Introduction
Vascular smooth muscle cell (VSMC) proliferation
plays a key role in the pathogenesis of vascular diseases,
including arteriosclerosis and restenosis after vein grafting or
coronary intervention (1–3). Consequently, antiproliferative
strategies have been employed to successfully prevent the
development of vascular proliferative disease. Injury causes the
release of multiple growth factors and cytokines that stimulate
cell proliferation via multiple signalling mechanisms (4). Following angioplasty,
platelet-derived growth factor (PDGF) has been shown to be an
important signal that contributes to the initiation of the early
cellular response to injury. Therefore, the identification of novel
compounds that inhibit PDGF-dependent cell proliferation have the
potential to improve existing therapeutic strategies and limit late
cardiovascular complications, such as in-stent restenosis and
bypass graft failure (5).
Gastrodia elata Bl, a traditional herbal
medicine, has been widely used in China and Japan for thousands of
years (6). Gastrodin
(p-hydroxymethylphenyl-β-D-glucopyranoside) is the main bioactive
component of Gastrodia elata Bl, and has been widely used
clinically for the treatment of neurasthenia, dizziness, epilepsy,
migraine, headache and dementia. Previous studies have shown that
gastrodin has neuroprotective pharmacological effects (7). Gastrodin protects against
hypoxia-induced toxicity in primary cultures of rat cortical
neurons (8), rescues impairments
of synaptic plasticity induced by lead in the rat hippocampus
(9), suppresses the accumulation
of calcium in PC12 cells induced by high glucose treatment and
decreases cell apoptosis in the PC12 cell line following I/R injury
in vitro (10), and it
improves learning behaviour in a rat model of Alzheimer’s disease
induced by intra-hippocampal Aβ 1–40 injection (11). However, little is known about the
effects of gastrodin on cardiovascular diseases and neointima
formation, and the workings of the related signalling mechanisms
remain unclear. Therefore, we addressed whether gastrodin
attenuates VSMC proliferation induced by PDGF-BB in vitro
and/or neointima formation in the carotid artery following wire
injury in vivo.
Materials and methods
Materials
Gastrodin was purchased from Sigma-Aldrich (St.
Louis, MO, USA). Recombinant mouse PDGF-BB was purchased from
ProSpec (Rehovot, Israel). The antibodies used to detect the total
levels and the phosphorylation of ERK1/2, p38, AKT, GSK-3β and
STAT3 were obtained from Cell Signaling Technology (Danvers, MA,
USA). Antibodies specific for p27Kip1, glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) and proliferating cell nuclear antigen (PCNA)
were also purchased from Cell Signaling Technology. Anti-SM22α and
anti-smooth muscle-α-actin (SMA) were purchased from Abcam
(Cambridge, MA, USA). Anti-desmin and anti-smoothelin were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Complete protease inhibitor, PhosSTOP, Cell Proliferation
ELISA, 5-bromo-2′-deoxyuridine (BrdU) (colorimetric) and Cell
Proliferation Reagent WST-1 kits were purchased from Roche
Diagnostics GmbH (Mannheim, Germany). Other reagents were purchased
from Sigma-Aldrich, except where specified. For the in vitro
studies, gastrodin was dissolved in phosphate-buffered saline
(PBS), and PBS alone served as a control.
Cell culture
Rat VSMCs were isolated enzymatically from the
thoracic aortas of Sprague-Dawley rats. These cells were cultured
in DMEM/F12 medium containing 10% fetal bovine serum and were
identified as VSMCs by smooth muscle-α-actin (SMA) immunostaining,
as previously described (12,13). VSMCs were grown to 60–80%
confluence and were serum-starved for 24 h. Three independent
experiments were analysed for all data shown. VSMCs from passages 5
to 12 were used for the experiments in this study.
Cell proliferation and DNA synthesis
assay
VSMCs (5×103/well) were seeded in a
96-well microplate, grown to 60% confluence and serum-starved for
24 h. Following preincubation with gastrodin for 1 h, the cells
were treated with PDGF-BB (20 ng/ml) for 48 h. Cell proliferation
and DNA synthesis were assessed using commercial non-radioactive
colorimetric WST-1 and BrdU incorporation assay kits (Roche)
according to the manufacturer’s instructions. The cell
proliferation reagent WST-1 was used to measure the accumulation of
the number of viable VSMCs based on the cleavage of tetrazolium
salts incubated in the culture medium. DNA synthesis in VSMCs was
assessed by the incorporation of BrdU.
Flow cytometric analysis of cell cycle
distribution
Cells were incubated with propidium iodide (PI)
staining buffer and were then analysed using a flow cytometer
(FACScan; BD Biosciences, Franklin Lakes, NJ, USA). G0/G1, S and
G2/M cell percentages were counted using the Multicycle AV software
(Phoenix Flow Systems, San Diego, CA, USA).
Western blotting
VSMCs were treated with gastrodin (200 μg/ml) for 2
h prior to incubation with 20 ng/ml PDGF-BB for the indicated time.
The VSMCs were lysed in RIPA buffer with a protease cocktail and a
phosphatase cocktail (Roche). Cell extracts were used for SDS-PAGE
and were then transferred to Immobilon-FL transfer membranes
(Millipore) and probed with various antibodies. The protein bands
were then incubated with a secondary IRDye®
800CW-conjugated antibody and detected with an Odyssey Imaging
System.
