Introduction
Alzheimer’s disease (AD) is the most common
neurodegenerative disease causing adult dementia. The pathological
feature of AD is progressive neuronal degeneration in separate
areas of the forebrain, including the hippocampus and associated
cortices (1). Oxidative stress
has also been implicated in the pathophysiological mechanisms
underlying AD (2). Thus, the
regulation of oxidative stress is important in AD patients.
Microglia are the primary immune cells of the brain
and play an essential role in the regulation of the immune response
triggered by damaged cells (3).
Microglial cells are considered ‘brain macrophages’ because they
scavenge dying cells in the brain. However, chronic activation of
these cells leads to the production of many pro-inflammatory
cytokines and inflammatory mediators, such as nitric oxide (NO),
tumor necrosis factor-α (TNF-α), interleukin (IL)-1β and IL-6
(4). Therefore, the regulation of
microglial activation has been regarded as an important therapeutic
approach for the treatment of inflammatory neurodegenerative
diseases. A number of studies have been conducted using BV2 cells,
an immortalized murine microglial cell line. In addition to
microglia, the hippocampus, an essential area for memory function,
also has an important function pertinent in neurodegenerative
diseases. Hippocampal HT22 cells are derived from the mouse
hippocampus and have been used as a model demonstrating the
mechanism of glutamate-induced neuronal cytotoxicity (5). Glutamate causes neuronal cell death
within the nervous system. Moreover, it induces oxidative injury by
suppressing the cellular uptake of cysteine through the
cysteine/glutamate transport system, which in turn induces the
depletion of glutathione and the accumulation of reactive oxygen
species (ROS) (6). Thus,
prevention of glutamate-induced oxidative damage in hippocampal
cells may be an effective approach for arresting the progression of
neurodegenerative disorders.
Nuclear erythroid-2 related factor 2 (Nrf-2) tightly
regulates ROS levels (7). Nrf-2
is a redox-sensitive transcription factor modulating the cellular
defense signaling pathway against toxic stimuli in several types of
cells and tissues. This function of Nrf-2 has been demonstrated in
several studies, including one which showed that Nrf-2 knockout
mice were more vulnerable to oxidative damage (8). Under oxidative stress, the
cytoplasmic Kelch-like ECH-associated protein 1 (Nrf-2/Keap1)
perceives changes in the cellular environment, and its ability to
promote Nrf-2 turnover is reduced; then, Nrf-2 translocates into
the nucleus and upregulates the expression of target genes
containing the antioxidant response element (ARE) (9). Heme oxygenase-1 (HO-1) belongs to
the family of ARE-containing genes and it is considered to be
regulated by Nrf-2. HO-1 is a stress-inducible protein that
protects cells against inflammatory and oxidative stimuli. It
catalyzes the oxidation of the heme molecule and decomposes the
pro-oxidant heme into iron, carbon monoxide (CO), biliverdin (BV)
and bilirubin (BR) in the brain and in other tissues (10). The end-products of the HO-1
enzymatic process have anti-inflammatory activities which protect
cells from oxidative stress. HO-1 has been implicated in
aging-related neurodegenerative diseases including stroke,
amyotrophic lateral sclerosis, Parkinson’s disease (PD) and AD
(11). Moreover, studies have
demonstrated the neuroprotective aspects of HO-1 expression under
different experimental conditions (12,13).
Bambusae Caulis in Taeniam (BC) is the stem
of Phyllostachys nigra var. henonis or
Phyllostachys bambusoides (Family: Poaceae) and is a
well-known traditional herbal medicine in the Orient (14). It has been used as a folk remedy
for treating hypertension and cardiovascular disease (15). Oral doses of 5,000 mg/kg or less
of BC did not produce toxic effects in rats, and the minimal lethal
dose was discovered to be over 5,000 mg/kg body weight for both
genders (16). However, a limited
number of reports on the efficacy of the anti-inflammatory and
neuroprotective functions of BC exist. Therefore, we examined
whether Bambusae Caulis in Taeniam ethyl acetate fraction
(BCE) induces HO-1 expression using the Nrf2 signaling pathway in
order to regulate the neuroprotective and anti-neuroinflammatory
effects in microglial BV2 and hippocampal HT22 cells.
Materials and methods
Cell culture
Microglial BV2 and hippocampal HT22 cell lines
obtained from the American Type Culture Collection (Rockville, MD,
USA) were grown as monolayers in Dulbecco’s modified Eagle’s medium
(DMEM; Gibco-BRL, Carlsbad, CA, USA) supplemented with 10 or 5%
heat-inactivated fetal bovine serum (FBS; Gibco-BRL). The cells
were incu bated at 37°C in a humidified atmosphere containing 5%
CO2. To avoid changes in cell characteristics caused by
extended periods of cell culture, all experiments were conducted
with cells between passages 15 and 25. Each cell suspension was
subcultured by trypsin/EDTA treatment every 2 days in order to
maintain exponential growth.
Cell viability assay
The cells were plated in 24-well plates at a density
of 27times;105cells/well.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
50 μg/ml) was added to each well. The plates were incubated
for 4 h at 37°C in a 5% CO2 atmosphere, after which the
supernatant was removed and the formazan crystals that had formed
in the viable cells were solubilized with dimethyl sulfoxide
(DMSO). The absorbance of each well was then read at 570 nm using
an enzyme-linked immunosorbent assay (ELISA) reader (Wallace,
Boston, MA, USA).
