Introduction
Hepatocellular carcinoma (HCC) is one of the most
common and recurrent malignancies worldwide (1). Although the surgical techniques and
various nonsurgical treatment modalities have improved, none of
these therapies has significantly improved the extremely poor
prognosis and the overall 5-year survival rate worldwide is only 2%
(2). It is imperative that novel
strategies against HCC are identified.
Hepatitis B virus X protein (HBx) is an HBV-encoded
protein with multiple functions in transcription pathways, signal
transduction, cell cycle progress and HBV replication (3). Studies have demonstrated that HBx
could inhibit hepatocarcinoma cell growth via inducing apoptosis.
In our previous studies, we showed that treatment with Ad-HBx
intratumorally could not only induce apoptosis but also suppress
angiogenesis (4).
HCC is a typical hypervascular tumor and tumor
angiogenesis is required for both growth and metastasis of HCC
(5,6). Most patients show disease recurrence
that rapidly progresses to the advanced stages with vascular
invasion and multiple intrahepatic metastases (7). Our laboratory demonstrated that
implantation of mesenchymal stem cells (MSCs) that are genetically
modified with adenovirus vector encoding interleukin-12 (IL-12)
could significantly suppress HCC tumor growth in vivo via
inhibiting tumor angiogenesis (8).
Not only neovascular endothelial cells that are
critical components of the tumor microenvironment, but also the
immunocytes in the tumor stroma can affect tumor prognosis through
participating innate and adaptive immunity (9). Many studies showed that HBx could
elicit cytotoxic T lymphocyte (CTL) antitumor immune responses.
Vaccines based on HBx full-length sequence or specific epitopes
could trigger significant immune reactions (10–13). Our previous studies showed that
immunotherapy with Ad-HBx vaccine induced a massive accumulation of
CD8+ T cells at the tumor site and accordingly elicit
the host CTL response against HBV-associated HCC (11). Intratumoral injections with Ad-HBx
induced not only apoptosis of HCC cells but also lymphocytes
infiltration in the tumor stroma.
IL-12 as a heterodimeric cytokine, has potent
anti-tumor activity and anti-metastatic effect. A large number of
animal experiments demonstrated that IL-12 could inhibit tumor
angiogenesis and participate in immunoregulation (14,15). IL-12 significantly promoted the NK
cell and T cell proliferation, induced the production of IFN-γ and
stimulated naive CD4+ T cells to differentiate toward
the Th1 phenotype (16–18). Recently, combination IL-12 with
other treatment options was proved to be a better anti-tumor effect
with lower side-effect (19–21).
In this study, to further investigate the regulatory
effect of IL-12 on HBx mediated intervention of hepatoma
microenvironment especially on intervention of neovessels and
immune microenvironment, we constructed the recombinant adenovirus
carrying HBx and mIL-12 named Ad-HBx-mIL-12.
Materials and methods
Cell lines and adenovirus
preparation
Hepa1-6 (mouse HCC) cell line, which was purchased
from the American Type Culture Collection, was cultured in DMEM
medium (Gibco-BRL) with 10% fetal bovine serum (FBS; Gibco-BRL) and
10 μg/ml gentamicin sulfate at 37°C in 5% CO2.
Ad-HBx-mIL-12 is an E1, E3-deleted recombinant adenoviruse (rAd)
expressing the HBx and murine IL-12. Ad-HBx, Ad-mIL-12 and Ad-null
are E1, E3-deleted rAd expressing the HBx, murine IL-12 and no
transgene, respectively. All recombinant adenoviruses were
constructed and conserved by our laboratory.
Hepa1-6 cell cycle and apoptosis assay in
vitro
Hepa1-6 cells were plated into 6-well plates at
5.0×105 cells/well, overnight. Then, cells were left
untreated or infected with Ad-null, Ad-mIL-12, Ad-HBx and
Ad-HBx-mIL-12. After 48 h, cell cycle profiles (MOI 10) and
apoptosis (MOI 30) were analyzed by flow cytometer (Beckman
Coulter) after propidium iodide (PI) staining or Annexin
V-phycoerythrin/PI staining, respectively.
In vivo studies
Female syngeneic C57BL/6 mice, 6–8 weeks old, were
obtained from Beijing Hua Fu Kang biological technology company and
maintained in pathogen-free conditions. All procedures were
reviewed and approved by the Institute Animal Care and Use
Committee. Mice were challenged subcutaneously with
5×106 Hepa1-6 cells in the right flank. When tumors had
reached the desired size (100 mm3), mice were grouped
randomly (n=10) and treatment was initiated. Ad-null, Ad-HBx,
Ad-mIL-12 and Ad-HBx-mIL-12, diluted in 100 μl sterile 1x PBS
(Sigma-Aldrich), were administered 4 times, 5 days apart,
intratumorally, at the dose of 108 plaque-forming units
(pfu). Tumor dimensions were measured with calipers every 3 days
and tumor volumes were calculated according to the formula: V =
length × width2 × π/6.
