Introduction
The importance of autoimmune-mediated mechanisms in
the pathogenesis of myocarditis and dilated cardiomyopathy has been
highlighted in recent studies (1,2).
Inflammatory cytokines activate the immune systems of patients or
experimental animals via direct cytotoxic effects and by
stimulating T and B cells. Rat experimental autoimmune myocarditis
(EAM) resembles human giant cell myocarditis (3). Studies using EAM models have shown
that cytokines such as interleukin (IL)-1 and tumor necrosis factor
(TNF)-α play an important role in mediating cardiac dysfunction
(4,5).
IL-1 is an important cytokine and is mainly secreted
by monocytes, macrophages, dendritic cells, B cells and NK cells
(6–8). IL-1 comprises two distinct
molecules, IL-1α and IL-1β, with high sequence homology and
indistinguishable biological activity. Both IL-1α and IL-1β bind to
two primary receptors: type I IL-1 receptor (IL-1RI) and IL-1RII
(9,10). The binding of IL-1RI to IL-1
recruits IL-1Racp to form a heterodimeric receptor, which transmits
a signal. In contrast, IL-1RII serves as a decoy receptor as it
lacks an intracellular domain and does not transmit a signal
(11). The function of IL-1 can
be blocked by two physiological mechanisms: the IL-1 receptor
antagonist (IL-1RA) associates with the IL-1RI to prevent the
binding of IL-1 and restricts the recruitment of IL-1RAcp; IL-1RII,
acting as a binding protein, binds to IL-1 but does not induce
signal transduction as it lacks an intracellular domain. IL-1RII
can also interact with IL-1RAcp (12–14). IL-1RA has been well studied over
recent years and is now being used to treat a variety of diseases
(15–18). However, there are few studies on
IL-1RII, particularly with respect to cardiovascular disease.
Furthermore, IL-17-producing cells (Th17 cells) have been
identified, and studies have shown that Th17 cells play a central
role in the pathogenesis of autoimmune diseases. The role of
IL-1RII in driving Th17 commitment in EAM rats is unknown.
Therefore, the aims of the present study were to
examine the effect of hydrodynamics-based delivery (19,20) of a plasmid encoding IL-1RII-Ig on
EAM rats and to evaluate its possible mechanism.
Materials and methods
Animals
Eight-week-old male Lewis rats were purchased from
Beijing Vital River Lab Animal Technology Co., Ltd. (Beijing,
China) and maintained in our animal facility. All animals were
treated in accordance with the Guidelines for Animal Experiments as
laid out by the Laboratory Animal Center of Xiamen University,
Fujian, China.
Induction of EAM
Cardiac myosin was a kind gift from the Division of
Cardiology, Niigata University Graduate School of Medical and
Dental Sciences, Niigata, Japan (3). For the induction of EAM, each rat
was immunized with 0.2 ml of an emulsion containing cardiac myosin
and an equal volume of complete Freund's adjuvant supplemented with
10 mg/ml Mycobacterium tuberculosis H37RA (Difco
Laboratories, Detroit, MI, USA) by a single s.c. injection in both
footpads on day 0.
Construction of the plasmid DNA for gene
transfer
First, the plasmid vector, pCAGGS-IgGFc, containing
SwaI and NotI restriction sites was prepared. The
control plasmid, pCAGGS-rat signal peptide (SP)-IgGFc, containing
the SP region of the secretory leukocyte protease inhibitor, was
constructed as previously described (18). To construct the pCAGGS-IL-1RII-Ig
plasmid, rat IL-1RII was amplified from EAM heart cDNA using the
following primers, 5′-TTCATTTAAATGTTCATCTTGCTTGTGTTA-3′ and
5′-GCATCGCGGCCGCGGAAGAAACTTCTTTGA-3′. Rat SP was amplified from EAM
heart cDNA using the following primers, 5′-GCCTTCACCATGAAGTAAAG-3′
and 5′-TGTATCCAACAGCATTTCCTTA-3′; and inserted into the vector,
pCAGGS-IgGFc, using SwaI and NotI sites. E.
coli JM109-competent cells were then transformed and the
recombinant plasmids were isolated using an E.Z.N.A. Plasmid Giga
kit (Omega Bio-Tek, USA).