Measurement of TNF-α, IL-1β and IL-6
concentrations
Cells were incubated first with various
concentrations of BCE for 1 h and then with lipopolysaccharide
(LPS) for 16 h. Following the 24-h incubation, TNF-α, IL-1β and
IL-6 levels in the culture media were quantified using an
enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems,
Minneapolis, MN, USA) according to the manufacturer’s
instructions.
Measurement of ROS
To evaluate the levels of intracellular ROS, cells
were treated with CM-H2DCFDA, an indicator of general oxidative
stress, for 1 h at 37°C under 5% CO2. The cells were
then harvested and washed three times with phosphate-buffered
saline (PBS). The fluorescence intensity was then measured by
confocal microscopy, microplate fluorimetry and flow cytometry at
an excitation wavelength of 488 nm and an emission wavelength of
525 nm. Data analyses were performed using the CXP 2.0 software
package (Beckman Coulter).
Transient transfection and dual
luciferase assay
Cells were transfected with an ARE-reporter plasmid
(Stratagene, Grand Island, NY, USA) using the FuGENE-HD reagent
(Roche Applied Sciences) according to the manufacturer’s
instructions. Then, a Renilla luciferase control plasmid, pRL-CMV
(Promega Corporation, Madison, WI, USA), was co-transfected as an
internal control for verification of transfection efficiency.
Twenty-four hours after transfection, the cells were incubated with
BCE for 1 h. Luciferase activity was assayed using a dual
Luciferase assay kit (Promega Corporation) according to the
manufacturer’s instructions. Luminescence was measured using a
microplate luminometer (Wallac 1420 multilabel counter;
Perkin-Elmer, Downers Grove, IL, USA).
Western blot analysis
Cells were harvested in ice-cold lysis buffer
consisting of 1% Triton X-100, 1% deoxycholate and 0.1% SDS. The
protein content of the cell lysates was then determined using
Bradford reagent (Bio-Rad, Hercules, CA, USA). Total proteins in
each sample (50 μg) were resolved by 7.5% SDS-polyacrylamide
gel electrophoresis (SDS-PAGE), transferred to a polyvinylidene
difluoride (PVDF) membrane and incubated with the appropriate
antibodies. The proteins were then visualized using an enhanced
chemiluminescence detection system (Amersham Biosciences,
Piscataway, NJ, USA) with horseradish peroxidase-conjugated
anti-rabbit or anti-mouse secondary antibodies. Images were
acquired using an ImageQuant 350 analyzer (Amersham
Biosciences).
Quantitative real-time polymerase chain
reaction (qRT-PCR)
Total RNA was isolated from the cells using the
RNAspin Mini RNA Isolation kit (GE Healthcare, Buckinghamshire, UK)
according to the manufacturer’s instructions. The cDNA was
synthesized from 1 μg of the total RNA using the Maxime RT
PreMix (Intron Biotechnology, Seongnam, Korea) and anchored
oligo(dT)15-primers. Real-time PCR was performed using a
Chromo4™ instrument (Bio-Rad) using the SYBR-Green master mix
(Applied Biosystems, Foster City, CA, USA). The relative amount of
target mRNA was determined using an established comparative
threshold (Ct) method by normalizing the target mRNA Ct values to
those for glyceraldehyde 3-phos phate dehydrogenase (GAPDH)
(ΔCt).
Statistical analyses
The data are expressed as the means ± standard error
(SE). Each experiment was repeated at least three times.
Statistical analysis was performed using SPSS version 16.0 software
(SPSS, Inc., Chicago, IL, USA). Significant differences were
evaluated using one- or two-way ANOVA followed by the Dunn’s post
hoc test. P-values <0.05 were considered to represent
statistically significant differences.
Results
Effects of BCE on microglial BV2 and
hippocampal HT22 cell viability
Microglial cells play an essential role in the
central nervous system, and hippocampal cells are important to
memory function (17,18). Therefore, it is of note that BCE
does not produce cytotoxicity in microglial BV2 and hippocampal
HT22 cells. To examine the potential cytotoxic properties of BCE,
we first measured the effect of BCE on cell viability in BV2 and
HT22 cells using the MTT assay. Cells were treated with 10, 20, 40,
60 and 80 μg/ml of BCE for 24 h, followed by incubation with
a working solution of MTT for 4 h at 37°C. BCE did not affect cell
viability in either microglial BV2 or hippocampal HT22 cells
(Fig. 1A and B). Thus, our data
suggest that BCE is non-toxic in doses ranging from 10–80
μg/ml. Based on these results, we selected 10–80
μg/ml of BCE for use in subsequent experiments.
