Introduction
Bone is continuously remodeled through bone
formation by osteoblasts and bone resorption by osteoclasts. A
balance of bone remodeling is important for maintaining skeletal
strength and density (1).
However, an imbalance in bone remodeling caused by excessive
osteoclastic bone resorption leads to bone destructive diseases,
such as osteoporosis (2).
Osteoclasts originate from hematopoietic precursor
cells of the monocyte/macrophage series and differentiate into
bone-resorbing multinucleated cells by fusion of mononuclear cells.
The osteoclastic process consists of multiple steps, including
osteoclastogenesis, activation, and apoptosis (3). Osteoclasts differentiate from
preosteoclasts in the presence of receptor activator of nuclear
factor-κB ligand (RANKL). Binding of RANKL to its receptor
activates tumor necrosis factor receptor-associated factor 6
(TRAF6), which is induced through the activation of nuclear
factor-κB (NF-κB) via the inhibitor of NF-κB (IκB) kinase and
mitogen-activated protein kinases (MAPK) (4). In addition, activated extracellular
signal-regulated kinase (ERK) can phosphorylate c-Fos, which
subsequently phosphorylates the nuclear factor of activated T cells
(NFATc) transcription factor (5).
Therefore, RANKL induces transcription factors required for the
regulation of genes involved in osteoclast differentiation. These
osteoclasts are characterized by expression of the markers
tartrate-resistant acid phosphatase (TRAP), matrix
metalloproteinase-9 (MMP-9), cathepsin-K, and calcitonin receptor
(CTR) (6).
Osteoclasts subsequently undergo apoptosis, which is
programmed cell death characterized by cell chromatin condensation,
cell shrinkage, and nuclear fragmentation (7). Osteoclast apoptosis is related to
bone remodeling and the treatment of bone diseases (8). Osteoclasts possess general apoptotic
mechanisms. The Fas receptor has been identified in mouse and human
osteoclasts and in RAW 264.7 cells, and functions through a Fas
signaling pathway (9) that is
regulated by the Bcl-2 protein, which in turn controls the release
of cytochrome c from mitochondria. Caspase proteins are activated
via cytochrome c, and apoptosis is typically defined by activated
caspase-3 (10). The relative
rate of pro-apoptotic and anti-apoptotic events determines
susceptibility to death signals.
In recent years natural compounds that inhibit bone
resorption have been studied for their ability to reduce the rate
of osteoclast differentiation or the number of activated
osteoclasts, or to induce osteoclast apoptosis. Among these, agents
that inhibit osteoclast differentiation have been proposed to
prevent and treat osteoporosis (11). Prior studies associated with
inhibition of osteoclastogenesis can be divided into two groups. It
was reported that osteoclasts interact closely with osteoblasts.
RANKL, a member of the TNF family, is a significant factor in
osteoclast differentiation. RANKL is expressed by
osteoblasts/stromal cells, and its expression is increased by
factors including 1,25(OH)2D3,
prostaglandinE2 (PGE2), parathyroid hormone
(PTH), and interleukin-11 (IL-11). Osteoprotegein (OPG) is a decoy
receptor for RANKL that negatively regulates bone resorption by
binding to RANKL. Thus, in many environments, osteoclastogenesis is
regulated indirectly by osteoblasts/stromal cells (12). Therefore, natural extracts such as
soybean and Scutellaria radix used in the treatment of
osteoblasts have indirect inhibitory effects on osteoclastogenesis.
An alternative method for demonstrating direct effects on the
inhibition of osteoclastogenesis is through blockage of signaling
pathways induced by RANKL in RAW 264.7 cells (13,14).
Silk is a well known fibrous protein produced by the
silkworm that has been used traditionally as textile fibers and
surgical sutures for humans (15). It is composed of two kinds of
proteins, a filament core protein (fibroin) and a gum-like coating
protein (sericin) that surrounds the fibroin fibers to cement them
together. Silk fibroin consists of heavy and light chain
polypeptides of ∼390 and ∼25 kDa, respectively, linked by a
disulfide bond at the C-termini of the two subunits through
hydrophobic interactions. The hydrophobic blocks tend to form
β-structures or crystals through hydrogen bonding and hydrophobic
interactions, forming the basis for the tensile strength of silk
fibroin (16). Hydrolysates of
silk fibroin are water-soluble peptides that have been investigated
as food and dietary supplements. Hydrolysate silk fibroin contains
18 kinds of amino acids including Gly (45.9%), Ala (30.3%), Ser
(12.1%), Tyr (5.3%) and Val (1.8%) (17,18). Numerous studies have documented a
range of effects of hydrolysate silk fibroin, including
moisturizing (19), wrinkle
improvement, inhibition of tyrosinase (20), and apoptosis (21), and biomedical applications such as
a matrix for mammalian cell culture and enzyme immobilization
(22), a scaffold for bone
substitution (23), a wound
dressing (24), and a drug
delivery carrier (25). Silk
fibroin hydrolysate has also been used in biomedical materials,
such as bone, cartilage, and ligaments (26). In a previous study, we
demonstrated the indirect effect of silk fibroin on the inhibition
of osteoclastogenesis in RAW 264.7 cells (27). These results led us to investigate
the direct effect of hydrolysate silk fibroin on
osteoclastogenesis.
Therefore, in this study we investigated the effect
of hydrolysate silk fibroin on signaling pathways involving ERK1/2
and NF-κB and the induction of apoptosis. Mouse RAW 264.7 cells
were grown to fully differentiate into osteoclasts and expression
levels of genes such as MMP-9, TRAP, cathepsin-K and CTR, which are
well known markers for osteoclasts, were also measured.
Materials and methods
Preparation of extract from silk
fibroin
Raw silk (Bombyx mori L.) cocoons reared on a
farm affiliated with the Rural Development Administration of Korea
were used as the raw materials. The raw materials were degummed
twice with 0.5% on the weight of fiber (OWF) Marseilles soap and a
0.3% OWF sodium carbonate solution at 100°C for 1 h and then washed
with distilled water. Degummed silk fibroin fibers were dissolved
in a mixed solution of CaCl2, H2O, and
ethanol at 95°C for 5 h. This calcium chloride/silk fibroin mixed
solution was filtered twice through a Miracloth (Calbiochem, San
Diego, CA, USA) quick filter. For the desalting of the calcium
chloride/silk fibroin mixed solution, gel filtration column
chromatography was performed on a GradiFrac system (Amersham
Parmacia Biotech, Tokyo, Japan) equipped with a UV-1 detector
operating at 210 nm. A commercially available and prepacked
Sephadex G-25 column (800 × 40 mm i.d. Amersham Parmacia Biotech)
was used. Distilled water was used as the elution solvent at a flow
rate of 25 ml/min; the sample injection volume was 200 ml, and the
fraction volume was 30 ml.
Enzymatic hydrolysis and
fractionation
A proteolytic enzyme, actinase from Streptomyces
griseus (Kaken Chem Co., Tokyo, Japan), was used for enzymatic
degradation. The silk fibroin solution and 5% actinase with respect
to the weight of the fibroin were mixed under nitrogen gas at 55°C
for 12 h. Then, the solution was heated in a boling water bath to
stop the enzyme reaction and centrifuged at 5,000 rpm for 10 min.