Expression and distribution of IL-RII
mRNA in the rat
Twenty-five rats were divided into two groups: the
control group (n=7) receiving no immunization and the EAM group
(n=18) receiving immunization on day 0. EAM rats were sacrificed on
day 14 (n=6), day 21 (n=6), and day 28 (n=6), respectively, and the
organs (heart, liver, spleen and kidney) were harvested. To detect
the biodistribution and the time course of IL-1RII expression,
total RNA was isolated from the tissue. cDNA synthesis and
real-time PCR of IL-1RII and GAPDH were carried out.
Plasmid DNA injection techniques
Thirty-two rats were randomly divided into two study
groups and one control group, The normal group consisted of rats
not immunized and not injected (n=11). The 21 immunized rats were
divided into two study groups. The IL-1RII group consisted of
immunized rats injected with pCAGGS-IL-1RII-IgFc-(GLU)-tag (n=11),
and the SP group consisted of immunized rats injected with
pCAGGS-SP-IgFc-(GLU)-tag (n=10). Each immunized rat was injected
with 800 μg pCAGGS-IL-1RII-IgFc-(GLU)-tag or
pCAGGS-SP-IgFc-(GLU)-tag dissolved in the appropriate volume of
Ringer's solution via the tail vein within 15 sec (~80 ml/kg body
weight) on day 6. We used the method of hydrodynamics-based gene
transfer, as this method is believed to be the most effective for
facilitating elevated concentration levels.
Plasmid mRNA expression and chimeric
GLU-tag protein measurement
Rats were injected with plasmid
pCAGGS-IL-1RII-IgFc-(GLU)-tag (n=3) or pCAGGS-SP-IgFc-(GLU)-tag
(n=3) with immunization on day 6 and sacrificed on day 7. IL-RII-Ig
or SP-Ig mRNA expression in liver tissues was examined to evaluate
the efficiency of the hydrodynamics-based gene transfer (19). Total RNA was isolated from the
livers, and cDNA was synthesized using M-MLV reverse transcriptase
and oligo(dT). The RT products were amplified by PCR using TaqDNA
polymerase (both were from Fermantas, USA). All PCR products were
resolved using ethidium bromide-stained 2% agarose gels.
To measure plasma concentrations of
IL-1RII-IgFc-GLU-tag proteins during the treatment course, blood
samples were taken on day 7 (n=7), day 12 (n=6), and day 17 (n=7)
respectively, after the hydrodynamics-based gene transfer on day 6.
GLU concentrations were measured using a GLU RIA kit (Daiichi
Radioisotope Laboratories, Tokyo, Japan). Chimeric protein
concentrations were calculated using a GLU-tag.
Echocardiography
Echocardiography was performed on day 16 using a
14-MHz probe (Vivid 7; General Electric, USA). The left ventricular
(LV) end-diastolic diameter (LVEDd), left ventricular end-systolic
diameter (LVEDs), interventricular septal thickness (IVS), LV
posterior wall thickness (LVPW), LV fractional shortening (LVFS),
and the LV ejection fractions (LVEF) were calculated from the
M-mode echocardiograms.
Evaluation of histopathology
All rats were sacrificed on day 17. The heart weight
(without atria) and the body weight were measured, and the ratio of
heart weight-to-body weight (g/g) was calculated. Hearts were fixed
in 10% formalin, paraffin-embedded, and cut into 4-μm transverse
sections for Masson's trichrome staining. The area of the entire
heart and the regions affected by myocarditis (regions showing
myocardial necrosis, inflammatory cell infiltration and myocardial
fibrosis) were calculated using image analysis software (Image-Pro
Plus v. 6.0; Image-Pro, USA).
Relative expression of markers of heart
failure and IL-1-related cytokines in the heart
Total RNA was isolated from the apex of the heart on
day 17. To evaluate the effects of gene therapy, the levels of two
specific heart failure markers, atrial natriuretic peptide (ANP)
and brain natriuretic peptide (BNP), were measured using
quantitative real-time RT-PCR. IL-1-related cytokines in the heart
tissues were also examined including IL-1β, prostaglandin E2
synthases (PGEs), cyclooxygenase (COX-2), and monocyte chemotactic
protein-1 (MCP-1) (Table I).