Carotid artery wire injury model
All animal experimental protocols were performed
according to institutional guidelines on animal welfare and were
approved by the Ethics Committee at Renmin Hospital of Wuhan
University. Eight-week-old male C57/BL6 mice were fed normal rodent
chow or chow containing 0.09% gastrodin (w/w) for 14 days prior to
wire injury. With this chow, the mice were fed ∼150 mg of
gastrodin/kg/day. For the wire injury surgery, the mice were
anesthetised with an intraperitoneal injection of sodium
pentobarbital (90 mg/kg). The left carotid artery was exposed and
looped on the external branch using an 8-0 silk suture. An
angiotomy was performed in the internal carotid artery, and a
straight metal guide wire (0.38 mm in diameter, C-SF-15-15; Cook,
Bloomington, IN, USA) was inserted toward the aortic arch and then
passed forward and withdrawn 5 times with a rotating motion, as
previously described (14). The
contralateral carotid artery served as a control. The mice were
maintained on their respective chow until they were euthanised and
processed for morphological studies at the indicated time points
following the initial surgery.
Histological and morphometric
analyses
The carotid arteries were harvested 28 days
post-injury. The animals were euthanised with an intraperitoneal
injection of excessive sodium pentobarbital. Subsequently, the
arteries were removed, fixed with 4% paraformaldehyde in PBS for at
least 16 h and paraffin-embedded without further dissection. Serial
sections (5 μm) were created across the site of injury, ∼300 μm
from the branches of the left carotid artery. The areas of the
intima and media were measured in H&E-stained sections in a
blinded manner by a single observer using Image-Pro Plus 6.0
software (Media Cybernetics). A mean value was determined after
assessing at least 4 sections from each mouse. Neointima formation
was defined as the ratio of the intimal area to that of the medial
area (I/M). For the PCNA immunohistochemical analysis, the sections
were preincubated with 5% normal goat serum and then incubated with
anti-PCNA monoclonal antibody (1:100, no. 2586, CST). The sections
were then incubated with a biotinylated secondary
streptavidin-horse-radish peroxidase antibody followed by
diaminobenzidine (DAB kit; Dako), after which the sections were
counterstained with haematoxylin. For the negative controls, the
primary antibody was replaced with PBS. The mean value from at
least 3 sections/mouse was used to quantitatively represent the
number of PCNA-positive cells that were present in the injured
vessel walls.
Statistical analysis
The results were expressed as mean ± SEM.
Statistical analysis was performed by one-way analysis of variance
(ANOVA), followed by Dunnett’s multiple comparison tests. A P-value
<0.05 was considered to indicate statistically significant
differences.
Results
Role of gastrodin in VSMC
proliferation
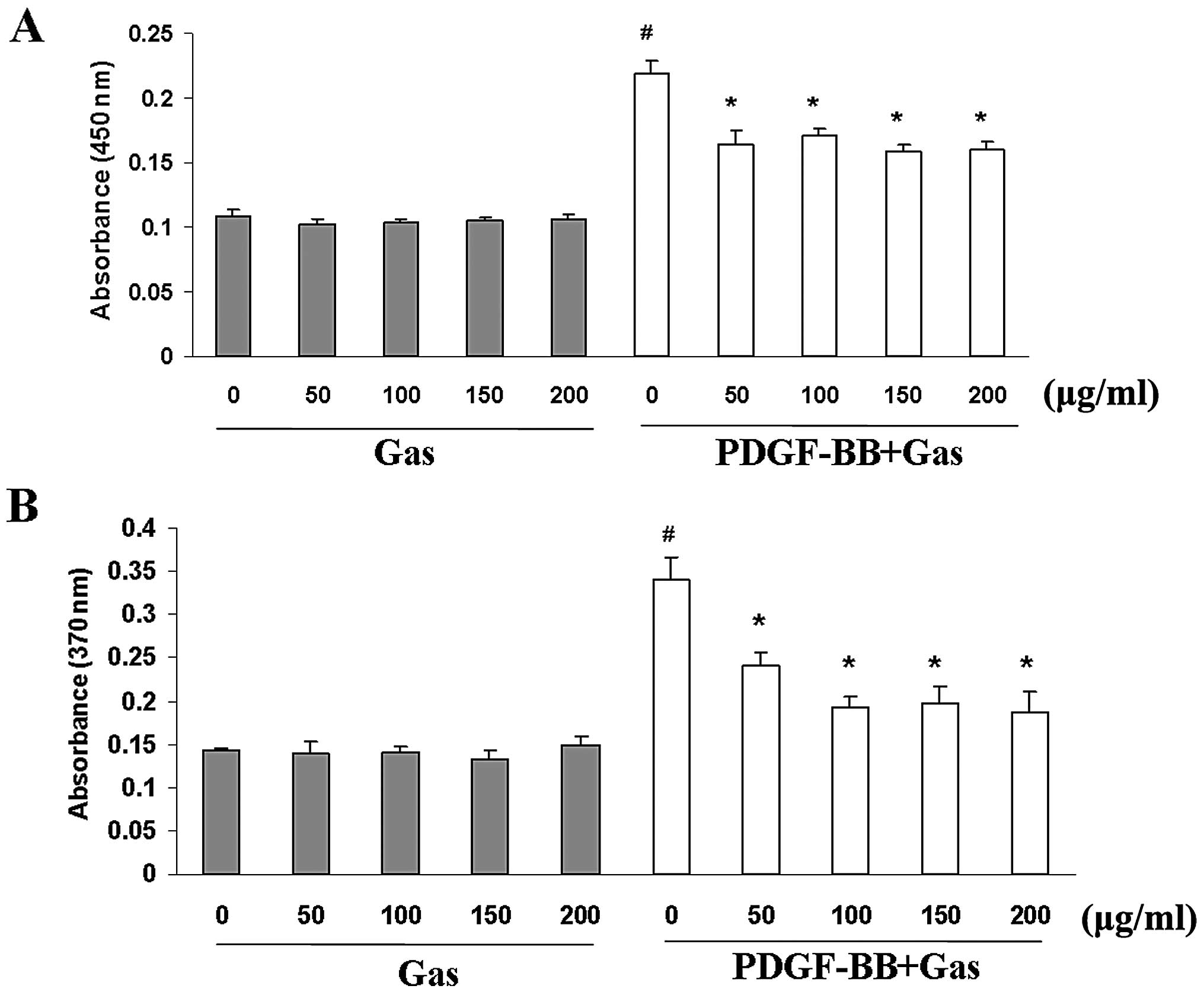
We first investigated the effect of gastrodin on
proliferation using a WST-1 cell proliferation assay. Gastrodin and
PDGF-BB were dissolved in serum-free medium for these experiments.
