evaluate
the benefit of adjuvant therapy over surgery alone.
In recent years, three pivotal studies carried out
in the US, Europe and Japan, respectively, demonstrated the
efficacy on survival of three different strategies of adjuvant
treatment. The INT-0116 trial showed that post-operative
chemoradiotherapy with 5-fluorouracil + leucovorin (5FU-LV)
prolonged the survival of patients radically resected for
adenocarcinoma of the stomach or gastroesophageal junction
(34). The MAGIC trial
demonstrated an improvement in disease-free survival (DFS) and
overall survival (OS) when 3 cycles of ECF (epirubicin, cisplatin,
5FU) were administered before and after surgery in patients with
gastric or lower esophageal cancer (35). The ACTS-GC group in Japan found
that 1 year of treatment with the oral fluoropyimidine S-1
prolonged the survival of Asian patients who had undergone a D2
dissection for stage II–III gastric cancer (36).
Cisplatin and 5FU are the most commonly used drugs
for gastric cancer and are considered milestones for chemotherapy
both in metastatic and in an adjuvant setting. However, the best
regimen for adjuvant treatment remains under evaluation, as the
best duration is undefined. Oxaliplatin was recently demonstrated
to be as active as cisplatin in metastatic gastric disease
(37), less toxic and more
manageable. Thus, it may replace cisplatin in multi-drug
regimens.
The association of 5FU-LV and oxaliplatin is a very
active regimen in metastatic colorectal cancer and is the standard
adjuvant chemotherapy for stage III colon cancer, since it
demonstrated clear survival advantages over 5FU-LV alone (38,39).
Based on this evidence, patients with stage
II–III–IV gastric cancer who had undergone radical surgery were
treated with adjuvant FOLFOX-4; here, we report our experience.
Materials and methods
Fifty-four patients referred to our institution
between October 2002 and June 2008 were enrolled for the present
study. All patients, after radical surgery for gastric cancer,
received adjuvant FOLFOX-4 (oxaliplatin 85 mg/m2, Day 1;
LV 100 mg/m2 i.v., Days 1 and 2; 5FU 400
mg/m2 i.v. bolus, Days 1 and 2; 5FU 600 mg/m2
in 22 h i.v. continuous infusion, Days 1 and 2; every 14 days) for
8 or 12 cycles.
Patients were monitored at each cycle of
chemotherapy by history, physical examination and complete
biochemistry; blood count was performed once a week during
chemotherapy. Toxicity was graded according to the National Cancer
Institute Common Toxicity Criteria, version 3.0.
Staging procedures performed prior to the beginning
of the treatment included blood cell count, biochemistry, blood CEA
level and thorax-abdomen-pelvis CT scan. Follow-up visits were
scheduled every 4 months during the first year and every 6 months
thereafter. Esophagogastroduodenoscopy and thorax-abdomen-pelvis CT
scan were performed yearly, and hematology, biochemistry and blood
CEA levels were repeated every 4 or 6 months. Bone scan, PET/CT
scan or magnetic resonance were executed only when clinically
required.
DFS was defined as the time elapsed between the date
of surgery and the occurrence of local relapse, distant metastases
or death without recurrence; OS was defined as the time elapsed
between the date of surgery and the date of death. Estimation of
likelihood events for relapse or death was calculated according to
the Kaplan-Meier method (40).
Statistical differences between curves were calculated using the
log-rank test (41). Multivariate
analysis was performed using the Cox proportional hazard model
(42) to estimate the hazard ratio
and relative 95% confidence intervals (95% CI) for each covariate.
A p-value of ≤0.05 was considered statistically significant.
Statistical analysis was performed with SPSS (version 16.0; SPSS,
Inc., Chicago, IL, USA).
Results
The main patient characteristics are reported in
Table I. They were mostly male,
with a median age of 61 years. More than half of the patients
received a total gastrectomy. The median value of pre-chemotherapy
hemoglobin was 12.1 g/dl in men and 11.7 g/dl in women. Thirteen
patients started adjuvant chemotherapy having <11 g/dl of
hemoglobin.
 | Table I.Patients and tumor
characteristics. |
Table I.
Patients and tumor
characteristics.
| n | Percentage |
|---|
| Gender | | |
| Male | 41 | 75.9 |
| Female | 13 | 24.1 |
| Age
(median/range) | 61 | 33–77 |
| Primary tumour
site | | |
| Cardia | 4 | 7.4 |
| Fundus | 3 | 5.6 |
| Body | 33 | 61.1 |
|
Antrum/pylorus | 12 | 22.2 |
| Gastric
stump | 2 | 3.7 |
| Gastrectomy | | |
| Total | 30 | 55.6 |
| Partial | 24 | 44.4 |
| Basal hemoglobin
(n=51) | | |
| <11 mg/ml | 14 | 27.5 |
| ≥11 mg/ml | 37 | 72.5 |
The primary tumor was located mostly in the
mid-lower part of the stomach; ∼75% were poorly differentiated. The
median number of the examined lymph nodes was 19 (range 2–40);
92.6% of the patients had positive lymph nodes and 87% of the cases
were stage III or IV (T4 primary or N3 nodal status). Detailed
pathological tumor characteristics are listed in Table II.
 | Table II.Pathological characteristics of the
primary tumor. |
Table II.
