Introduction
Zinc (Zn) is an essential trace element that serves
as the active center of approximately 300 enzymes and participates
in physiological functions including immune function, neurosensory
function, protein metabolism, wound healing and sexual function
(1,2). Zn plays important roles as a growth
co-factor, an immunomodulator and a cytoprotectant with
antioxidant, antiapoptotic and anti-inflammatory effects (3,4).
Therefore, Zn depletion results in numerous types of symptoms
including taste disorder, hyperkeratotic skin change, wound healing
disturbance and testicular dysfunction.
Zn deficiency is also frequently observed in
patients with chronic liver diseases (CLD), including chronic
hepatitis and liver cirrhosis (5–8).
Serum Zn levels inversely correlate with the severity of hepatic
fibrosis (7). Zn depletion in
patients with liver cirrhosis is closely related to the progression
of hepatic encephalopathy. Zn deficiency usually leads to a
decrease in the activity of ornitine transcarbamylase, a key
Zn-containing enzyme for the urea cycle. This consequently causes
the accumulation of ammonia and seems to account for the
pathogenesis of hepatic encephalopathy (9). Zn administration has favorable
effects on the inhibition of hepatic fibrosis, activating
collagenase, which belongs to a zinc-metallo-enzyme, and/or
diminishing prolyl hydroxylase, which contributes to collagen
synthesis (10). Zn
supplementation also relieves hepatic encephalopathy. In addition,
we previously elucidated that oral administration of polaprezinc, a
complex of zinc and l-carnosine, markedly improved
necroinflammation in the liver of hepatitis C virus (HCV)-related
CLD, reducing iron overload in the liver (11).
It has been widely established that persistent HCV
infection leads to metabolic abnormalities including insulin
resistance, hepatic steatosis and hypertriglycemia (12,13).
Impaired signaling of the insulin receptor substrate (IRS) by the
HCV core protein is considered to trigger insulin resistance in
patients with HCV-related CLD (14). Previous studies revealed that
insulin resistance is significantly associated with body mass index
(BMI) (15), hepatic steatosis
(16), hepatic fibrosis (17,18)
and/or iron overload (19) in
patients with chronic hepatitis C (CH-C).
On the other hand, Zn also plays crucial roles in
the secretion and activation of insulin (20). Therefore, abnormalities of Zn
metabolism seem to be associated with the pathogenesis of type 2
diabetes mellitus (DM) (21). A
recent report revealed that oral administration of a Zn complex
improved glucose tolerance in an experimental model of type 2 DM
(22).
However, little is known about the relationship
between insulin resistance and Zn deficiency in patients with
HCV-related CLD. The primary purpose of this study was to
investigate whether or not Zn deficiency contributes to insulin
resistance in patients with HCV-related CLD.
Materials and methods
Study population
Forty-eight non-diabetic patients with CH-C, who had
HCV-RNA detectable in the sera by polymerase chain reaction (PCR)
and showed histological characteristics consistent with chronic
hepatitis, were randomly selected for this study. Informed consent
was obtained from the enrolled patients.
Laboratory assessments
The severity of obesity was evaluated using BMI.
Insulin resistance was determined by the Homeostasis model for
assessment of insulin resistance (HOMA-IR) method using the
following equation: HOMA-IR = fasting insulin (μU/ml) x fasting
glucose (mg/dl)/405 (23). Serum
alanine aminotransferase (ALT) levels were assessed as a
serological parameter of necroinflammation in the liver. Serum
ferritin levels were examined as a serological hallmark of iron
storage in the liver. Blood samples were drawn in the morning with
fasting on the basis of circadian serum Zn levels (24). Zn deficiency was defined as a serum
Zn level <65 μg/dl, which corresponds to the normal lower limit
of normal serum Zn. Quantitative detection of serum HCV-RNA was
performed by Amplicor-HCV monitor assay (Roche Molecular
Diagnostics, Tokyo, Japan) (25).
