Introduction
Cerebral ischemia is considered a major cause of
morbidity and mortality worldwide. Excitotoxicity is a major
pathophysiological mechanism associated with stroke-induced
inflammation and oxidative stress-associated disruption of the
blood-brain barrier, resulting in enhancing neuronal cell death
during ischemic stroke (1).
Recombinant tissue plasminogen activator (t-PA) is the preferred
treatment for acute ischemic stroke, however, due to a limited time
window few patients are able to receive this therapy. Furthermore,
t-PA treatment is likely to increase the risk of hemorrhage
(2). Therefore, development of
alternative therapies for ischemic stroke is important.
Mounting evidence indicates that potential neural
stem/progenitor cells are present in various brain regions outside
of the subventricular zone (SVZ) and subgranular zone of the
hippocampal dentate gyrus (3,4).
Stroke-induced neurogenesis has also been demonstrated in the adult
human brain, even in patients of advanced age (5–7),
indicating potential involvement of neural stem cells (8) and neurogenesis-regulating factors
(9). Various factors regulate
neurogenesis during development. One developmental molecule of
particular interest in this regard is retinoic acid (RA), which is
involved in the normal development of the central nervous system
(10) and is also critical in the
adult brain (11,12). RA expression and signaling
continues in the postnatal and adult brain, stimulating neonatal
SVZ and adult hippocampal neurogenesis (13–16). The hypothesis that RA is crucial
in the transcriptional model after nerve injury is supported by
indirect evidence regarding the regulation of trauma-related genes,
by observations of retinoid receptors and binding proteins after
nerve injury and by dorsal root ganglia, retinal and spinal cord
explant cultures (17).
Retinoid-binding proteins are expressed in the SVZ-olfactory bulb
pathway and RA receptors persist in the olfactory bulb (18). Transplantation of neural cells
obtained from RA-treated cynomolgus monkey embryonic stem cells
successfully enhanced the motor function of hemiplegic mice with
experimental brain injury (19).
Acupuncture has been used to treat neurologic
conditions, and acupuncture reportedly enhances cell proliferation
in the neurogenic area (hippocampal dentate gyrus and the SVZ of
the lateral ventricle walls) in pathological conditions (20–22), which is associated with enhanced
brain function (23,24). The LI11 (Quchi) and ST36 (Zusanli)
acupoints have been selected for brain injury rehabilitation.
Electroacupuncture (EA) at these acupoints has the potential to
stimulate cell proliferation and reduce brain injury (20,21,25–27). However, the mechanisms underlying
the effects of acupuncture are poorly understood. Thus, whether or
not the neuroprotective effects of EA are mediated by regulation of
the RA signaling pathway should be investigated.
To extend the clinical observations of the potential
neuroprotective effect of EA and help to establish a scientific
foundation for further research, the effect of EA on the infarct
volume, neurological functional recovery, and the expression of RA
mRNA and protein were examined in this study. EA was found to
decrease the infarct volume, promote neurological functional
recovery and regulate the expression of RA mRNA and protein. This
finding suggests that promoting neurological functional recovery
through modulating RA expression in the post-ischemic brain is one
of the mechanisms by which EA can be effective in the treatment of
ischemic stroke.
Materials and methods
Reagents
The Raldh1, Raldh2 and β-actin primers were
purchased from Sangon Biotech (Shanghai) Co., Ltd. (Shanghai,
China). Raldh1 and Raldh2 antibodies, horseradish peroxidase
(HRP)-conjugated secondary antibodies and the antibody against
β-actin were obtained from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Any other chemicals used, unless otherwise
stated, were obtained from Sigma Chemical Company (St. Louis, MO,
USA).
Animals
Healthy and pathogen-free male Sprague-Dawley rats
(n=216), weighing 220–280 g, were purchased from SLAC Laboratory
Animal Co., Ltd., Shanghai, China (Laboratory Animal Use
Certificate no. SCXK(SH)2007000509570) and raised in a sterile
environment. The care and use of the laboratory animals complied
with the Guidance Suggestions for the Care and Use of Laboratory
Animals, enforced by the Ministry of Science and Technology, China
(2006) (28).
Animal model of transient middle cerebral
artery occlusion
Animals were housed in a 12-h reverse light-dark
cycle and were provided with food and water ad libitum. Rats
were anesthetized with 10% chloral hydrate (3.5 ml/kg body weight).
