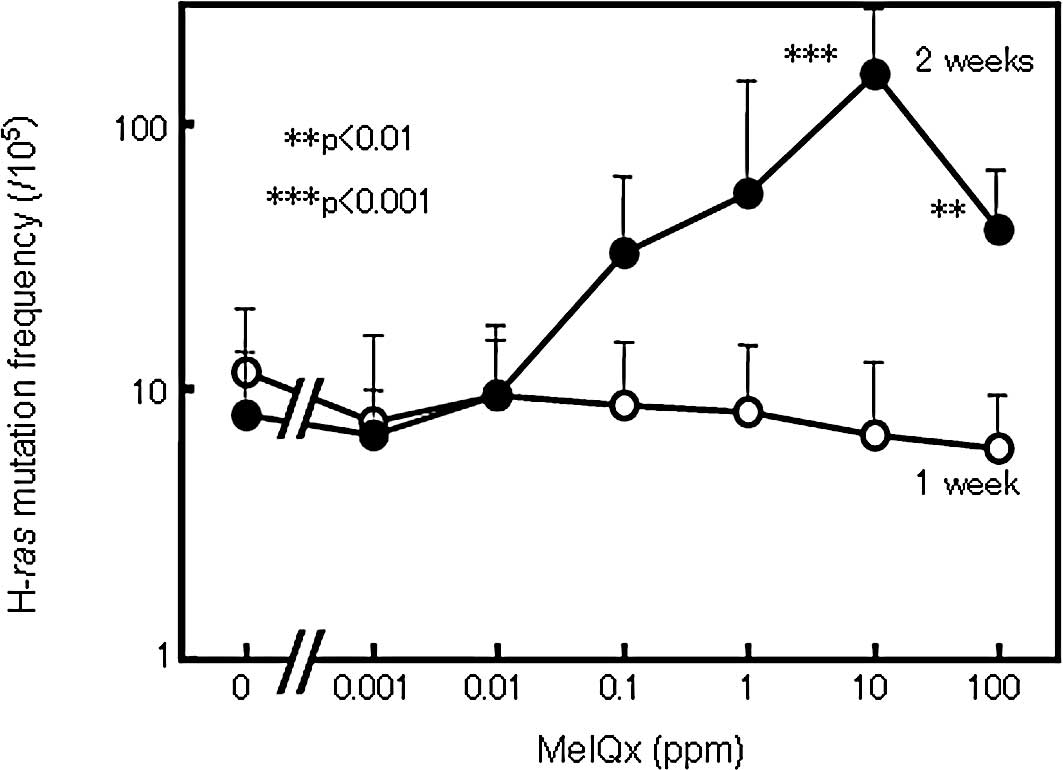
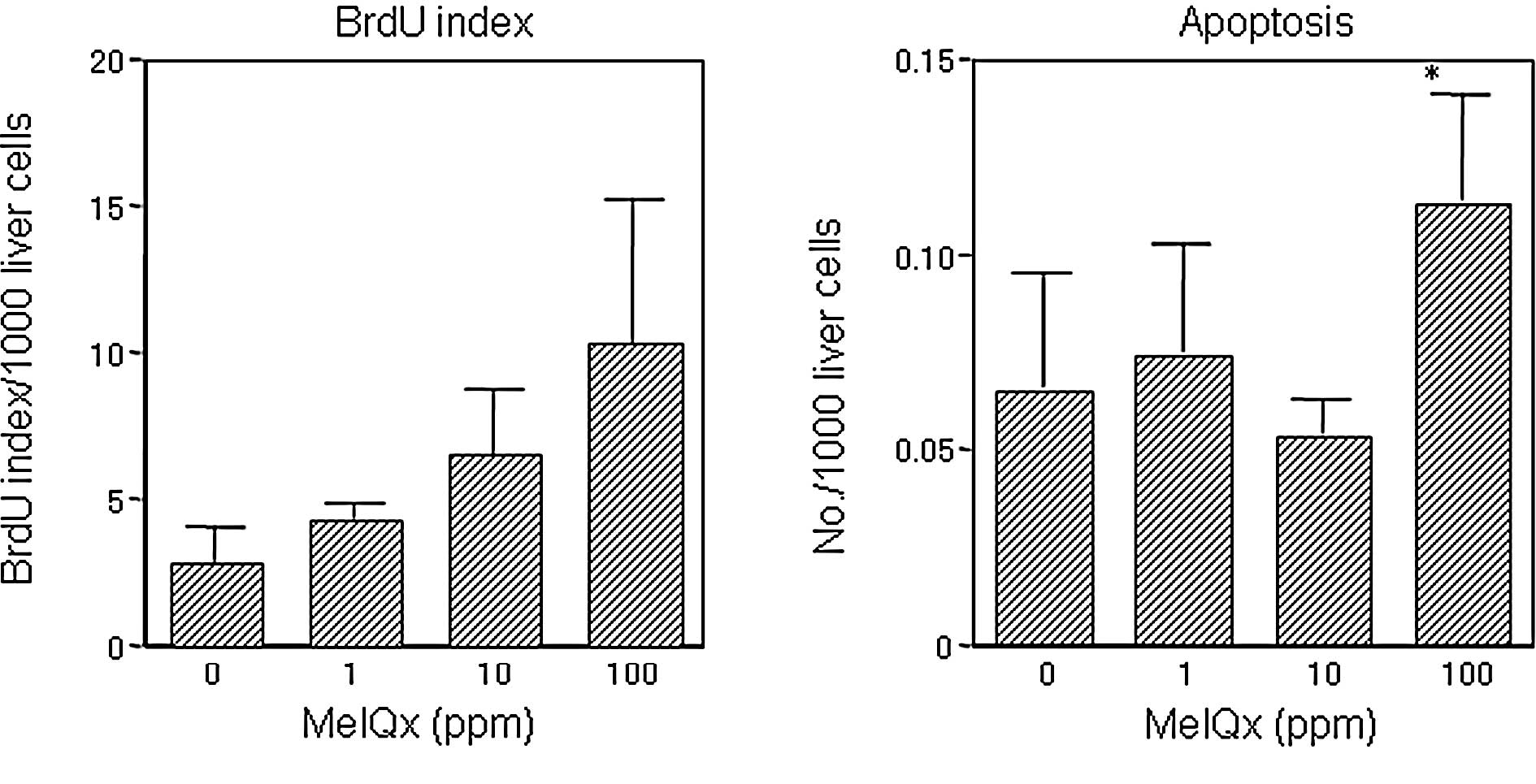
|
1.
|
Sistonen L and Alitalo K: Activation of
c-ras oncogenes by mutations and amplification. Ann Clin Res.
18:297–303. 1986.PubMed/NCBI
|
|
2.
|
Orita M, Suzuki Y, Sekiya T, et al: Rapid
and sensitive detection of point mutations and DNA polymorphisms
using the polymerase chain reaction. Genomics. 5:874–879. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Winter E, Yamamoto F, Almoguera C, et al:
A method to detect and characterize point mutations in transcribed
genes: amplification and overexpression of the mutant c-Ki-ras
allele in human tumor cells. Proc Natl Acad Sci USA. 82:7575–7579.
1985. View Article : Google Scholar
|
|
4.
|
Myers RM, Lumelsky N, Lerman LS, et al:
Detection of single base substitutions in total genomic DNA.
Nature. 313:495–498. 1985. View
Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Conner BJ, Reyes AA, Morin C, et al:
Detection of sickle cell beta S-globin allele by hybridization with
synthetic oligonucleotides. Proc Natl Acad Sci USA. 80:278–282.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Ehlen T and Dubeau L: Detection of ras
point mutations by the polymerase chain reaction using
mutation-specific, inosine-containing oligonucleotide primers.
Biochem Biophys Res Commun. 160:441–447. 1989. View Article : Google Scholar
|
|
7.
|
Ichikawa T, Yano Y, Uchida M, et al: The
activation of K-ras gene at an early stage of lung tumorigenesis in
mice. Cancer Lett. 107:165–170. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Yano T, Yuasa M, Murakami A, et al: The
detection of chemically initiated cells having the mutation of
K-ras gene at an early stage of lung carcinogenesis in mice. Anal
Biochem. 244:187–189. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Kawasaki K, Suzuki T, Ueda M, et al: CC to
TT mutation in the mitochondrial DNA of normal skin: relationship
to ultraviolet light exposure. Mutat Res. 22:35–43. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Takeda S, Ichii S and Nakamura Y:
Detection of K-ras mutation in sputum by mutant-allele-specific
amplification (MASA). Hum Mutat. 2:112–117. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Liang TJ, Hasegawa K, Munoz SJ, et al:
Hepatitis B virus precore mutation and fulminant hepatitis in the
United States. A polymerase chain reaction-based assay for the
detection of specific mutation. J Clin Invest. 93:550–555. 1994.