Recycling HPLC was performed to fractionate the enzyme-hydrolyzed
silk fibroin on a JAI-908-C60 HPLC apparatus (Japan Analytical
Industry Co., Tokyo, Japan) equipped with a JAI refractive-index
and UV detectors operating at 220 nm. Both a commercially available
and prepacked PVA HP-GPC column (JAI-GEL GS-220, 100 cm × 5 cm
i.d.) and an ODS-BP column (JAI-GEL, 100 cm × 5 cm i.d.) were
employed. Water was used as the eluting solvent at a flow rate of 3
ml/min; the sample injection volume was 20 ml. The hydrolysate silk
fibroin was concentrated and stored at −20°C until use.
Cell culture and induction of osteoclast
differentiation
Mouse monocyte/macrophage RAW 264.7 cells were
purchased from the American Type Culture Collection (ATCC,
Manassas, VA, USA) and maintained in DMEM supplemented with 10% FBS
and 1% antibiotics in a humidified atmosphere of 5% CO2
at 37°C, with a media change every three days. In order to induce
differentiation into osteoclasts, RAW 264.7 cells were resuspended
in DMEM supplemented with 10% FBS and 50 ng/ml RANKL (Petrotech
Inc., Seoul, Korea) and plated in a 24-well plate. Multinucleated
osteoclasts were observed starting on differentiation Day 3.
Cell viability assay
RAW 264.7 cells were treated with varying doses
(0.001, 0.01, 0.1, 1 and 10 mg/ml) of hydrolysate silk fibroin for
24, 48 and 72 h. After treatment, the cells were incubated with the
MTT solution for 3 h at 37°C. The supernatants were carefully
aspirated, 200 μl DMSO was added to each well, and the plates were
agitated to dissolve the crystal product. Absorbance was measured
at 570 nm using the VersaMax multi-well plate reader (Molecular
Devices Corp., Sunnyvale, CA, USA).
Western blot analysis
RAW 264.7 cells were washed twice with ice-cold PBS
and lysed with extraction buffer containing 20 mM Tris, pH 8, 150
mM NaCl, 10 mM sodium phosphate, 100 μM sodium vanadate, 100 μM
ammonium molybdate, 10% glycerol, 0.1% Nonidet P-40, 0.1% SDS, and
1X protease and phosphatase inhibitors. Insoluble materials were
removed by centrifugation at 12,000 rpm for 20 min. The total
concentration of extracted proteins was determined using the
Bradford method (28). The
proteins in the supernatants (25 μg/lane) were electrophoretically
separated on SDS-polyacrylamide gels (10–12% SDS-PAGE) and
transferred to nitrocellulose membranes. Blots were blocked in 5%
skim milk diluted in PBS-T buffer (PBS containing 0.5% Tween-20)
for 1 h and then incubated overnight with polyclonal antibodies
against TRAF6, Erk1/2, C-Fos, NFATc1, IκBα, NF-κB, MMP-9, CTR,
cathepsin-K, poly ADP-ribose polymerase (PARP), cytochrome c,
Bcl-2, Bax, caspase-3, and β-actin (Cell Signaling Technology,
Beverly, MA, USA). To detect the antigen-bound antibody, the blots
were treated with secondary antibody conjugated with horseradish
peroxidase (HRP) coupled anti-IgG. Immunoreactive proteins were
visualized by an enhanced chemiluminescence (ECL) detection system,
and band intensity was quantified by densitometry (Biomaging
systems ver.4.8, UVP Inc., San Gabriel, CA, USA).
Annexin V/propidium iodide (PI) flow
cytometric analysis
Phosphatidylserine exposed on the outside of
apoptotic cells was determined by an Annexin V-fluorescein
isothiocyanate (FITC) apoptosis kit (Roche Molecular Diagnostics,
Mannheim, Germany). To detect apoptosis and necrosis induced by
silk fibroin in RAW 264.7 cells, the cells were treated with
different concentrations of hydrolysate silk fibroin (0.001, 0.01,
0.1, 1 and 10 mg/ml) for 72 h. After treatment the cells were
harvested, washed twice with ice-cold PBS, and centrifuged. The
cells were then stained with 100 μl Annexin V-FITC labeling
solution supplemented with 20 μl Annexin V-FITC labeling reagent,
20 μl PI solution, and 1 ml incubation buffer at room temperature
for 15 min in the dark. Annexin V-FITC and PI emissions were
detected in the FL1 and FL2 channels of a FACScan flow cytometer
(BD Biosciences, San Jose, CA, USA) using emission filters of 525
and 575 nm, respectively. The percentage distribution of intact
(Annexin V-FITC−/PI−), early apoptotic
(Annexin V-FITC+/PI−), late apoptotic
(Annexin V-FITC+/PI+), and necrotic cells
(Annexin V-FITC−/PI+) was calculated using
the CellQuest™ software (BD Biosciences).
Statistical analysis
Each experiment was performed in triplicate and the
results are expressed as the mean ± SD. Statistical analysis was
performed using the SPSS software. Analysis of variance was
performed using ANOVA procedures. Significant differences
(P<0.05) between the means were determined by the Duncan's
multiple range tests.
Results
Effect of hydrolysate silk fibroin on RAW
264.7 cell viability
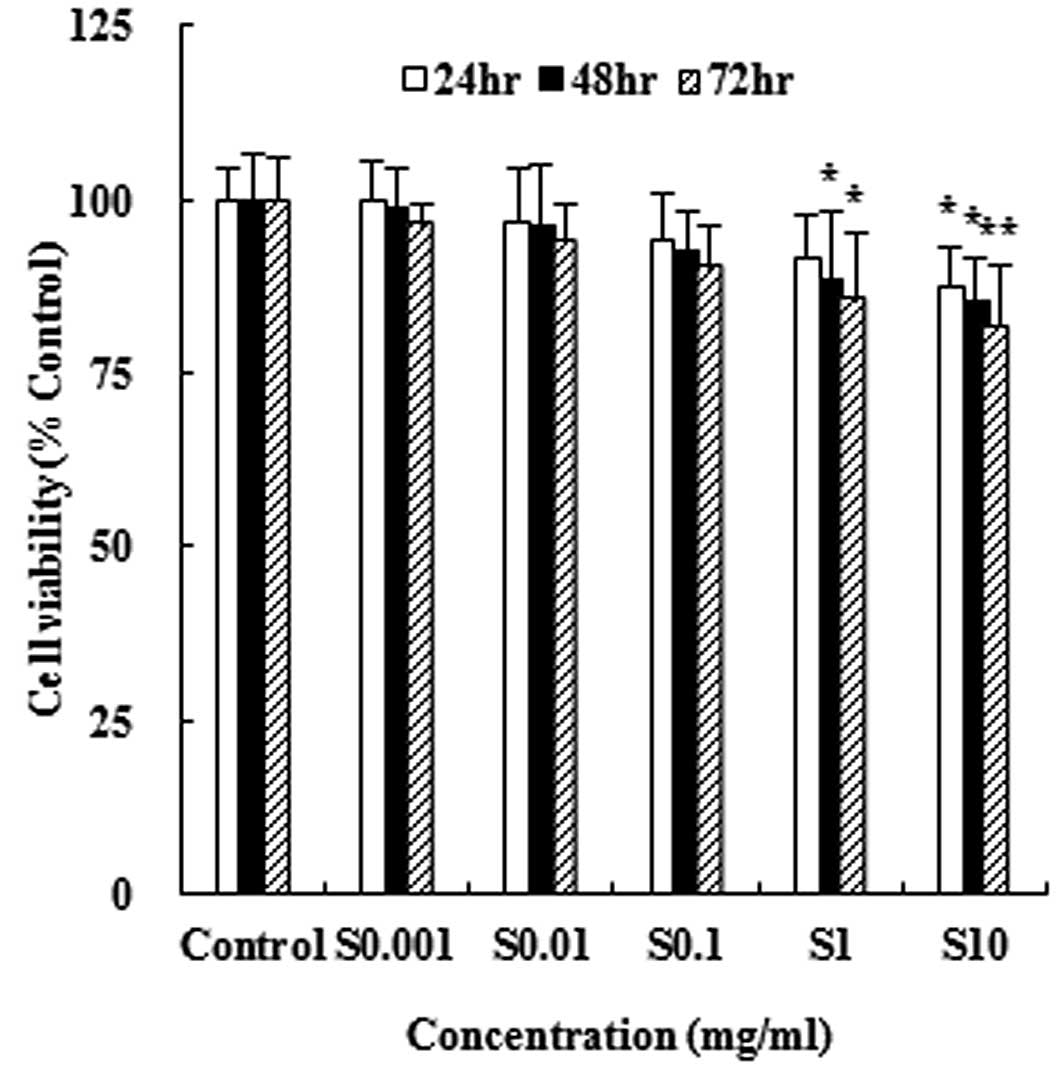
In order to measure the cytotoxic effect of
hydrolysate silk fibroin on the murine monocyte/macrophage cell
line RAW 264.7, we treated cells with different concentrations of
hydrolysate silk fibroin for 24, 48 and 72 h and measured cell
viability using the MTT assay. As shown in Fig. 1, hydrolysate silk fibroin
significantly reduced the viability of RAW 264.7 cells in a dose-
and time-dependent manner. However, the extent of inhibition did
not exceed 50%.