Tumor microenvironment analysis
For FACS analysis, we prepared single-cell
suspensions of tumors from untreated or treated mice. Briefly,
tumors were minced using a sterile razor blade and digested with
collagenase I. For extracellular staining of immune markers,
1×106 of freshly prepared cells were stained with
different combinations of fluorochrome-coupled antibodies to CD4,
CD8, CD11b, CD11c, F4/80 and Gr1 (BD Biosciences). Fluorescence
data were collected on FACScalibur and analyzed using cell quest
software (BD Biosciences).
Immunohistochemistry
Three days after the completion of treatment, the
mice were sacrificed for histological analysis. The tissues were
fixed in 4% paraformaldehyde. Primary tumors were embedded in
paraffin and cut into 3–5 μm sections. The apoptotic cells within
the tumor sections were evaluated by TUNEL staining, using DeadEnd™
Fluorometric TUNEL System (Promega). Apoptosis index was determined
by counting the number of apoptotic cells and dividing by the total
number of cells in the field (5 high power fields/slide). Frozen
tumor tissues were stained for the quantification of microvessel
density (MVD) using a monoclonal rabbit anti-mouse
CD31-phcoerythrin conjugate (Santa Cruz Biotechnology, Inc.).
Toxicity evaluation
To investigate potential side effects or toxicity in
the treated mice, they were observed continuously for relevant
indexes such as weight loss, ruffled fur, diarrhea, anorexia,
cachexia, skin ulceration or toxic deaths. After fixing in 4%
neutral buffered formalin solution more than 24 h, the tissues of
heart, liver, spleen, lung, kidney and brain were embedded in
paraffin. Slices of 3–5 mm were stained with hematoxylin and eosin
(H&E) and observed in double blinded manner.
Statistical analysis
SPSS 17.0 was used for statistical analysis. Data
were expressed as the mean ± SE. The statistical analysis in all
the experiments was performed using one-way analysis of variance
(ANOVA) or unpaired Student’s t-test. P-values <0.05 were
considered significant.
Results
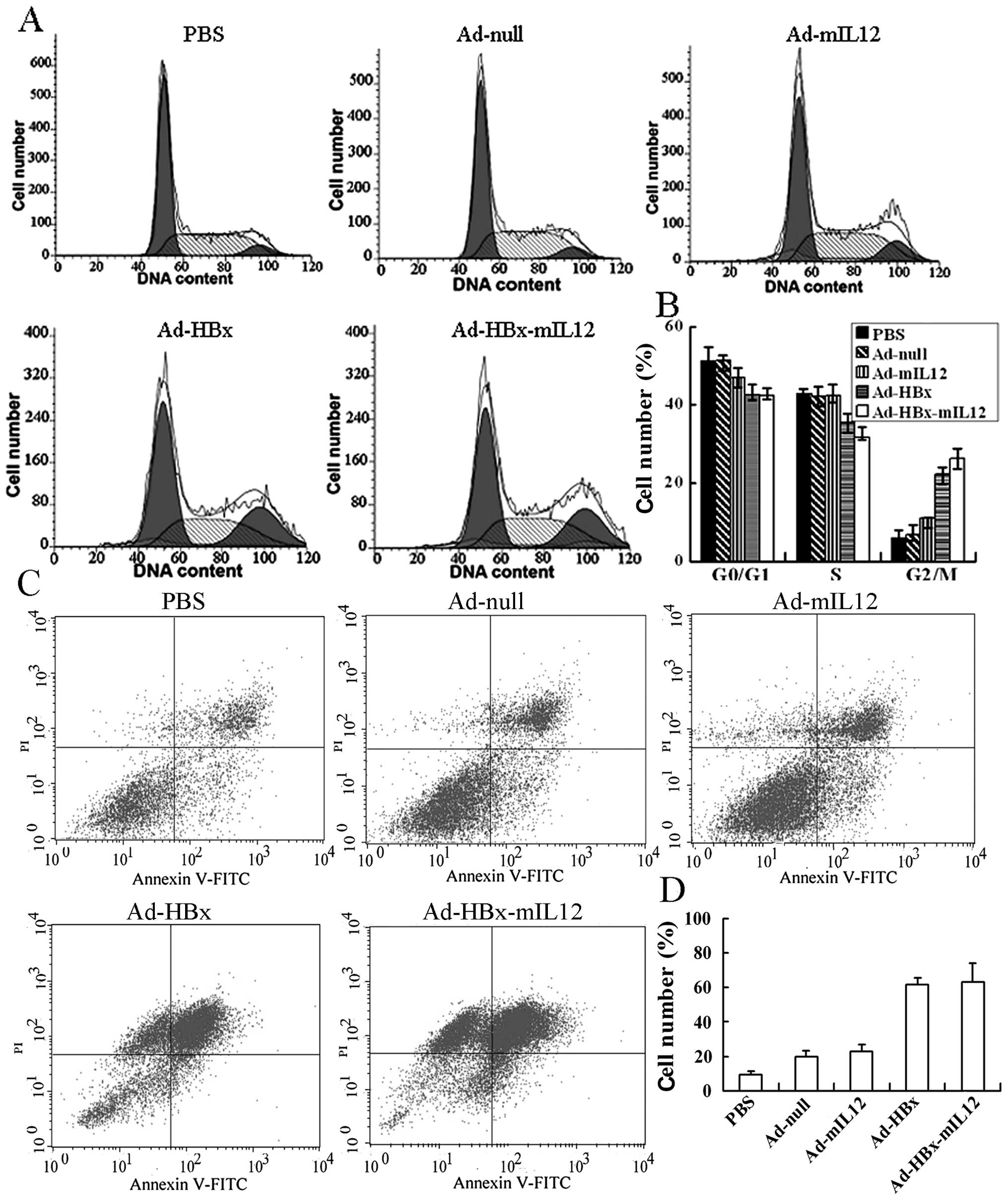
HBx-mIL-12 induces HCC cells to cell
cycle arrest and apoptosis in vitro
Hepa1-6 cells were left untreated or infected by