Quiescent cells were pre-treated with gastrodin (50–200 μg/ml;
Sigma-Aldrich) for 1 h prior to PDGF-BB stimulation (20 ng/ml;
ProSpec). PDGF-BB stimulation increased VSMC proliferation by
∼2.5-fold compared with the control group at 48 h. Gastrodin
markedly inhibited VSMC proliferation in a dose-dependent manner
(Fig. 1A). BrdU incorporation
assays were used to further investigate the effects of gastrodin on
DNA synthesis. Gastrodin significantly inhibited the BrdU
incorporation induced by PDGF-BB stimulation in a dose-dependent
manner (Fig. 1B). Moreover,
gastrodin treatment in the absence of PDGF-BB did not decrease the
viability of the VSMCs or their incorporation of BrdU compared with
the control cells, indicating that gastrodin treatment at these
concentrations has no cytotoxic effects on VSMCs, and that the
inhibitory effect of gastrodin targets DNA synthesis rather than
cytotoxicity to cause the loss of cellular DNA (Fig. 1A).
Role of gastrodin in cell cycle
progression
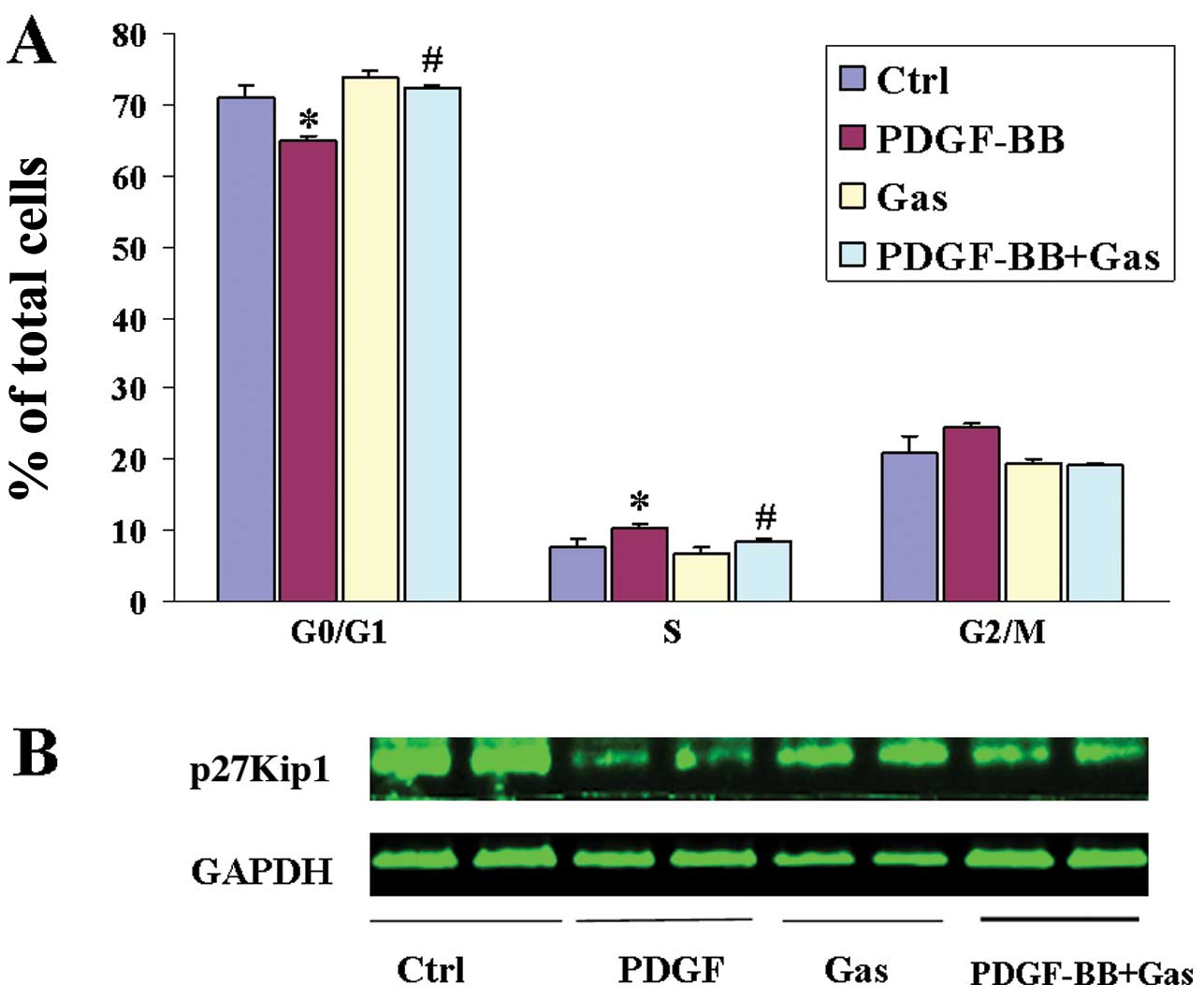
Cell proliferation is controlled by progression
through the cell cycle, which is regulated by many proliferative
signalling cascades. Progression through the cell cycle requires
the activation of cyclin/cyclin-dependent protein kinase (CDK)
complexes, which are regulated by CDK inhibitory proteins (CKIs)
such as p27Kip1. We analysed the effects of gastrodin treatment on
the cell cycle stage distribution of VSMCs. As shown by flow
cytometry, PDGF-BB stimulation significantly increased the
percentage of cells in S-phase and decreased the percentage of
those in the G0/G1 phases, whereas gastrodin (at a concentration of
200 μg/ml) significantly decreased the number of S-phase cells and
increased the fraction of G0/G1-phase cells among the VSMCs
(Fig. 2A). These data indicate
that gastrodin can prevent S-phase entry in VSMCs. We next analysed
the expression of p27Kip1 by western blotting. p27Kip1 was found to
be constitutively expressed in quiescent VSMCs, and its expression
was downregulated following PDGF-BB stimulation. By contrast,
pretreatment with gastrodin markedly restored p27Kip1 expression
(Fig. 2B).