Pathological characteristics of the
primary tumor.
| n | Percentage |
|---|
| T primarya | | |
| 1 | 1 | 1.8 |
| 2 | 5 | 9.3 |
| 3 | 38 | 70.4 |
| 4 | 10 | 18.5 |
| N primarya | | |
|
N− | 4 | 7.4 |
| N1 | 31 | 57.4 |
| N2 | 14 | 25.9 |
| N3 | 5 | 9.3 |
| UICC-TNM
stagea | | |
| Ib | 1 | 1.8 |
| II | 6 | 11.1 |
| IIIa | 22 | 40.7 |
| IIIb | 14 | 25.9 |
| IVb | 11 | 20.4 |
| Examined lymph
nodes | | |
| ≤15 | 23 | 42.6 |
| >15 | 31 | 57.4 |
| Histology | | |
|
Adenocarcinoma | 44 | 81.5 |
| Signet ring
cell | 5 | 9.3 |
| Mucinous
adenocarcinoma | 4 | 7.4 |
| Other | 1 | 1.8 |
| Grading | | |
| Well
differentiated | 6 | 11.1 |
| Intermediate | 7 | 13.0 |
| Poorly
differentiated | 41 | 75.9 |
The median time elapsed from surgery to the
commencement of adjuvant chemotherapy was 6 weeks (range 3–14); 14
(25.9%) patients started chemotherapy ≥8 weeks from surgery.
A total of 463 cycles of chemotherapy were
administered; the median number of the received cycles of FOLFOX-4
was 8 (range 1–12). Twenty-two and 16 patients completed the 8 or
12 prescribed cycles, respectively; 3 patients progressed during
chemotherapy, 1 patient died 14 days after the 4th cycle of
chemotherapy, in 7 cases FOLFOX-4 was stopped due to toxicity (3
due to hematological, 1 for severe mucositis and 3 due to both
hematological and gastrointestinal adverse events) and 5 patients
refused to complete the scheduled number of cycles.
Overall chemotherapy was well tolerated, and
registered adverse events were comparable to those reported with
adjuvant FOLFOX-4 in colorectal cancer. Grade 3–4 hematological
toxicity was observed in 57% (neutropenia), 2% (thrombocytopenia)
and 2% (anemia) of the patients. One patient received 5 blood
transfusions because of grade 4 anemia. Peripheral neuropathy was
experienced by 46% of the patients, but it was grade 4 only in 2%
of the cases. Grade 3 gastrointestinal toxicity occurred only in 5
cases. Table III presents in
detail the worse adverse events experienced per patient.
 | Table III.Worse experienced toxicity among the
54 treated patients. |
Table III.
Worse experienced toxicity among the
54 treated patients.
| Adverse event | G0
| G1
| G2
| G3
| G4
|
|---|
| n | % | n | % | n | % | n | % | n | % |
|---|
| Nausea | 33 | 61.1 | 20 | 37.0 | 1 | 1.8 | - | - | - | - |
| Vomiting | 39 | 72.2 | 9 | 16.7 | 4 | 7.4 | 2 | 3.7 | - | - |
| Diarrhea | 27 | 50.0 | 20 | 37.0 | 5 | 9.3 | 2 | 3.7 | - | - |
| Stomatitis | 43 | 79.6 | 6 | 11.1 | 4 | 7.4 | 1 | 1.8 | - | - |
| Neutropenia | 15 | 27.8 | 5 | 9.3 | 3 | 5.6 | 21 | 38.9 | 10 | 18.5 |
| Anemia | 12 | 22.2 | 28 | 51.8 | 13 | 24.1 | - | - | 1 | 1.8 |
|
Thrombocytopenia | 18 | 33.3 | 31 | 57.4 | 4 | 7.4 | 1 | 1.8 | - | - |
| Peripheral
neuropathy | 29 | 53.7 | 21 | 38.9 | 3 | 5.6 | - | - | 1 | 1.8 |
| Asthenia | 38 | 70.4 | 12 | 22.2 | 3 | 5.6 | 1 | 1.8 | - | - |
After a median follow-up of 33.1 months (range
3.5–79.4) from surgery, 17 (31.5%) patients relapsed (4 peritoneal
carcinosis, 2 liver, 2 perianastomotic, 2 lung, 1 suprarenal gland
and 6 multiple sites) and 17 (31.5%) sucuumbed to the disease. The
mean observed DFS and OS were 49.7 months (range 40.7–58.8) and
57.9 months (range 49.6–66.2), respectively. The estimated 3-year
DFS and 3-year OS were 57 and 67%, respectively. The causes of
death were metastatic disease in 12 cases, cardiovascular event in
4 and sudden death in 1 case. The median number of days elapsed
from the end of chemotherapy and the date of the 5 deaths without
recurrence was 428 days (range 14–1,104); 2 patients died of
myocardial infarction and ictus, respectively, within 60 days from
the end of chemotherapy.
