Introduction
Gastric cancer is one of the most common
malignancies and the major cause of cancer-related death worldwide
(1). Surgical resection is the
mainstay of treatment for gastric cancer. However, in spite of
advances in diagnostic techniques and surgical procedures, the
prognosis after resection has remained unsatisfactory due to a high
incidence of postoperative recurrence (2). The identification of variables in
gastric tumor biology may lead to a more precise assessment of
outcome and response to therapy.
Endoglin (CD105) is a 180-kDa homodimeric membrane
glycoprotein and the receptor complex of transforming growth
factor-β1 (3). The CD105 antibody
binds preferentially to activated endothelial cells that
participate in tumor angiogenesis, and CD105-stained endothelial
cells are found on peritumoral and intratumoral vessels. Many
studies have examined CD105 expression in a variety of solid
tumors, including lung, gastrointestinal, liver, gynecological,
prostate and breast (4–8). CD105 is a more specific and sensitive
microvessel marker than other traditional panendothelial markers,
such as CD31, CD34 and von Willebrand factor. Furthermore, in a
variety of solid cancers, CD105 overexpression was consistently
associated with poor prognosis and presence of distant metastasis
(3). Thus, it has been known that
CD105 is an established marker of proliferating tumor blood vessels
and a potential predictor of prognosis.
As for gastric cancer, a few studies have
demonstrated that overexpression of CD105 in gastric cancer may be
associated with shorter overall survival (9,10),
although non-curative cases with distant metastasis or peritoneal
dissemination were included in these studies. To date, the
prognostic value of CD105 in gastric cancer patients after radical
surgery has not yet been elucidated. Unfavorable outcomes may be
attributed to recurrences that arise from undetectable
micrometastases. Without tumor angiogenesis, tumors cannot
metastasize to distant organs. Therefore, we hypothesize that the
evaluation of neovasculature may be helpful in discriminating the
probability of recurrence and poor prognosis in radically resected
gastric cancer.
The combination of biomarkers may improve the
ability to identify cancer patients at high risk of disease.
Therefore, adding an angiogenic factor to the evaluation of CD105
may provide a more clinically useful biomarker than either alone.
Matrix metalloproteases (MMPs), which represent a major family of
extracellular proteases that target a variety of molecules, are
up-regulated in several conditions that accompany angiogenesis.
Some family members are implicated in promoting angiogenesis by
remodeling the perivascular extracellualar matrix (ECM) and
liberating angiogenic factors from the ECM (11). Most MMPs are produced by stromal
cells, such as fibrobalsts, endothelial cells and inflammatory
cells, whereas MMP-7 is primarily expressed by cancer cells
(12). These findings may
therefore implicate that MMP-7 acts as a specific signal from
cancer cells to the stromal cell components necessary for tumor
angiogenesis. In fact, the overexpression of MMP-7 is associated
with advanced stage and unfavorable prognosis in a variety of
tumors (13). Recently, we also
reported that MMP-7-expressing tumors have aggressive phenotypes in
gastric cancer (14).
In the present study, we investigated the
significance of vessels recognized by CD105 as endothelial markers
by immunohistochemical staining in 132 cases of curatively resected
gastric cancer and analyzed the relationship between microvessel
density (MVD) by CD105 and clinical outcomes, including
relapse-free survival and the recurrence pattern after surgery. In
addition, we used previously acquired data on MMP-7 to evaluate the
expression of MMP-7 as an angiogenic factor in relation to the
prognostic implication and CD105 expression.
Materials and methods
Clinical materials
Primary gastric adenocarcinoma specimens were
obtained from 132 patients who underwent curative resection at
Fukushima Medical University between January 1991 and December
2004. Written informed consent was obtained from all patients
before surgery. No patients received chemotherapy or radiotherapy
before surgery. Each patient underwent D1 or D2 lymphandectomy. The
mean number of examined lymph nodes was 27.71 (15–60) and the mean
number of metastatic nodes was 3.59 (0–32). Clinical and
pathological status was defined according to the Japanese
Classification of Gastric Cancer (15). Histological type was divided into
differentiated and undifferentiated type as described previously
(14). Routine chemotherapy had
been administered to the patients with advanced-stage disease after
surgery, but no radiation treatment was carried out in any of the
patients included in our study. The endpoint of follow-up was the
date of the last contact and the date of death or recurrence
through March 2009. The median follow-up time was 1,957 days (range
18–6,407). At the end of our study, 46 (34.8%) patients had died,
30 (22.7%) of them directly from gastric cancer after recurrence.