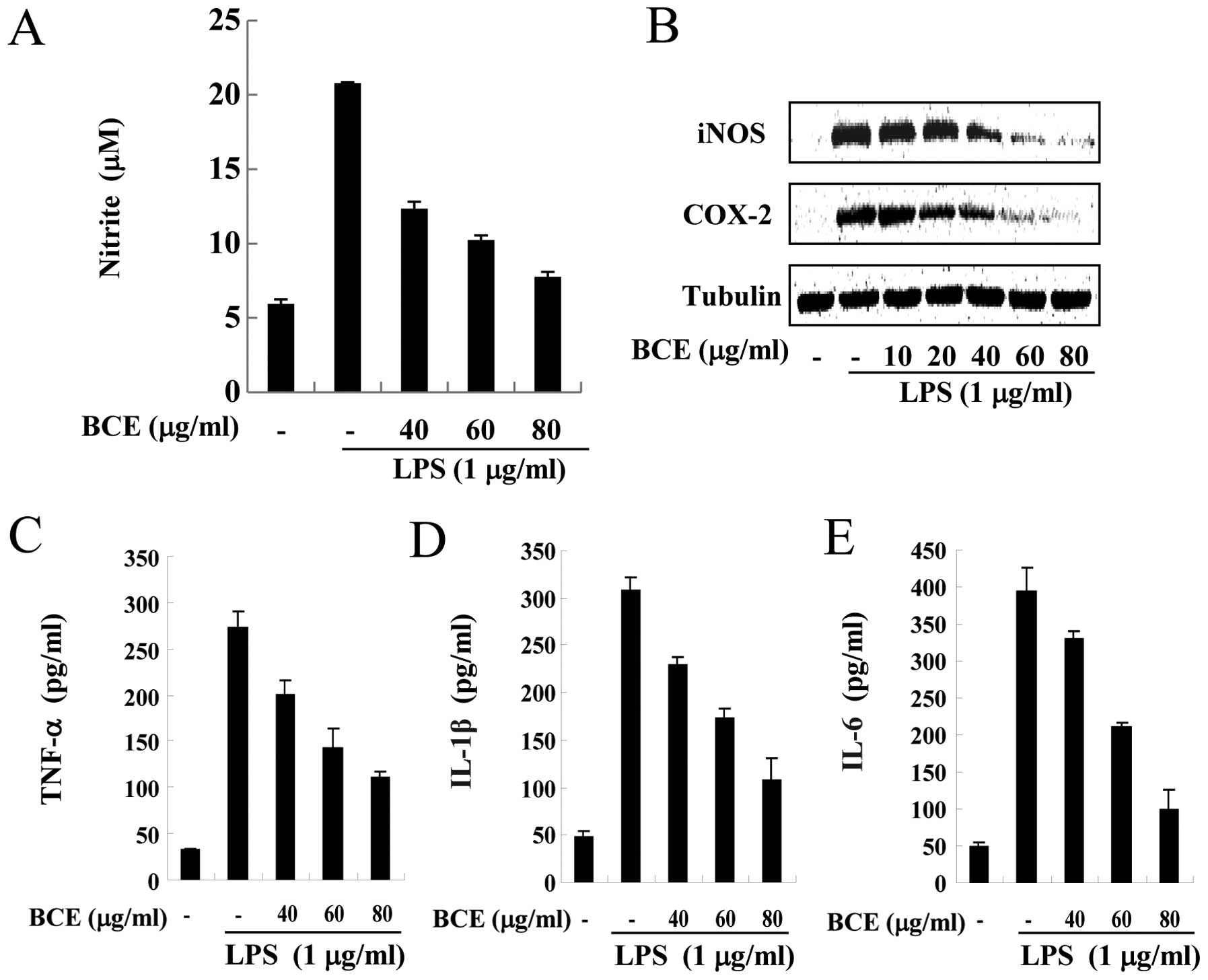
BCE suppresses LPS-induced
pro-inflammatory mediators and cytokines in microglial BV2
cells
Under inflammatory conditions, such as exposure to
LPS, activated microglia produce pro-inflammatory mediators and
cytokines, including NO, TNF-α, IL-1β and IL-6 (19). Since overproduction of these
pro-inflammatory mediators and cytokines causes acute damage in
cells, their production must be tightly controlled. Therefore, we
investigated whether BCE regulates the expression of these
inflammatory mediators and cytokines using western blotting and
ELISA assays. Microglial BV2 cells were treated with BCE (40, 60
and 80 μg/ml) for 1 h and/or LPS at a dose of 1 μg/ml
for 16 h. NO production increased ∼4-fold only in LPS-stimulated
cells (Fig. 2A). However, the
cells pre-treated with BCE demonstrated lower NO production when
compared to cells treated with LPS alone, in a dose-dependent
manner. Production of LPS-induced pro-inflammatory cytokines,
including TNF-α (Fig. 2C), IL-1β
(Fig. 2D) and IL-6 (Fig. 2E), decreased in the BCE-treated
cells in a concentration-dependent manner. Next, we monitored the
levels of inducible NO synthase (iNOS) and cyclooxygenase (COX)-2
by western blotting. Microglial BV2 cells were pre-treated with 0,
10, 20, 40, 60 and 80 μg/ml of BCE and then incubated for 24
h with LPS (1 μg/ml). Levels of iNOS and COX-2 protein
decreased in the BCE-treated BV2 cells, compared to those exposed
only to LPS (Fig. 2B). These
results indicated that BCE inhibited the LPS-induced production of
pro-inflammatory mediators in microglial BV2 cells.