Body temperature was maintained using a 37°C water-recirculating
pad. Transient middle cerebral artery occlusion (tMCAO) was
produced for 120 min using the external carotid artery insertion
method as described previously (29). Briefly, the left common carotid
artery was exposed through a midline incision, and the internal and
external carotid arteries were separated. A 3.0-mm nylon
monofilament with a tip rounded by heat was placed in the external
carotid artery and advanced through the internal carotid artery
until resistance occurred. The monofilament was left in place for
120 min and then removed under anesthesia. Animals were observed
until recovery from anesthesia. The sham group was treated in the
same way, although tMCAO was not induced. This protocol produced
infarcts involving the striatum and cortex, with a mortality rate
of ~30%. The rats were subsequently randomized into 3 experimental
groups: EA group (n=72), model group (n=72) and sham group (n=72).
The rats were tested at 1, 7, 14 and 28 days subsequent to tMCAO.
Eighteen rats were decapitated at each time point in each
experimental group, after the neurological function was tested. Six
rats were used for triphenyltetrazolium hydrochloride (TTC)
staining, 6 rats were used for western blotting (Raldh1 and Raldh2
protein measurement), and the remaining 6 were used for Raldh1 and
Raldh2 mRNA measurement.
Treatment
In the EA group, EA was applied to acupoints for
hemiplegia. The acupoint prescription included ST36 (Zusanli, 5 mm
below the head of fibula under the knee joint and 2 mm lateral to
the anterior tibial tubercle) and LI11 (Quchi, in the depression
lateral to the anterior aspect of the radius joint of the forelimb)
at a depth of 5 mm into the skin with a stainless needle measuring
0.25×20 mm in length with a guide-tube for 20 min (Wujiang Shenli
Medical & Health Material Co., Ltd., Wujiang, China). Electric
stimulation was generated using an electrical stimulator (Huatuo
SDZ-II; Suzhou Medical Appliance Factory Co., Ltd., China) for 20
min and the stimulation parameter exhibited disperse-dense waves of
a frequency of 5/20 Hz (28.5 msec/15 msec pulse duration) and a
current density of 2–4 mA. Model and sham groups did not undergo
any EA, which was administered once a day for a period of 4
weeks.
Behavioral testing
Neurological function deficits were evaluated using
the HomeCageScan system at 1, 7, 14 and 28 days after tMCAO. Each
rat was placed in a separate cage identical to its home-cage, with
fresh bedding, food and water. A video camera (Sony DCR-HC62) was
positioned perpendicular to the long axis of the cage so that the
field of view included the entire length of the cage. The rats were
acclimatized to their new environment for 1 h and activity was
recorded for 24 h (12-hour light/dark cycle) under standard
fluorescent lighting. The rats were then returned to their
home-cages. The full length of each video was analyzed using
HomeCageScan (version 3.0; Clever Systems, Inc.) for the
quantification of rat motor deficits, specifically quantifying
behaviors including grooming, feeding, walking and rearing. The sum
of the time spent in each behavior was calculated, yielding an
index of total activity.
Measurement of infarct volume
Infarct volume was assessed at 1, 7, 14 and 28 days
after tMCAO using TTC staining. Animals were sacrificed by 10%
chloral hydrate overdose, brains were rapidly removed, sectioned
coronally at 2-mm intervals from the frontal pole and immersed in
TTC (2%) at 37°C for 20 min, followed by formaldehyde (4%) for 15
min. The hemispheric infarcted area in each section was calculated
by subtracting the area of normal TTC-stained brain in the
ipsilateral cortex from the contralateral area. The data were
analyzed with a computer-based image analysis system (Adobe
Photoshop 8.0). The sum of the areas of all the sections was
calculated and then multiplied by 2 mm, yielding the volume.
RNA extraction and RT-PCR analysis
A total of 72 rats (n=6/group) were sacrificed by
decapitation at 1, 7, 14 and 28 days after tMCAO. The ischemic zone
of rat brain was rapidly removed and stored at −80°C for subsequent
analysis. Total RNA from brain tissue was isolated with TRIzol
reagent (Invitrogen). Oligo (dT)-primed RNA (1 μg) was
reverse-transcribed with SuperScript II reverse transcriptase
(Promega) according to the manufacturer’s instructions. The
obtained cDNA was used to determine the mRNA amount of Raldh1 or
Raldh2 by PCR with Taq DNA polymerase (Fermentas). β-actin was used
as an internal model. The primers used in these reactions are
listed in Table I. The amplified
products were analyzed by 1.5% agarose gel electrophoresis. Optical
density ratios for Raldh1, Raldh2 and β-actin were used for the
semi-quantitative analyses.
 | Table IPT-PCR primers. |
Table I
PT-PCR primers.