The HCV genotype was determined by the HCV-RNA Genotyping assay
system (Home Brew SRL Inc., Tokyo, Japan) (26).
Evaluation of hepatic fibrosis and
steatosis
The grades of hepatic fibrosis were determined by
the New Inuyama classification system, which is a standard
criterion for the histological assessment of chronic hepatitis in
Japan (27). The stages in chronic
hepatitis were classified from F0 through F3.
F0 was defined as no fibrosis in the liver, while
F4 was defined as liver cirrhosis. The enrolled patients
were divided into two groups on the basis of hepatic fibrosis:
early-stage (F0 and F1) and late-stage
(F2 and F3).
The severity of hepatic steatosis was evaluated in
accordance with the classification proposed by Brunt and colleagues
(28): grade 0, no steatosis;
grade 1, <33% of hepatocytes with steatosis; grade 2, 33–66% of
hepatocytes affected.
Statistical analyses
Data values are represented as means ± standard
deviation (SD). The Mann-Whitney U test was applied for the
comparison of continuous variables. The linear regression analysis
was used to analyze the relation of the serum ferritin levels with
serum Zn and ALT levels, or the values of HOMA-IR. p-values
<0.05 were considered to indicate a significant difference
between groups.
Results
Demographic features of the enrolled
patients with CH-C
Demographic characteristics are shown in Table I. Of the enrolled patients, 32
(67%) had a HCV genotype of 1b, 11 (23%) had a genotype of 2a and 5
(10%), 2b, respectively. There were no significant differences in
the serum Zn levels among the enrolled patients with the three HCV
genotypes (genotype 1b, 79.5±15.1; 2a, 71.9±9.8; 2b, 70.4±7.0).
Serum Zn levels were not associated with the load of HCV-RNA
(r=0.0074, p=0.6230).
 | Table I.Demographic, viral and histological
characteristics of 48 patients with chronic hepatitis C. |
Table I.
Demographic, viral and histological
characteristics of 48 patients with chronic hepatitis C.
|
Characteristics | Data |
|---|
| Age (range) | 59±11 (23–76) |
| Gender
(male/female) | 34/14 |
| Body mass index
(range) | 23.7±2.6
(17.9–30.6) |
| Genotype | |
| 1b | 32 (67%) |
| 2a | 11 (23%) |
| 2b | 5 (10%) |
| Hepatic
fibrosis | |
|
F0 | 1 (2%) |
|
F1 | 20 (42%) |
|
F2 | 6 (12%) |
|
F3 | 21 (44%) |
| Hepatic
steatosis | |
| Grade 0 | 27 (57%) |
| Grade 1 | 16 (33%) |
| Grade 2 | 5 (10%) |
| Zinc
deficiency | 7 (15%) |
Of the 48 patients with CH-C, 1 had no fibrosis
(F0) in the liver and 20 patients had stage
F1 fibrosis. Six and 21 patients with CH-C fulfilled
stage F2 and F3 fibrosis, respectively.
Therefore, the enrolled patients were divided into 2 groups: 21
patients with CH-C in the early stage of hepatic fibrosis and 27
patients with CH-C in the late stage of hepatic fibrosis.
Severity of hepatic steatosis in the enrolled
patients was evaluated as follows: 27 (57%) out of the 48 patients
with CH-C had no hepatic steatosis (grade 0). Sixteen (33%) and 5
(10%) patients had hepatic steatosis of grade 1 and grade 2,
respectively.
Seven out of the 48 (15%) patients with CH-C
fulfilled the criteria for Zn deficiency. These 7 patients with
CH-C had hepatic fibrosis of stage F2 or
F3.