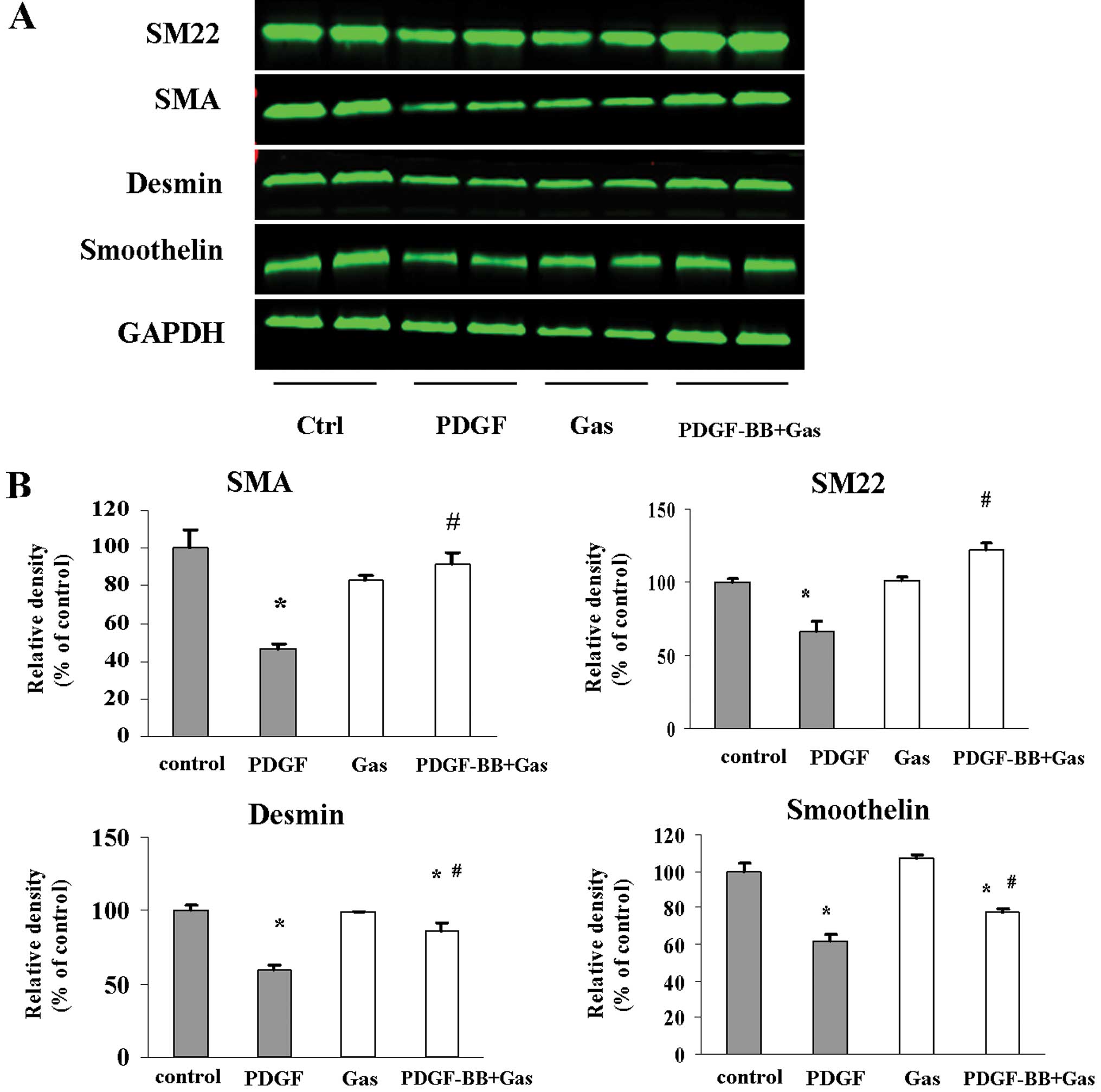
Role of gastrodin in VSMC phenotype
switching
In response to the vascular injury environment,
VSMCs can dedifferentiate into a proliferative phenotype that is
characterised by increased proliferation and decreased expression
of smooth muscle markers, such as SMA, SM22α, desmin and
smoothelin. Thus, we examined whether gastrodin modulates the
phenotype of VSMCs in culture. Following pretreatment with
gastrodin (200 μg/ml) for 2 h, quiescent VSMCs were stimulated with
PDGF (20 ng/ml) for 48 h in the presence/absence of gastrodin.
Western blotting was performed to detect the expression of SMA,
smoothelin, SM22α and desmin. PDGF-BB stimulation reduced the
protein levels of SMA, SM22α and desmin (Fig. 3). However, pretreatment with
gastrodin reversed the repressive effect of PDGF-BB stimulation on
the expression of SM-α-actin, smoothelin and desmin. These data
indicate that gastrodin inhibits the ability of VSMCs to switch
into the proliferative phenotype in vitro.