After an initial denaturation step of 10 min at 95°C, a 2-step
cycling procedure (denaturation at 95°C for 15 sec, annealing and
extension at 60°C for 1 min) was used for 40 cycles. Melting curve
analysis was performed immediately after the amplification step.
The levels of gene expression were normalized to that of GAPDH.
Relative changes in the expression of these molecules was assessed
by comparative analysis of the quantitative real-time PCR results
using the ΔΔCT method (21).
 | Table IPrimers used for RT-PCR and
quantitative RT-PCR. |
Table I
Primers used for RT-PCR and
quantitative RT-PCR.
| Gene | Sense primer | Antisense primer |
|---|
| ANP |
5′-atggatttcaagaacctgctaga-3′ |
5′-gctccaatcctgtcaatcctac-3′ |
| BNP |
5′-gatgattctgctcctgcttttc-3′ |
5′-gccatttcctctgacttttctc-3′ |
| IL-1β |
5′-gctagtgtgtgatgttcccattag-3′ |
5′-cttttccatcttcttctttgggta-3′ |
| PGEs |
5′-gtgatggagaacagccaggt-3′ |
5′-gaggaccacgaggaaatgtatc-3′ |
| COX-2 |
5′-tgtgatattctcaaacaggagcat-3′ |
5′-aaggaggatggagttgttgtagag-3′ |
| MCP-1 |
5′-ctgtctcagccagatgcagttaat-3′ |
5′-tatgggtcaagttcacattcaaag-3′ |
| IL-6 |
5′-ccgagtagacctcatagtgacctt-3′ |
5′-cctattgaaaatctgctctggtct-3′ |
| TGF-β |
5′-tcagacattcgggaagcagtg-3′ |
5′-attccgtctccttggttcagc-3′ |
| RORγt |
5′-tctggaagctgtgggataga-3′ |
5′-gaggagcctgtggagaaatac-3′ |
| IL-17 |
5′-tactcatccctcaaagttcagtgt-3′ |
5′-ctcttgctggatgagaacagaat-3′ |
| GAPDH |
5′-atcaccatcttccaggagcga-3′ |
5′-agccttctccatggtggtgga-3′ |
Spleen cell culture with serum containing
IL-1RII-Ig
To prepare serum containing IL-1RII-Ig or SP-Ig for
spleen cell culture, normal rats were injected with 800 μg of
pCAGGS-IL-1RII-IgGFc or pCAGGS-SP-IgGFc dissolved in the
appropriate volume of Ringer's solution (~80 ml/kg body weight) via
the tail vein within 15 sec, and the serum was collected after 24
h. Spleens were obtained from EAM rats on day 17 and cultured at a
density of 6×106 cells/ml on 35 mm-well dishes in 2 ml
of RPMI-1640 medium supplemented with 10% fetal calf serum (FCS).
Shortly after culture, spleen cells were stimulated with rat IL-1α
(final concentration, 10 ng/ml; PeproTech, UK) and 100 μl of
IL-1RII-Ig-GLU-tag-containing serum obtained from an
IL-1RII-Ig-treated normal rat or the same amount of an
Ig-GLU-tag-containing serum from an SP-Ig-treated normal rat
(22).
Detection of Th17 cell-derived molecules
using quantitative real-time RT-PCR
Spleen cells were collected after 24 h of culture at
37°C, total RNA was isolated, and cDNA was synthesized as described
above. The relative expression of IL-6, transforming growth
factor-β (TGF-β), retinoic acid-related orphan nuclear receptor
(RORγt) and IL-17 mRNA was measured by quantitative real-time
RT-PCR (Table I). The ΔΔCT method
was used to quantitate gene expression.
Statistical analysis
Statistical analysis was performed using the
unpaired Student's t-test or one-way ANOVA, and the Bonferroni
multiple comparison test. Differences were considered significant
at P<0.05. The heart weight-to-body weight ratio, area of
myocarditis, echocardiography and hemodynamic parameters, and the
data obtained from quantitative RT-PCR were expressed as means ±
SEM.