The relapse-free 5-year survival rate was 59.8%. Of the 132
patients, 33 (25%) showed recurrence during the postoperative
follow-up period. Hematogenous, peritoneal and locoregional
recurrences were observed in 22, 12 and 11 cases, respectively.
Some patients had overlapping recurrence.
Immunohistochemistry
All specimens were fixed in formalin and embedded in
paraffin. Serial sections (4 μm) were deparaffinized in
xylene and hydrated through a graded series of ethanol. After the
sections were rinsed in phosphate-buffered saline (PBS), endogenous
peroxidase was blocked with 0.3% H2O2 in
methanol for 30 min. Antigens were retrieved by autoclaving
sections on slides in 0.01 M (pH 6.0) citrate buffer for 10 min for
MMP-7, or by incubation with Proteinase K for 5 min for CD105.
After being rinsed in PBS, the sections were incubated with each
primary antibody overnight at 4°C. The primary antibodies were
anti-CD105 (clone SN6h; R&D Systems, Minneapolis, MN, USA;
1:40) and anti-MMP-7 (clone 141-7B2; Daiichi Fine Chemical, Toyama,
Japan; 1:200). A further wash in PBS was followed by treatment with
peroxidase-labeled polymer conjugated to goat anti-mouse
immunogloblins (Envison+ kit; Dako, Glostrup, Denmark)
as the secondary antibody for 30 min at room temperature. The
staining was visualized with diaminobenzidine (DAB), followed by
counterstaining with hematoxylin.
Sections were considered positive for MMP-7 when
>5% of tumor cells were stained in the cytoplasm or cell
membrane. Assessment of the staining was evaluated by two
independent pathologists without knowledge of the clinical status
of the patients.
Quantification of MVD
The MVD recognized by CD105 was evaluated under
light microscopy according to the procedure described by Weidner
et al (16). Briefly, after
scanning the sections at low magnifications (x40), three tumor
areas with the greatest number of distinctly highlighted
micovessels (‘hot spot’) were selected. The number of vessels was
counted in the hot spots at high magnifications (x200), and the
average counts of the fields were recorded. Each brown-stained
endothelial cell or endothelial cell cluster, which was clearly
separate from the adjacent micovessels, tumor cells and connective
tissue elements was considered a single, countable microvessel.
Statistical analysis
The Student's t-test and one-way ANOVA were used to
compare means in groups. We used the Bonferoni test to compare
multiple pairs. Cumulative survival was estimated by the
Kaplan-Meier method, and differences between survival curves were
analyzed by the log-rank test. The influence of each variable on
survival was analyzed by the multivariate analysis of Cox
proportional hazard model. To identify the independent predictors
for each recurrence pattern, the factors found to be significant in
univariate analysis were included in subsequent multivariate
logistic regression analysis. Differences at p<0.05 were
considered significant. All statistical analyses were performed
using SPSS 11.0 software (SPSS Inc., Chicago, IL, USA).
Results
CD105 and MMP-7 expression in gastric
cancer
In normal gastric tissues, CD105 staining was hardly
observed in any vessels (Fig. 1A).
CD105-stained microvessels were frequently observed in microvessels
of tumoral tissues (Fig. 1B–D).
The mean MVD value as assessed by CD105 was 37.20±23.72 (mean ± SD,
median 32.55, range 3.67–94.33). As shown in Table I, higher MVD was significantly
correlated with deeper depth of invasion (p=0.001), presence of
lymphatic invasion (p=0.022), presence of venous invasion
(p=0.001), presence of lymph node metastasis (p<0.001) and
advanced stage (p<0.001). No correlation was found between MVD
and age, gender and histological type.
 | Table I.MVD by CD105 and clinicopathological
factors in patients with gastric cancer. |
Table I.