Correlation between serum ferritin levels
and serum Zn levels, HOMA-IR values or serum ALT levels
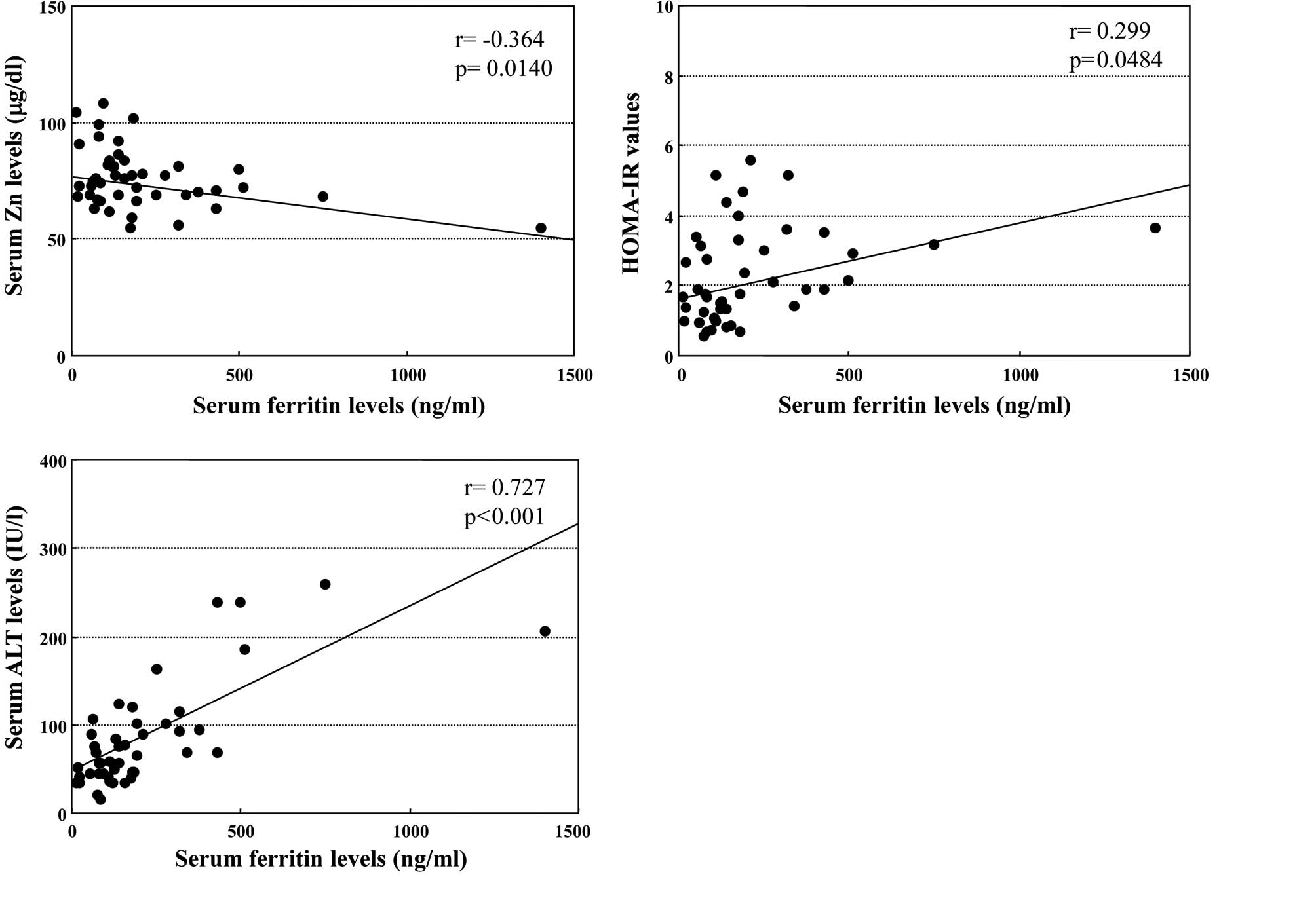
The relationship between serum feritin and Zn levels
in the enrolled patients with CH-C was examined. As shown in
Fig. 1A, the serum ferritin levels
were inversely correlated with the serum Zn levels (r=−0.364,
p=0.0140). The correlation was independent of HCV genotype or load
of HCV-RNA (data not shown).