Ad-null, Ad-mIL-12, Ad-HBx and Ad-HBx-mIL-12. After 48 h, cell
cycle profiles (MOI 10) and apoptosis (MOI 30) were analyzed by
flow cytometer. Results showed that Ad-HBx and Ad-HBx-mIL-12
induced a significant accumulation of cells in G2/M phase
accompanied by a decrease in the percentage of cells in G0/G1 phase
and S phase (Fig. 1A). The
percentages of Ad-HBx and Ad-HBx-mIL-12 infected Hepa1-6 cells in
G2/M phase were 22.25±1.8% and 26.15±2.1%, respectively, and higher
than those of untreated, Ad-null and Ad-mIL-12 infected cells
(P<0.01) (Fig. 1B). With
increasing MOI, flow cytometric analysis revealed that Ad-HBx and
Ad-HBx-mIL-12 infected cells underwent significant apoptosis
(Fig. 1C). The percentages of
apoptosis in Ad-HBx and Ad-HBx-mIL-12 infected groups were
63.17±5.2% and 61.68±3.1%, respectively, and higher than those of
untreated, Ad-null and Ad-mIL-12 infected groups (P<0.01)
(Fig. 1D). According to our flow
cytometric analysis, the cell cycle arrest and apoptosis in Ad-HBx
and Ad-HBx-mIL-12 infected groups have no significant deviation.
These results indicate that HBx could induce HCC cells to G2/M
arrest and apoptosis, whereas mIL-12 has no function in inducing
cell cycle arrest and apoptosis of HCC cells in vitro.
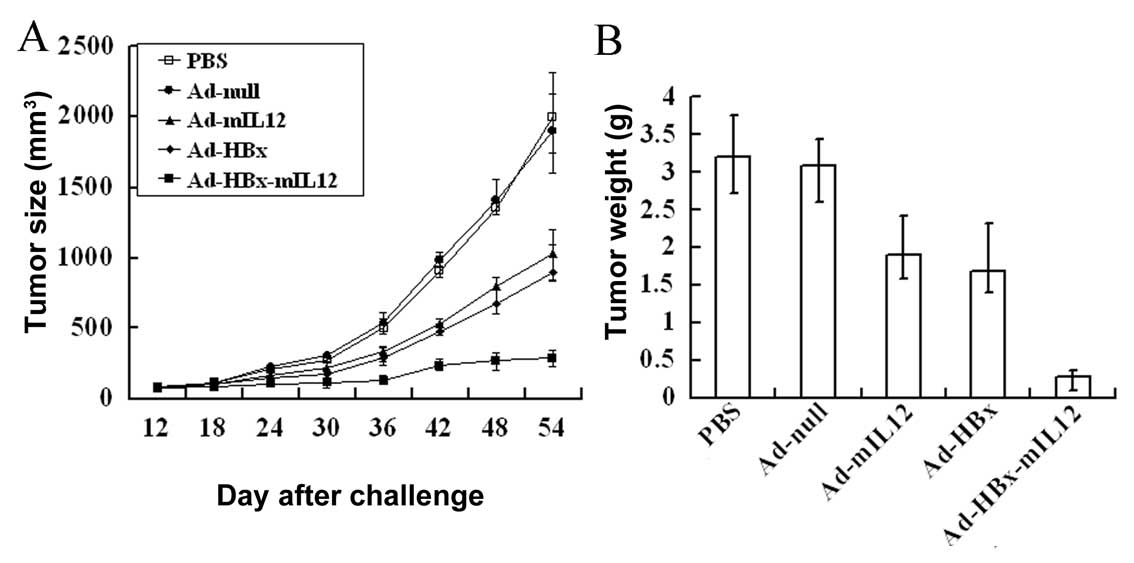
HBx-mIL-12 inhibits the growth of HCC in
vivo
A murine hepatic carcinoma model was established to
investigate the anti-tumor effect of Ad-HBx-mIL-12 in vivo.
When tumors had reached the desired size (100 mm3),
Ad-null, Ad-HBx, Ad-mIL-12, Ad-HBx-mIL-12 or PBS was administered
intratumorally. Alterations in tumor growth were monitored every 3
days (Fig. 2A). The Ad-HBx-mIL-12
group exhibited effective inhibition of tumor growth, compared to
the other groups (P<0.01). On Day 54 after the Hepa1-6 cells
were challenged, the mice were sacrificed and the tumor weights
were measured (Fig. 2B). We
observed that treatment with Ad-HBx-mIL-12 resulted in average
tumor weight reductions of 85.6±4.9%, 85.1±2.8%, 75.8±6.1% and
72.8±5.6% compared to PBS, Ad-null, Ad-mIL-12 and Ad-HBx,
respectively (P<0.01). These results indicate that Ad-HBx-mIL-12
can effectively suppress tumor growth.