After relapse, 11 (64.7%) patients received further
treatment (chemotherapy or radiotherapy); 4 patients received 2 or
more lines of chemotherapy for metastatic disease.
Upon univariate analysis (Table IV), females and patients who
received <8 cycles of adjuvant chemotherapy had a significantly
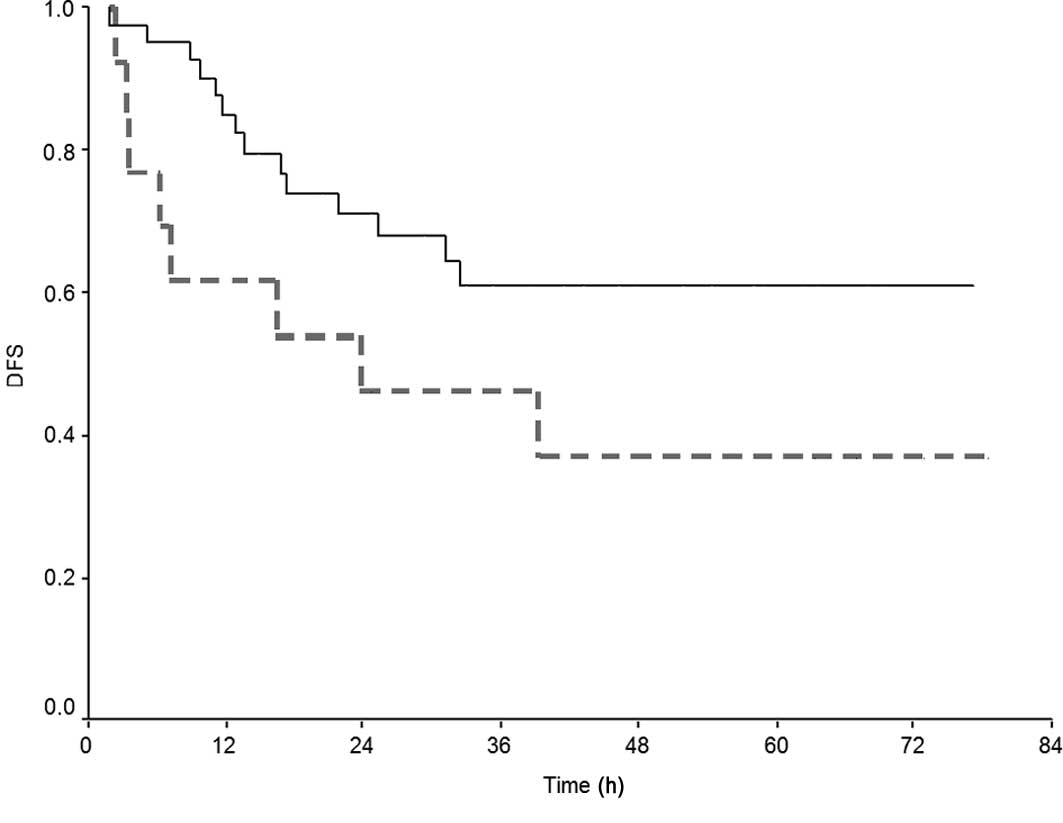
worse probability of DFS and OS. Kaplan-Meier curves for DFS and
OS, according to delivered cycles of FOLFOX-4, are depicted in
Fig. 1A and B. The interval
between surgery and the initiation of adjuvant treatment did not
affect the long-term outcome.
 | Table IV.Univariate analysis. |
Table IV.