Moreover, serum ferritin levels were significantly
associated with serum HOMA-IR values in the enrolled patients
(r=0.299, p=0.0484, Fig. 1B),
regardless of HCV genotype or load of HCV-RNA. On the other hand,
the linear regression analysis showed a close relationship between
serum ferritin and ALT concentrations in patients with CH-C
(r=0.727, p<0.001, Fig.
1C).
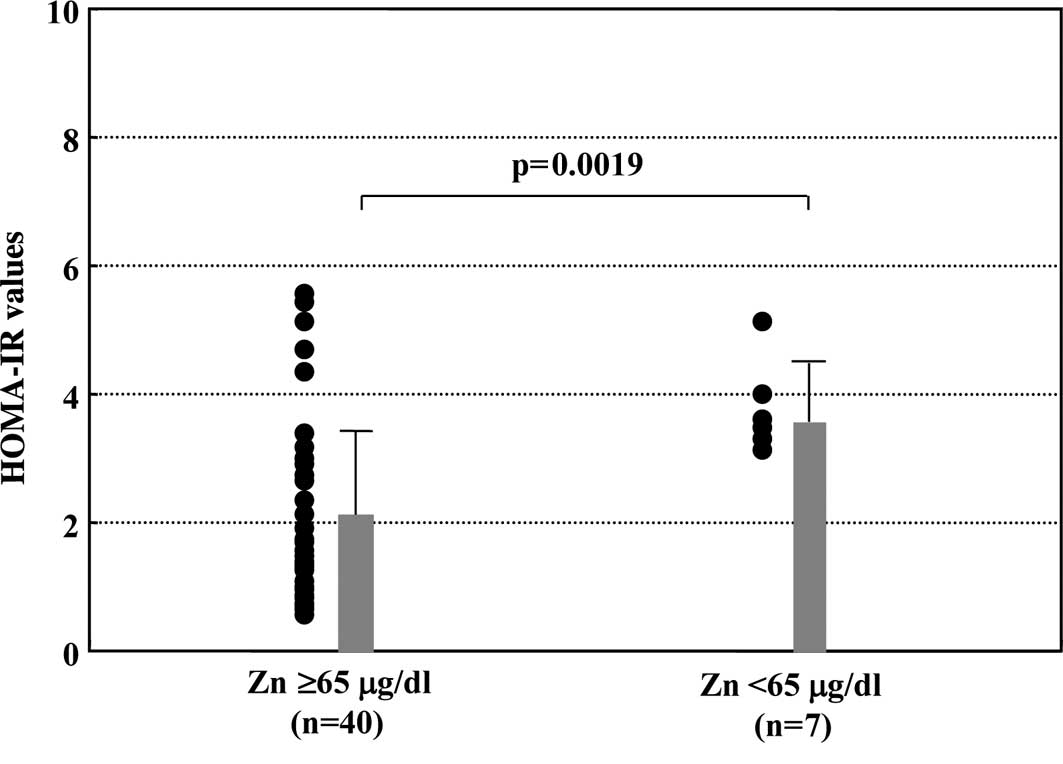
Comparison of insulin resistance between
Zn-deficient and normal Zn groups
The values of HOMA-IR were compared between the
group with Zn-deficiency and the group whose Zn levels were within
a normal range. As shown in Fig.
2, the mean value of HOMA-IR was significantly higher in the
Zn-deficient than that in the normal Zn group (3.76±0.66 vs.
2.08±1.35, p=0.0019).
Relationship between hepatic fibrosis and
serum Zn levels or HOMA-IR values
The relationship between the severity of hepatic
fibrosis and serum Zn levels or insulin resistance in the enrolled
patients with CH-C was investigated. CH-C patients at late stages
of hepatic fibrosis (F2 and F3) had
significantly lower serum Zn levels than those at early stages of
hepatic fibrosis (F0 and F1) (73±13 vs. 81±13
μg/dl, p=0.0366, Fig. 3A).