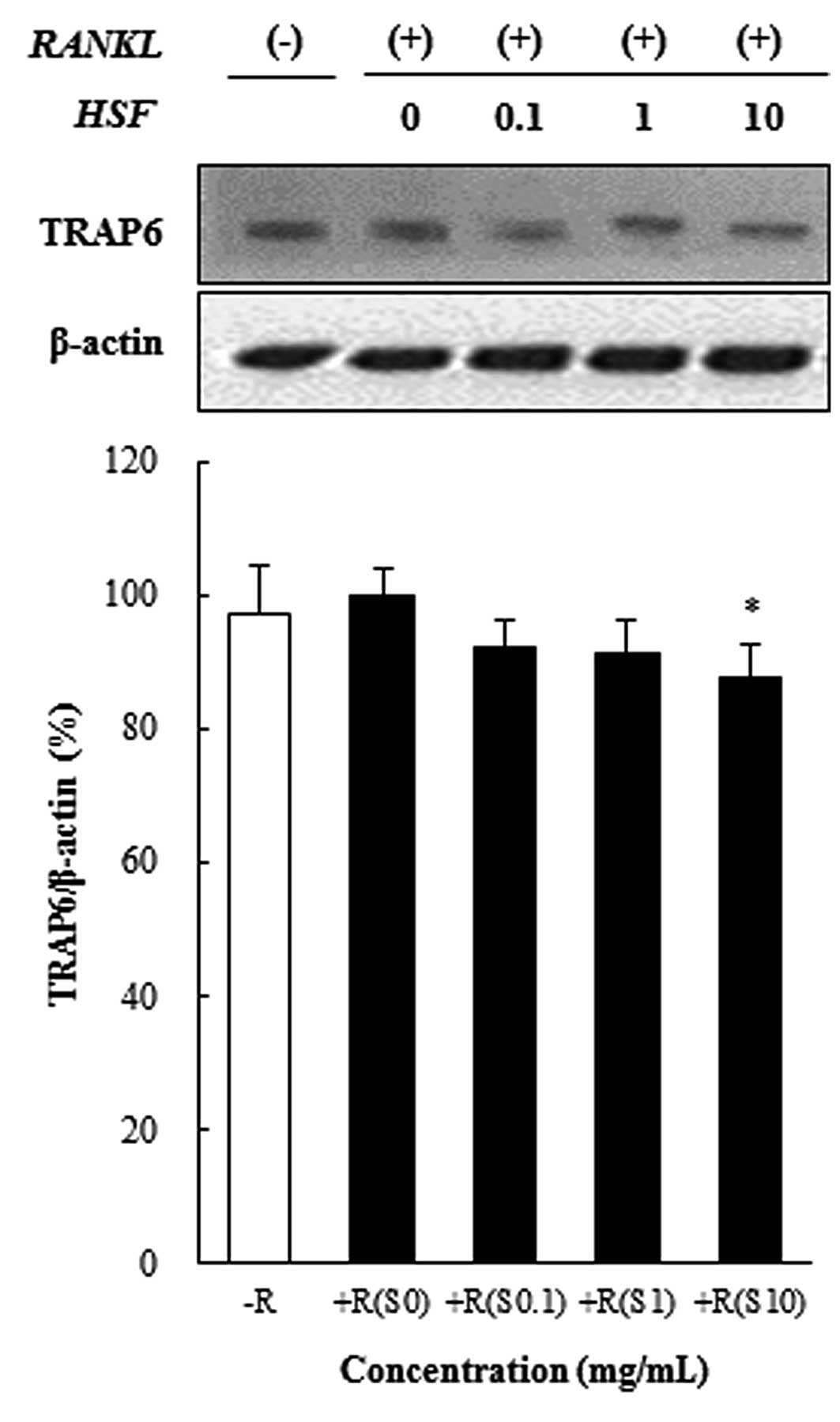
Hydrolysate silk fibroin inhibits
osteoclast differentiation in RAW 264.7 cells
To investigate the effect of hydrolysate silk
fibroin on osteoclastogenesis of RAW 264.7 cells, cells were grown
in the presence of RANKL (50 ng/ml) to induce the activation of
TRAF6 through the binding of RANKL to RANK. Activated TRAF6 is
known to induce the expression of c-Fos, NFATc1, and NF-κB, which
are essential for osteoclastogenesis. Hydrolysate silk fibroin
significantly inhibited the RANKL-induced activation of TRAF6 in
preosteoclasts, as shown in Fig.
2.