Univariate analysis.
| Variable | Probability of DFS
(%)a | P-value | Probability of OS
(%)a | P-value |
|---|
| Age, in years | | 0.20 | | 0.10 |
| ≤65 | 61.1 | | 75.0 | |
| >65 | 50.0 | | 55.6 | |
| Gender | | 0.01 | | 0.02 |
| Male | 65.8 | | 78.1 | |
| Female | 30.7 | | 38.5 | |
| Primary tumor | | 0.80 | | 0.60 |
| pT1 | 100.0 | | 100.0 | |
| pT2 | 60.0 | | 60.0 | |
| pT3 | 57.9 | | 71.1 | |
| pT4 | 50.0 | | 60.0 | |
| Nodal status | | 0.40 | | 0.09 |
| pN0 | 75.0 | | 100.0 | |
| pN1 | 64.5 | | 74.2 | |
| pN2 | 42.9 | | 57.1 | |
| pN3 | 40.0 | | 40.0 | |
| UICC-TNM stage | | 0.50 | | 0.10 |
| I–II | 71.4 | | 85.7 | |
| III | 58.3 | | 72.2 | |
| IV | 45.4 | | 45.5 | |
| No. of examined
nodes | | 0.08 | | 0.20 |
| ≤15 | 69.6 | | 73.9 | |
| >15 | 48.4 | | 64.5 | |
| Grading | | 0.50 | | 0.90 |
| G1–G2 | 69.2 | | 69.2 | |
| G3 | 53.7 | | 68.2 | |
| Basal
hemoglobin | | 0.90 | | 0.90 |
| <11 mg/ml | 46.1 | | 61.5 | |
| ≥11 mg/ml | 53.0 | | 71.1 | |
| Interval
surgery-chemotherapy | | 0.90 | | 0.90 |
| ≤8 weeks | 55.5 | | 65.0 | |
| >8 weeks | 64.3 | | 78.6 | |
| No. of received
cycles of CT | | 0.05 | | 0.007 |
| ≥8 | 63.4 | | 78.1 | |
| <8 | 38.5 | | 38.5 | |
Upon multivariate analysis, in a model including
age, gender and TNM stage as adjusting covariates, females showed a
2.6-fold risk of recurrence and death (p=0.04). The risk of death
was significantly affected by age (HR=4.1, p=0.01) and the number
of delivered cycles of chemotherapy (HR=2.78, p=0.04). However, in
a model excluding the patients who died for causes other than the
tumor, age lost significance (Table
V). The interactions gender-stage and gender-number of
delivered cycles were not significant in the Cox model.
 | Table V.Multivariate analysis. |
Table V.
Multivariate analysis.
| Variable | Disease-free
survival
| Overall survival
|
|---|
| Hazard
ratioa | P-value (95%
CI) | Hazard
ratioa | P-value (95%
CI) |
|---|
| Age, in years | | 0.10 | | 0.01b |
| ≤65 | 1c | | 1c | |
| >65 | 2.0 | (0.8–5.2) | 4.1b | (1.3–12.6)b |
| Gender | | 0.04 | | 0.04 |
| Male | 1c | | 1c | |
| Female | 2.6 | (1.1–6.4) | 2.6b | (1.0–7.0) |
| UICC-TNM stage | | 0.60 | | 0.07 |
| I-II | 1c | | 1c | |
| III | 1.6 | (0.3–7.3) | 2.3 | (0.3–19.2) |
| IV | 2.3 | (0.4–13.8) | 8.5 | (0.8–90.5) |
| No. of examined
nodes | | 0.10 | | 0.35 |
| N+
≤15 | 1c | | 1c | |
| N+
>15 | 1.9 | (0.8–4.7) | 1.5 | (0.6–4.2) |
| No. of received
cycles of CT | | 0.20 | | 0.04 |
| ≥8 | 1c | | 1c | |
| <8 | 1.7 | (0.7–4.2) | 2.78 | (1.1–7.1) |
Discussion
The optimum regimen and the optimal duration of
adjuvant or neo-adjuvant chemotherapy after radical surgery of
patients with gastric cancer remains in debate. The three pivotal
trials demonstrated that 5 cycles of 5FU-FA + radiotherapy
(34), or 1 year of oral
fluoropyrimidine (36), or 6
cycles of poly-chemotherapy (ECF) (35), produce survival benefit over
surgery alone.
Evidence suggests that the duration of adjuvant
chemotherapy is considered an independent prognostic factor for DFS
(43) or mortality risk (44) in colon and ovarian cancer (45). Our analysis suggested that a short
duration of adjuvant chemotherapy (<8 cycles) is detrimental for
DFS and OS of patients surgically operated on for gastric
cancer.