However, there were no significant differences in the HOMA-IR
values between early- and late-stage hepatic fibrosis patients
(2.19±1.55 vs. 2.44±1.30, p=0.5527, Fig. 3B).
Other factors contributing to insulin
resistance in patients with CH-C
Recent reports revealed that BMI (15) and hepatic steatosis (16) also affect insulin resistance in
patients with CH-C. We confirmed that the mean HOMA-IR value in
CH-C patients with hepatic steatosis of grade 2 was significantly
higher than that in those without hepatic steatosis (3.88±1.08 vs.
1.90±1.20, p=0.0044). However, there was no significant correlation
between the HOMA-IR value and BMI (r=0.015, p=0.9203).
Discussion
In this study, we revealed that insulin resistance
in patients with CH-C is closely associated with Zn deficiency
(Fig. 2). Zn deficiency was found
in 15% of the patients with chronic hepatitis. To our knowledge,
this is the first report that describes the relationship between
insulin resistance and Zn deficiency in patients with CH-C.
Previously, Furutani and colleagues revealed that insulin
resistance was characterized by iron accumulation in the liver
(19). We confirmed the
correlation between insulin resistance and iron overload in
patients with CH-C and postulated that iron overload may derive
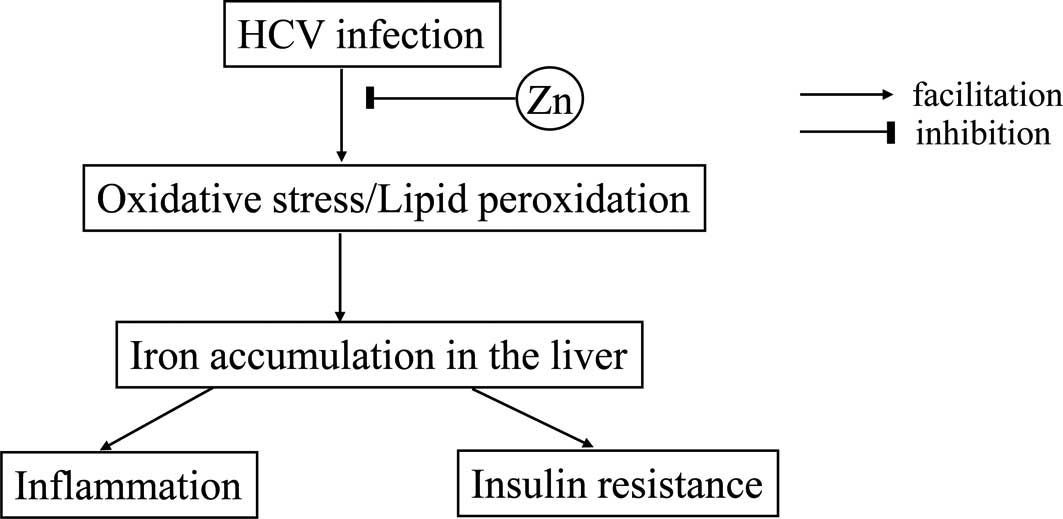
from Zn deficiency. The data obtained in the present study suggest
the possible mechanism of insulin resistance in patients with CH-C
(Fig. 4).
It is well recognized that chronic HCV infection
eventually leads to reactive oxygen species in the liver (29). The oxidative stress induced by HCV
infection consequently facilitates iron storage in the liver. On
the other hand, Zn plays crucial roles in antioxidative actions and
confers protective effects on hepatocytes (3,4). Zn
deficiency has been shown to result in attenuation of antioxidative
properties and thereby a significant elevation in the serum
ferritin level, which corresponds to iron accumulation in the
liver. Iron overload in the liver eventually evokes insulin
resistance (19), as well as
necroinflammation (30) in the
liver.