BCE protects hippocampal HT22 cells
against glutamate-induced cell toxicity
Glutamate exposure leads to neuronal cell death in
the nervous system (6). Glutamate
exposure increases the generation of ROS, which, due to its
reactive properties, causes cell death in severe cases. Therefore,
we examined the neuroprotective properties of BCE in
glutamate-stimulated hippocampal HT22 cells. The hippocampal HT22
cells were pre-treated with BCE (10–80 μg/ml) for 1 h and
then incubated with 5 mM glutamate for 24 h. As shown in Fig. 3A, only 50% of the cells treated
with glutamate remained viable, compared to cells not treated with
glutamate. However, treatment with 10–80 μg/ml of BCE
improved the viability of glutamate-stimulated cells gradually, in
a dose-dependent manner. Due to the unstable nature of ROS, their
overproduction may cause neuronal cell toxicity (20). Therefore, we investigated whether
glutamate-induced ROS production was reduced by BCE in hippocampal
HT22 cells. We observed that ROS production increased in
glutamate-treated hippocampal HT22 cells (Fig. 3B). However, cells treated with
10–80 μg/ml of BCE prior to glutamate exposure exhibited a
gradual reduction in ROS production, down to the same level as
cells that had not been stimulated with glutamate. Taken together,
these results lead us to conclude that BCE has cytoprotective and
ROS-attenuating effects in hippocampal HT22 cells. Thus, BCE is
regarded as a potential therapeutic treatment against the oxidative
effects of glutamate.
BCE reduces ROS production in microglial
BV2 and hippocampal HT22 cells
ROS is a by-product of oxygen metabolism in aerobic
organisms and is considered an essential second messenger at low
concentrations. However, higher concentrations of ROS have been
reported to be harmful to cellular macromolecules (21). When there is an imbalance between
the ROS generation and removal systems, neuronal cell damage
occurs. Moreover, ROS are implicated in a number of
neurodegenerative diseases including ischemia, PD and AD, since ROS
participate in chain reactions. Hence, we examined the effect of
BCE on the elimination of ROS in LPS-stimulated microglial BV2
cells and glutamate-stimulated hippocampal HT22 cells using flow
cytometry. ROS production was significantly increased in microglial
BV2 cells stimulated by treatment with 1 μg/ml LPS for 12 h,
compared to cells not treated with LPS (Fig. 4A). However, ROS production was
reduced in LPS-stimulated cells pre-treated with BCE (80
μg/ml). The same experiment was conducted in hippocampal
HT22 cells. ROS production in hippocampal HT22 cells exposed to 5
mM glutamate for 12 h also increased, up to ∼3 times that of cells
not stimulated with glutamate, but pre-treatment of the hippocampal
HT22 cells with 80 μg/ml of BCE decreased ROS production
considerably (Fig. 4B). These
results suggest that BCE exerts neuroprotective and
anti-neuroinflammatory effects through the restriction of ROS
production.
BCE treatment increases the mRNA and
protein expression levels of HO-1 in microglial BV2 and hippocampal
HT22 cells
HO-1 has been related to anti-inflammatory effects
in recent studies (11,12). HO-1 is also regarded as a cellular
oxidative stress sensor since it responds to changes in the
cellular redox status (13).
Expression of HO-1 is restricted in normal brain neurons and
neuroglia, but various inflammatory stimuli are known to induce
HO-1 expression (22). An
increase in HO-1 expression may protect cells by producing BV and
BR, which are by-products of heme metabolism (10). To assess the effect of BCE on HO-1
protein expression, microglial BV2 and hippocampal HT22 cells were
treated with 80 μg/ml of BCE for varying lengths of time
(0–24 h). The HO-1 protein levels were increased by BCE treatment,
especially in cells treated with BCE for 8 h (Fig. 5A and E). Next, we tested the
protein expression level of HO-1 in both microglial BV2 and
hippocampal HT22 cells treated with 0, 10, 20, 40, 60 and 80
μg/ml of BCE and 0 and 20 μM of CoPP (HO-1 activator)
for 8 h. HO-1 protein expression increased in the presence of BCE,
in a concentration-dependent manner (Fig. 5B and F). To confirm the effect of
BCE on the expression of HO-1 protein, we examined the HO-1 mRNA
level using qRT-PCR. The mRNA level of HO-1 increased in a
dose-dependent manner in the presence of BCE (Fig. 5D and H). This data indicated that
BCE induces HO-1 expression in microglial BV2 and hippocampal HT22
cells. In other words, BCE may protect cells against inflammatory
stimuli since it induces the expression of HO-1, which has
well-known anti-inflammatory and antioxidant effects.