MVD by CD105 and clinicopathological
factors in patients with gastric cancer.
| Patients n (%) | MVD by CD105 mean
(SD) | p-value |
|---|
| Age | | | 0.251 |
| >65 | 70 (53) | 34.96 (24.13) | |
| <65 | 62 (47) | 39.73 (23.19) | |
| Gender | | | 0.837 |
| Male | 89 (67) | 37.49 (25.16) | |
| Female | 43 (33) | 36.58 (20.71) | |
| Histological
type | | | 0.779 |
| Differentiated | 63 (48) | 36.59 (26.00) | |
|
Undifferentiated | 69 (52) | 37.75 (21.61) | |
| Depth of
invasion | | | 0.005 |
| T1 | 41 (31) | 27.44 (23.08) | |
| T2 | 54 (41) | 42.61 (23.26) | |
| T3 | 37 (28) | 40.11 (22.33) | |
| Lymphatic
invasion | | | 0.022 |
| Present | 107 (81) | 39.47 (23.23) | |
| Absent | 25 (19) | 27.48 (23.79) | |
| Venous invasion | | | 0.001 |
| Present | 94 (71) | 41.49 (24.56) | |
| Absent | 38 (29) | 26.53 (17.72) | |
| LN metastasis | | | <0.001 |
| Positive | 70 (53) | 46.64 (23.46) | |
| Negative | 62 (47) | 26.53 (19.18) | |
| Stage | | | <0.001 |
| I | 60 (45) | 25.20 (18.48) | |
| II | 28 (21) | 49.50 (21.76) | |
| III | 44 (34) | 45.73 (23.97) | |
| MMP-7 expression | | | 0.019 |
| Positive | 95 (72) | 40.06 (24.13) | |
| Negative | 37 (28) | 29.84 (21.22) | |
| Recurrence | | | 0.003 |
| Yes | 33 (25) | 47.76 (25.15) | |
| No | 99 (75) | 33.68 (22.26) | |
The positive expression of MMP-7 (Fig. 1F–H), which existed in the cytoplasm
and membrane of cancer cells, was found in 95 of the 132 cases
(72%), while MMP-7 expression was rarely detected in normal
endothelial cells (Fig. 1E).
Higher MVD was also associated with positive MMP-7 expression
(p=0.019).
Prognostic significance of MVD by CD105
and MMP-7 expression
The median rate of MVD was 32.55, and the value of
35 was chosen as the cut-off point. High and low MVD was observed