| Gene | Primer
sequence | Annealing
temperature (°C) |
|---|
| Raldh1 | Forward
5′-CTTCTTTGTCCAGCCCACAGTCT-3′ | |
| Reverse
5′-GTTCACCCAGTTCTCGTCCATTT-3′ | 55 |
| Raldh2 | Forward
5′-GTCGTCAACATTCTGCCAGGGTAT-3′ | |
| Reverse
5′-CTGCTCCACAGCGTAGTCCAAGTC-3′ | 56 |
| β-actin | Forward
5′-ACTGGCATTGTGATGGACTC-3′ | |
| Reverse
5′-CAGCACTGTGTTGGCATAGA-3′ | 55 |
Western blot analysis
The ischemic zone of rat brain was sectioned, and
the corresponding samples were homogenized with a homogenizer in
lysis buffer (NP-40 lysis buffer and 100 mM PMSF). The homogenates
were centrifuged at 14,000 rpm for 30 min at 4°C. The supernatant
was collected and the total protein content was determined using a
Micro BCA protein assay kit with bovine serum albumin as the
standard (Pierce Chemical, Rockford, IL, USA). Equal amounts of
protein (20 μg) were boiled in loading buffer and separated by 12%
SDS-PAGE gel under reducing condition using 80 V for 1 h. The
proteins were then electrophoretically transferred onto
nitrocellulose membranes using the iBlot Western Detection
Stack/iBlot Dry Blotting system (Invitrogen). Membranes were
blocked for 30 min with agitation at RT in SuperBlock T20 (TBS)
blocking buffer (Thermo Scientific, Rockford, IL, USA). Membranes
were washed in TBS with 0.25% Tween-20 (TBST) and exposed to
primary antibodies against Raldh1 or Raldh2 (1:1,000) overnight at
4°C on a rocking platform or rotator. β-actin (1:1,000) was also
measured as an internal control for protein loading. After
membranes were washed in TBST, secondary horseradish peroxidase
(HRP)-conjugated antibodies (anti-goat) were added at 1:5,000
dilution for 1 h at room temperature and the membranes were washed
again in TBST. The antibody-bound protein bands were then detected
with ECL, and images were captured using a Bio-Rad ChemiDoc XRS+
(Bio-Rad, Hercules, CA, USA). The ratio of gray scale values of the
target protein to the internal control was used to measure the
relative amount of Raldh1 and Raldh2.
Statistical analysis
Data are presented as the means ± SD for the
indicated number of independently performed experiments.
Comparisons between multiple groups were conducted with one-way
ANOVA, whereas within each group data were analyzed with analysis
of intraclass variance analysis. Differences between the 2 groups
were assessed using the Student’s t-test. P-values of <0.05 and
<0.01 were considered as significant and highly significant
differences, respectively.
Results
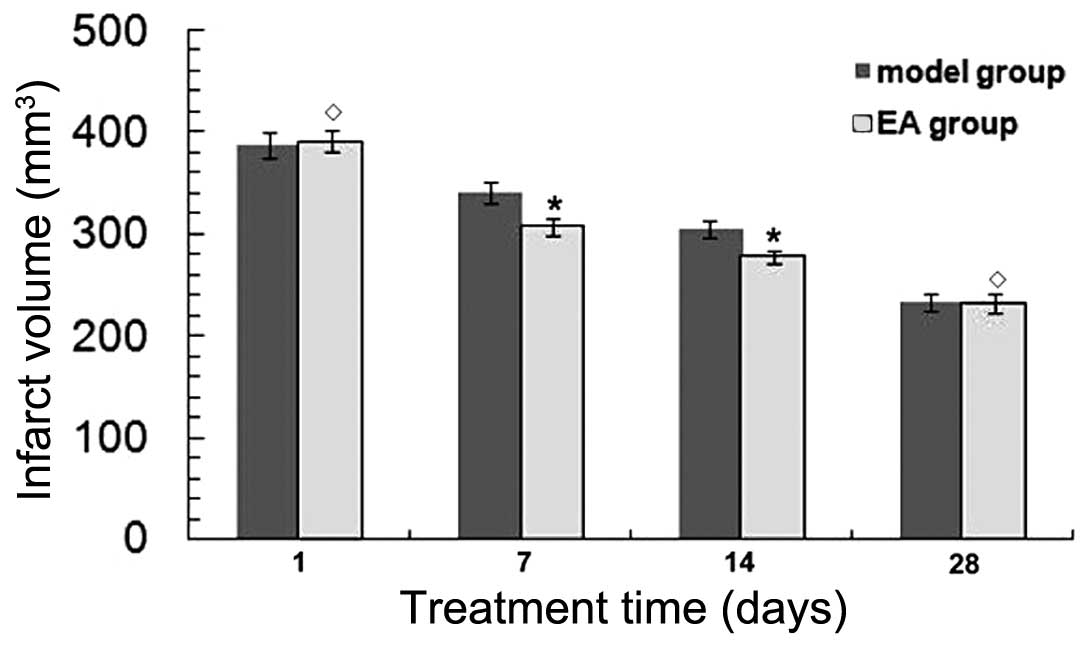
RA reduces infarct volume in rats
following tMCAO
TTC staining was performed to determine the infarct
volumes in brain sections at 1, 7, 14 and 28 days after tMCAO. The
infarct volume was found to be decreased in a time-dependent manner
in both the EA and model groups (P<0.05) (Fig. 1). At 7 and 14 days, the infarct
volume was significantly decreased in the EA group (P<0.05) vs.
the model group. No significant differences were detected in the
infarct volume between the EA and model groups at 1 and 28 days
after tMCAO (P>0.05), indicating that rats were able to recover
naturally after tMCAO.