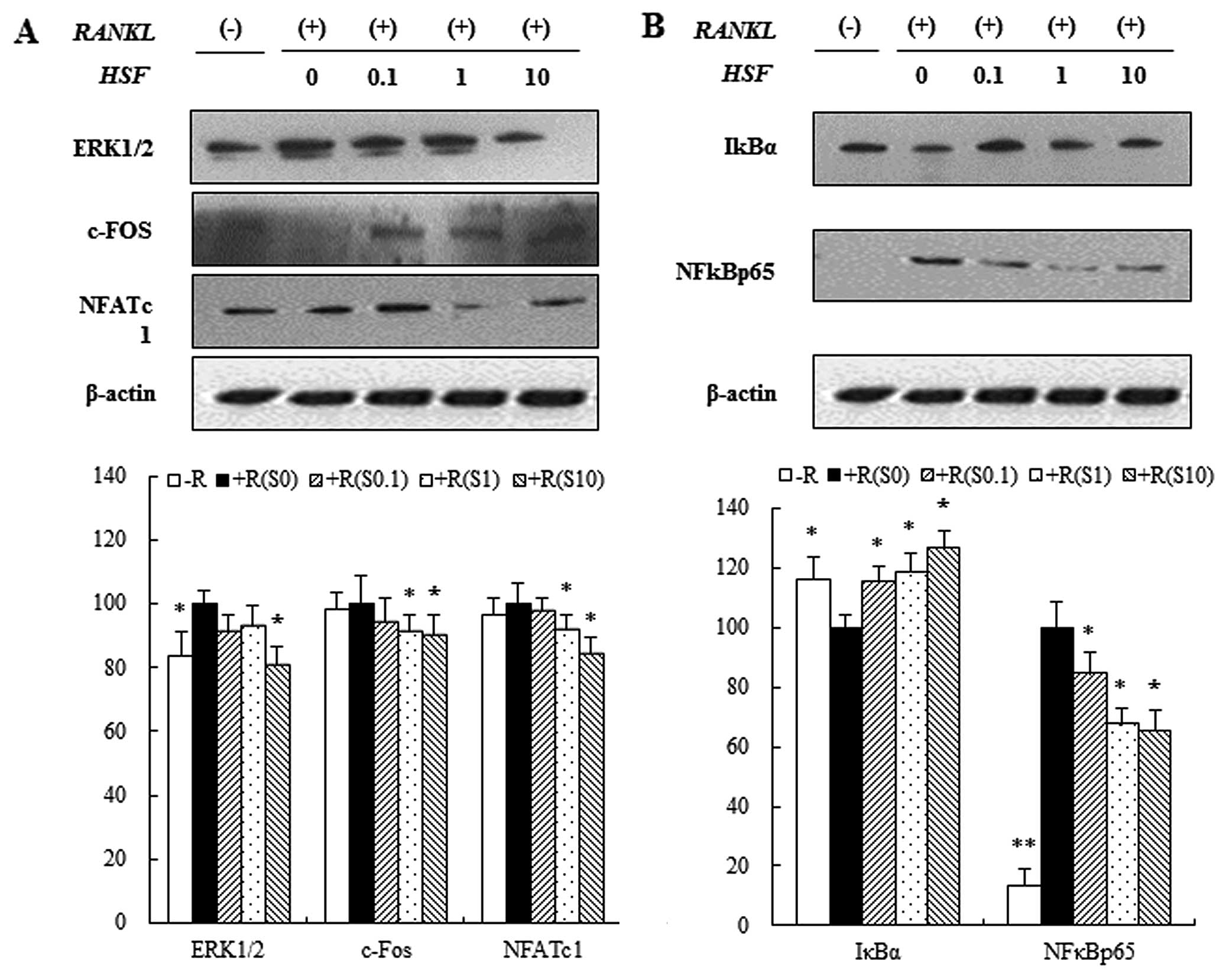
Effects of hydrolysate silk fibroin on
the ERK signaling pathway, NF-κBp65 subunit, and IκB in RAW 264.7
cells
Three mitogen-activated protein kinases (p38, ERK
and JNK) have been implicated in the mediation of RANKL-regulated
osteoclastogenesis (29). To
investigate the signaling pathways by which hydrolysate silk
fibroin inhibits osteoclastogenesis, we evaluated the effect of
hydrolysate silk fibroin on the signaling pathways of ERK and
NF-κB. Through the ERK1/2 signaling pathway, RANKL (50 ng/ml)
induced the expression of ERK1/2 in RAW 264.7 cells. This
expression of ERK1/2 was attenuated by hydrolysate silk fibroin.
Since activated ERK1/2 induces expression of c-Fos and NFATc1
proteins, which are essential for osteoclastogenesis, we examined
the effects of hydrolysate silk fibroin on the expression of c-Fos
and NFATc1. RANKL induced the expression of c-Fos and NFATc1, but
treatment with hydrolysate silk fibroin significantly inhibited
RANKL-induced c-Fos and NFATc1 expression (Fig. 3A). Activation of NF-κB is also
involved in osteoclastogenesis (30). NF-κB, a key transcription factor
for osteoclastogenesis, is activated via the IκB kinase signaling
pathway (12) and translocation
of the NF-κBp65 subunit into the nucleus is induced by RANKL. Our
data showed that hydrolysate silk fibroin inhibited NF-κBp65
expression and degradation of IκBα in a dose-dependent manner
(Fig. 3B).
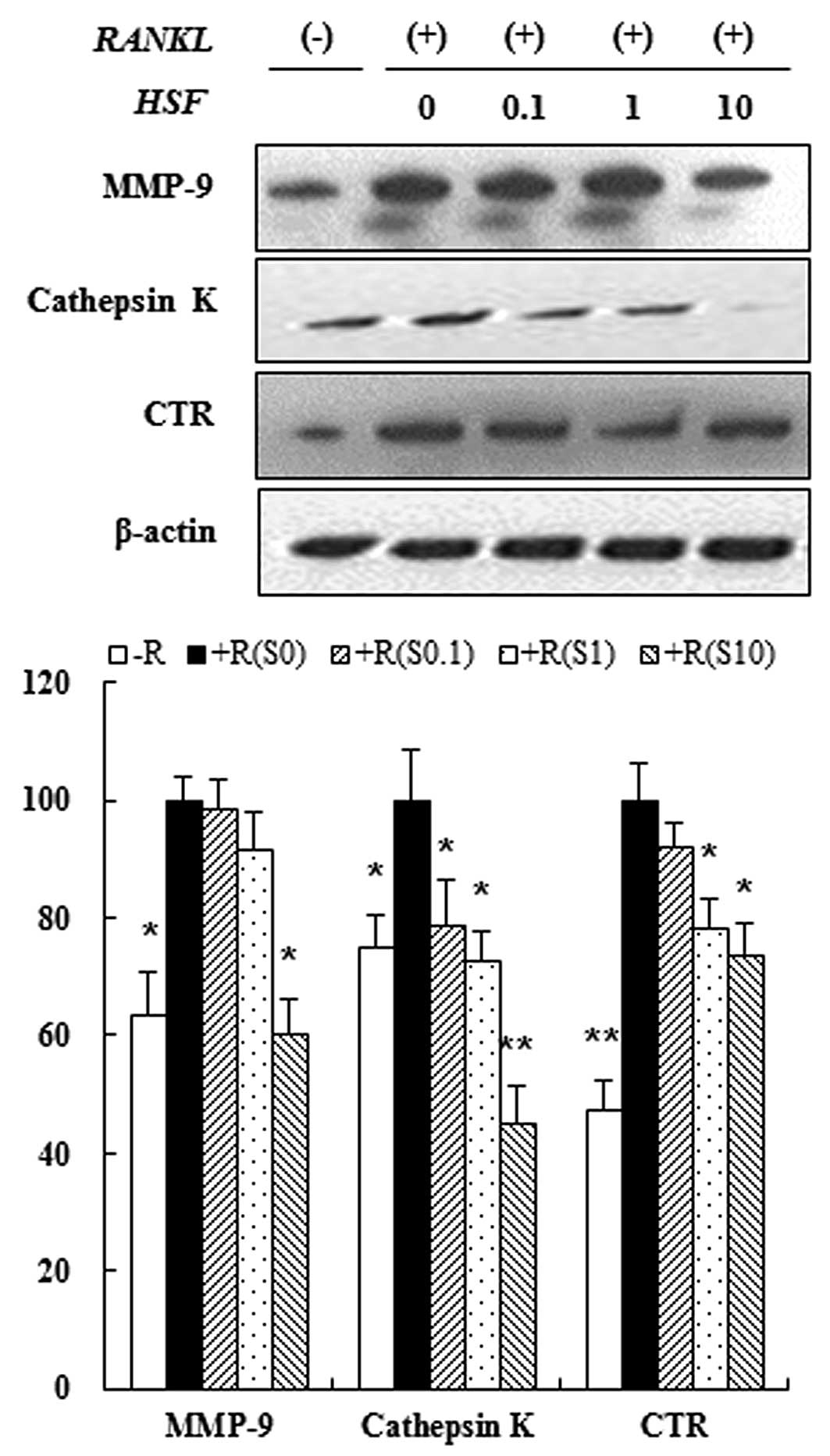
Hydrolysate silk fibroin inhibited
expression of osteoclast-specific proteins
Osteoclastogenesis is associated with upregulation
of specific proteins in response to RANKL (31). Therefore, we assessed the
inhibitory effect of hydrolysate silk fibroin on osteoclastogenesis
by evaluating the expression of osteoclast-related proteins such as
MMP-9, cathepsin-K and CTR. Immunoblot analysis showed that the
expression of osteoclastic protein markers was increased by RANKL,
but treatment with hydrolysate silk fibroin inhibited expression of
these proteins in a dose-dependent manner (Fig. 4). These results indicate that
hydrolysate silk fibroin has a specific effect on the regulation of
proteins that are induced during osteoclastogenesis.