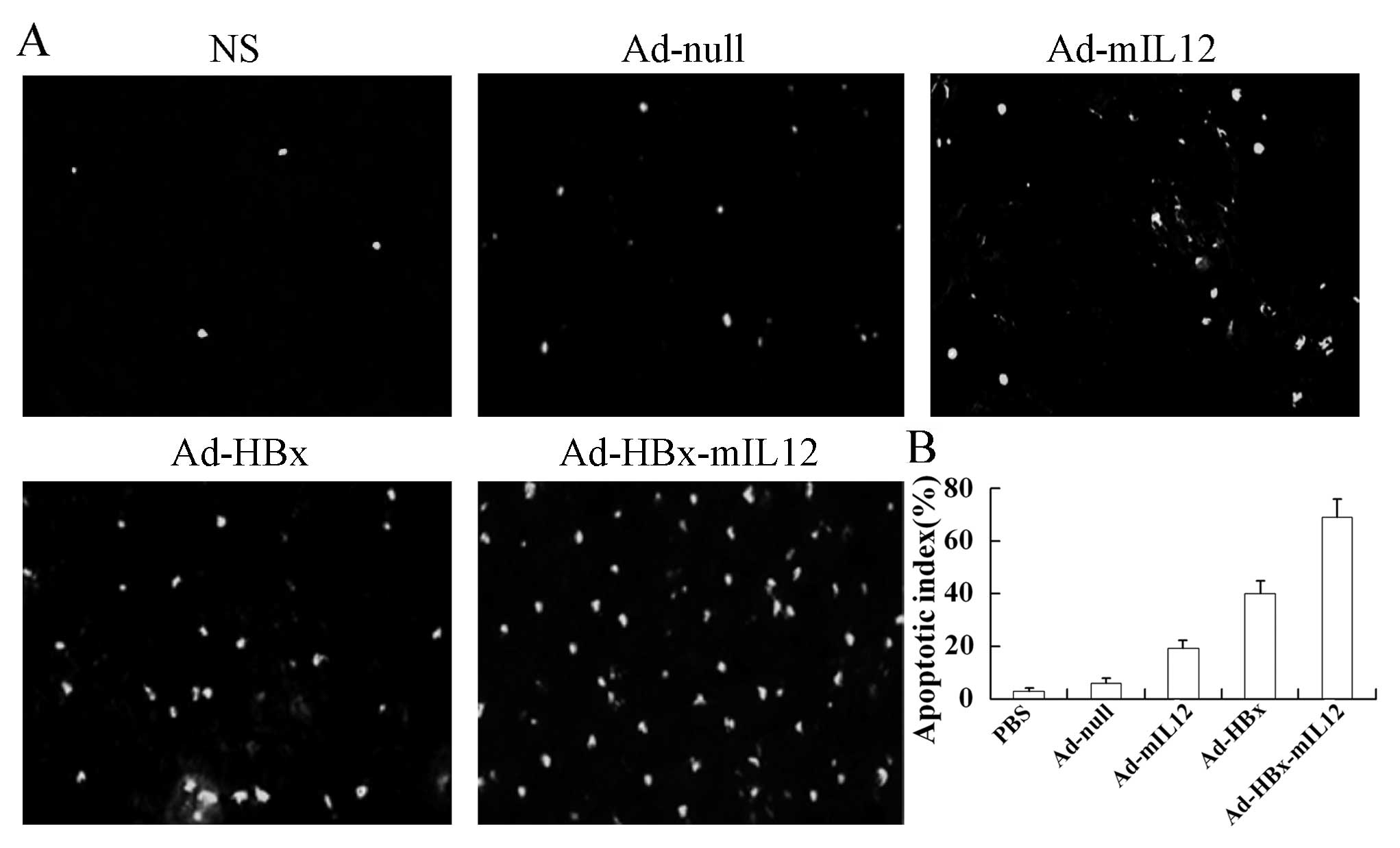
HBx-mIL-12 induces apoptosis of HCC cells
in vivo
In order to estimate the apoptosis in tumor tissues,
TUNEL staining was applied to the tumor sections. We observed more
apoptotic cells in the tumor sections of mice treated with
Ad-HBx-mIL-12 than with PBS, Ad-null, Ad-mIL-12 and Ad-HBx
(Fig. 3A). The apoptosis index
also showed that the Ad-HBx-mIL-12 displayed the highest indices
among all the groups (Fig.
3B).