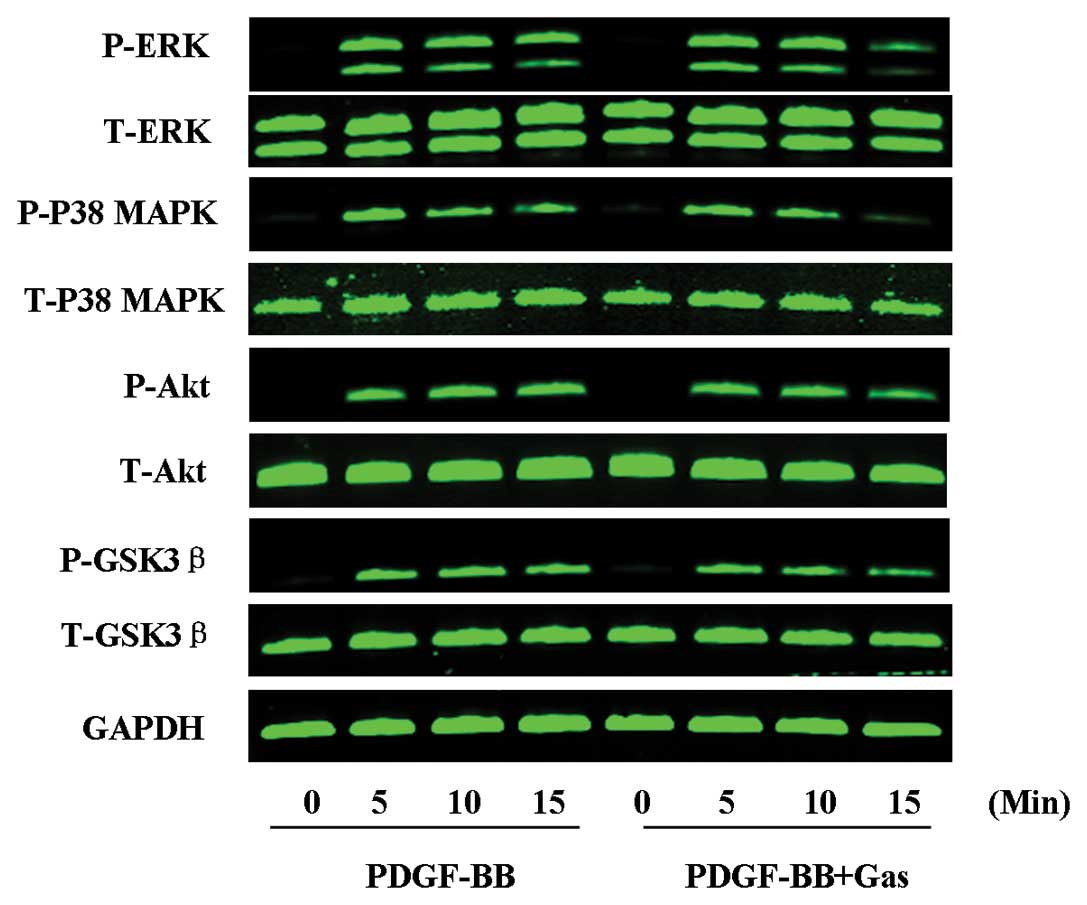
Role of gastrodin in signalling pathways
induced by PDGF-BB
We next examined the signalling pathway(s) involved
in the inhibitory effect of gastrodin on VSMC proliferation in
response to PDGF-BB stimulation. ERK1/2, p38 MAPK, Akt and GSK3β
phosphorylation were each increased at 5 min post-PDGF-BB
treatment, and this effect persisted until 15 min post-PDGF-BB
treatment. Whereas PDGF-BB stimulation induced the phosphorylation
of ERK1/2, p38 MAPK, Akt and GSK3β, these modifications were
significantly inhibited by gastrodin at 15 min post-treatment. The
total protein levels of these signalling molecules did not change
significantly during the course of stimulation with PDGF-BB in the
presence or absence of gastrodin (Fig. 4). These data suggest that the MAPK
and Akt signalling pathways are involved in the suppressive effects
of gastrodin on the proliferation of VSMCs in response to
PDGF-BB.
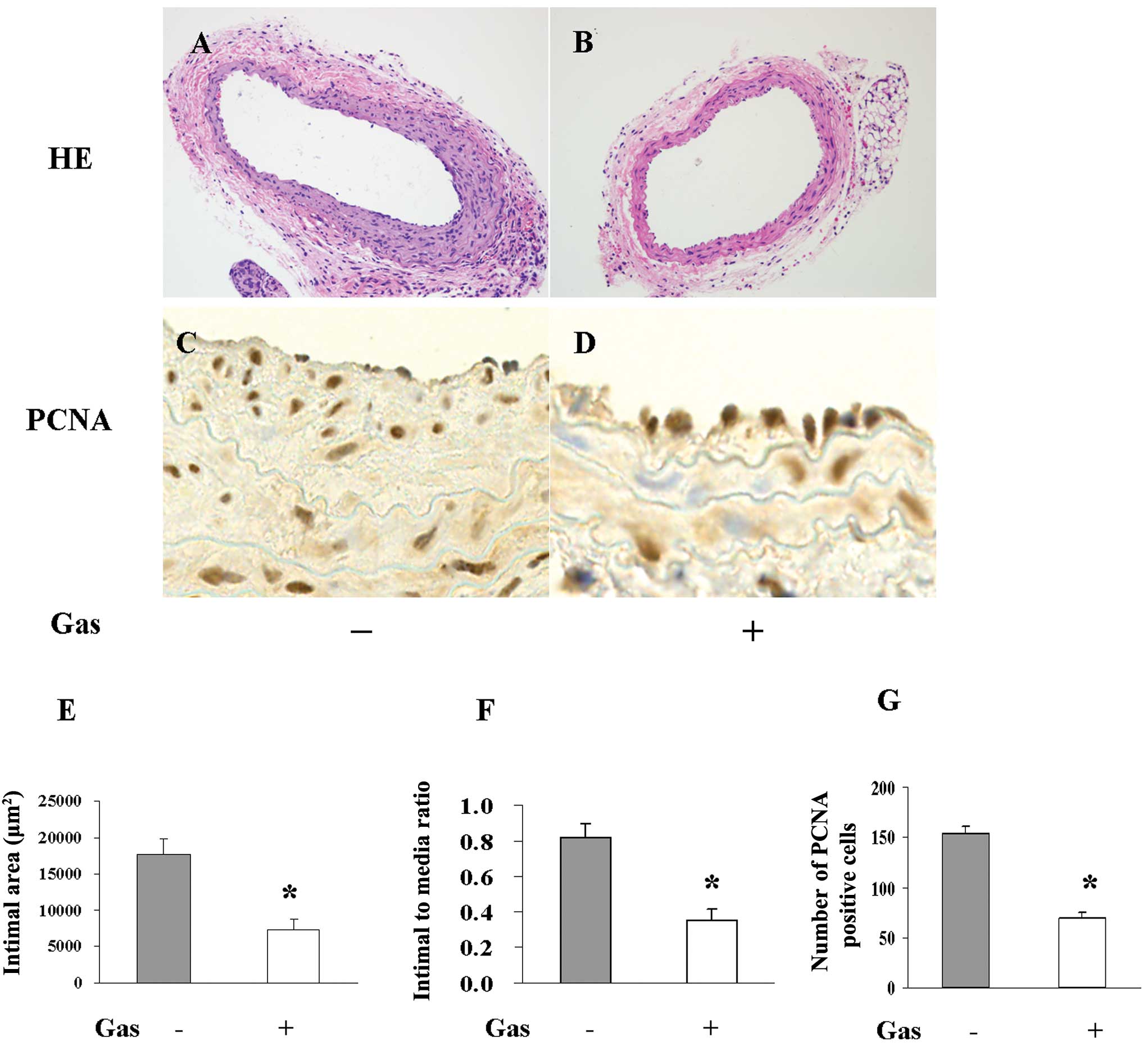
Role of gastrodin in neointima formation
and cell proliferation in vivo
We observed that gastrodin could inhibit the
proliferation of VSMCs, influence their cell cycle progression,
reverse their phenotypic switch and suppress the signalling
pathways induced by PDGF-BB. These findings suggest a potent
therapeutic application for gastrodin against neointima formation
in response to injury. To confirm this hypothesis, the left carotid
arteries of mice were injured with a metal guide wire to induce a
model of neointima hyperplasia. Neointima thickening 28 days
following wire injury was reduced by 50% in the gastrodin chow-fed
group compared to the normal chow-fed group. VSMC proliferation
in vivo was characterised according to immunohistochemical
staining with a PCNA antibody. Gastrodin chow consumption
significantly diminished the proliferation response induced by
arterial injury, as the number of PCNA-positive cells was more than
2-fold greater (P<0.01) in the normal chow-fed group than in the
gastrodin chow-fed group (Fig.
5).
Discussion
The present study demonstrates for the first time
that gastrodin treatment inhibits VSMC proliferation induced by
PDGF-BB in vitro and markedly suppresses neointimal
hyperplasia following wire injury in vivo. These protective
effects may be associated with a wide spectrum of signalling
pathways, including MAPKs and Akt/GSK3β. These observations suggest
that gastrodin may have beneficial effects for protecting against
the neointima formation associated with arteriosclerosis and
restenosis following percutaneous coronary intervention (PCI) and