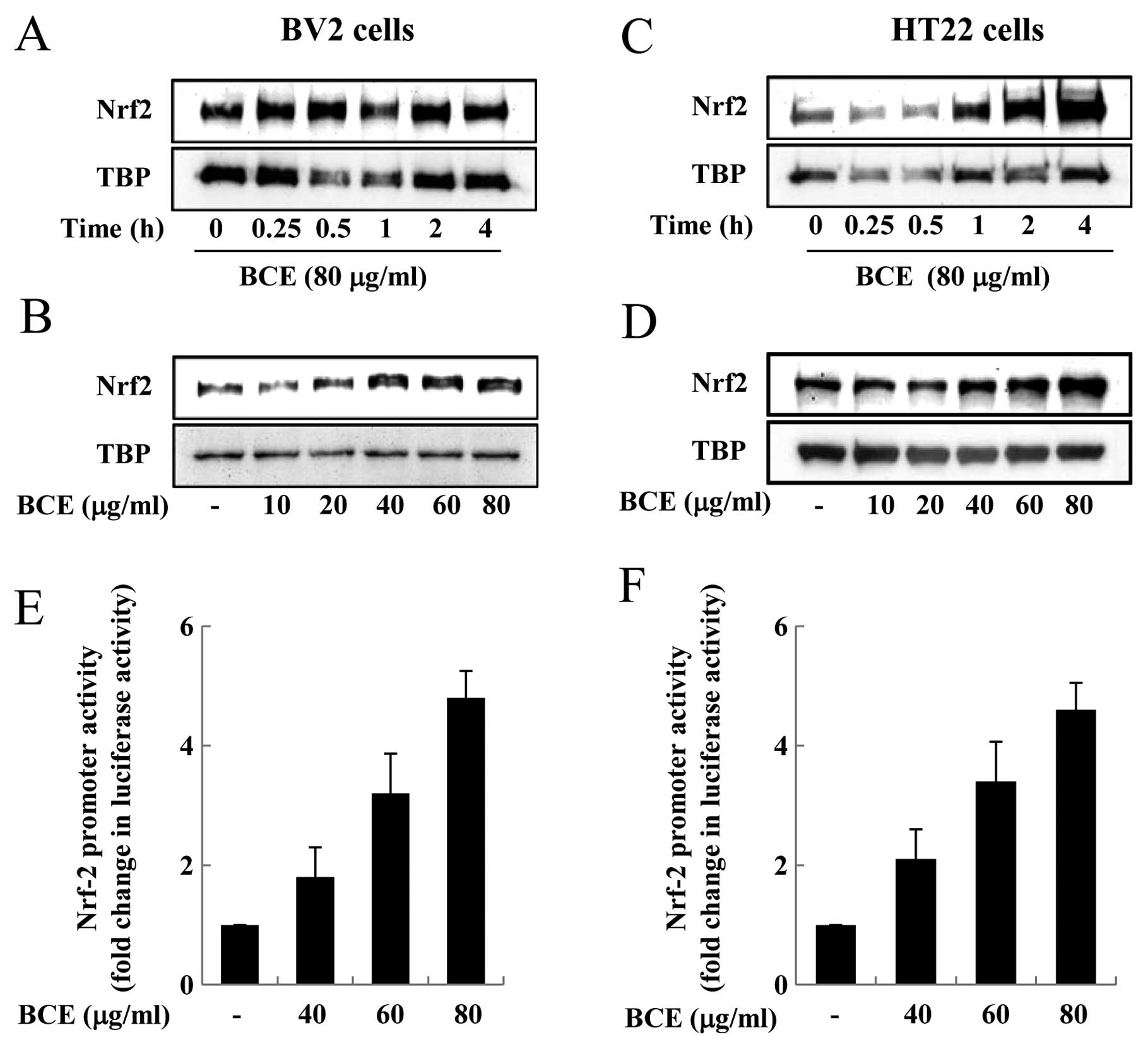
Effects of BCE on the accumulation and
transactivation function of Nrf-2 in microglial BV2 and hippocampal
HT22 cells
Nrf-2 exists in an inactive form in the cytoplasm
when bound by Keap1. However, when exposed to inflammatory stimuli,
such as LPS and ROS, Nrf-2 is released from Keap1 and translocates
from the cytoplasm into the nucleus (21). Nrf-2 plays a critical role in the
induction of a number of genes associated with the antioxidant
response, such as HO-1, since Nrf-2 is a transcription factor that
binds to ARE in the promoter region of target genes (21). Nrf-2 is an important player in the
upregulation of cellular stress-inducible genes, and the
accumulation of Nrf-2 in the nucleus endows the cell with the
adaptive response capability critical for maintaining normal
cellular functions (23). We
aimed to observe the effect of BCE on Nrf-2 nuclear accumulation
and transactivation in microglial BV2 and hippocampal HT22 cells.
First, microglial BV2 cells were treated with 80 μg/ml of
BCE for 0.25–4 h. BCE treatment increased nuclear accumulation of
Nrf-2 in these cells, especially in those treated with BCE for 0.5
h (Fig. 6A). When cells were
treated with varying concentrations of BCE (10, 20, 40, 60 and 80
μg/ml), while keeping the treatment time constant at 0.5 h,
Nrf-2 nuclear accumulation was induced by BCE in a dose-dependent
manner (Fig. 6B). Next, we
performed the same experiments in hippocampal HT22 cells, treating
them with 10–80 μg/ml of BCE for 4 h. Nuclear accumulation
of Nrf-2 increased with increasing doses of BCE, especially in the
cells treated with BCE for 4 h (Fig.
6C). The nuclear accumulation of Nrf-2 increased with
increasing doses of BCE, in a concentration-dependent manner
(Fig. 6D). To examine whether BCE
also induces Nrf-2-mediated transactivation of target genes, we
performed reporter gene analysis using an ARE-containing luciferase
reporter construct. Microglial BV2 and hippocampal HT22 cells were
transfected with the Nrf-2-responsive luciferase vector and then
treated with 0, 40, 60, or 80 μg/ml of BCE. We observed that
the luciferase activity increased with increasing doses of BCE,
indicating an increase in Nrf-2-responsive promoter activity
(Fig. 6E and F). This data
indicated that BCE induces Nrf-2 translocation from the cytoplasm
into the nucleus and that Nrf-2 binds the ARE in the promoter
region. This Nrf-2-mediated transactivation is involved in the
expression of a number of genes, including HO-1, involved in
anti-inflammatory and antioxidant pathways. Therefore, we
hypothesized that BCE induces the expression of HO-1 through the
Nrf-2-ARE pathway.