Results
IL-1RII expression and distribution in
EAM rats
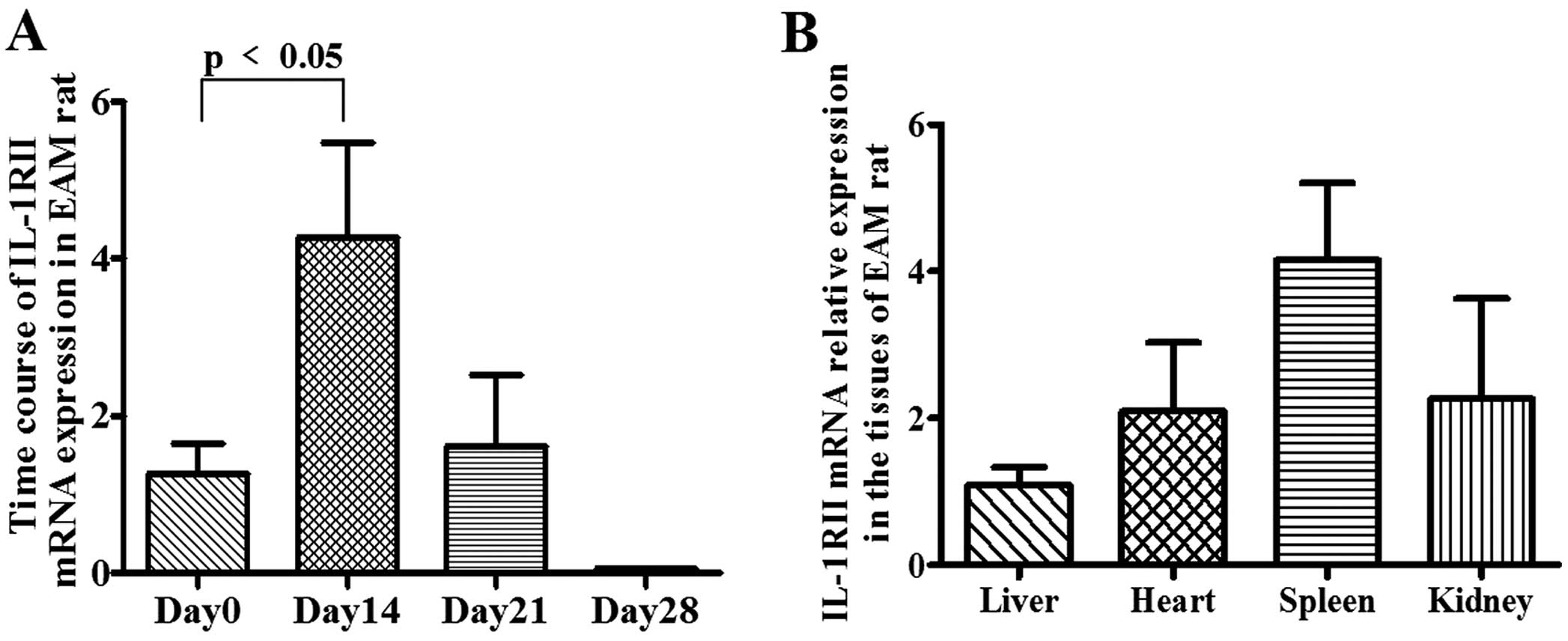
The mRNA expression of IL-1RII in the hearts of EAM
rats was increased, peaking at 4.269±1.208-fold on day 14 (vs. day
0, <0.05) and gradually decreasing to 1.61±0.9-fold on day 21
and 0.056±0.009-fold on day 28 (Fig.
1A). The distribution of IL-1RII in the liver, heart, spleen
and kidney of EAM rats showed on day 14 that IL-1RII gene
expression was highest in the spleen and lowest in the liver
(Fig. 1B).