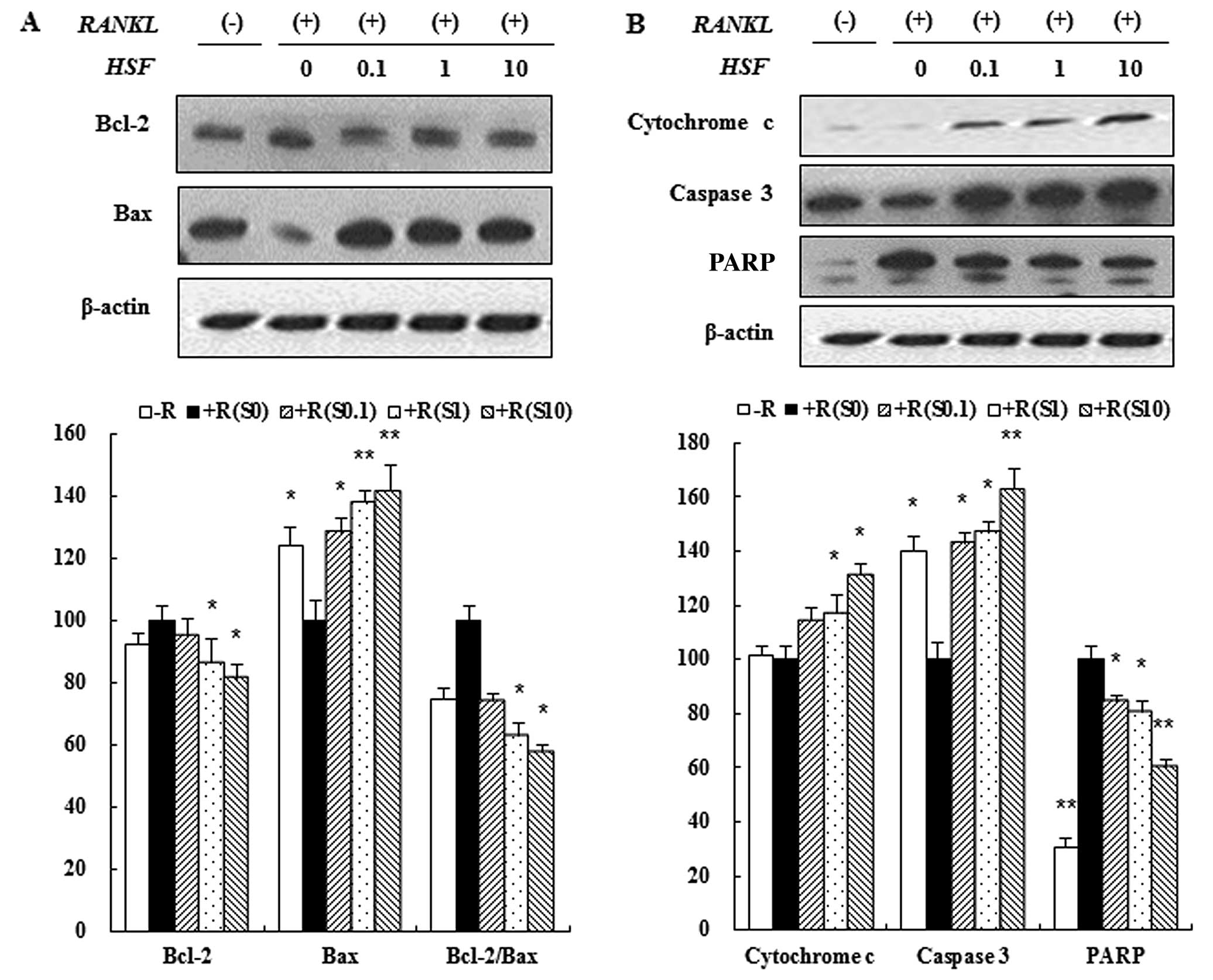
Hydrolysate silk fibroin induced
apoptosis of osteoclasts
Apoptosis is characterized by activation of a
caspase cascade that can be induced by the release of cytochrome c.
We evaluated the effect of hydrolysate silk fibroin on caspase-3
activation and cytochrome c release in osteoclasts. RAW 264.7 cells
were incubated in the absence or presence of RANKL with hydrolysate
silk fibroin (0.1, 1 and 10 mg/ml) for 72 h. Proteins were analyzed
by immunoblotting and quantified by densitometric analysis.
Caspase-3 activity was induced by hydrolysate silk fibroin at high
concentrations (10 mg/ml) (Fig.
5B). Additionally, the release of cytochrome c into the
cytoplasm was increased by hydrolysate silk fibroin in a
dose-dependent manner (Fig. 5B).
Hydrolysate silk fibroin induced the cleavage/activation of
caspase-3 and PARP, and decreased the expression of anti-apoptotic
proteins such as Bcl-2 in a dose-dependent manner. In contrast,
expression of Bax was increased by Hydrolysate silk fibroin in a
dose-dependent manner (Fig.
5A).