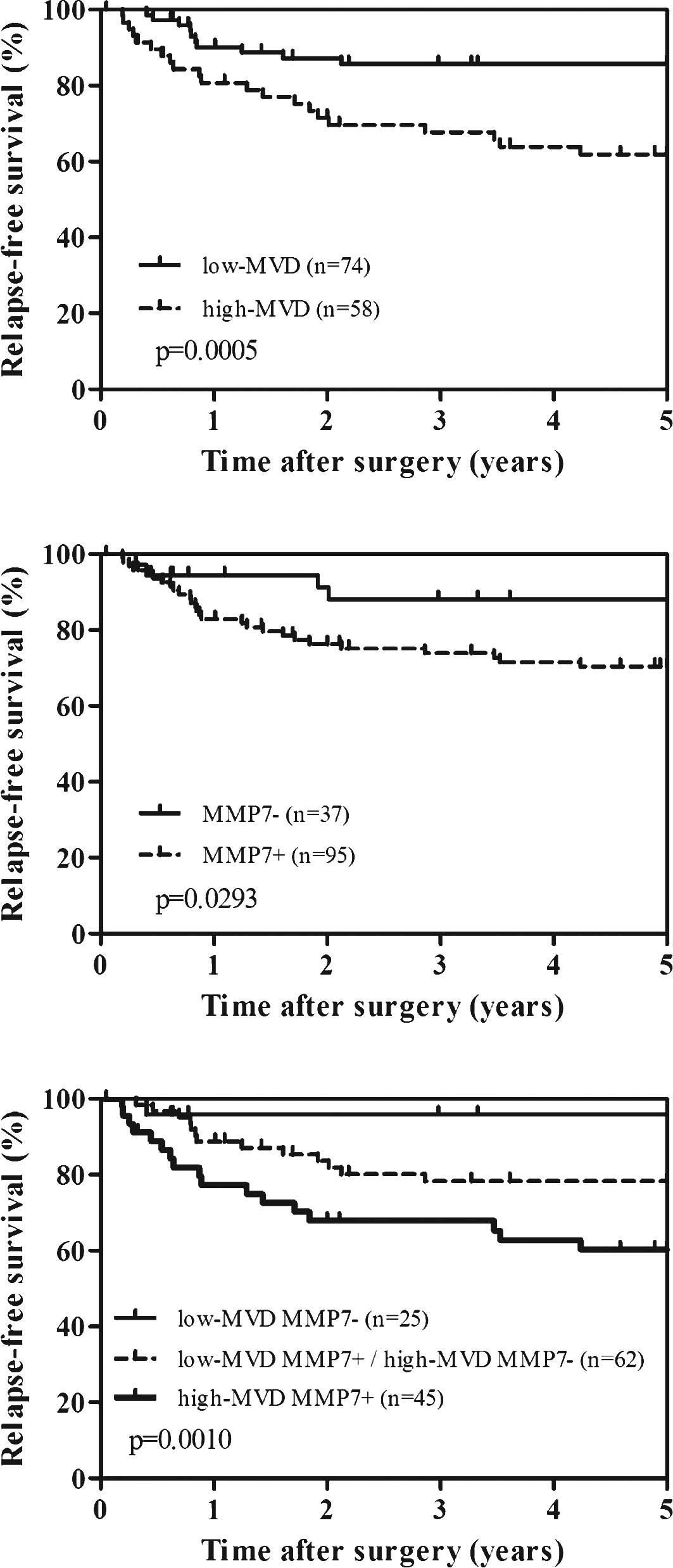
in 58 (43.9%) and 74 (56.1%) patients, respectively. Fig. 2A shows the survival curves of the
patients according to high or low MVD; the survival rates were
evaluated by log-rank test. High-MVD patients were significantly
associated with worse relapse-free survival compared to low-MVD
patients (p=0.0005). Similarly, patients with MMP-7 expression also
showed significant worse relapse-free survival (p=0.0293) (Fig. 2B). We further performed the
combined analysis of MVD and MMP-7 expression to predict
recurrence. As shown in Fig. 2C,
high-MVD and positive MMP-7 patients had the worst prognosis,
whereas low-MVD and negative MMP-7 patients had the most favorable
prognosis (p=0.0010). However, combined analysis of MVD and MMP-7
was not superior to that of MVD alone in both univariate and
multivariate analysis.
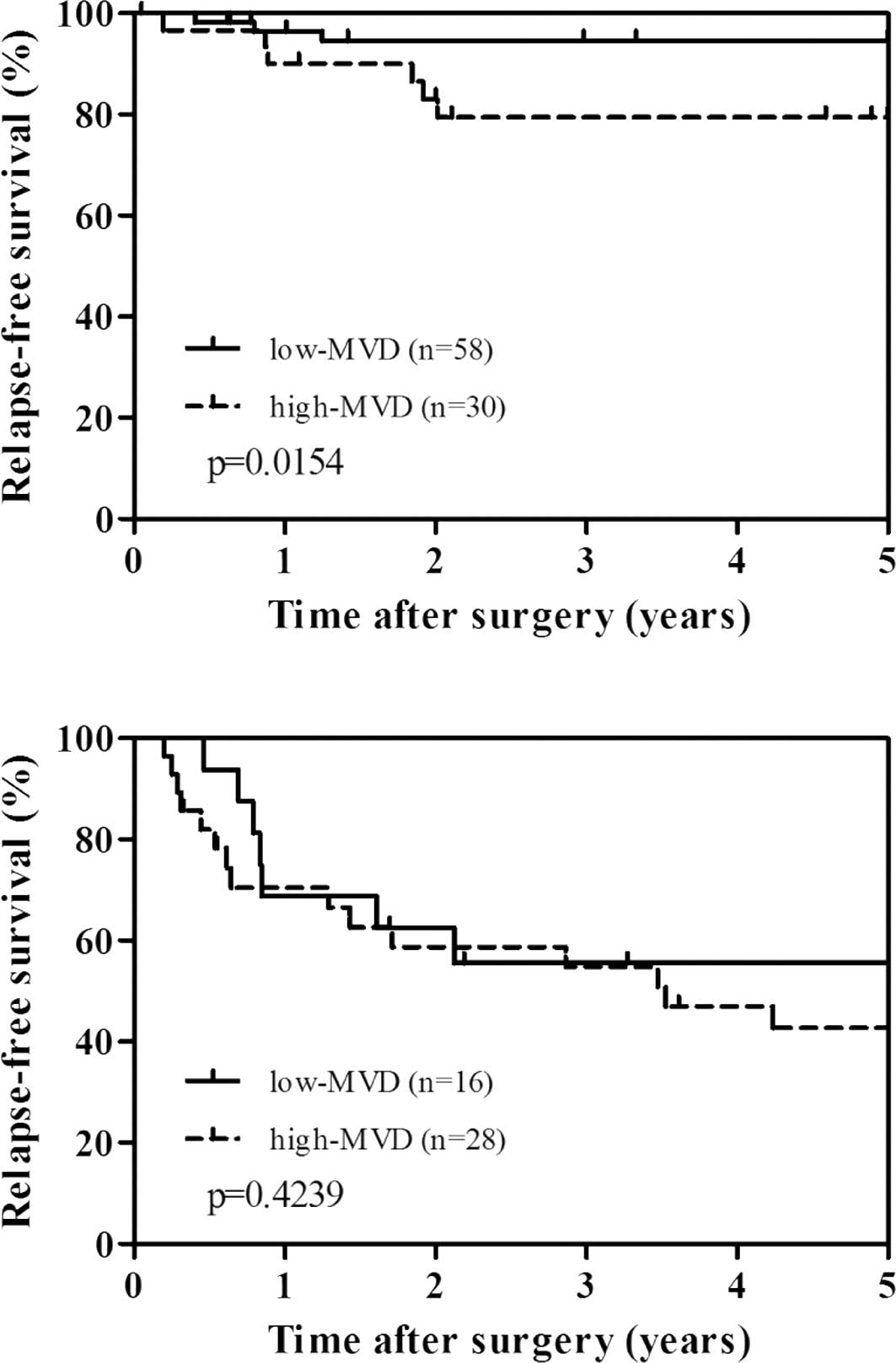
To evaluate the potential use of MVD and MMP-7 as a
clinical biomarker, we performed stratified analysis by stage of
disease (Fig. 3A and B). There was
a significant difference between high MVD and relapse-free survival
in stage I–II cases (p=0.0154), but not in stage III cases
(p=0.4239). However, when stratified by stage of disease, MMP-7
expression was not significantly related to relapse-free survival
(data not shown).
The prognostic relevance of MVD and MMP-7 was
assessed using univariate and subsequent multivariate Cox
proportional hazard model adjusted for the established clinical
prognostic factors in all cases and stage I–II cases, respectively
(Table IIA and B). The
multivariate analysis revealed that depth of invasion [p<0.001;
hazard ratio (HR)=2.001; 95% confidence interval (CI) 1.396–2.868]