Plasma IL-1RII-Ig-GLU-tag protein
levels
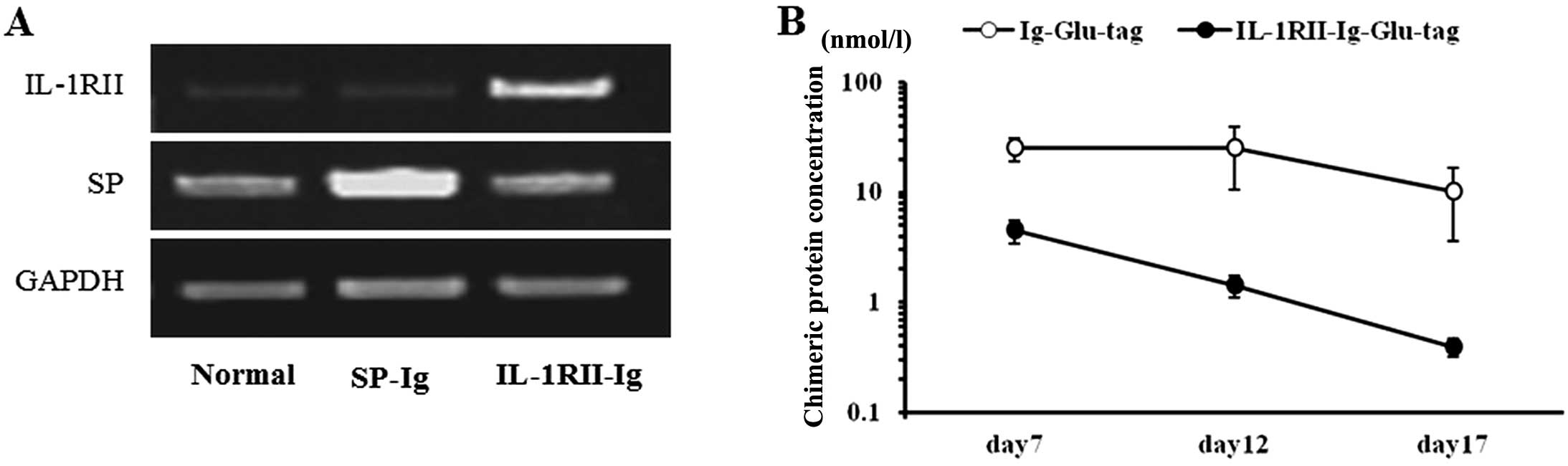
The expression of injected plasmid IL-1RII or SP in
the liver was examined using RT-PCR. The results showed that
expression of IL-1RII or SP significantly increased 24 h after
injection of the recombinant plasmid IL-1RII or SP (Fig. 2A). During the course of treatment,
plasma IL-1RII-Ig-GLU-tag protein levels in rats injected with
pCAGGS-IL-1RII-Ig on day 6 increased to 4.49±1.1 nmol/l (mean ±
SEM) on day 7 and gradually decreased on days 12 and 17 to
1.43±0.33 and 0.4±0.08 nmol/l, respectively. Plasma Ig-GLU-tag
protein levels in the pCAGGS-SP-Ig control rats increased to
25.28±5.97 nmol/l on day 7 and decreased on days 12 and 17 to
25.02±14.33 and 10.18±6.55 nmol/l, respectively (Fig. 2B). It has been reported that
IL-1RII (0.5 to 1.5 nmol/l and 0.007 to 0.014 nmol/l) suppresses
the production of PGEs from human chondrocytes stimulated by IL-1β
in vitro (23). These
results indicated that continuous effective delivery of the
IL-1RII-Ig protein for >17 days can be achieved in rats by
hydrodynamics-based gene transfer.
Echocardiography and hemodynamic
parameters
The LVEDd, LVEDs and LVPW values in the IL-1RII-Ig
group were significantly smaller than these values in the SP-Ig
group. The LV fractional shortening (LVFS%) and LV ejection
fraction (LVEF%) in the IL-1RII-Ig group were significantly higher
than those in the SP-Ig group (Table
II). This indicated that the degree of heart failure in the
IL-1RII group was relieved while that in the SP-Ig group was
not.
 | Table IIResults of the echocardiograph. |
Table II
Results of the echocardiograph.