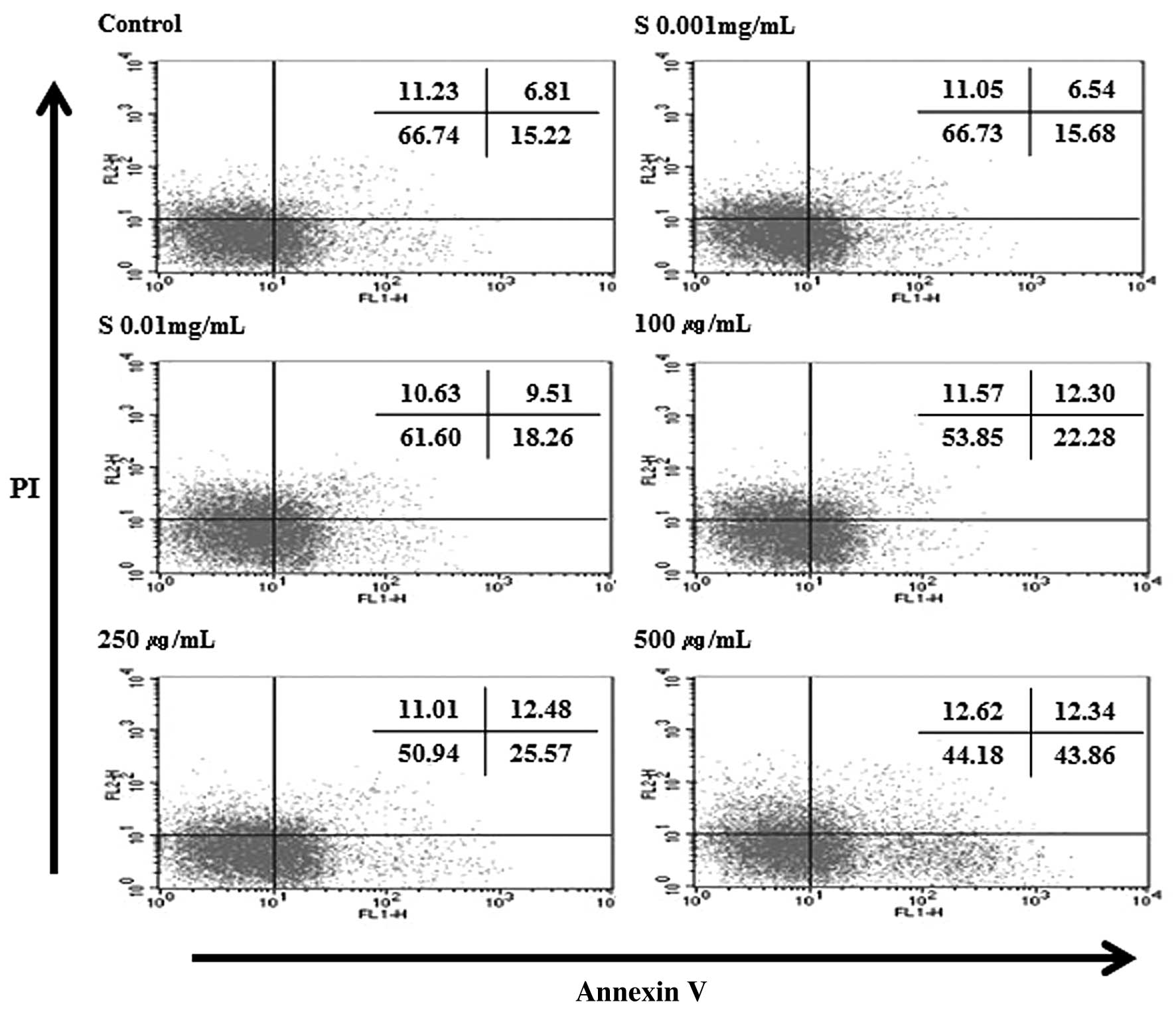
Hydrolysate silk fibroin induces
apoptosis in RAW 264.7 cells by Annexin V/PI analysis
To ascertain the mechanism of cell death (apoptosis
or necrosis), RAW 264.7 cells were treated with different
concentrations of hydrolysate silk fibroin (0.001, 0.01, 0.1, 1 and
10 mg/ml) for 72 h, and then stained with Annexin V-FITC and PI.
This protocol is based on the observation that, soon after
initiating apoptosis, membrane phosphatidylserine (PS) translocates
from the inner face of the plasma membrane to the cell surface
where it can be easily detected by staining with a fluorescent
conjugate of Annexin V, a protein that has a very strong affinity
for PS. Simultaneous staining with Annexin V-FITC and PI non-vital
dye makes it possible to distinguish between intact, early
apoptotic, late apoptotic, and necrotic cells. The results
indicated that hydrolysate silk fibroin decreased the percentage of
live cells and increased the number of early apoptotic cells. When
the concentrations increased, the percentage of normal cells
decreased from 65.01% (control) to 48.75% (10 mg/ml). However the
percentage of apoptotic cells increased from 25.17% (control) to
39.96% (10 mg/ml) (Fig. 6). These
results indicate that the decrease in live cells is due at least in
part to the effect of hydrolysate silk fibroin on inducing
apoptosis.
Discussion
There is growing interest about the use of natural
extracts to prevent loss of bone mass by inhibiting
osteoclastogenesis and bone resorptive activity, and/or inducing
osteoclast apoptosis. Silk fibroin possesses several biological and
pharmacological activities including reducing cholesterol,
hypoglycemia, and growth of osteoblasts, and enhancing bone
formation (32). A previous study
reported that silk fibroin inhibits osteoclastogenesis. This study
further demonstrated that osteoclastogenesis is indirectly
inhibited by osteoblasts/stromal cells; however, there are no
studies showing direct inhibition of osteoclastogenesis.
Therefore, the aims of this study were to evaluate
the effects of hydrolysate silk fibroin on osteoclastogenesis and
the induction of apoptosis. We found that hydrolysate silk fibroin
is a potent inhibitor of osteoclasts in RANKL-stimulated RAW 264.7
cells. As shown in Fig. 3, the
inhibitory effect of hydrolysate silk fibroin was demonstrated by
measuring the RANKL-induced expression of osteoclast-related
proteins such as MMP-9, cathepsin-K, and CTR, which are required
for the bone resorptive activity of mature osteoclasts. MMP-9 and
cathepsin-K are highly expressed in osteoclasts. MMP-9 can initiate
the osteoclastic resorption process by removing the collagenous
layer from the bone surface (33), while cathepsin-K plays an
essential role in osteoclastic bone resorption (34,35). Therefore, these results suggest
that hydrolysate silk fibroin could inhibit the resorbing capacity
of osteoclasts.
The effects of hydrolysate silk fibroin on the
signaling mechanism of RANKL were clearly demonstrated. The
inhibitory effect of hydrolysate silk fibroin on the
differentiation stage results from suppression of RANKL-induced
ERK1/2 signaling and expression of NF-κBp65. Binding of RANKL to
RANK stimulates TRAF6 and various transcription factors (36). TRAF6 activation subsequently leads
to the activation of MAP kinases (ERK, JNK and p38) and NF-κB,
which induces the expression of proteins required for
osteoclastogenesis (37). Several
transcription factors have been suggested to regulate induction of
genes involved in RANKL-induced osteoclastogenesis (38). Importantly, RANKL specifically and
strongly induces NFATc1, the master regulator of
osteoclastogenesis, and this induction is dependent on c-Fos
pathways (39). RANKL has been
shown to elevate the level of c-Fos expression in osteoclasts, and
our data indicated that hydrolysate silk fibroin inhibits this
RANKL-induced c-Fos expression (Fig.