vein grafting.
Gastrodin, the main bioactive component of
Gastrodia elata Bl, has potent protective effects against
the nervous system and has been commonly prescribed by Chinese
practitioners for the treatment of neurasthenia, dizziness,
epilepsy, migraine, headache and dementia. Although gastrodin
treatment has been shown to have many neuroprotective
pharmacological effects, little is known about the effects of
gastrodin on vascular disease and cell proliferation. Luo et
al (15) reported that
gastrodin could inhibit VSMC proliferation in vitro, as VSMC
numbers and H3-TdR incorporation decreased significantly
in a dose-dependent manner following treatment with gastrodin or an
injection with Gastrodia elata Bl. However, that report did
not determine whether gastrodin could inhibit the VSMC
proliferation induced by mitogens, which is an important component
of the pathological process of various vascular diseases (5,16–18). In the current study, we
demonstrated that gastrodin treatment suppressed the increased cell
number and DNA incorporation resulting from PDGF-BB stimulation.
Moreover, cell proliferation is controlled by a large number of
signalling proteins that regulate the mitotic cell cycle. p27Kip1,
a CDK inhibitory protein (CKI), plays a key role in controlling the
cell cycle in response to various pathophysiological processes
(19–21). In quiescent cells, p27Kip1 is
highly translated and demonstrates stable expression. Upon
mitogenic stimulation, p27Kip1 is rapidly downregulated, which
enables the activation of CDK/cyclin complexes and the subsequent
transcriptional activation of genes required for the G1/S
transition and the initiation of DNA replication (20). However, the effect of gastrodin on
the modulation of the cell cycle was not examined. In this study,
we found that gastrodin can influence the G/S transition of the
cell cycle and stabilise p27Kip1, which is degraded following
PDGF-BB stimulation. In addition to acting as a VSMC mitogen,
PDGF-BB is a potent negative regulator of VSMC differentiation.
VSMCs are remarkably plastic in response to the local environmental
changes induced by vascular injury (22). The dedifferentiation of VSMCs is
associated with dramatically increased cell proliferation,
migration and synthetic capacity, and it plays a critical role in
neointimal hyperplasia. In our study, we found that gastrodin
treatment could restore the suppression of smooth muscle
(SM)-marker gene expression induced by PDGF-BB, indicating that
gastrodin significantly affects the manifestation of the VSMC
phenotype in vitro.