The significance of the timing of the commencement
of adjuvant chemotherapy in many solid tumors remains
controversial. In colon cancer, a German study found a positive
effect of early (<27 days) administration of adjuvant
chemotherapy on DFS, but not on survival (46). Chau et al (47) reported a significant OS advantage
for patients who started adjuvant chemotherapy within 8 weeks from
surgery; this latter finding was confirmed by Hershman et al
in elderly patients (>65 years); however, disease-specific
mortality was found to significantly worsen only after a longer
than 3-month interval (48). In
breast cancer, two large retrospective analyses (49,50)
failed to demonstrate any effect of early initiation of adjuvant
chemotherapy on survival. However, starting adjuvant chemotherapy
within 21 days from surgery has been suggested to be favorable for
pre-menopausal patients with receptor-negative tumors (51). One of the reasons why some
oncologists prefer pre-operative chemotherapy is that surgery for
gastric cancer is demanding, post-operative complications are
frequent and convalescence is often long-lasting, not allowing for
the timely commencement of chemotherapy. Most trials have 6
(36) or 8 weeks (19–21,34)
from surgery as eligibility criteria for entry, thus they do not
include patients who cannot be randomized within this time
interval. Our results suggested that starting adjuvant chemotherapy
more than 2 months after surgery does not affect long-term outcome
and does not nullify the effect of adjuvant chemotherapy following
radical surgery.
Patients who receive radical resection for gastric
cancer usually have low values of hemoglobin due to pre-operative
bleeding, prolonged fasting and impaired iron absorption. Anemia is
frequently reported as a negative predictor of response to
chemoradiotherapy (52–54). In early gastric cancer,
pre-operative hemoglobin of less than 12 g/dl has been associated
with poorer survival (55), and in
a metastatic setting, hemoglobin of less than 10 g/dl has been
correlated with worse response rate and increased risk of death
(56). In our patients, starting
adjuvant chemotherapy with hemoglobin values of less than 11.0 g/dl
did not affect the number of delivered cycles of FOLFOX-4 or
long-term prognosis. However, basal hemoglobin of less than 11 g/dl
was associated with increased hematological toxicity, since 53.8%
of these patients experienced grade ≥2 anemia during
chemotherapy.
Although the combination of 5FU and oxaliplatin has
been extensively studied in metastatic gastric cancer (57), there are a few reports regarding
the adjuvant setting mainly in Chinese populations (58,59).
To our knowledge, our series is the largest in the literature to
address the feasibility and tolerability of FOLFOX-4 regimen in
Caucasian patients surgically operated on for gastric cancer. Grade
3–4 hematological toxicity registered in our patients is comparable
to that reported in the Mosaic study (38) in patients operated on for colon
cancer, neutropenia being the most frequent adverse event.
Gastrointestinal tolerability was good, since less than 4% of the
patients experienced grade 3 vomiting or diarrhea. Severe
peripheral neuropathy occurred in 1 patient, much less than
reported in the Mosaic study (12.4%), probably because the median
number of delivered cycles of chemotherapy was lower (8 vs. 12
cycles).
The prognostic role of gender in solid tumors is
questionable and is often attributed to chance. However, in gastric
cancer some experimental evidence suggests that estrogen-positive
tumors are more frequent in women and more aggressive (60,61).
The reason for such behavior remains poorly understood; however, it
can be explained by a crosstalk between estrogen receptors and
epidermal growth factor receptor pathways in gastric cancer cells
(62). In our study, we confirmed
that women have a significantly worse prognosis and that the
increased risk of recurrence at multivariate analysis is
independent from other prognostic factors (the interactions
gender-stage and gender-number of delivered cycles were not
significant). Thus, it may be speculated that it is due to
intrinsic growth characteristics of the tumor cells.
In conclusion, our experience suggests that FOLFOX-4
is a feasible and manageable regimen as adjuvant treatment and
represents a valid option when a platinum + fluoropyrimidine scheme
is indicated for radically resected gastric cancer patients.
Moreover, although demonstrated in a limited number of patients,
the present study suggests that at least 4 months of adjuvant
chemotherapy should be administered to obtain the most beneficial
effect and that a short interval between surgery and the
commencement of adjuvant chemotherapy is not fundamental for the
effect of adjuvant chemotherapy on prognosis.
References
|
1.
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar
|
|
2.
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar
|
|
3.
|
Kajitani T: The general rules for the
gastric cancer study in surgery and pathology. Part I. Clinical
classification. Jpn J Surg. 11:127–139. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Dent DM, Madden MV and Price SK:
Randomized comparison of R1 and R2 gastrectomy for gastric
carcinoma. Br J Surg. 75:110–112. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Robertson CS, Chung SC, Woods SD, Griffin
SM, Raimes SA, Lau JT and Li AK: A prospective randomized trial
comparing R1 subtotal gastrectomy with R3 total gastrectomy for
antral cancer. Ann Surg. 220:176–182. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Cuschieri A, Weeden S, Fielding J, et al:
Patient survival after D1 and D2 resections for gastric cancer:
long-term results of the MRC randomized surgical trial. Surgical
Co-operative Group. Br J Cancer. 79:1522–1530. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Bonenkamp JJ, Hermans J, Sasako M, et al:
Extended lymph-node dissection for gastric cancer. N Engl J Med.