and lymph node metastasis (p<0.001; HR=5.274; 95% CI
1.797–15.479) were independently associated with a relapse-free
survival in all cases. When restricting the analysis to stage I–II
cases, high MVD was the only independent prognostic factor
associated with relapse-free survival (p=0.028; HR=4.582; 95% CI
1.184–17.737). However, MMP-7 expression did not predict survival
independently.
 | Table II.Univariate and multivariate Cox
regression analysis of relapse-free survival. |
Table II.
Univariate and multivariate Cox
regression analysis of relapse-free survival.
A, All cases (stage
I–III cases).
|
|---|
| Variable |
Comparison/referent | Univariate
| Multivariate
|
|---|
| | p-value | HR | 95% CI | p-value |
|---|
| MVD by CD105 | High/low | 0.001 | | | NS |
| MMP-7 |
Positive/negative | 0.038 | | | NS |
| Age | >60/<60 | 0.076 | | | |
| Gender | Female/male | 0.729 | | | |
| Histological
type |
Undifferentiated/differentiated | 0.417 | | | |
| Depth of
invasion | T3/T1-T2 | <0.001 | 2.001 | 1.396–2.868 | <0.001 |
| Lymph node
metastasis | Present/absent | <0.001 | 5.274 | 1.797–15.479 | 0.002 |
B, Stage I–II
cases.
|
|---|
| Variable |
Comparison/referent | Univariate
| Multivariate
|
|---|
| | p-value | HR | 95% CI | p-value |
|---|
| MVD by CD105 | High/low | 0.028 | 4.582 | 1.184–17.737 | 0.028 |
| MMP-7 |
Positive/negative | 0.657 | | | |
| Age | >60/<60 | 0.127 | | | |
| Gender | Female/male | 0.831 | | | |
| Histological
type |
Undifferentiated/differentiated | 0.721 | | | |
| Depth of
invasion | T3/T1–T2 | 0.680 | | | |
| Lymph node
metastasis | Present/absent | 0.045 | | | NS |
Relation between recurrence patterns and
MVD
The relations between the recurrence patterns and
MVD counts are shown in Fig. 4.