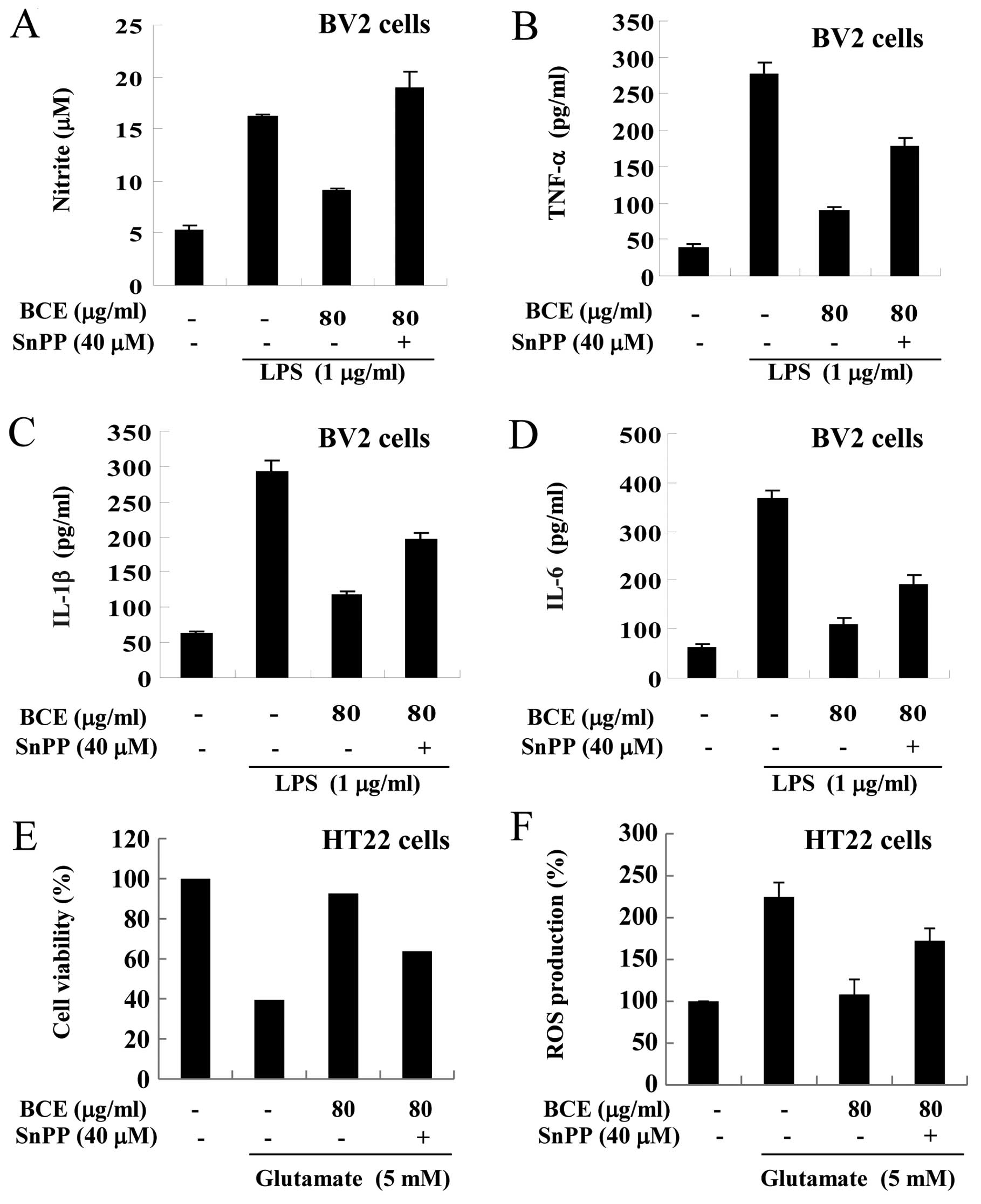
Confirmation of the anti-inflammatory and
antioxidant effects induced by BCE
In order to confirm that the anti-inflammatory and
antioxidant effects of BCE occurred through the HO-1 signaling
pathway, we conducted experiments using a selective inhibitor of
HO-1 (SnPP). First, we examined the expression levels of
pro-inflammatory cytokines in microglial BV2 cells. After treatment
with 80 μg/ml of BCE, microglial BV2 cells were exposed to
SnPP (40 μM) and/or LPS (1 μg/ml) (Fig. 7A–D). The production of NO
increased in only the LPS-stimulated cells and decreased in cells
pre-treated with BCE (80 μg/ml) (Fig. 7A). These effects were reversed in
SnPP-treated cells. A similar reversal was observed in the
production of TNF-α, IL-1β and IL-6 (Fig. 7B–D). Next, we examined whether the
cytoprotective effects of BCE were HO-1-dependent. After treatment
with 80 μg/ml of BCE, hippocampal HT22 cells were exposed to
SnPP (40 μM) and/or glutamate (5 mM). The viability of cells
was reduced in cells treated with glutamate alone, and the
glutamate-induced decrease in cell viability was rescued with BCE
treatment; however, this rescue was absent in SnPP-treated cells
(Fig. 7E). Taken together, these
results demonstrate that the mechanism behind BCE cytoprotective
and ROS reduction activity is dependent on HO-1 activity.
Discussion
Recent studies suggest that neuroinflammation
contributes to the development of neurodegenerative diseases
(11,12). Furthermore, several studies have
shown that anti-inflammatory reactions play an important role in
reducing cytotoxicity (24,25). The results of our study showed
that BCE may protect hippocampal HT22 and microglial BV2 cells from
glutamateand LPS-induced cytotoxicity, respectively. To investigate
whether BCE has neuroprotective and anti-neuroinflammatory effects,
we conducted two types of experiments. We determined whether BCE
had antioxidant effects (i.e., removal of ROS), and in the second
experiment we determined whether BCE had anti-inflammatory effects
(i.e., decrease of pro-inflammatory cytokines, including TNF-α,
IL-1β and IL-6). We discovered that BCE exerted both an antioxidant
and an anti-inflammatory effect in neuronal cells.