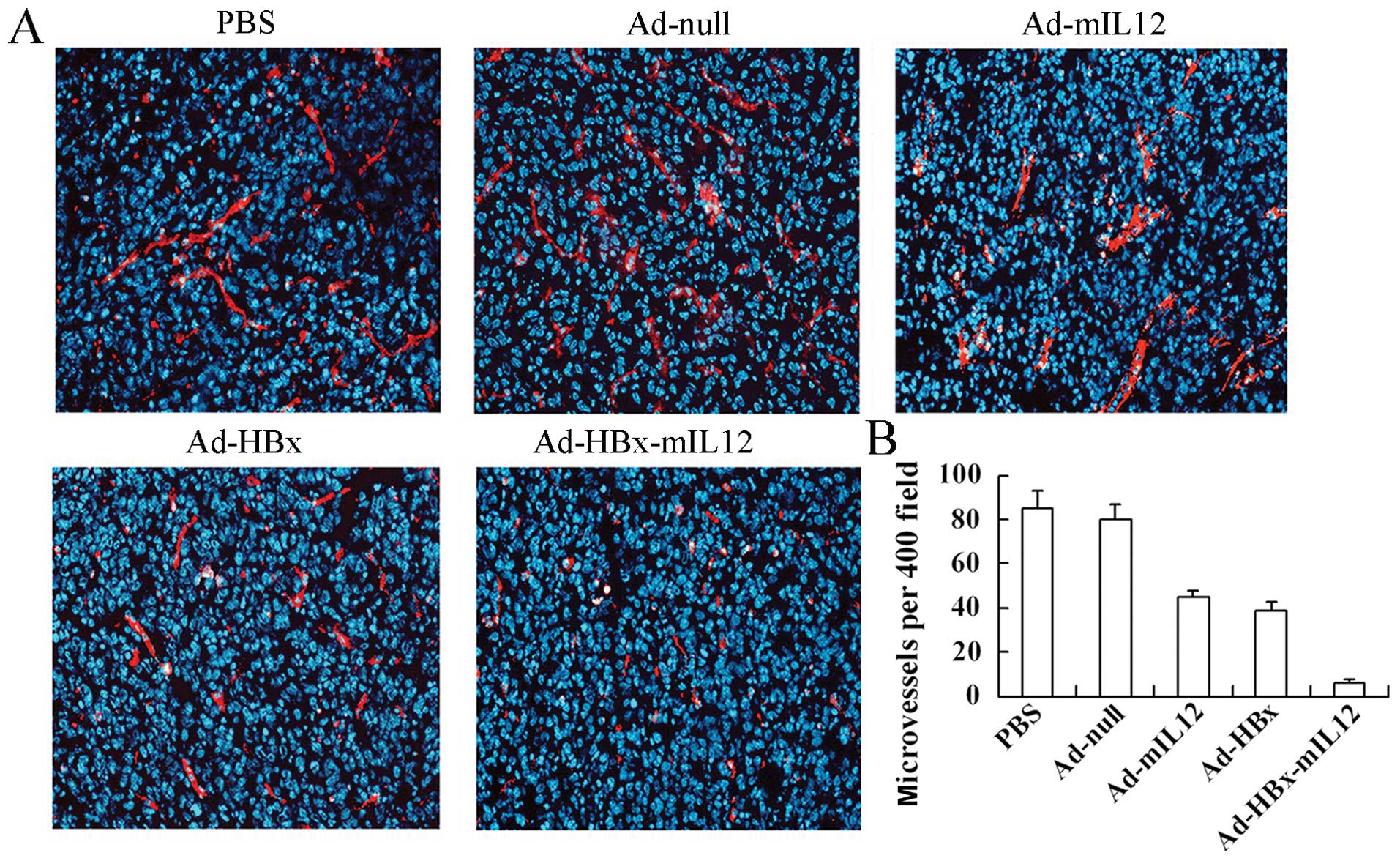
HBx-mIL-12 inhibits vascular endothelial
cell growth in vivo
We further investigated whether Ad-HBx-mIL-12 also
had inhibitory effects on tumor-stromal cells besides HCC cells
within treated tumor tissue. Angiogenic blood vessels within tumor
tissue were detected by CD31 immunohistochemistry. The frozen
sections were stained for angiogenic blood vessels using a
monoclonal rabbit anti-mouse CD-31-phcoerythrin conjugate and
counterstained with DAPI (Fig.
4A). Tumors of the control groups (including PBS, Ad-null,
Ad-mIL-12 and Ad-HBx groups) showed larger microvessel count than
those of Ad-HBx-mIL-12 group (Fig.
4B).
HBx-mIL-12 causes a significant increase
in tumor-infiltrating CD8+ T-cell
To further assess the role of immune modulation in
the observed antitumor effects mediated by Ad-HBx-mIL-12 treatment,
we analyzed the modulation of tumor microenvironment. The ratio of
T cells within the tumor microenvironment is considered indicative
of the effect of adaptive immune responses on tumor progression and
metastasis (19,20). We investigated the subsets of
tumor-infiltrating T-cell populations by staining with anti-CD4-PE
and CD8-fluorescein isothiocyanate. Although Ad-HBx-mIL-12
treatment did not induce significant change in the ratio of
CD4+ T-cells, we observed an increase in the
infiltration of total CD8+ T cells in the tumor stroma,
as shown by flow cytometric analysis (Fig. 5A). These effects probably result
from HBx and mIL-12 mediating host CTL response.