4A). We therefore investigated whether hydrolysate silk fibroin
reduced the activation of NFATc1 in osteoclastogenesis in RAW 264.7
cells. As shown in Fig. 4A,
hydrolysate silk fibroin reduced the transcriptional activity of
NFATc1. These results suggest that hydrolysate silk fibroin
suppressed NTATc1 by inhibiting the upregulation of c-Fos in
response to RANKL. When RANKL-stimulated RAW 264.7 cells were
treated with hydrolysate silk fibroin, MMP-9 expression was
inhibited in a response similar to that of NFATc1 expression.
NFATc1 was proposed to act as a downstream mediator of increased
MMP-9 expression during osteoclastogenesis (40). Therefore, our results suggest that
the inhibitory effect of hydrolysate silk fibroin on MMP-9 may be
responsible for the downregulation of NFATc1 activation.
As mentioned above, NF-κB is another transcription
factor that is activated by the RANKL signaling pathway in
osteoclastogenesis. In the absence of RANKL, NF-κB is retained in
the cytoplasm as a complex with the IκB protein. However, when the
cells are stimulated by RANKL, the IκB kinase (IKK) complex
phosphorylates IκB and the phosphorylated IκB is targeted by the
proteasome and subsequently degraded, leading to the activation of
NF-κB signaling. The NF-κBp65 subunit is separated from IκBα and
enters the nucleus where it enhances transcription of target genes
(41). Therefore, inhibition of
NF-κB translocation might be an effective approach to target
osteoclastogenesis. In our study, hydrolysate silk fibroin
inhibited RANKL-induced IκB degradation and activation of NF-κBp65
in the cytoplasm. These findings raise the possibility that silk
fibroin prevents the translocation of the NF-κBp65 subunit through
the blockade of phosphorylation-induced IκBα degradation.
In addition to its effects on osteoclastogenesis,
hydrolysate silk fibroin also induced apoptosis in osteoclasts.
Osteoclasts are characterized by multinuclearity, an actin ring
structure, and acidic cell conditions during resorption.
Osteoclasts can be inhibited by disrupting the actin ring,
triggering their apoptosis, and/or inhibiting expression/activity
of proteins required for their function (42). The classical Fas signaling pathway
involving mitochondrial release of cytochrome c and activation of
caspase-3 has been identified in mouse and human osteoclasts and in
RAW 264.7 cells (9). Therefore,
we investigated the effect of hydrolysate silk fibroin on the
activation/expression of apoptotic signaling molecules in
osteoclasts by immunoblot analysis of RAW 264.7 cells. As shown in
Fig. 5, the Fas signaling pathway
was induced by hydrolysate silk fibroin. In addition,
anti-apoptotic Bcl-2 was downregulated and pro-apoptotic Bax was
upregulated by treatment with hydrolysate silk fibroin. These
results suggest that hydrolysate silk fibroin has antiresorptive
activity by both inhibiting osteoclastogenesis and inducing
apoptosis of osteoclasts. Although our findings are preliminary and
have certain limitations, our study may provide new insight toward
understanding the mechanisms of osteoclastogenesis and
apoptosis.
In conclusion, we suggest that hydrolysate silk
fibroin modulates expression of specific genes in osteoclasts
through downregulation of transcription factors such as NFATc1 and
NF-κB, and also induces apoptosis of osteoclasts. Therefore,
hydrolysate silk fibroin may be a useful natural compound for
reducing osteoclastogenesis and preventing bone resorption.
Acknowledgements
This study was supported by 2012
Post-doctoral Fellowship Program (PJ906973022012) of National
Academy Science, Rural Development Administration, Republic of
Korea.
References
|
1.
|
AM ParfittBone remodeling and bone loss:
Understanding the pathophysiology of osteoporosisClin Obstet
Gynecol30789811198710.1097/00003081-198712000-000043319313
|
|
2.
|
WJ BoyleWS SimonetDL LaceyOsteoclast
differentiation and
activationNature423337342200310.1038/nature0165812748652
|
|
3.
|
K MatsuoN IrieOsteoclast-osteoblast
communicationArch Biochem
Biophys473201209200810.1016/j.abb.2008.03.027
|
|
4.
|
BG DarnayBB AggarwalSignal transduction by
tumour necrosis factor and tumour necrosis factor related ligands
and their receptorsAnn Rheum
Dis5812113199910.1136/ard.58.2008.i210577967
|
|
5.
|
T KogaM InuiK InoueSH KimA SuematsuE
KobayashiT IwataH OhnishiT MatozakiT KodamaCostimulatory signals
mediated by the ITAM motif cooperate with RANKL for bone
homeostasisNature428758763200410.1038/nature0244415085135
|
|
6.
|
O AnusaksathienC LaplaceX LiY RenL PengSR
GoldringDL GalsonTissue-specific and ubiquitous promoters direct
the expression of alternatively spliced transcripts from the
calcitonin receptor geneJ Biol
Chem2762266322674200110.1074/jbc.M00710420011309373
|
|
7.
|
BF BoyceK WrightSV ReddyBA KoopB StoryR
DevlinRJ LeachGD RoodmanJJ WindleTargeting simian virus 40 T
antigen to the osteoclast in transgenic mice causes osteoclast
tumors and transformation and apoptosis of
osteoclastsEndocrinology1365751575919957588333
|
|
8.
|
DE HughesBF BoyceApoptosis in bone
physiology and diseaseMol
Pathol50132137199710.1136/mp.50.3.1329292147
|
|
9.
|
X WuMA McKennaX FengTR NagyJM
McDonaldOsteoclast apoptosis: the role of fas in vivo and in
vitroEndocrinology1445544555552003
|
|
10.
|
L XingBF BoyceRegulation of apoptosis in
osteoclasts and osteoblastic cellsBiochem Biophys Res
Commun328709720200510.1016/j.bbrc.2004.11.07215694405
|
|
11.
|
GA RodanTJ MartinTherapeutic approaches to
bone
diseasesScience28915081514200010.1126/science.289.5484.150810968781
|
|
12.
|
T YamashitaZ YaoF LiQ ZhangIR BadellEM
SchwarzS TakeshitaEF WagnerM NodaK MatsuoNF-kappaB p50 and p52
regulate receptor activator of NF-kappaB ligand (RANKL) and tumor
necrosis factor-induced osteoclast precursor differentiation by
activating c-fos and NFATc1J Biol
Chem2821824518253200710.1074/jbc.M610701200
|
|
13.
|
KH ParkWC JuYH ChoConditioned medium of
soybean extract treated osteoblasts inhibits RANKL induced
differentiation of osteoclastsFASEB J3964702010
|
|
14.
|
JM ShinCK ParkEJ ShinTH ChoIK HwangEffects
of Scutellaria radix extract on osteoblast differentiation
and osteoclast formationKorean J Food Sci Tech406746792008
|
|
15.
|
G WangH YangM LiS LuX ChenX CaiThe use of
silk fibroin/hydroxyapatite composite co-cultured with rabbit
bone-marrow stromal cells in the healing of a segmental bone
defectJ Bone Joint Surg
Br92320325201010.1302/0301-620X.92B2.2260220130332
|
|
16.