To date, several types of oxidative stress markers,
including 8-hydroxy-2’-deoxyguanosine (8-OHdG), malondialdehyde
(MDA), 4-hydroxy-2’-nonenal (4-HNE) and thioredoxin, have been
identified. On the other hand, superoxide dismutase (SOD) and
glutathione peroxidase are well recognized as useful hallmarks for
scavengers of oxidative stress. Patients with CH-C usually show
increased serum and liver levels of these oxidant products as well
as attenuation of antioxidant defenses. Oxidative stress
sequentially leads to lipid peroxidation, hepatic steatosis and
finally hepatocarcinogenesis in patients with HCV-related CLD
(31).
The hypothesis that oxidative stress by persistent
HCV infection enhances excessive iron accumulation in the liver is
supported by a recent study (32).
Fujita and colleagues revealed that hepatic 8-OHdG counts in
patients with CH-C were significantly correlated with serum
ferritin levels, indicating that oxidative stress caused by HCV
infection is strongly associated with iron overload.
In the present study, we determined that
hyperferritinemia, which reflects iron storage in the liver, in the
patients with CH-C may be responsible for the Zn deficiency
(Fig. 1). The decrease in the
activity of copper/zinc-SOD due to Zn deficiency may account for
iron overload in patients with CH-C.
An inverse correlation between serum ferritin and Zn
levels is also observed in patients with β-thalassemia (33), which fulfills the criteria for
secondary iron overload syndrome. Notably, iron overload and Zn
deficiency in these patients may play a part in the pathogenesis of
impaired glucose tolerance (34).
Hyperferritinemia primarily seems to be responsible
for the down-regulation of hepcidin, which has been recognized as a
peptide hormone modulating iron uptake from the small intestine
(35) and/or up-regulation of
transferrin receptors (36). Miura
and colleagues (35) recently
revealed that a decrease in the activity of hepcidin caused by
HCV-induced oxidative stress was primarily derived from i) the
stabilization of the negative hepcidin regulators, hypoxia
inducible factor (HIF) 1α and HIF2α, and ii) the hypoacetylation of
histone and subsequent inhibition of the binding of two positive
regulators, CCAT/enhancer-binding protein α (C/EBPα) and the signal
transducer and activator of transcription 3 (STAT3), to the
hepcidin promoter.
A close relationship between insulin resistance and
iron overload has been observed in patients with type 2 DM
(37). We also revealed that iron
overload participated in insulin resistance in patients with CH-C
(Fig. 1). Moreover, we confirmed
that iron overload in patients with CH-C eventually caused liver
damage (Fig. 1).
It has been well established that the severity of
hepatic fibrosis is inversely related to serum Zn levels (11). Previous reports affirm that the
degree of hepatic fibrosis is associated with insulin resistance in
patients with HCV-related CLD (12,17,18).
However, we did not confirm the relationship between hepatic
fibrosis and insulin resistance in patients with CH-C. Other
factors including iron overload may contribute to insulin
resistance in patients with CH-C.
The data described above allowed us to predict that
Zn supplementation may facilitate protection against oxidative
stress caused by HCV infection, subsequently reduce hepatic iron
accumulation and thereby improve insulin resistance. Further
clinical trials are required to investigate this prediction.
In conclusion, Zn deficiency is likely to contribute
to iron overload in patients with CH-C. Therefore, Zn deficiency
seems to be an important factor contributing to insulin resistance
in patients with CH-C.
Abbreviations:
|
ALT,
|
alanine aminotransferase;
|
|
CH-C,
|
chronic hepatitis C;
|
|
HCV,
|
hepatitis C virus;
|
|
CLD,
|
chronic liver disease;
|
|
DM,
|
diabetes mellitus;
|
|
HOMA,
|
homeostasis model assessment;
|
|
IR,
|
insulin resistance;
|
|
8-OHdG,
|
8-hydroxy-2′-deoxyguanosine;
|
|
SOD,
|
superoxide dismutase;
|
|
Zn,
|
zinc
|
References
|
1.
|
McClain CJ, Kasarskis EJ and Allen JJ:
Functional consequences of zinc deficiency. Prog Food Nutr Sci.