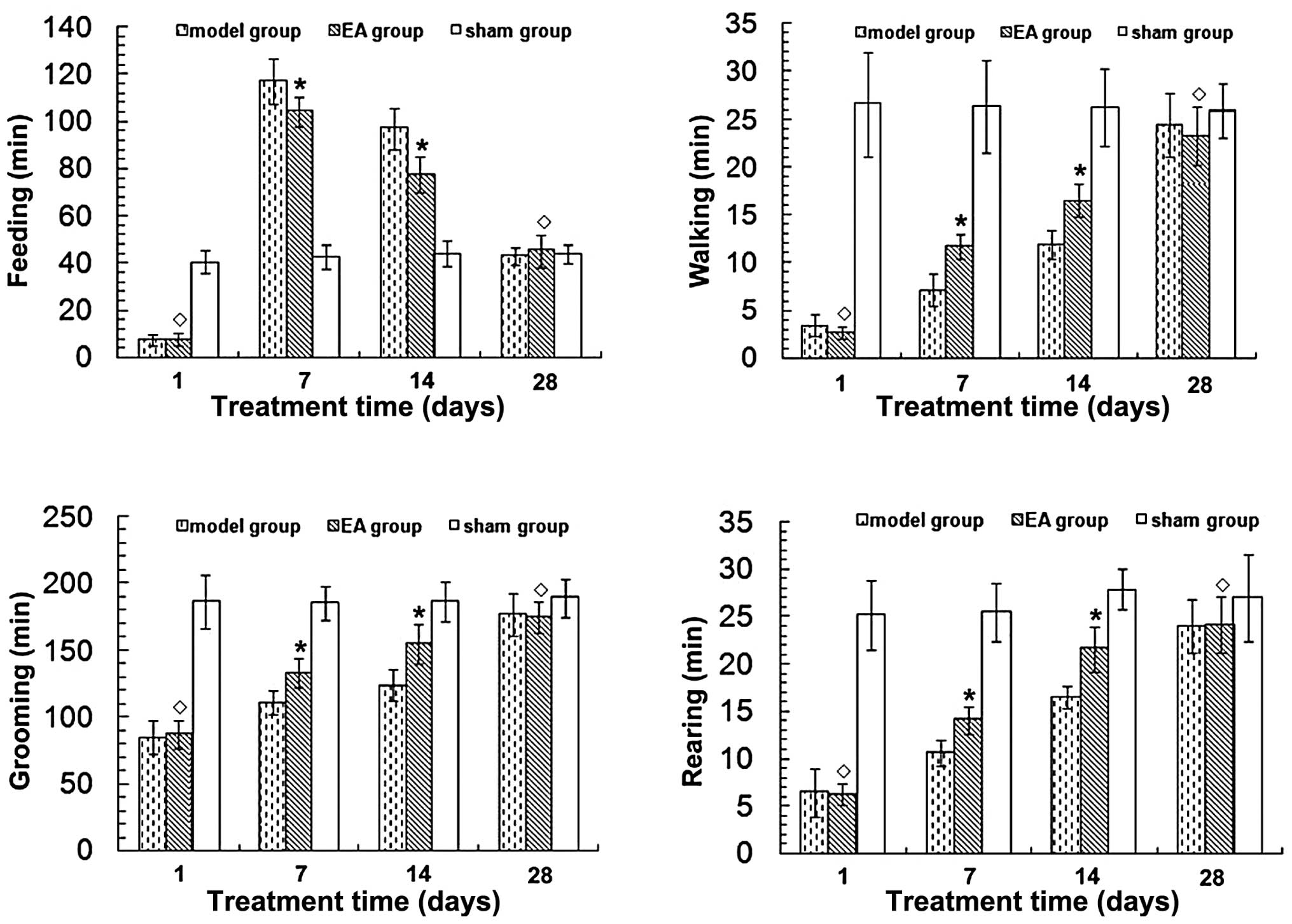
RA improves neurological function in rats
following tMCAO
Cerebral cortex infarction caused neurological
function deficits in rats, mainly manifested as right forelimb
paralysis. To quantify this deficit, 24-h video recordings were
analyzed using a software program designed for rat home-cage
behavioral assessment. Neurological function was found to have
improved in a time-dependent manner in the EA and model groups
(P<0.05) (Fig. 2). Time spent
walking, rearing and grooming was increased, while time spent
feeding was reduced in the EA group 7 and 14 days after tMCAO
(P<0.05) compared with the model group. No significant
differences in neurological function were observed between the EA
and model groups at 1 and 28 days after tMCAO (P>0.05). No
neurological function deficits were evident in the sham group.