|
C ZhouF ConfalonieriM JacquetR PerassoZ
LiJ JaninSilk fibroin: structural implications of a remarkable
amino acid
sequenceProteins44119122200110.1002/prot.107811391774
|
|
17.
|
ED KimT BayaraaEJ ShinCK
HyunFibroin-derived peptides stimulate glucose transport in normal
and insulin-resistant 3T3-L1 adipocytesBiol Pharm
Bull32427433200910.1248/bpb.32.42719252290
|
|
18.
|
M TsukadaG FreddiN MinouraG
AllaraPreparation and application of porous silk fibroin materialsJ
Appl Polym Sci54507514199410.1002/app.1994.070540411
|
|
19.
|
A NishidaM YamadaT KanazawaY TakashimaK
OuchiH OkadaUse of silk protein, sericin, as a sustained-release
material in the form of a gel, sponge and filmChem Pharm
Bull5814801486201010.1248/cpb.58.148021048340
|
|
20.
|
N KatoS SatoA YamanakaH YamadaN FuwaM
NomuraSilk protein, sericin, inhibits lipid peroxidation and
tyrosinase activityBiosci Biotechnol
Biochem62145147199810.1271/bbb.62.1459501526
|
|
21.
|
C AcharyaSK GhoshSC KunduSilk fibroin
protein from mulberry and non-mulberry silkworms: cytotoxicity,
biocompatibility and kinetics of L929 murine fibroblast adhesionJ
Mater Sci Mater
Med1928272836200810.1007/s10856-008-3408-318322779
|
|
22.
|
N MinouraS AibaY GotohM TsukadaY
ImaiAttachment and growth of cultured fibroblast cells on silk
protein matricesJ Biomed Mater
Res2912151221199510.1002/jbm.8202910088557723
|
|
23.
|
S SofiaMB McCarthyG GronowizaDL
KaplanFunctionalized silk-based biomaterials for bone formationJ
Biomed Mater
Res54139148200110.1002/1097-4636(200101)54:1%3C139::AID-JBM17%3E3.0.CO;2-711077413
|
|
24.
|
DH RohSY KangJY KimYB KwonH Young KweonKG
LeeYH ParkRM BaekCY HeoJ ChoeWound healing effect of silk
fibroin/alginate-blended sponge in full thickness skin defect of
ratJ Biomed Mater Res17547552200616691353
|
|
25.
|
T HanawaA WatanabeT TsuchiyaR IkomaM
HidakaM SugiharaNew oral dosage form for elderly patients. II.
Release behavior of benfotiamine from silk fibroin gelChem Pharm
Bull (Tokyo)43872876199510.1248/cpb.43.8727553973
|
|
26.
|
LG GriffithG NaughtonTissue engineering -
current challenges and expanding
opportunitiesScience29510091014200210.1126/science.106921011834815
|
|
27.
|
JH YeoKH ParkWC JuJA LeeGG LeeSO WooSM
HanHY KweonSS KimYH ChoInhibitory effect of conditioned medium of
silk fibroin-treated osteoblasts in osteoclast differentiationJ
Korean Soc Food Sci Nutr37992997200810.3746/jkfn.2008.37.8.992
|
|
28.
|
MM BradfordA rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye bindingAnal
Biochem72248254197610.1016/0003-2697(76)90527-3942051
|
|
29.
|
K MatsuzakiN UdagawaN TakahashiK
YamaguchiH YasudaN ShimaT MorinagaY ToyamaY YabeK
HigashioOsteoclast differentiation factor (ODF) induces
osteoclast-like cell formation in human peripheral blood
mononuclear cell culturesBiochem Biophys Res
Commun246199204199810.1006/bbrc.1998.85869600092
|
|
30.
|
E JimiS AkiyamaT TsurukaiN OkahashiK
KobayashiN UdagawaT NishiharaN TakahashiT SudaOsteoclast
differentiation factor acts as a multifunctional regulator in
murine osteoclast differentiation and functionJ
Immunol163434442199910384146
|
|
31.
|
SE LeeKM WooSY KimH KimK KwackZH LeeH
KimThe phosphatidylinositol 3-kinase, p38, and extracellular
signal-regulated kinase pathways are involved in osteoclast
differentiationBone307177200210.1016/S8756-3282(01)00657-311792567
|
|
32.
|
GH AltmanF DiazC JakubaT CalabroRL HoranJ
ChenH LuJ RichmondDL KaplanSilk-based
biomaterialsBiomaterials24401416200310.1016/S0142-9612(02)00353-812423595
|
|
33.
|
P ReponenC SahlbergC MunautI ThesleffK
TryggvasonHigh expression of 92-kD type IV collagenase (gelatinase
B) in the osteoclast lineage during mouse developmentJ Cell
Biol12410911102199410.1083/jcb.124.6.10918132709
|
|
34.
|
BD GelbGP ShiHA ChapmanRJ
DesnickPycnodysostosis, a lysosomal disease caused by cathepsin K
deficiencyScience27912361238199610.1126/science.273.5279.12368703060
|
|
35.
|
T IshikawaM KamiyamaN Tani-IshiiH SuzukiY
IchikawaY HamaguchiN MomiyamaH ShimadaInhibition of osteoclast
differentiation and bone resorption by cathepsin K antisense
oligonucleotidesMol Carcinog328491200110.1002/mc.106711746820
|
|
36.
|
K KanazawaA KudoTRAF2 is essential for
TNF-alpha-induced osteoclastogenesisJ Bone Miner
Res20840847200510.1359/JBMR.04122515824857
|
|
37.
|
ZH LeeH KimSignal transduction by receptor
activator of nuclear factor kappa B in osteoclastsBiochem Biophys
Res Commun305211214200310.1016/S0006-291X(03)00695-812745060
|
|
38.
|
H TakayanagiS KimT KogaH NishinaM IsshikiH
YoshidaA SaiuraM IsobeT YokochiJ InoueInduction and activation of
the transcription factor NFATc1 (NFAT2) integrate RANKL signaling
in terminal differentiation of osteoclastsDev
Cell3889901200210.1016/S1534-5807(02)00369-612479813
|
|
39.
|
G KarsentyEF WagnerReaching a genetic and
molecular understanding of skeletal developmentDev
Cell2389406200210.1016/S1534-5807(02)00157-011970890
|
|
40.
|
N IshidaK HayashiM HoshijimaT OgawaS KogaY
MiyatakeM KumegawaT KimuraT TakeyaLarge scale gene expression
analysis of osteoclastogenesis in vitro and elucidation of NFAT2 as
a key regulatorJ Biol
Chem2774114741156200210.1074/jbc.M20506320012171919
|
|
41.
|
K MatsuoDL GalsonC ZhaoL PengC LaplaceKZ
WangMA BachlerH AmanoH AburataniH IshikawaNuclear factor of
activated T-cells (NFAT) rescues osteoclastogenesis in precursors
lacking c-fosJ Biol
Chem2792647526480200410.1074/jbc.M31397320015073183
|
|
42.
|
PH SternAntiresorptive agents and
osteoclast apoptosisJ Cell
Biochem10110871096200710.1002/jcb.2131117407157
|