9:185–226. 1985.PubMed/NCBI
|
|
2.
|
McClain CJ, Marsano L, Burk RF and Bacon
B: Trace metals in liver disease. Semin Liver Dis. 11:321–339.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Prasad AS: Zinc and immunity. Mol Cell
Biochem. 188:63–69. 1998. View Article : Google Scholar
|
|
4.
|
Powell SR: The antioxidant properties of
zinc. J Nutr. 130:S1447–S1454. 2000.
|
|
5.
|
Vallee BL, Wacker WEC, Bartholomay AF,
Robin ED, Vallee RL and Wacker WE: Zinc metabolism in hepatic
dysfunction. I Serum zinc concentrations in Laennëc’s cirrhosis and
their validation by sequential analysis. N Engl J Med. 255:403–408.
1956.
|
|
6.
|
Bode JC, Hanisch P, Henning H, Koenig W,
Richter FW and Bode C: Hepatic zinc content in patients with
chronic active and chronic persistent hepatitis. Hepatology.
8:1650–1659. 1988.PubMed/NCBI
|
|
7.
|
Moriyama M, Matsumura H, Fukushima A, et
al: Clinical significance of evaluation of serum zinc
concentrations in C-viral chronic liver disease. Dig Dis Sci.
51:1967–1977. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Stamoulis I and Kouraklis G: Zinc and
liver: an active interaction. Dig Dis Sci. 52:1595–1612. 2007.
View Article : Google Scholar
|
|
9.
|
Riggio O, Merli M, Capocaccia L, et al:
Zinc supplementation reduces blood ammonia and increases liver
ornithine transcarbamylase activity in experimental cirrhosis.
Hepatology. 16:785–789. 1992. View Article : Google Scholar
|
|
10.
|
Gimenez A, Pares A, Alie S, et al:
Fibrogenic and collagenolytic activity in
carbon-tetrachloride-injured rats: beneficial effects of zinc
administration. J Hepatol. 21:292–298. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Himoto T, Hosomi N, Nakai S, et al:
Efficacy of zinc administration in patients with hepatitis C
virus-related chronic liver disease. Scand J Gastroenterol.
42:1078–1087. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Fartoux L, Poujol-Robert A, Guechot J,
Wendum D, Poupon R and Serfaty L: Insulin resistance is a cause of
steatosis and fibrosis progression in chronic hepatitis C. Gut.
254:1003–1008. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Mirandola S, Realdon S, Iqbal J, et al:
Liver microsomal triglyceride transfer protein is involved in
hepatitis C liver steatosis. Gastroenterology. 130:1661–1669. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Aytug S, Reich D, Sapiro LE, Berstein D
and Begum N: Impaired IRS-1/PI3-kinase signaling in patients with
HCV: a mechanism for increased prevalence of type diabetes.
Hepatology. 38:1384–1392. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Maeno T, Okumura A, Ishikawa T, et al:
Mechanisms of increased insulin resistance in non-cirrhotic
patients with chronic hepatitis C virus infection. J Gastroenterol
Hepatol. 18:1358–1363. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Camma C, Bruno S, Di Marco V, et al:
Insulin resistance is associated with steatosis in nondiabetic
patients with genotype 1 chronic hepatitis C. Hepatology. 43:64–71.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Moucari R, Asselah T, Cazals-Hatem D, et
al: Insulin resistance in chronic hepatitis C: association with
genotypes 1 and 4, serum HCV-RNA levels and liver fibrosis.
Gastroenterology. 134:416–423. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Hui JM, Sud A, Farrell GC, et al: Insulin
resistance is associated with chronic hepatitis C and virus
infection fibrosis progression. Gastroenterology. 125:1695–1704.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Furutani M, Nakashima T, Hirohama A, et
al: Insulin resistance/β cell function and serum ferritin level in
non-diabetic patients with hepatitis C virus infection. Liver Int.