340:908–914. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
The Gastrointestinal Tumor Study Group:
Controlled trial of adjuvant chemotherapy following curative
resection for gastric cancer. Cancer. 49:1116–1122. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Higgins GA, Amadeo JH, Smith DE, Humphrey
EW and Keehn RJ: Efficacy of prolonged intermittent therapy with
combined 5-FU and methyl-CCNU following resection for gastric
carcinoma. A Veterans Administration Surgical Oncology, Group
report. Cancer. 52:1105–1112. 1983. View Article : Google Scholar
|
|
10.
|
Engstrom PF, Lavin PT, Douglass HO Jr and
Brunner KW: Postoperative adjuvant 5-fluorouracil plus methyl-CCNU
therapy for gastric cancer patients. Eastern Cooperative Oncology
Group study (EST 3275). Cancer. 55:1868–1873. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Allum WH, Hallissey MT and Kelly KA:
Adjuvant chemo-therapy in operable gastric cancer. 5 year follow-up
of first British Stomach Cancer Group trial. Lancet. 1:571–574.
1989.PubMed/NCBI
|
|
12.
|
Coombes RC, Schein PS, Chilvers CE, et al:
A randomized trial comparing adjuvant fluorouracil, doxorubicin,
and mitomycin with no treatment in operable gastric cancer.
International Collaborative Cancer Group. J Clin Oncol.
8:1362–1369. 1990.
|
|
13.
|
Krook JE, O’Connell MJ, Wieand HS, et al:
A prospective, randomized evaluation of intensive-course
5-fluorouracil plus doxorubicin as surgical adjuvant chemotherapy
for resected gastric cancer. Cancer. 67:2454–2458. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Grau JJ, Estape J, Alcobendas F, Pera C,
Daniels M and Teres J: Positive results of adjuvant mitomycin-C in
resected gastric cancer: a randomised trial on 134 patients. Eur J
Cancer. 29A:340–342. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Lise M, Nitti D, Marchet A, et al: Final
results of a phase III clinical trial of adjuvant chemotherapy with
the modified fluorouracil, doxorubicin and mitomycin regimen in
resectable gastric cancer. J Clin Oncol. 13:2757–2763.
1995.PubMed/NCBI
|
|
16.
|
Macdonald JS, Fleming TR, Peterson RF, et
al: Adjuvant chemotherapy with 5-FU, adriamycin, and mitomycin-C
(FAM) versus surgery alone for patients with locally advanced
gastric adenocarcinoma: A Southwest Oncology Group study. Ann Surg
Oncol. 2:488–494. 1995. View Article : Google Scholar
|
|
17.
|
Neri B, de Leonardis V, Romano S, et al:
Adjuvant chemotherapy after gastric resection in node-positive
cancer patients: a multicentre randomised study. Br J Cancer.
73:549–552. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Cirera L, Balil A, Batiste-Alentorn E, et
al: Randomized clinical trial of adjuvant mitomycin plus tegafur in
patients with resected stage III gastric cancer. J Clin Oncol.
17:3810–3815. 1999.PubMed/NCBI
|
|
19.
|
Bajetta E, Buzzoni R, Mariani L, et al:
Adjuvant chemotherapy in gastric cancer: 5-year results of a
randomised study by the Italian Trials in Medical Oncology (ITMO)
Group. Ann Oncol. 13:299–307. 2002.PubMed/NCBI
|
|
20.
|
De Vita F, Giuliani F, Orditura M, et al:
Adjuvant chemotherapy with epirubicin, leucovorin, 5-fluorouracil
and etoposide regimen in resected gastric cancer patients: a
randomized phase III trial by the Gruppo Oncologico Italia
Meridionale (GOIM 9602 Study). Ann Oncol. 18:1354–1358. 2007.
|
|
21.
|
Di Costanzo F, Gasperoni S, Manzione L, et
al: Adjuvant chemotherapy in completely resected gastric cancer: a
randomized phase III trial conducted by GOIRC. J Natl Cancer Inst.
100:388–398. 2008.PubMed/NCBI
|
|
22.
|
Hermans J, Bonenkamp JJ, Boon MC, Bunt AM,
Ohyama S, Sasako M and van de Velde CJ: Adjuvant therapy after
curative resection for gastric cancer: meta-analysis of randomized
trials. J Clin Oncol. 11:1441–1447. 1993.PubMed/NCBI
|
|
23.
|
Hermans J and Benekamp K: In replay. J
Clin Oncol. 12:879–880. 1994.
|
|
24.
|
Earle CC and Maroun JA: Adjuvant
chemotherapy after curative resection for gastric cancer in
non-Asian patients: revisiting a meta-analysis of randomised
trials. Eur J Cancer. 35:1059–1064. 1999. View Article : Google Scholar
|
|
25.
|
Mari E, Floriani I, Tinazzi A, et al:
Efficacy of adjuvant chemotherapy after curative resection for
gastric cancer: a meta-analysis of published randomised trials. A
study of the GISCAD (Gruppo Italiano per lo Studio dei Carcinomi
dell’ Apparato Digerente). Ann Oncol. 11:837–843. 2000.
|
|
26.
|
Gianni L, Panzini I, Tassinari D, Mianulli
AM, Desiderio F and Ravaioli A: Meta-analyses of randomized trials
of adjuvant chemotherapy in gastric cancer. Ann Oncol.