The mechanisms by which gastrodin exerts its
anti-proliferative effects remain unclear. Dai et al
(7) reported that gastrodin could
inhibit the expression of inducible NO synthase, cyclooxygenase-2
and proinflammatory cytokines in cultured LPS-stimulated microglia
via the MAPK pathways. MAPKs have been shown to play important
roles in PDGF-BB-induced cell proliferation in many cell types
(23). It has also been reported
that PDGF-BB induces the phosphorylation of Elk-1 through ERK1/2,
and that phosphorylated Elk-1 competes with SRF for binding to CArG
elements within the promoters of SMC marker genes, such as SM22α
and SMA (24). Therefore, we
investigated the effect of gastrodin treatment on the
phosphorylation of three MAPKs induced by PDGF-BB stimulation in
VSMCs. We observed that the phosphorylation of ERK1/2 and p38 was
rapidly and markedly induced in VSMCs following PDGF-BB
stimulation, whereas gastrodin pretreatment suppressed these
effects, indicating that the inhibition of MAPK signalling is
involved in the anti-proliferative effects of gastrodin.
Previous studies have demonstrated that growth
factors such as PDGF-BB facilitate the phosphorylation of Akt in
VSMCs. Moreover, Akt signalling regulates cell survival and
proliferation in a variety of cell types (25,26). Accordingly, the transfection of a
dominant-negative Akt (DN-Akt) mutant was found to attenuate
hyperplasia through the inhibition of VSMC proliferation and the
reversal of VSMC dedifferentiation (27,28). It is well known that GSK3β is an
Akt substrate and that activated Akt can phosphorylate GSK3β and
decrease its catalytic activity (29). GSK3β phosphorylates cyclin D1 at
T286, which directs its proteolytic degradation. The catalytic
activity of GSK3β is inhibited by Akt-dependent phosphorylation,
and Akt-mediated GSK3β phosphorylation stabilises cyclin D1
(30). Akt activation also
mediates the ubiquitination and degradation of p27Kip1 in response
to mitogen stimulation via the upregulation of SKP2, a key
component of the SCFSKP2 ubiquitin ligase complex.
Moreover, Bonnet et al (31) demonstrated that the inhibition of
Akt/GSK3β/NFAT signalling in response to PDGF could suppress VSMC
proliferation and reverse injury-induced remodelling. In line with
previous reports, we observed a marked increase in Akt activity and
the phosphorylation of its substrate GSK3β in PDGF-BB-stimulated
VSMCs. Furthermore, pretreatment with gastrodin facilitated the
dephosphorylation of Akt and GSK3β, and stabilised p27Kip1. These
results indicate that the Akt/GSK3β signalling pathway is partially
involved in the inhibitory effects of gastrodin treatment on VSMC
proliferation.
In conclusion, this study has clearly demonstrated
that gastrodin treatment attenuates PDGF-BB-induced VSMC
proliferation in vitro and injury-induced neointima
formation in vivo. Gastrodin protects against VSMC
proliferation by preventing the G/S cell cycle transition,
suppressing the VSMC proliferative phenotypic switch and by
inhibiting ERK1/2, p38 and Akt/GSK3β signalling. Thus, we believe
that gastrodin is a strong candidate for effective therapy in the
prevention of vascular proliferative disease.
Acknowledgements
This study was supported by grants
from the Natural Science Foundation of Hubei Province of China (no.
2010CDB06203); the Major Subject of Health Department of Hubei
Province of China (no. JX4A02); the National Natural Science
Foundation of China (no. 81000342); the Special Funds for Basic
Research and Operating Expenses of Central University (no.
4101032)
References
|
1.
|
AC DoranN MellerCA McNamaraRole of smooth
muscle cells in the initiation and early progression of
atherosclerosisArterioscler Thromb Vasc
Biol28812819200810.1161/ATVBAHA.107.15932718276911
|
|
2.
|
GK OwensMS KumarBR WamhoffMolecular
regulation of vascular smooth muscle cell differentiation in
development and diseasePhysiol
Rev84767801200410.1152/physrev.00041.200315269336
|
|
3.
|
H HaoG GabbianiML Bochaton-PiallatArterial
smooth muscle cell heterogeneity: implications for atherosclerosis
and restenosis developmentArterioscler Thromb Vasc
Biol2315101520200310.1161/01.ATV.0000090130.85752.ED12907463
|
|
4.
|
MN BabapulleMJ EisenbergCoated stents for
the prevention of restenosis: Part
ICirculation10627342740200210.1161/01.CIR.0000038982.49640.7012438301
|
|
5.
|
A LevitzkiPDGF receptor kinase inhibitors
for the treatment of restenosisCardiovasc
Res65581586200510.1016/j.cardiores.2004.08.00815664384
|
|
6.
|
CJ BulpittY LiPF BulpittJ WangThe use of
orchids in Chinese medicineJR Soc
Med100558563200710.1258/jrsm.100.12.55818065708
|
|
7.
|
JN DaiY ZongLM ZhongYM LiW ZhangLG BianQL
AiYD LiuJ SunD LuGastrodin inhibits expression of inducible NO
synthase, cyclooxygenase-2 and proinflammatory cytokines in
cultured LPS-stimulated microglia via MAPK pathwaysPLoS
One6e21891201110.1371/journal.pone.002189121765922
|
|
8.
|
X XuY LuX BieProtective effects of
gastrodin on hypoxia-induced toxicity in primary cultures of rat
cortical neuronsPlanta
Med73650654200710.1055/s-2007-98152317583824
|
|
9.
|
W YongTR XingS WangL ChenP HuCC LiHL WangM
WangJT ChenDY RuanProtective effects of gastrodin on lead-induced
synaptic plasticity deficits in rat hippocampusPlanta
Med7511121127200910.1055/s-0029-118545219291610
|
|
10.
|
CP HoiYP HoL BaumAH ChowNeuroprotective
effect of honokiol and magnolol, compounds from Magnolia
officinalis, on beta-amyloid-induced toxicity in PC12
cellsPhytother Res2415381542201010.1002/ptr.317820878707
|
|
11.