LPS is an endotoxin and is found in gram-negative
bacteria, as a constituent of the outer membrane. It induces innate
immunity, including production of pro-inflammatory mediators such
as NO, TNF-α and IL-6 (26). We
discovered that BCE significantly decreased the production of
pro-inflammatory mediators and cytokines in LPS-stimulated
microglial BV2 cells (Fig. 2).
Notably, a previous study revealed that BC extract had
anti-inflammatory effects in a murine model of asthma (27). These data suggested that BC has
strong anti-inflammatory properties in neurons and in airway
cells.
ROS are essential molecules that regulate the redox
status and growth of cells (28).
ROS are highly reactive as they have at least one unpaired
electron. Therefore, excessive production of ROS may promote
harmful reactions that may cause both reversible and irreversible
tissue damage. ROS are implicated in a number of disorders
including neurological diseases, cancer, Parkinson’s and
Alzheimer’s disease (29). The
level of ROS is important since excessive amount of ROS cause
oxidative stress and cell damage by inducing harmful reactions
(28). Therefore, the
ROS-scavenging activity of candidate substances is a suitable
barometer of the potential antioxidant effects. To validate the
antioxidant effects of BCE in microglial BV2 and hippocampal HT22
cells, we examined whether BCE reduces the production of ROS using
flow cytometry. Our data showed that BCE provides a cytoprotective
effect through the reduction of ROS levels in both hippocampal HT22
and microglial BV2 cells (Fig.
4).
A previous study reported that the upregulation of
HO-1 protects cells by degrading pro-oxidant heme to
oxidantscavenging bile pigments, including BV and BR. Moreover, it
focused on the relationship between the neuroprotective effects and
HO-1 signaling (12). In this
study, we confirmed that BCE induces HO-1 expression through the
Nrf-2 signaling pathway (Figs. 5
and 6). We discovered that BCE
induces the upregulation of HO-1, both at the protein and mRNA
levels using western blotting and qRT-PCR (Fig. 5). Further, to identify whether BCE
induces Nrf-2 translocation from the cytosol to the nucleus, we
examined the Nrf-2 level in the nuclear fraction and the promoter
activity by using ARE-reporter plasmid. Results showed that BCE
significantly induced the expression of HO-1 and translocation of
Nrf-2 (Fig. 6).
The data presented in Fig. 7 clearly demonstrate a decisive
role for HO-1 enzymatic activity in the neuroprotective and
anti-neuroinflammatory effects exerted by BCE. We used an HO-1
inhibitor (SnPP) to confirm that the protective effects of BCE were
due to HO-1 signals. The anti-neuroinflammatory effect of BCE was
reversed by SnPP in microglial BV2 cells (Fig. 7A–D). In addition, the protective
effects of BCE on cell viability and ROS production was also
reversed by SnPP in hippocampal HT22 cells (Fig. 7E and F). Thus, it is possible that
neuroprotective and anti-neuroinflammatory effects of BCE may occur
through HO-1 signaling pathways.
In conclusion, the findings of this study suggest
that BCE exhibits neuroprotective and anti-neuroinflammatory
effects in murine microglial BV2 and hippocampal HT22 cells.
Furthermore, we determined that these effects occur through the
Nrf-2/HO-1-mediated pathway. Our results provide a new insight for
understanding the neuroprotective mechanisms of BCE. These effects
of BCE, coupled with its low toxicity, point to a strong potential
for use in the treatment of patients suffering from
neurodegenerative diseases such as Alzheimer’s disease.
Acknowledgements
This research was supported by the
Basic Science Research Program through the National Research
Foundation of Korea (NRF) funded by the Ministry of Education,
Science and Technology (2012R1A12005031).
References
|
1.
|
DJ SelkoeThe molecular pathology of
Alzheimer’s diseaseNeuron64874981991
|
|
2.
|
J VinaA LloretE GiraldoMC BadiaMD
AlonsoAntioxidant pathways in Alzheimer’s disease: possibilities of
interventionCurr Pharm Des17386138642011
|
|
3.
|
ST DheenC KaurEA LingMicroglial activation
and its implications in the brain diseasesCurr Med
Chem1411891197200710.2174/09298670778059796117504139
|
|
4.
|
VH PerryThe influence of systemic
inflammation on inflammation in the brain: implications for chronic
neurodegenerative diseaseBrain Behav
Immun18407413200410.1016/j.bbi.2004.01.00415265532
|
|
5.
|
GS JeongDS LeeTO KwonHS LeeRB AnYC
KimCytoprotective constituents of the heartwood of Caesalpinia
sappan on glutamate-induced oxidative damage in HT22 cellsBiol
Pharm Bull32945949200919420770
|
|
6.
|
TH MurphyM MiyamotoA SastreRL SchnaarJT
CoyleGlutamate toxicity in a neuronal cell line involves inhibition
of cystine transport leading to oxidative
stressNeuron215471558198910.1016/0896-6273(89)90043-32576375
|
|
7.