12:1178–1180. 2001.PubMed/NCBI
|
|
27.
|
Panzini I, Gianni L, Fattori PP, et al:
Adjuvant chemotherapy in gastric cancer: a meta-analysis of
randomized trials and a comparison with previous meta-analyses.
Tumori. 88:21–27. 2002.PubMed/NCBI
|
|
28.
|
Hu JK, Chen ZX, Zhou ZG, et al:
Intravenous chemotherapy for resected gastric cancer: meta-analysis
of randomized controlled trials. World J Gastroenterol.
8:1023–1028. 2002.PubMed/NCBI
|
|
29.
|
Janunger KG, Hafstrom L and Glimelius B:
Chemotherapy in gastric cancer: a review and updated meta-analysis.
Eur J Surg. 168:597–608. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Liu TS, Wang Y, Chen SY and Sun YH: An
updated meta-analysis of adjuvant chemotherapy after curative
resection for gastric cancer. Eur J Surg Oncol. 34:1208–1216. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Zhao SL and Fang JY: The role of
postoperative adjuvant chemotherapy following curative resection
for gastric cancer: a meta-analysis. Cancer Invest. 26:317–325.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Sun P, Xiang JB and Chen ZY: Meta-analysis
of adjuvant chemotherapy after radical surgery for advanced gastric
cancer. Br J Surg. 96:26–33. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Oba K: Efficacy of adjuvant chemotherapy
using tegafur-based regimen for curatively resected gastric cancer:
update of a meta-analysis. Int J Clin Oncol. 14:85–89. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Macdonald JS, Smalley SR, Benedetti J, et
al: Chemoradiotherapy after surgery compared with surgery alone for
adenocarcinoma of the stomach or gastroesophageal junction. N Engl
J Med. 345:725–730. 2001. View Article : Google Scholar
|
|
35.
|
Cunningham D, Allum WH, Stenning SP, et
al: Perioperative chemotherapy versus surgery alone for resectable
gastroesophageal cancer. N Engl J Med. 355:11–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Sakuramoto S, Sasako M, Yamaguchi T, et
al: Adjuvant chemotherapy for gastric cancer with S-1, an oral
fluoropyrimidine. N Engl J Med. 357:1810–1820. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Cunningham D, Starling N, Rao S, et al:
Capecitabine and oxaliplatin for advanced esophagogastric cancer. N
Engl J Med. 358:36–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Andre T, Boni C, Navarro M, et al:
Improved overall survival with oxaliplatin, fluorouracil and
leucovorin as adjuvant treatment in stage II or III colon cancer in
the MOSAIC trial. J Clin Oncol. 27:3109–3116. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Kuebler JP, Wieand HS, O’Connell MJ, et
al: Oxaliplatin combined with weekly bolus fluorouracil and
leucovorin as surgical adjuvant chemotherapy for stage II and III
colon cancer: results from NSABP C-07. J Clin Oncol. 25:2198–2204.
2007. View Article : Google Scholar
|
|
40.
|
Kaplan EL and Meier P: Nonparametric
estimation for incomplete observations. J Am Stat Assoc.
53:457–481. 1958. View Article : Google Scholar
|
|
41.
|
Peto R and Peto J: Asymptotically
efficient rank invariant test procedures. J R Stat Soc.
135A:185–206. 1972.
|
|
42.
|
Cox DR: Regression models and life tables
(with discussion). J R Stat Soc. 4B:187–220. 1972.
|
|
43.
|
Qiu MZ, Teng KY, Ruan DY, et al: Impact of
adjuvant chemotherapy duration on 3-year disease-free survival of
colorectal carcinoma patients after radical resection. Chin J
Cancer. 28:743–748. 2009.PubMed/NCBI
|
|
44.
|
Neugut AI, Matasar M, Wang X, et al:
Duration of adjuvant chemotherapy for colon cancer and survival
among the elderly. J Clin Oncol. 24:2368–2375. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Yen MS, Twu NF, Lai CR, Horng HC, Chao KC
and Juang CM: Importance of delivered cycles and nomogram for
intraperitoneal chemotherapy in ovarian cancer. Gynecol Oncol.