23:294–299. 2003.
|
|
20.
|
Chausmer AB: Zinc, insulin and diabetes. J
Am Coll Nutr. 17:109–115. 1998. View Article : Google Scholar
|
|
21.
|
Kinlaw WB, Levine AS, Morley JE, Silvis SE
and McClain CJ: Abnormal zinc metabolism in type II diabetes
mellitus. Am J Med. 75:273–277. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Adachi Y, Yoshida J, Kodera Y, et al: Oral
administration of a zinc complex improves type 2 diabetes and
metabolic syndrome. Biochem Biophys Res Commun. 351:165–170. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Mattews DR, Hosker JP, Rudenski AS, Naylor
BA, Trecher DF and Turner RC: Homeostasis model assessment: insulin
resistance and beta-cell function from fasting plasma glucose and
insulin concentration in man. Diabetologia. 28:412–429. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Lifschitz MD and Henkin RI: Circadian
variation in copper and zinc in man. J Appl Physiol. 31:88–92.
1971.PubMed/NCBI
|
|
25.
|
Lau JY, Davis GL, Kniffen J, et al:
Significance of serum hepatitis C virus RNA levels in chronic
hepatitis C. Lancet. 341:1501–1504. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Simmonds P, Alberti A, Alter HJ, et al: A
proposed system for the nomenclature of hepatitis C viral
genotypes. Hepatology. 19:1321–1324. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Ichida F, Tsuji T, Omata M, et al: New
Inuyama classification: new criteria for histological assessment of
chronic hepatitis. Int Hepatol Commun. 6:112–119. 1996. View Article : Google Scholar
|
|
28.
|
Brunt EM, Janney CG, Di Bisceglie AM,
Neuschwander-Tetri BA and Bacon BR: Nonalcoholic steatohepatitis: a
proposal for grading and staging the histological lesions. Am J
Gastroenterol. 94:2467–2474. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Okuda M, Li K, Beard MR, Showalter LA,
Scholle F, Lemon SM and Weinman SA: Mitochondrial injury, oxidative
stress and antioxidant gene expression are induced by hepatitis C
virus core protein. Gastroenterology. 122:366–375. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Hayashi H, Takikawa T, Nishimura N, Yano
M, Isomura T and Sakamoto N: Improvement of serum aminotransferase
levels after phlebotomy in patients with chronic active hepatitis
C. Am J Gastroenterol. 89:986–989. 1994.PubMed/NCBI
|
|
31.
|
Koike K and Miyoshi H: Oxidative stress
and hepatitis C viral infection. Hepatol Res. 34:65–73. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Fujita N, Horiike S, Sugimoto R, et al:
Hepatic oxidative DNA damage correlates with iron overload in
chronic hepatitis C patients. Free Radic Biol Med. 42:352–362.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
De Sanctis V, Gamberini MR, Borgatti L,
Atti G, Vullo C and Bagni B: Alpha and beta cell evaluation in
patients with thalassaemia intermedia and iron overload. Postgrad
Med J. 61:963–967. 1985.PubMed/NCBI
|
|
34.
|
Dehshal MH, Hooghooghi AH, Kebrayaeezadeh
A, et al: Zinc deficiency aggravates abnormal glucose metabolism in
thalassemia major patients. Med Sci Monit. 13:CR235–239.
2007.PubMed/NCBI
|
|
35.
|
Miura K, Taura K, Kodama Y, Schnabl B and
Brenner DA: Hepatitis C virus-induced oxidative stress suppresses
hepcidin expression through increased histone deacetylase activity.
Hepatology. 48:1420–1429. 2008. View Article : Google Scholar
|
|
36.
|
Mifuji R, Kobayashi Y, Ma N, et al: Role
of transferrin receptor 2 in hepatic accumulation of iron in
patients with chronic hepatitis C. J Gastroenterol Hepatol.
21:144–151. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Fernandez-Real JM, Lopez-Bermejo A and
Ricart W: Cross-talk between iron metabolism and diabetes.
Diabetes. 51:2348–2354. 2002. View Article : Google Scholar : PubMed/NCBI
|