EA treatment regulates the expression of
Raldh1 and Raldh2 after tMCAO
To explore the mechanism of the potential
neuroprotective effect of EA, RT-PCR and western blot analysis were
carried out to examine the mRNA and protein expression in the
ischemic brain after tMCAO. In the model group, Raldh1 and Raldh2
mRNA was greatly increased at 7 days, peaked at 14 days and then
gradually decreased (Fig. 3).
Additionally, in the model group, higher Raldh1 and Raldh2 mRNA
levels were found at 7 and 14 days (P<0.05) compared with the
sham group. In the EA group, the Raldh1 and Raldh2 mRNA levels were
higher than those in the model group at 7 and 14 days (P<0.05).
No significant differences were detected in the Raldh1 and Raldh2
mRNA expression levels between the EA and model groups at 1 and 28
days after tMCAO (P>0.05), and the pattern of protein expression
of Raldh1 and Raldh2 was similar to their respective mRNA levels
(Fig. 4). These findings suggest
that EA promotes neurological functional recovery in rats following
tMCAO through the regulation of expression of the RA family.
Discussion
Neural stem cells from the adult forebrain SVZ give
rise to olfactory bulb interneurons, while those in the hippocampal
dentate gyrus generate new neurons in the granule cell layer
(18). Brain insults such as
seizure, stroke and trauma activate neural stem cells to
proliferate more rapidly, migrate into injured regions and form new
neurons and glia (30–36).
Retinoids, in particularly the active metabolite of
vitamin A, RA, is known to stimulate neonatal SVZ and adult
hippocampal neurogenesis (14–16). Retinol and its derivatives exert
their biological actions via specific nuclear receptors (RARs and
RXRs) that regulate gene transcription (37). RA receptors also interact with
other nuclear receptors that possess neuroprotective properties,
such as 1,25-dihydroxyvitamin D (9) that is neuroprotective against stroke
(38) and RXR receptors that form
heterodimers with the vitamin D3 receptor (RXR-VDR). RXR is also
able to form a dimer with thyroid hormone receptors, and thyroid
hormone derivatives are protective against infarction (39). Thus, the neuroprotective effects
of retinol and RA may be mediated, at least in part, by their
formation of heterodimers with other nuclear receptors. In addition
to its effect on nuclear receptors, retinol exerts acute effects on
other potentially protective biological sites including calcium
channels and gap junction channels, as well as having antioxidant
properties. Calcium channel antagonists and antioxidants protect
the brain from ischemia in animal models of stroke (40). RA also induced the expression of
midkine, which is protective against brain ischemia (41).
RA is synthesized from retinol in a two-step pathway
involving oxidation first to retinaldehyde, then to RA. The former
step is predominantly catalyzed by retinol dehydrogenases, the
latter step by retinaldehyde dehydrogenases (Raldhs), which are
encoded by Raldh1, Raldh2 and Raldh3 expressed in non-overlapping
patterns. Raldh3 is expressed only in the eye and facial
region/ventral retina and the ganglionic eminence of the
telencephalon. Later in development, this enzyme can be detected by
zymography in a variety of sites, including the liver and adult
skin. Raldh1 is present in only the dorsal retina, ventral
mesencephalon and projections from here to the corpus striatum and
the otic vesicle regions (data not shown). Although Raldh2 is
absent from most of the brain proper, it is strongly expressed in
the leptomeninges that surround it (42). In the present study, we found that
the Raldh1 and Raldh2 mRNA levels of the EA and model groups were
greatly increased at 7 days, peaked at 14 days and gradually
decreased at 28 days after tMCAO. A marked increase was observed in
the Raldh1 and Raldh2 mRNA levels at 7 and 14 days in the EA group
(P<0.05) compared with the model group. Expression of the Raldh1
and Raldh2 protein was similar to that of Raldh1 and Raldh2
mRNA.