|
T NguyenP NioiCB PickettThe
Nrf2-antioxidant response element signaling pathway and its
activation by oxidative stressJ Biol
Chem2841329113295200910.1074/jbc.R90001020019182219
|
|
8.
|
T JiangZ HuangJY ChanDD ZhangNrf2 protects
against As(III)-induced damage in mouse liver and bladderToxicol
Appl Pharmacol240814200910.1016/j.taap.2009.06.01019538980
|
|
9.
|
W LiAN KongMolecular mechanisms of
Nrf2-mediated antioxidant responseMol
Carcinog4891104200910.1002/mc.20465
|
|
10.
|
MD MainesThe heme oxygenase system: a
regulator of second messenger gasesAnnu Rev Pharmacol
Toxicol37517554199710.1146/annurev.pharmtox.37.1.5179131263
|
|
11.
|
HM SchipperHeme oxygenase-1 in Alzheimer
disease: a tribute to Moussa YoudimJ Neural
Transm118381387201110.1007/s00702-010-0436-120563825
|
|
12.
|
HM SchipperW SongH ZukorJR HascaloviciD
ZeligmanHeme oxygenase-1 and neurodegeneration: expanding frontiers
of engagementJ
Neurochem110469485200910.1111/j.1471-4159.2009.06160.x19457088
|
|
13.
|
HM SchipperHeme oxygenase expression in
human central nervous system disordersFree Radic Biol
Med3719952011200410.1016/j.freeradbiomed.2004.09.015
|
|
14.
|
M ShibataY YamatakeM SakamotoM KanamoriK
TakagiPhamacological studies on bamboo grass (1). Acute toxicity
and anti-inflammatory and antiulcerogenic activities of
water-soluble fraction (Folin) extracted from Sasa albomarginata
Makino et ShibataNihon Yakurigaku Zasshi714814901975(In
Japanese)
|
|
15.
|
GC BrownJJ NeherInflammatory
neurodegeneration and mechanisms of microglial killing of
neuronsMol
Neurobiol41242247201010.1007/s12035-010-8105-920195798
|
|
16.
|
DH ShinJY ShinSH KimJH KimHJ ChungJC
KimSingle oral dose toxicity study of Bambusae caulis in
Taeniam in ratsJ Toxicol and Public Health203253282004(In
Korean)
|
|
17.
|
Y NakamuraQS SiK
KataokaLipopolysaccharide-induced microglial activation in culture:
temporal profiles of morphological change and release of cytokines
and nitric oxideNeurosci
Res3595100199910.1016/S0168-0102(99)00071-1
|
|
18.
|
J LiuL LiWZ SuoHT22 hippocampal neuronal
cell line possesses functional cholinergic propertiesLife
Sci84267271200910.1016/j.lfs.2008.12.00819135458
|
|
19.
|
L MedaMA CassatellaGI SzendreiL Otvos JrP
BaronM VillalbaD FerrariF RossiActivation of microglial cells by
beta-amyloid protein and
interferon-gammaNature374647650199510.1038/374647a07715705
|
|
20.
|
J FangH QinT SekiH NakamuraK TsukigawaT
ShinH MaedaTherapeutic potential of pegylated hemin for reactive
oxygen species-related diseases via induction of heme oxygenase-1:
results from a rat hepatic ischemia/reperfusion injury modelJ
Pharmacol Exp Ther339779789201110.1124/jpet.111.185348
|
|
21.
|
KA JungMK KwakThe Nrf2 system as a
potential target for the development of indirect
antioxidantsMolecules1572667291201010.3390/molecules1510726620966874
|
|
22.
|
PA DenneryRegulation and role of heme
oxygenase in oxidative injuryCurr Top Cell
Regul36181199200010.1016/S0070-2137(01)80008-X10842752
|
|
23.
|
YJ SurhJK KunduMH LiHK NaYN ChaRole of
Nrf2-mediated heme oxygenase-1 upregulation in adaptive survival
response to nitrosative stressArch Pharm
Res3211631176200910.1007/s12272-009-1807-819727608
|
|
24.
|
T Ben-HurO Ben-MenachemV FurerO EinsteinR
Mizrachi-KolN GrigoriadisEffects of proinflammatory cytokines on
the growth, fate, and motility of multipotential neural precursor
cellsMol Cell
Neurosci24623631200310.1016/S1044-7431(03)00218-514664813
|
|
25.
|
CT EkdahlJH ClaasenS BondeZ KokaiaO
LindvallInflammation is detrimental for neurogenesis in adult
brainProc Natl Acad Sci
USA1001363213637200310.1073/pnas.223403110014581618
|
|
26.
|
T SeoS ChaTI KimJS LeeKM
WooPorphyromonas gingivalis-derived
lipopolysaccharide-mediated activation of MAPK signaling regulates
inflammatory response and differentiation in human periodontal
ligament fibroblastsJ
Microbiol50311319201210.1007/s12275-012-2146-x
|
|
27.
|
J RaS LeeHJ KimYP JangH AhnJ
KimBambusae Caulis in Taeniam extract reduces
ovalbumin-induced airway inflammation and T helper 2 responses in
miceJ Ethnopharmacol128241247201010.1016/j.jep.2010.01.023
|
|
28.
|
KJ DaviesOxidative stress: the paradox of
aerobic lifeBiochem Soc Symp6113119958660387
|
|
29.
|
H MaedaT AkaikeOxygen free radicals as
pathogenic molecules in viral diseasesProc Soc Exp Biol
Med198721727199110.3181/00379727-198-43309C1656471
|