114:415–419. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Arkenau HT, Bermann A, Rettig K,
Strohmeyer G and Porschen R: 5-Fluorouracil plus leucovorin is an
effective adjuvant chemotherapy in curatively resected stage III
colon cancer: long-term follow-up results of the adjCCA-01 trial.
Ann Oncol. 14:395–399. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Chau I, Norman AR, Cunningham D, et al: A
randomised comparison between 6 months of bolus
fluorouracil/leucovorin and 12 weeks of protracted venous infusion
fluorouracil as adjuvant treatment in colorectal cancer. Ann Oncol.
16:549–557. 2005. View Article : Google Scholar
|
|
48.
|
Hershman D, Hall MJ, Wang X, Jacobson JS,
McBride R, Grann VR and Neugut AI: Timing of adjuvant chemotherapy
initiation after surgery for stage III colon cancer. Cancer.
107:2581–2588. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Cold S, During M, Ewertz M, Knoop A and
Moller S: Does timing of adjuvant chemotherapy influence the
prognosis after early breast cancer? Results of the Danish Breast
Cancer Cooperative Group (DBCG). Br J Cancer. 93:627–632. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Jara SC, Ruiz A, Martin M, et al:
Influence of timing of initiation of adjuvant chemotherapy over
survival in breast cancer: a negative outcome study by the Spanish
Breast Cancer Research Group (GEICAM). Breast Cancer Res Treat.
101:215–223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Colleoni M, Bonetti M, Coates AS, et al:
Early start of adjuvant chemotherapy may improve treatment outcome
for premenopausal breast cancer patients with tumors not expressing
estrogen receptors. The International Breast Cancer Study Group. J
Clin Oncol. 18:584–590. 2000.
|
|
52.
|
Box B, Lindsey I, Wheeler JM, et al:
Neoadjuvant therapy for rectal cancer: improved tumor response,
local recurrence, and overall survival in nonanemic patients. Dis
Colon Rectum. 48:1153–1160. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Lee SD, Park JW, Park KS, et al: Influence
of anemia on tumor response to preoperative chemoradiotherapy for
locally advanced rectal cancer. Int J Colorectal Dis. 24:1451–1458.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
54.
|
Ferrandina G, Distefano M, Smaniotto D, et
al: Anemia in patients with locally advanced cervical carcinoma
administered preoperative radiochemotherapy: association with
pathological response to treatment and clinical outcome. Gynecol
Oncol. 103:500–505. 2006. View Article : Google Scholar
|
|
55.
|
Shen JG, Cheong JH, Hyung WJ, Kim J, Choi
SH and Noh SH: Pretreatment anemia is associated with poorer
survival in patients with stage I and II gastric cancer. J Surg
Oncol. 91:126–130. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Park SH, Lee J, Lee SH, et al: Anemia is
the strongest prognostic factor for outcomes of
5-fluorouracil-based first-line chemotherapy in patients with
advanced gastric cancer. Cancer Chemother Pharmacol. 57:91–96.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
De Vita F, Orditura M, Matano E, et al: A
phase II study of biweekly oxaliplatin plus infusional
5-fluorouracil and folinic acid (FOLFOX-4) as first-line treatment
of advanced gastric cancer patients. Br J Cancer. 92:1644–1649.
2005.PubMed/NCBI
|
|
58.
|
Fang Y, Wang YJ, Li F and Li J:
Oxaliplatin in combination with calcium folinate and fluorouracil
as neoadjuvant chemotherapy in the treatment of advanced gastric
cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 9:510–512.
2006.PubMed/NCBI
|
|
59.
|
Zhao L, Li XY, Bai CM and Chen SC:
Post-operative adjuvant treatment with oxaliplatin, fluorouracil,
and leucovorin for local advanced gastric cancer. Zhonghua Yi Xue
Za Zhi. 88:1264–1266. 2008.PubMed/NCBI
|
|
60.
|
Matsui M, Kojima O, Kawakami S, Uehara Y
and Takahashi T: The prognosis of patients with gastric cancer
possessing sex hormone receptors. Surg Today. 22:421–425. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
61.
|
Harrison JD, Morris DL, Ellis IO, Jones JA
and Jackson I: The effect of tamoxifen and estrogen receptor status
on survival in gastric carcinoma. Cancer. 64:1007–1010. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
62.
|
Koullias GJ, Kouraklis GP, Raftopoulos IS,
Davaris PS, Papadopoulos SA and Golematis BC: Increased estrogen
receptor and epidermal growth factor receptor gene product
co-expression in surgically resected gastric adenocarcinomas. J
Surg Oncol. 63:166–171. 1996. View Article : Google Scholar : PubMed/NCBI
|