Behavior is known to be the ultimate and most
complex output of the brain. To quantify neurological function
deficit, 24-h video recordings were conducted and digitally
analyzed. The assessment of locomotor deficits using this method
has been established in models of Huntington’s and prion disease
(43), with a similar approach
having been described recently in a rat ICH model (44). Compared with traditional methods
of behavioral assessment in stroke models, this digital approach
has a number of benefits (45–47). First, it requires minimal
investigator participation, reducing labor costs and the potential
for bias. Second, it facilitates the analysis of night-time
behavior. The validity of daytime testing of nocturnal animals in
stroke models has never been established and is questionable given
the behavioral inhibition and cognitive disturbances observed in
rats during light phase testing. Third, this digital approach
measures behaviors that are directly relevant to the animal’s
functional status. Most importantly, however, it eliminates the
variability inherent in measuring brief episodes of behavior in
animals stressed by environmental variation and human
interference.
In this study, we found that time spent walking,
rearing and grooming was markedly increased, whereas time spent
feeding was reduced with locomotor activity improvement in the EA
group at 7 and 14 days after tMCAO, compared with that in the model
group (P<0.05). The changes we found in the neurological
function were concomitant with improvements in RA mRNA and protein
expression. These results therefore suggest that the administration
of EA is likely to promote neurological functional recovery and
regulate the expression of RA mRNA and protein. To the best of our
knowledge, this is the first study to report that EA regulates RA
expression.
It was also demonstrated that there was no
significant difference in the infarct volume, neurological
function, expression of RA mRNA and protein between the EA and
model groups at 28 days after tMCAO (P>0.05), indicating that
rats were able to recover naturally after tMCAO. Spontaneous
recovery may be due to neurite regeneration and synapse remodeling,
as well as a reduction in brain edema. A series of plasticity
responses after tMCAO, including the release of neurotrophic
factors, synthesis of neuron-specific proteins and rise of synaptic
excitation, is involved in the process of functional recovery
(48). Additionally, our study
examined the correlation between neurogenesis and the use of young
rats in behavioral recovery. The findings of this study are in
contrast with the reality that the elderly population is most often
affected by strokes and these people, unlike young healthy rodents,
also have a multiplicity of other disease co-morbidities, such as
hypertension and diabetes that along with increased age, are likely
to limit or alter the neurogenic response post-stroke. Indeed, aged
animals exhibit stroke-induced neurogenesis, however, their
response is ‘muted’ compared with young animals (49,50). Although the spontaneous recovery
of neurological function deficit may occur after tMCAO, the
administration of EA significantly promoted neural function
recovery and decreased infarct volume. Raldh1 and Raldh2 mRNA and
protein expression was increased by EA treatment following tMCAO
compared to the model group.
In conclusion, administration of EA decreased the
infarct volume, promoted neurological functional recovery and
demonstrated that this neuroprotective effect is attributed, at
least in part, to the expression of RA mRNA and protein. These
findings likely provide an experimental basis for the treatment of
ischemic stroke by electroacupuncture.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 30901935) and the Key Laboratory
for Orthopedics and Sports Rehabilitation of the Ministry of
Education, China.
References
|
1
|
Lo EH, Dalkara T and Moskowitz MA:
Mechanisms, challenges and opportunities in stroke. Nat Rev
Neurosci. 4:399–415. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Madden K: Optimal timing of thrombolytic
therapy in acute ischaemic stroke. CNS Drugs. 16:213–218. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Willaime-Morawek S and van der Kooy D:
Cortex- and striatum-derived neural stem cells produce distinct
progeny in the olfactory bulb and striatum. Eur J Neurosci.
27:2354–2362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakagomi T, Taguchi A, Fujimori Y, Saino
O, Nakano-Doi A, Kubo S, Gotoh A, Soma T, Yoshikawa H, Nishizaki T,
Nakagomi N, Stern DM and Matsuyama T: Isolation and
characterization of neural stem/progenitor cells from post-stroke
cerebral cortex in mice. Eur J Neurosci. 29:1842–1852. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang
Y, Shen J, Mao Y, Banwait S and Greenberg DA: Evidence for
stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci
USA. 103:13198–13202. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Macas J, Nern C, Plate KH and Momma S:
Increased generation of neuronal progenitors after ischemic injury
in the aged adult human forebrain. J Neurosci. 26:13114–13119.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Minger SL, Ekonomou A, Carta EM, Chinoy A,
Perry RH and Ballard CG: Endogenous neurogenesis in the human brain
following cerebral infarction. Regen Med. 2:69–74. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Landgren H and Curtis MA: Locating and
labeling neural stem cells in the brain. J Cell Physiol. 226:1–7.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hodge RD and Hevner RF: Expression and
actions of transcription factors in adult hippocampal neurogenesis.
Dev Neurobiol. 71:680–689. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ross SA, McCaffery PJ, Drager UC and De
Luca LM: Retinoids in embryonal development. Physiol Rev.