The mean MVD in patients without recurrence, patients with
peritoneal, hematogenous and locoregional recurrence was
33.68±22.26, 35.08±25.25, 50.55±23.77 and 58.82±26.10,
respectively. Higher MVD was significantly correlated with
hematogenous and locoregional recurrence (p<0.05 and p<0.01,
respectively). By contrast, no relationship was found between MMP-7
expression and any specific patterns of recurrence. To determine
whether any variable would provide a better estimate of relative
risks for the development of metastasis, univariate and subsequent
multivariate logistic analysis was applied. Covariants included
histological type, depth of invasion, lymphatic invasion, venous
invasion, lymph node metastasis, MVD and MMP-7 expression. As shown
in Table III, multivariate
analysis identified T3 (serosal invasion) tumor, presence of lymph
node metastasis and high-MVD as independent predictors of
recurrence. With respect to each recurrence pattern, depth of
invasion was associated with both hematogenous and peritoneal
recurrence. On the other hand, high MVD was associated with both
hematogenous and locoregional recurrence. However, histological
type, lymphatic or venous invasion and MMP-7 expression were not
associated with any specific recurrence pattern in multivariate
analysis.
 | Table III.Multivariate analysis for each
recurrence pattern by logistic regression. |
Table III.
Multivariate analysis for each
recurrence pattern by logistic regression.
|
Comparison/referent | OR | 95% CI | p-value |
|---|
| Any recurrence | | | | |
| Depth of
invasion | T3/T1–T2 | 2.325 | 1.418–3.812 | 0.001 |
| Lymph node
metastasis | Present/absent | 4.089 | 1.171–14.285 | 0.027 |
| MVD by CD105 | High/low | 2.882 | 1.025–8.108 | 0.045 |
| Locoregional
recurrence | | | | |
| MVD by CD105 | High/low | 15.208 | 1.886–122.662 | 0.011 |
| Hematogenous
recurrence | | | | |
| Depth of
invasion | T3/T1–T2 | 2.274 | 1.365–3.789 | 0.002 |
| MVD by CD105 | High/low | 5.718 | 1.875–17.442 | 0.002 |
| Peritoneal
recurrence | | | | |
| Depth of
invasion | T3/T1–T2 | 4.148 | 1.885–9.126 | <0.001 |
Discussion
Early diagnosis, surgical treatment with systematic
lymph node dissection and appropriate chemotherapy have improved
the survival of patients with gastric cancer (17). However, even after a curative
resection, tumor recurrences are likely to assume a variety of
forms in various organs. The prediction of risks for recurrences as
well as recurrence patterns after surgery could help the design of
better follow-up programmes and appropriate treatment strategies
for gastric cancer patients. Recurrences probably arise from the
growth of occult micro-metastases that have already been
established at the time of surgery. This may depend on the
biological nature of the resected tumor itself.
CD105 is a proliferation-associated and
hypoxia-inducible glycoprotein abundantly expressed in angiogenic
endothelial cells, and it is essential in angiogenesis. The
intensity of staining for CD105 is greater in blood vessel
endothelia within neoplastic than within normal tissues, indicating
that CD105 is a powerful marker of neovascularization in solid
malignancies (3).
Herein, we showed for the first time that MVD by
CD105 indicates recurrence in patients with resected gastric
cancer. Significant higher MVD was found in tumors with deeper
depth of invasion, presence of lymphatic and venous invasion,
presence of lymph node metastasis, advanced stage and tumor
recurrence. In survival analysis, high-MVD was significantly
correlated with worse relapse-free survival by univariate analysis.
When tumors were divided into stage I–II and stage III, high MVD
was also significantly associated with worse relapse-free survival
in stage I–II cases. Furthermore, in stage I–II cases, high MVD was
the only independent predictor for relapse-free survival by
multivariate analysis of Cox proportional hazard model. Regarding
the specific patterns of recurrence, high MVD was independently
related to locoregional and hematogenous recurrence by multivariate
logistic analysis.
MMP-7 plays a key role, not only in the degradation
of extracellular matrix (ECM), but also in the creation and
maintenance of a microenvironment that facilitates the growth and
angiogenesis of tumors (12,13).