|
X LiuM WangGastrodin improves learning
behavior in a rat model of Alzheimer’s disease induced by
intra-hippocampal Aβ 1–40 injectionMol Neurodegener7S152012
|
|
12.
|
L WangLH ZhuH JiangQZ TangL YanD WangC
LiuZY BianH LiGrape seed proanthocyanidins attenuate vascular
smooth muscle cell proliferation via blocking phosphatidylinositol
3-kinase-dependent signaling pathwaysJ Cell
Physiol2237137262010
|
|
13.
|
LH ZhuL WangD WangH JiangQZ TangL YanZY
BianXA WangH LiPuerarin attenuates high-glucose-and
diabetes-induced vascular smooth muscle cell proliferation by
blocking PKCbeta2/Rac1-dependent signalingFree Radic Biol
Med48471482201010.1016/j.freeradbiomed.2009.10.04019854265
|
|
14.
|
L LiHN ZhangHZ ChenP GaoLH ZhuHL LiX LvQJ
ZhangR ZhangZ WangSIRT1 acts as a modulator of neointima formation
following vascular injury in miceCirc
Res10811801189201110.1161/CIRCRESAHA.110.23787521474819
|
|
15.
|
H LuoJ XiaoS YuanH GuoThe effect of Tianma
injection on cultured vascular smooth muscle cells proliferationHua
Xi Yi Ke Da Xue Xue Bao2862651997(In Chinese)
|
|
16.
|
J WaltenbergerA UeckerJ KrollH FrankU
MayrJD BjorgeD FujitaA GazitV HombachA LevitzkiFD BöhmerA dual
inhibitor of platelet-derived growth factor beta-receptor and Src
kinase activity potently interferes with motogenic and mitogenic
responses to PDGF in vascular smooth muscle cells. A novel
candidate for prevention of vascular remodelingCirc
Res851222199910.1161/01.RES.85.1.12
|
|
17.
|
K KappertJ SparwelA SandinA SeilerU
SieboltsO LeppänenS RosenkranzA OstmanAntioxidants relieve
phosphatase inhibition and reduce PDGF signaling in cultured VSMCs
and in restenosisArterioscler Thromb Vasc
Biol2626442651200610.1161/01.ATV.0000246777.30819.8516990553
|
|
18.
|
GA FernsEW RainesKH SprugelAS MotaniMA
ReidyR RossInhibition of neointimal smooth muscle accumulation
after angioplasty by an antibody to
PDGFScience25311291132199110.1126/science.16534541653454
|
|
19.
|
DW StaceyThree observations that have
changed our understanding of cyclin D1 and p27Kip1 in cell cycle
controlGenes
Cancer111891199201010.1177/194760191140347521779442
|
|
20.
|
W WeiNG AyadY WanGJ ZhangMW KirschnerWG
Kaelin JrDegradation of the SCF component Skp2 in cell-cycle phase
G1 by the anaphase-promoting
complexNature428194198200410.1038/nature0238115014503
|
|
21.
|
MR KibbeTR BilliarE TzengGene therapy for
restenosisCirc Res86829833200010.1161/01.RES.86.8.829
|
|
22.
|
OG McDonaldGK OwensProgramming smooth
muscle plasticity with chromatin dynamicsCirc
Res10014281441200710.1161/01.RES.0000266448.30370.a017525382
|
|
23.
|
J AndraeR GalliniC BetsholtzRole of
platelet-derived growth factors in physiology and medicineGenes
Dev2212761312200810.1101/gad.165370818483217
|
|
24.
|
CP MackSignaling mechanisms that regulate
smooth muscle cell differentiationArterioscler Thromb Vasc
Biol3114951505201110.1161/ATVBAHA.110.22113521677292
|
|
25.
|
JA RomashkovaSS MakarovNF-kappaB is a
target of AKT in anti-apoptotic PDGF
signalingNature4018690199910.1038/4347410485711
|
|
26.
|
TF FrankeSI YangTO ChanK DattaA
KazlauskasDK MorrisonDR KaplanPN TsichlisThe protein kinase encoded
by the Akt proto-oncogene is a target of the PDGF-activated
phosphatidylinositol
3-kinaseCell81727736199510.1016/0092-8674(95)90534-07774014
|
|
27.
|
T KaimotoO YasudaM OhishiM MogiY TakemuraT
SuharaT OgiharaK FukuoH RakugiNifedipine inhibits vascular smooth
muscle cell dedifferentiation via downregulation of Akt
signalingHypertension56247252201010.1161/HYPERTENSIONAHA.110.14978120530298
|
|
28.
|
H MiyakeK MaedaN AsaiR ShibataH IchimiyaM
Isotani-SakakibaraY YamamuraK KatoA EnomotoM TakahashiT MuroharaThe
actin-binding protein Girdin and its Akt-mediated phosphorylation
regulate neointima formation after vascular injuryCirc
Res10811701179201110.1161/CIRCRESAHA.110.236174
|
|
29.
|
J LiangJM SlingerlandMultiple roles of the
PI3K/PKB (Akt) pathway in cell cycle progressionCell
Cycle2339345200310.4161/cc.2.4.43312851486
|
|
30.
|
JA DiehlM ChengMF RousselCJ SherrGlycogen
synthase kinase-3beta regulates cyclin D1 proteolysis and
subcellular localizationGenes
Dev1234993511199810.1101/gad.12.22.34999832503
|
|
31.
|
S BonnetR PaulinG SutendraP DromparisM
RoyKO WatsonJ NagendranA HaromyJR DyckED
MichelakisDehydroepiandrosterone reverses systemic vascular
remodeling through the inhibition of the Akt/GSK3-{beta}/NFAT
axisCirculation12012311240200919752325
|