80:1021–1054. 2000.PubMed/NCBI
|
|
11
|
McCaffery P, Zhang J and Crandall JE:
Retinoic acid signaling and function in the adult hippocampus. J
Neurobiol. 66:780–791. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Werner EA and Deluca HF: Retinoic acid is
detected at relatively high levels in the CNS of adult rats. Am J
Physiol Endocrinol Metab. 282:E672–E678. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Calza L, Giuliani A, Fernandez M, Pirondi
S, D’Intino G, Aloe L and Giardino L: Neural stem cells and
cholinergic neurons: regulation by immunolesion and treatment with
mitogens, retinoic acid, and nerve growth factor. Proc Natl Acad
Sci USA. 100:7325–7330. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haskell GT and LaMantia AS: Retinoic acid
signaling identifies a distinct precursor population in the
developing and adult forebrain. J Neurosci. 25:7636–7647. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang TW, Zhang H and Parent JM: Retinoic
acid regulates postnatal neurogenesis in the murine subventricular
zone-olfactory bulb pathway. Development. 132:2721–2732. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jacobs S, Lie DC, DeCicco KL, Shi Y,
DeLuca LM, Gage FH and Evans RM: Retinoic acid is required early
during adult neurogenesis in the dentate gyrus. Proc Natl Acad Sci
USA. 103:3902–3907. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mey J and McCaffery P: Retinoic acid
signaling in the nervous system of adult vertebrates.
Neuroscientist. 10:409–421. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Plane JM, Whitney JT, Schallert T and
Parent JM: Retinoic acid and environmental enrichment alter
subventricular zone and striatal neurogenesis after stroke. Exp
Neurol. 214:125–134. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ikeda R, Kurokawa MS, Chiba S, Yoshikawa
H, Ide M, Tadokoro M, Nito S, Nakatsuji N, Kondoh Y, Nagata K,
Hashimoto T and Suzuki N: Transplantation of neural cells derived
from retinoic acid-treated cynomolgus monkey embryonic stem cells
successfully improved motor function of hemiplegic mice with
experimental brain injury. Neurobiol Dis. 20:38–48. 2005.
View Article : Google Scholar
|
|
20
|
Kim EH, Jang MH, Shin MC, Lim BV, Kim HB,
Kim YJ, Chung JH and Kim CJ: Acupuncture increases cell
proliferation and neuropeptide Y expression in dentate gyrus of
streptozotocin-induced diabetic rats. Neurosci Lett. 327:33–36.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim EH, Kim YJ, Lee HJ, Huh Y, Chung JH,
Seo JC, Kang JE, Lee HJ, Yim SV and Kim CJ: Acupuncture increases
cell proliferation in dentate gyrus after transient global ischemia
in gerbils. Neurosci Lett. 297:21–24. 2001. View Article : Google Scholar
|
|
22
|
Liu Q, Yu J, Mi WL, Mao-Ying QL, Yang R,
Wang YQ and Wu GC: Electroacupuncture attenuates the decrease of
hippocampal progenitor cell proliferation in the adult rats exposed
to chronic unpredictable stress. Life Sci. 81:1489–1495. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng H, Yu J, Jiang Z, Zhang X, Liu C,
Peng Y, Chen F, Qu Y, Jia Y, Tian Q, Xiao C, Chu Q, Nie K, Kan B,
Hu X and Han J: Acupuncture improves cognitive deficits and
regulates the brain cell proliferation of SAMP8 mice. Neurosci
Lett. 432:111–116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Naylor AS, Bull C, Nilsson MK, Zhu C,
Bjork-Eriksson T, Eriksson PS, Blomgren K and Kuhn HG: Voluntary
running rescues adult hippocampal neurogenesis after irradiation of
the young mouse brain. Proc Natl Acad Sci USA. 105:14632–14637.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yun SJ, Park HJ, Yeom MJ, Hahm DH, Lee HJ
and Lee EH: Effect of electroacupuncture on the stress-induced
changes in brain-derived neurotrophic factor expression in rat
hippocampus. Neurosci Lett. 318:85–88. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao J, Wang S, Wang X and Zhu C:
Electroacupuncture enhances cell proliferation and neuronal
differentiation in young rat brains. Neurol Sci. 32:369–374. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jang MH, Shin MC, Lee TH, Lim BV, Shin MS,
Min BI, Kim H, Cho S, Kim EH and Kim CJ: Acupuncture suppresses
ischemia-induced increase in c-Fos expression and apoptosis in the
hippocampal CA1 region in gerbils. Neurosci Lett. 347:5–8. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
The Ministry of Science and Technology of
the People’s Republic of China. Guidance Suggestions for the Care
and Use of Laboratory Animals. 2006 09 30
|
|
29
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Arvidsson A, Collin T, Kirik D, Kokaia Z
and Lindvall O: Neuronal replacement from endogenous precursors in
the adult brain after stroke. Nat Med. 8:963–970. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Goings GE, Sahni V and Szele FG: Migration
patterns of subventricular zone cells in adult mice change after
cerebral cortex injury. Brain Res. 996:213–226. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lichtenwalner RJ and Parent JM: Adult
neurogenesis and the ischemic forebrain. J Cereb Blood Flow Metab.