View Article : Google Scholar
|
|
12.
|
Horikoshi T, Lenz HJ, Danenberg K, et al:
Quantitative determination of the ratio of mutated to normal ras
genes in the blood of leukemia patients by allele-specific PCR.
Leuk Res. 18:693–702. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Etoh T, Ueo H, Inoue H, et al: Clinical
significance of K-ras mutations in intraoperative tumor drainage
blood from patients with colorectal carcinoma. Ann Surg Oncol.
8:407–412. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Hashimoto T, Kobayashi Y, Ishikawa Y, et
al: Prognostic value of genetically diagnosed lymph node
micrometastasis in non-small cell lung carcinoma cases. Cancer Res.
60:6472–6478. 2000.PubMed/NCBI
|
|
15.
|
Felton JS and Knize MG: Occurrence,
identification, and bacterial mutagenicity of heterocyclic amines
in cooked food. Mutat Res. 259:205–217. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Snyderwine EG: Some perspectives on the
nutritional aspects of breast cancer research. Food-derived
heterocyclic amines as etiologic agents in human mammary cancer.
Cancer. 74:1070–1077. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
IARC Working Group on the evaluation of
carcinogenic risk to humans: MeIQx
(2-amino-3,8-demethylimidazo[4,5-f]quinoxaline). IARC
Monographs on the Evaluation of Carcinogenic Risks to Humans – Some
Naturally Occurring Substances: Food Items and Constituents,
Heterocyclic Aromatic Amines and Mycotoxins. 56. Armstrong B: IARC
Publisher; Lyon: pp. 221–228. 1993
|
|
18.
|
Murray S, Gooderham NJ, Boobis AR, et al:
Detection and measurement of MeIQx in human urine after ingestion
of a cooked meat meal. Carcinogenesis. 10:763–765. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Ushiyama H, Wakabayashi K, Hirose M, et
al: Presence of carcinogenic heterocyclic amines in urine of
healthy volunteers eating normal diet, but not of inpatients
receiving parenteral alimentation. Carcinogenesis. 12:1417–1422.
1991. View Article : Google Scholar
|
|
20.
|
Tannenbaum SE, Stillwell WG, Ji H, et al:
MeIQx (2-amino-3,8-demethylimidazo [4,5-f]quinoxaline):
metabolism in humans and urinary metabolites in human populations.
Heterocyclic Amines in Cooked Foods, Possible Human Carcinogens.
Asamson RH, Gustafsson JA, Ito N, Nagao M, Sugimura T, Wakabayashi
K and Yamazoe Y: Princeton Scientific Publishing Co. Inc.;
Princeton: pp. 197–206. 1993
|
|
21.
|
Totsuka Y, Fukutome K, Takahashi M, et al:
Presence of
N2-(deoxyguanosin-8-yl)-2-amino-3,8-dimethylimidazo[4,5-f]
quinoxaline (dG-C8-MeIQx) in human tissues. Carcinogenesis.
17:1029–1034. 1996.PubMed/NCBI
|
|
22.
|
Yamashita K, Adachi M, Kato S, et al: DNA
adducts formed by
2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline in rat liver:
dose-response on chronic administration. Jpn J Cancer Res.
81:470–476. 1990.
|
|
23.
|
Kato T, Ohgaki H, Hasegawa H, et al:
Carcinogenicity in rats of a mutagenic compound,
2-amino-3,8-dimethylimidazo[4,5-f] quinoxaline.
Carcinogenesis. 9:71–73. 1998.PubMed/NCBI
|
|
24.
|
Pfau W, Martin FL, Cole KJ, et al:
Heterocyclic aromatic amines induce DNA strand breaks and cell
transformation. Carcinogenesis. 20:545–551. 1999. View Article : Google Scholar : PubMed/NCBI
|