HBx-mIL-12 induces an increase in
tumor-infiltrating DCs and macrophages
It has been documented that tumor-infiltrating
dendritic cells (DC) and macrophages are potentially able to induce
an antitumor innate and adaptive immune response (9). We therefore assessed DCs
(CD11b+CD11c+) and tumor-infiltrating
macrophage (F4/80+CD11b+) populations by flow
cytometry, which showed an increase in DCs and macrophages in
tumors after local treatment with Ad-HBx-mIL-12 and Ad-mIL-12
(Fig. 5B and C). However, the
increased ratios of DCs and macrophages in Ad-mIL-12 and
Ad-HBx-mIL-12 treated tumors have no significant deviation. The
numbers of tumor-infiltrating DCs and macrophages in Ad-HBx and
Ad-null treated tumors have no significant increase compared with
that in PBS group. These results indicate that the increased ratios
of tumor-infiltrating DCs and macrophages potentially result from
IL-12 mediating the immune responses.
HBx-mIL-12 has no impact on the
percentage of MDSCs in tumor microenvironment
Myeloid-derived suppressor cells (MDSCs) in the
tumor microenvironment produce growth factors and angiogenic
factors critical for tumor progression (20,21). Thus, a large number of MDSCs in
tumor microenvironment have probably suppressive actions in the
treatment of tumor. We therefore assessed MDSCs
(Gr1+CD11b+) populations by flow cytometry,
which showed the percentage of MDSCs in Ad-HBx-mIL-12 treated
tumors did not have significant changes compared with control
groups (including PBS, Ad-null, Ad-mIL-12 and Ad-HBx groups)
(Fig. 5D). These result indicate
that Ad-HBx-mIL-12 have no effect on decreasing the percentage of
MDSCs in tumor microenvironment.
Discussion
In the present study, we demonstrated that
HBx-mIL-12 has more anti-tumor effect when compared with HBx or
IL-12, as shown in our previous studies (4). In vitro, we found that
Hepa1-6 cells infected with Ad-HBx and Ad-HBx-mIL-12 underwent G2/M
arrest and apoptosis. However IL-12 did not affect the function of
HBx. In vivo, we established a mouse tumor model and
performed intratumoral injection of Ad-HBx-mIL-12. We found that
although IL-12 did not affect the function of HBx in vitro,
combination of IL-12 with HBx induced higher apoptotic effect than
HBx or IL-12 in vivo. Combination of IL-12 with HBx led to
an almost complete eradication of HCC. The anti-tumor mechanisms of
HBx-mIL-12 are due not only to induction of cell cycle arrest and
apoptosis in HCC cells, but also effectively shift the tumor
microenvironment from pro-oncogenic to anti-tumor.
Although tumors are capable of autonomous growth,
their progression is highly influenced by their stromal component
(22). T cells,
tumor-infiltrating DC, tumor-associated macrophages and myeloid
cells are critical components of the tumor microenvironment and the
cells may exert either inhibiting or promoting effects on tumor
growth (23) through
participating innate and adaptive immunity (21,24,25). The ratio of T cells within the
tumor microenvironment is considered indicative of the effect of
adaptive immune responses on tumor progression and metastasis
(19). Abundant CD8+
T-cell infiltration in the tumor stroma is associated with a
favorable course of malignancy in cancer (26–29). Previous studies have demonstrated
that HBx could induce infiltration of CD8+ T lymphocytes
in HBx-positive tumors (11). In
addition, IL-12 can induce an efficient antitumor CD8+
CTL response which is regarded as the major mediators of the
natural host response against developing tumors (30,31). Thus, there is a hypothesis that
IL-12 can enhance CD8+ CTL response elicited by HBx. In
our study, increased T cell infiltration, especially
CD8+ cells, can be detected in tumor tissue of mice
treated with Ad-HBx-mIL-12 compared to control groups. This
observation is consistent with other studies, indicating that a
higher infiltration CD8+ ratio within the tumor
microenvironment has been correlated with a favorable response to
the treatment, as compared with unchanged ratios in
non-responders.
Many studies have definitively shown that
recruitment of various antigen-presenting cells, such as dendritic
cells (DCs) and macrophages, within tumors can initiate a cascade
of innate, adaptive or humoral immune responses against growing
tumors (20,32,33). Our study showed an increase in the
infiltration of DCs and macrophages in the tumor stroma, after
local treatment with Ad-HBx-mIL-12 and Ad-mIL-12. However, the
increased ratios of DCs and macrophages in Ad-mIL-12 and
Ad-HBx-mIL-12 treated tumors have no significant deviation. These
results indicate that the increased ratios of tumor-infiltrating
DCs and macrophages potentially result from IL-12 mediating the
immunological reaction. Indeed, our findings support the view that
IL-12, a key cytokine in cancer immunoprevention, seems to induce
infiltration of activated DCs and macrophages to tumor and
different organs (34,35).
In our study, treatment with Ad-HBx-mIL-12 reduced
the number of angiogenic blood vessels within tumor tissues
compared with control group. Studies have shown that MDSCs in the
tumor microenvironment produce growth factors and angiogenic
factors critical for tumor progression (21). However, the percentage of MDSCs in
treated tumors did not decrease compared with control groups. The
anti-angiogenic property of IL-12 was well documented in different
in vitro and in vivo studies (36,37). In our previous study, treatment
with Ad-HBx apparently reduced the number of angiogenic blood
vessels within HCC tumor tissue (4). Thus, the mechanism of the
anti-angiogenic effect of Ad-HBx-mIL-12 in the present study may
result from IL-12 and HBx mediated the anti-angiogenic effects.