26:1–20. 2006. View Article : Google Scholar
|
|
33
|
Parent JM, Valentin VV and Lowenstein DH:
Prolonged seizures increase proliferating neuroblasts in the adult
rat subventricular zone-olfactory bulb pathway. J Neurosci.
22:3174–3188. 2002.PubMed/NCBI
|
|
34
|
Parent JM, Vexler ZS, Gong C, Derugin N
and Ferriero DM: Rat forebrain neurogenesis and striatal neuron
replacement after focal stroke. Ann Neurol. 52:802–813. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Parent JM, Yu TW, Leibowitz RT, Geschwind
DH, Sloviter RS and Lowenstein DH: Dentate granule cell
neurogenesis is increased by seizures and contributes to aberrant
network reorganization in the adult rat hippocampus. J Neurosci.
17:3727–3738. 1997.PubMed/NCBI
|
|
36
|
Zhang RL, Zhang ZG, Zhang L and Chopp M:
Proliferation and differentiation of progenitor cells in the cortex
and the subventricular zone in the adult rat after focal cerebral
ischemia. Neuroscience. 105:33–41. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liden M and Eriksson U: Understanding
retinol metabolism: structure and function of retinol
dehydrogenases. J Biol Chem. 281:13001–13004. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Y, Chiang YH, Su TP, Hayashi T,
Morales M, Hoffer BJ and Lin SZ: Vitamin D(3) attenuates cortical
infarction induced by middle cerebral arterial ligation in rats.
Neuropharmacology. 39:873–880. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Doyle KP, Suchland KL, Ciesielski TM,
Lessov NS, Grandy DK, Scanlan TS and Stenzel-Poore MP: Novel
thyroxine derivatives, thyronamine and 3-iodothyronamine, induce
transient hypothermia and marked neuroprotection against stroke
injury. Stroke. 38:2569–2576. 2007. View Article : Google Scholar
|
|
40
|
Sato Y, Meller R, Yang T, Taki W and Simon
RP: Stereo-selective neuroprotection against stroke with vitamin A
derivatives. Brain Res. 1241:188–192. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Harvey BK, Shen H, Chen GJ, Yoshida Y and
Wang Y: Midkine and retinoic acid reduce cerebral infarction
induced by middle cerebral artery ligation in rats. Neurosci Lett.
369:138–141. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
McCaffery P and Drager UC: Regulation of
retinoic acid signaling in the embryonic nervous system: a master
differentiation factor. Cytokine Growth Factor Rev. 11:233–249.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Steele AD, Jackson WS, King OD and
Lindquist S: The power of automated high-resolution behavior
analysis revealed by its application to mouse models of
Huntington’s and prion diseases. Proc Natl Acad Sci USA.
104:1983–1988. 2007.PubMed/NCBI
|
|
44
|
Otero L, Zurita M, Aguayo C, Bonilla C,
Rodríguez A and Vaquero J: Video-Tracking-Box linked to Smart
software as a tool for evaluation of locomotor activity and
orientation in brain injured rats. J Neurosci Methods. 188:53–57.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Roedel A, Storch C, Holsboer F and Ohl F:
Effects of light or dark phase testing on behavioural and cognitive
performance in DBA mice. Lab Anim. 40:371–381. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Spruijt BM and DeVisser L: Advanced
behavioural screening: automated home cage ethology. Drug Discov
Today Technol. 3:231–237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen L, Zhang X, Chen-Roetling J and Regan
RF: Increased striatal injury and behavioral deficits after
intracerebral hemorrhage in hemopexin knockout mice. J Neurosurg.
114:1159–1167. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Stichel CC and Muller HW: Experimental
strategies to promote axonal regeneration after traumatic central
nervous system injury. Prog Neurobiol. 56:119–148. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gao P, Shen F, Gabriel RA, Law D, Yang E,
Yang GY, Young WL and SU H: Attenuation of brain response to
vascular endothelial growth factor-mediated angiogenesis and
neurogenesis in aged mice. Stroke. 40:3596–3600. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Leasure JL and Grider M: The effect of
mild post-stroke exercise on reactive neurogenesis and recovery of
somatosensation in aged rats. Exp Neurol. 226:58–67. 2010.
View Article : Google Scholar : PubMed/NCBI
|