MMP-7 accelerates the proliferation of human umbilical vein
endothelial cells in vitro (18). Another study demonstrated that
MMP-7-induced angiogenesis in a mouse model was inhibited by an
MMP-7 specific antisense oligonucleotide (19). Moreover, MMP-7 cleaves the
matrix-bound isoform of vascular endothelial growth factor, which
is one of the most powerful mediators of angiogenesis (20). These findings suggest that MMP-7
potently promotes angiogenesis. In the present study, we revealed
that MMP-7 expression in tumors was significantly related to MVD
recognized by CD105, suggesting that MMP-7 may be involved in
neoangiogenesis in gastric cancer. Furthermore, patients with
MMP-7-positive tumor had shorter relapse-free survival. However,
multivariate analysis revealed that MMP-7 expression lost
independence of development of recurrence.
In the present study, we also revealed that higher
MVD was significantly correlated with lymph node status and
lymphatic invasion, suggesting that CD105 may be involved in
lymphatic metastasis. Recently, Clasper et al demonstrated
that CD105 was up-regulated in tumor lymphatic endothelial cells
(LEC) compared to normal LEC, using combined GeneChip microarray
and immunohistochemical analyses. The authors also revealed that
CD105 was not confined to the blood vasculature, but detected in
numerous LYVE-1-positive tumor lymphatics (21). Likewise, Yoshitomi et al
reported that CD105 was found in lymphatic endothelial cells of
pancreatic cancer tissue identified by staining with the D2-40
antibody (22). These results
suggest that CD105 expression in endothelial cells of small
capillary-like vessels consist of immature endothelial cells
induced, not only by tumor angiogenesis, but also by tumor
lymphangiogenesis. Our finding was consistent with the reports that
CD105 also exists in lymphatics, suggesting that CD105 may be a
potential predictor of lymphatic metastasis as well as hematogenous
metastasis.
These data suggest that CD105 is a candidate target
molecule for novel antitumor therapy based on the inhibition of
tumor neovasculature. In fact, anti-CD105 therapy has been
validated experimentally in several animal models (3,23,24).
Recently, targeting the angiogenic mediator vascular endothelial
growth factor has proven efficacious in several solid malignancies,
therefore, targeting the tumor-associated activated endothelial
cell directly may also be a successful strategy (25).
In conclusion, MVD recognized by CD105 may be a
useful predictor of tumor recurrence and specific site of
reccurence after surgical resection, and thereby may help to refine
therapeutic decisions in gastric cancer.
References
|
1.
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
2.
|
Moriguchi S, Maehara Y, Korenaga D,
Sugimachi K and Nose Y: Risk factors which predict pattern of
recurrence after curative surgery for patients with advanced
gastric cancer. Surg Oncol. 1:341–346. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Dallas NA, Samuel S, Xia L, Fan F, Gray
MJ, Lim SJ and Ellis LM: Endoglin (CD105): a marker of tumor
vasculature and potential target for therapy. Clin Cancer Res.
14:1931–1937. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Saad RS, Liu YL, Nathan G, Celebrezze J,
Medich D and Silverman JF: Endoglin (CD105) and vascular
endothelial growth factor as prognostic markers in colorectal
cancer. Mod Pathol. 17:197–203. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Mineo TC, Ambrogi V, Baldi A, Rabitti C,
Bollero P, Vincenzi B and Tonini G: Prognostic impact of VEGF,
CD31, CD34, and CD105 expression and tumour vessel invasion after
radical surgery for IB-IIA non-small cell lung cancer. J Clin
Pathol. 57:591–597. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Zijlmans HJ, Fleuren GJ, Hazelbag S, Sier
CF, Dreef EJ, Kenter GG and Gorter A: Expression of endoglin
(CD105) in cervical cancer. Br J Cancer. 100:1617–1626. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
El-Gohary YM, Silverman JF, Olson PR, Liu
YL, Cohen JK, Miller R and Saad RS: Endoglin (CD105) and vascular
endothelial growth factor as prognostic markers in prostatic
adenocarcinoma. Am J Clin Pathol. 127:572–579. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Li C, Guo B, Wilson PB, Stewart A, Byrne
G, Bundred N and Kumar S: Plasma levels of soluble CD105 correlate
with metastasis in patients with breast cancer. Int J Cancer.