In our study, treatment with Ad-HBx-mIL-12 not only
induced cell cycle arrest and apoptosis in HCC cells, also induced
a massive accumulation of immune cells (CD8+ T
leukocytes, macrophages and DC) and significant reduction of
vascular endothelial cell growth tumors in situ. HBx-mIL-12
could shift the tumor microenvironment from pro-oncogenic to
antitumor not only through recruitment immune cells but also
inhibiting stromal cell growth. Collectively, our data in the
present study suggest that local delivery of Ad-HBx-mIL-12 could
result in antitumor effects via inhibition of hepatoma cell growth
and intervention of tumor microenvironment.
Acknowledgements
This study is supported by the
National Natural Science Foundation of China (Grant no. 81101728),
the National Science and Technology Major Projects of New Drugs
(Grant no. 2012ZX09103301-036), the National Science and Technology
Major Project for Infectious Diseases Control (Grant no.
2012ZX10002014-004) and the Research Fund for the Doctoral Program
of Higher Education of China (Grant no. 20110181120086).
References
|
1.
|
RP BeasleyRocks along the road to the
control of HBV and HCCAnn
Epidemiol19231234200910.1016/j.annepidem.2009.01.01719344859
|
|
2.
|
K OkudaHepatocellular carcinomaJ
Hepatol32225237200010.1016/S0168-8278(00)80428-6
|
|
3.
|
H TangN OishiS KanekoS MurakamiMolecular
functions and biological roles of hepatitis B virus x proteinCancer
Sci97977983200610.1111/j.1349-7006.2006.00299.x16984372
|
|
4.
|
P ChengY LiL YangHepatitis B virus X
protein (HBx) induces G2/M arrest and apoptosis through sustained
activation of cyclin B1-CDK1 kinaseOncol Rep22110111072009
|
|
5.
|
T TianKJ NanSH WangPTEN regulates
angiogenesis and VEGF expression through phosphatase-dependent and
-independent mechanisms in HepG2
cellsCarcinogenesis3112111219201010.1093/carcin/bgq08520430845
|
|
6.
|
M FernandezD SemelaJ BruixI ColleM
PinzaniJ BoschAngiogenesis in liver diseaseJ
Hepatol50604620200910.1016/j.jhep.2008.12.011
|
|
7.
|
L LiuY CaoC ChenSorafenib blocks the
RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor
cell apoptosis in hepatocellular carcinoma model PLC/PRF/5Cancer
Res661185111858200610.1158/0008-5472.CAN-06-137717178882
|
|
8.
|
X ChenX LinJ ZhaoA tumor-selective
biotherapy with prolonged impact on established metastases based on
cytokine gene-engineered MSCsMol
Ther16749756200810.1038/mt.2008.318362930
|
|
9.
|
C GuiducciAP VicariS SangalettiG
TrinchieriMP ColomboRedirecting in vivo elicited tumor infiltrating
macrophages and dendritic cells towards tumor rejectionCancer
Res6534373446200515833879
|
|
10.
|
E ChunJ LeeHS CheongKY LeeTumor
eradication by hepatitis B virus X antigen-specific CD8+
T cells in xenografted nude miceJ
Immunol17011831190200310.4049/jimmunol.170.3.118312538674
|
|
11.
|
Y LiP ChengY WenT lymphocyte responses
against hepatitis B virus-related hepatocellular carcinoma induced
by adenovirus vaccine encoding HBxInt J Mol Med268698762010
|
|
12.
|
FX DingF WangYM LuMultiepitope
peptide-loaded virus-like particles as a vaccine against hepatitis
B virus-related hepatocellular
carcinomaHepatology4914921502200910.1002/hep.2281619206147
|
|
13.
|
YJ WangY HouH HuangGR LiuAP WhiteSL LiuTwo
oral HBx vaccines delivered by live attenuated Salmonella: both
eliciting effective anti-tumor immunityCancer
Lett2636776200810.1016/j.canlet.2007.12.02218226855
|
|
14.
|
A HombachC HeuserH AbkenSimultaneous
targeting of IL2 and IL12 to Hodgkin’s lymphoma cells enhances
activation of resting NK cells and tumor cell lysisInt J
Cancer1152412472005
|
|
15.
|
C HalinS RondiniF NilssonEnhancement of
the antitumor activity of interleukin-12 by targeted delivery to
neovasculatureNat
Biotechnol20264269200210.1038/nbt0302-26411875427
|
|
16.
|
LS PengML PenichetSL MorrisonA
single-chain IL-12 IgG3 antibody fusion protein retains antibody
specificity and IL-12 bioactivity and demonstrates antitumor
activityJ Immunol1632502581999
|
|
17.
|
JA HendrzakMJ BrundaAntitumor and
antimetastatic activity of interleukin-12Curr Top Microbiol
Immunol21365831996
|
|
18.
|
G TrinchieriImmunobiology of
interleukin-12Immunol Res17269278199810.1007/BF02786451
|
|
19.
|
Y NasuCH BangmaGW HullCombination gene
therapy with adenoviral vector-mediated HSV-tk+ GCV and
IL-12 in an orthotopic mouse model for prostate cancerProstate
Cancer Prostatic Dis44451200110.1038/sj.pcan.450049412497062
|
|
20.
|
CM CouglinKE SalhangM
WysockaInterleukin-12 and interleukin-18 synergistically induce
murine tumor regression which involves inhibition of angiogenesisJ
Clin Invest10114411452199810.1172/JCI15559502787
|
|
21.
|
BM PützerM HittWJ MullerInterleukin 12 and
B7-1 co-stimulatory molecule expressed by an adenovirus vector act
synergistically to facilitate tumor regressionProc Natl Acad Sci
USA94108891089519979380730
|
|
22.
|
JD BuiR UppaluriCS HsiehRD
SchreiberComparative analysis of regulatory and effector T cells in
progressively growing versus rejecting tumors of similar
originsCancer
Res6673017309200610.1158/0008-5472.CAN-06-055616849580
|
|
23.
|
M KortylewskiP SwiderskiA HerrmannIn vivo
delivery of siRNA to immune cells by conjugation to a TLR9 agonist
enhances antitumor immune responsesNat
Biotechnol27925932200910.1038/nbt.156419749770
|
|
24.
|
M KujawskiM KortylewskiH LeeA HerrmannH
KayH YuStat3 mediates myeloid cell-dependent tumor angiogenesis in
miceJ Clin Invest11833673377200810.1172/JCI3521318776941
|
|
25.
|
LM CoussensZ WerbInflammation and
cancerNature420860867200210.1038/nature0132212490959
|
|
26.
|
A MantovaniS SozzaniM LocatiP AllavenaA
SicaMacrophage polarization: tumor-associated macrophages as a
paradigm for polarized M2 mononuclear phagocytesTrends
Immunol23549555200210.1016/S1471-4906(02)02302-512401408
|
|
27.
|
PC RodriguezMS ErnstoffC HernandezArginase
I-producing myeloid-derived suppressor cells in renal cell
carcinoma are a subpopulation of activated granulocytesCancer
Res6915531560200910.1158/0008-5472.CAN-08-192119201693
|
|
28.
|
LA NorianPC RodriguezLA
O’MaraTumor-infiltrating regulatory dendritic cells inhibit
CD8+ T cell function via L-arginine metabolismCancer
Res6930863094200910.1158/0008-5472.CAN-08-282619293186
|
|
29.
|
SJ PiersmaES JordanovaMI van PoelgeestHigh
number of intraepithelial CD8+ tumor-infiltrating
lymphocytes is associated with the absence of lymph node metastases
in patients with large early-stage cervical cancerCancer
Res67354361200717210718
|
|
30.
|
AG MenonGJ FleurenEA AlphenaarA basal
membrane-like structure surrounding tumour nodules may prevent
intraepithelial leucocyte infiltration in colorectal cancerCancer
Immunol Immunother521211262003
|
|
31.
|
E SatoSH OlsonJ AhnIntraepithelial
CD8+ tumor-infiltrating lymphocytes and a high
CD8+/regulatory T cell ratio are associated with
favorable prognosis in ovarian cancerProc Natl Acad Sci
USA10218538185432005
|
|
32.
|
L ZhangJR Conejo-GarciaD
KatsarosIntratumoral T cells, recurrence, and survival in
epithelial ovarian cancerN Engl J
Med348203213200310.1056/NEJMoa02017712529460
|
|
33.
|
O SimmaE ZebedinN NeugebauerIdentification
of an indispensable role for tyrosine kinase 2 in CTL-mediated
tumor surveillanceCancer
Res69203211200910.1158/0008-5472.CAN-08-170519118004
|
|
34.
|
MN TorreroX XiaW HenkS YuS LiStat1
deficiency in the host enhances interleukin-12-mediated tumor
regressionCancer
Res6644614467200610.1158/0008-5472.CAN-05-355416618773
|
|
35.
|
H KanzlerFJ BarratEM HesselRL
CoffmanTherapeutic targeting of innate immunity with Toll-like
receptor agonists and antagonistsNat
Med13552559200710.1038/nm158917479101
|
|
36.
|
W BarchetV WimmenauerM SchleeG
HartmannAccessing the therapeutic potential of immunostimulatory
nucleic acidsCurr Opin
Immunol20389395200810.1016/j.coi.2008.07.00718652893
|
|
37.
|
CS TannenbaumN WickerD ArmstrongCytokine
and chemokine expression in tumors of mice receiving systemic
therapy with IL-12J Immunol15669369919968543822
|
|
38.
|
S AdrisE ChuluyanA BravoMice vaccination
with interleukin 12-transduced colon cancer cells potentiates
rejection of syngeneic non-organ-related tumor cellsCancer
Res6066966703200011118055
|
|
39.
|
CL NastalaHD EdingtonTG
McKinneyRecombinant IL-12 administration induces tumor regression
in association with IFN-gamma productionJ
Immunol1531697170619947913943
|
|
40.
|
AD LusterP LederIP-10, a -C-X-C-
chemokine, elicits a potent thymus-dependent antitumor response in
vivoJ Exp Med17810571065199310.1084/jem.178.3.10578350046
|