89:122–126. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Ding S, Li C, Lin S, Yang Y, Liu D, Han Y,
Zhang Y, Li L, Zhou L and Kumar S: Comparative evaluation of
microvessel density determined by CD34 or CD105 in benign and
malignant gastric lesions. Hum Pathol. 37:861–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Nikiteas NI, Tzanakis N, Theodoropoulos G,
Atsaves V, Christoni Z, Karakitsos P, Lazaris AC, Papachristodoulou
A, Klonaris C and Gazouli M: Vascular endothelial growth factor and
endoglin (CD-105) in gastric cancer. Gastric Cancer. 10:12–17.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Roy R, Zhang B and Moses MA: Making the
cut: protease-mediated regulation of angiogenesis. Exp Cell Res.
312:608–622. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Overall CM and Kleifeld O: Tumour
microenvironment – opinion: validating matrix metalloproteinases as
drug targets and anti-targets for cancer therapy. Nat Rev Cancer.
6:227–239. 2006.
|
|
13.
|
Ii M, Yamamoto H, Adachi Y, Maruyama Y and
Shinomura Y: Role of matrix metalloproteinase-7 (matrilysin) in
human cancer invasion, apoptosis, growth, and angiogenesis. Exp
Biol Med. 231:20–27. 2006.PubMed/NCBI
|
|
14.
|
Okayama H, Kumamoto K, Saitou K, Hayase S,
Kofunato Y, Sato Y, Miyamoto K, Nakamura I, Ohki S, Sekikawa K and
Takenoshita S: CD44v6, MMP-7 and nuclear Cdx2 are significant
biomarkers for prediction of lymph node metastasis in primary
gastric cancer. Oncol Rep. 22:745–755. 2009.PubMed/NCBI
|
|
15.
|
Japanese Gastric Cancer Association:
Japanese classification of gastric carcinoma, 2nd English edition.
Gastric Cancer. 1:10–24. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis – correlation in invasive
breast carcinoma. N Engl J Med. 324:1–8. 1991.
|
|
17.
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 374:477–490. 2009.
View Article : Google Scholar
|
|
18.
|
Huo N, Ichikawa Y, Kamiyama M, Ishikawa T,
Hamaguchi Y, Hasegawa S, Nagashima Y, Miyazaki K and Shimada H:
MMP-7 (matrilysin) accelerated growth of human umbilical vein
endothelial cells. Cancer Lett. 177:95–100. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Nishizuka I, Ichikawa Y, Ishikawa T,
Kamiyama M, Hasegawa S, Momiyama N, Miyazaki K and Shimada H:
Matrilysin stimulates DNA synthesis of cultured vascular
endothelial cells and induces angiogenesis in vivo. Cancer Lett.
173:175–182. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Lee S, Jilani SM, Nikolova GV, Carpizo D
and Iruela-Arispe ML: Processing of VEGF-A by matrix
metalloproteinases regulates bioavailability and vascular
patterning in tumors. J Cell Biol. 169:681–691. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Clasper S, Royston D, Baban D, Cao Y,
Ewers S, Butz S, Vestweber D and Jackson DG: A novel gene
expression profile in lymphatics associated with tumor growth and
nodal metastasis. Cancer Res. 68:7293–7303. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Yoshitomi H, Kobayashi S, Ohtsuka M,
Kimura F, Shimizu H, Yoshidome H and Miyazaki M: Specific
expression of endoglin (CD105) in endothelial cells of intratumoral
blood and lymphatic vessels in pancreatic cancer. Pancreas.
37:275–281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Shiozaki K, Harada N, Greco WR, Haba A,
Uneda S, Tsai H and Seon BK: Antiangiogenic chimeric anti-endoglin
(CD105) antibody: pharmacokinetics and immunogenicity in nonhuman
primates and effects of doxorubicin. Cancer Immunol Immunother.
55:140–150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Tsujie M, Tsujie T, Toi H, Uneda S,
Shiozaki K, Tsai H and Seon BK: Anti-tumor activity of an
anti-endoglin monoclonal antibody is enhanced in immunocompetent
mice. Int J Cancer. 122:2266–2273. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Fonsatti E, Nicolay HJ, Altomonte M, Covre
A and Maio M: Targeting cancer vasculature via endoglin/CD105: a
novel antibody-based diagnostic and therapeutic strategy in solid
tumours. Cardiovasc Res. 86:12–19. 2010. View Article : Google Scholar : PubMed/NCBI
|