| Normal (n=8) | SP-Ig (n=6) | IL-1RII-Ig
(n=7) |
|---|
| LVEDd (mm) | 6.410±0.1324 |
6.981±0.1419a |
6.235±0.1396e |
| LVEDs (mm) | 3.990±0.1147 |
4.680±0.1325b |
4.176±0.1038e |
| IVS (mm) | 1.314±0.04684 | 1.378±0.05494 | 1.282±0.04031 |
| LVPW (mm) | 1.442±0.04693 |
1.490±0.04161b |
1.353±0.03100d |
| LVFS (%) | 39.31±0.9265 | 29.23±1.429c | 36.67±1.415e |
| LVEF (%) | 75.46±1.057 | 61.83±2.149c | 70.11±1.519e |
Effect of in vivo treatment with plasmid
DNA encoding the IL-1RII-Ig gene
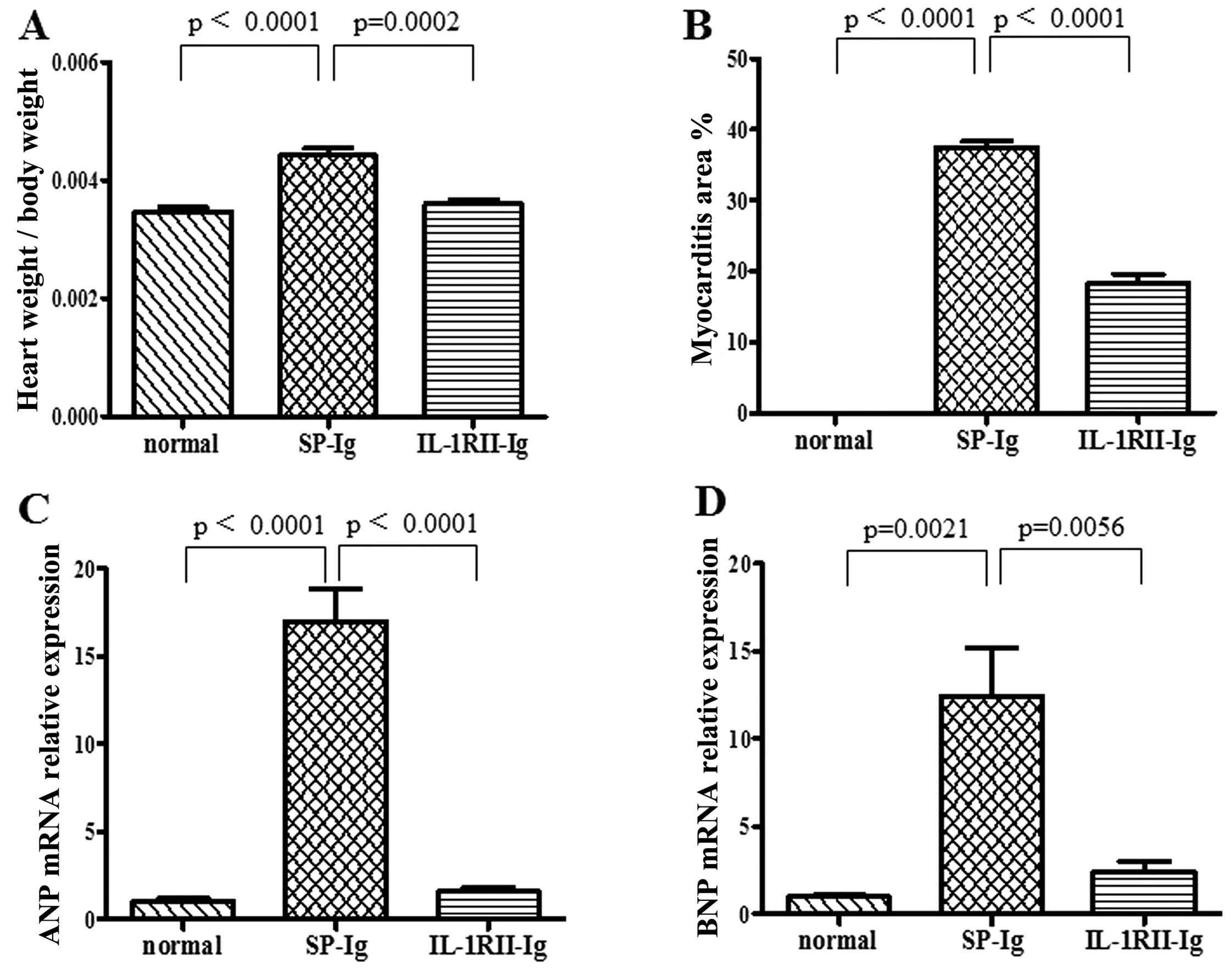
The heart-to-body weight ratio in the IL-1RII-Ig
group was significantly lower than that in the SP-Ig group
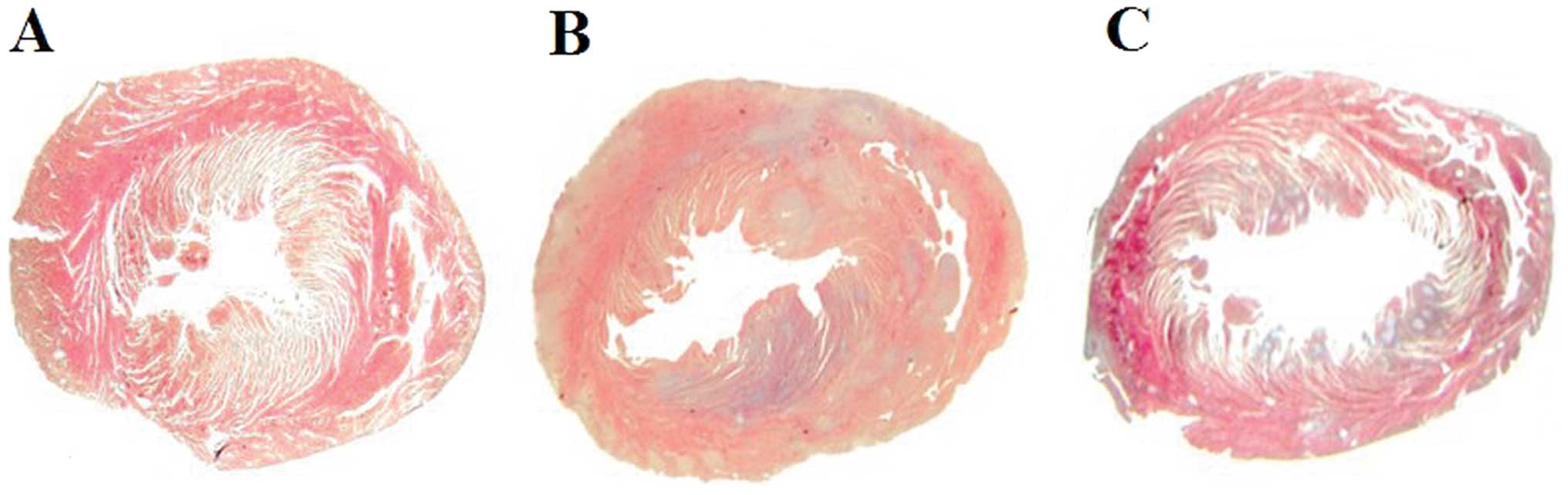
(0.36±0.01 vs. 0.44±0.02%; p=0.0002) (Fig. 3A). Many inflammatory cells and
fibroblasts had infiltrated the SP-Ig group hearts, but fewer
inflammatory cells were observed in the hearts of IL-1RII-Ig rats.
The area of myocarditis in the IL-1RII-Ig group was significantly
smaller when compered with that in the SP-Ig group (18.40±1.20 vs.
37.51±0.79%; p<0.0001) (Figs.
3B and 4). The relative
expression of ANP mRNA was significantly lower in heart tissues
from the IL-1RII-Ig group when compard with that in the SP-Ig group
(1.58±0.22 vs. 16.99±1.84; p<0.0001) (Fig. 3C). Expression of BNP mRNA was also
significantly lower in the IL-1RII-Ig group when compared with that
in the SP-Ig group (2.41±0.58 vs. 12.40±2.78; p=0.0056) (Fig. 3D).
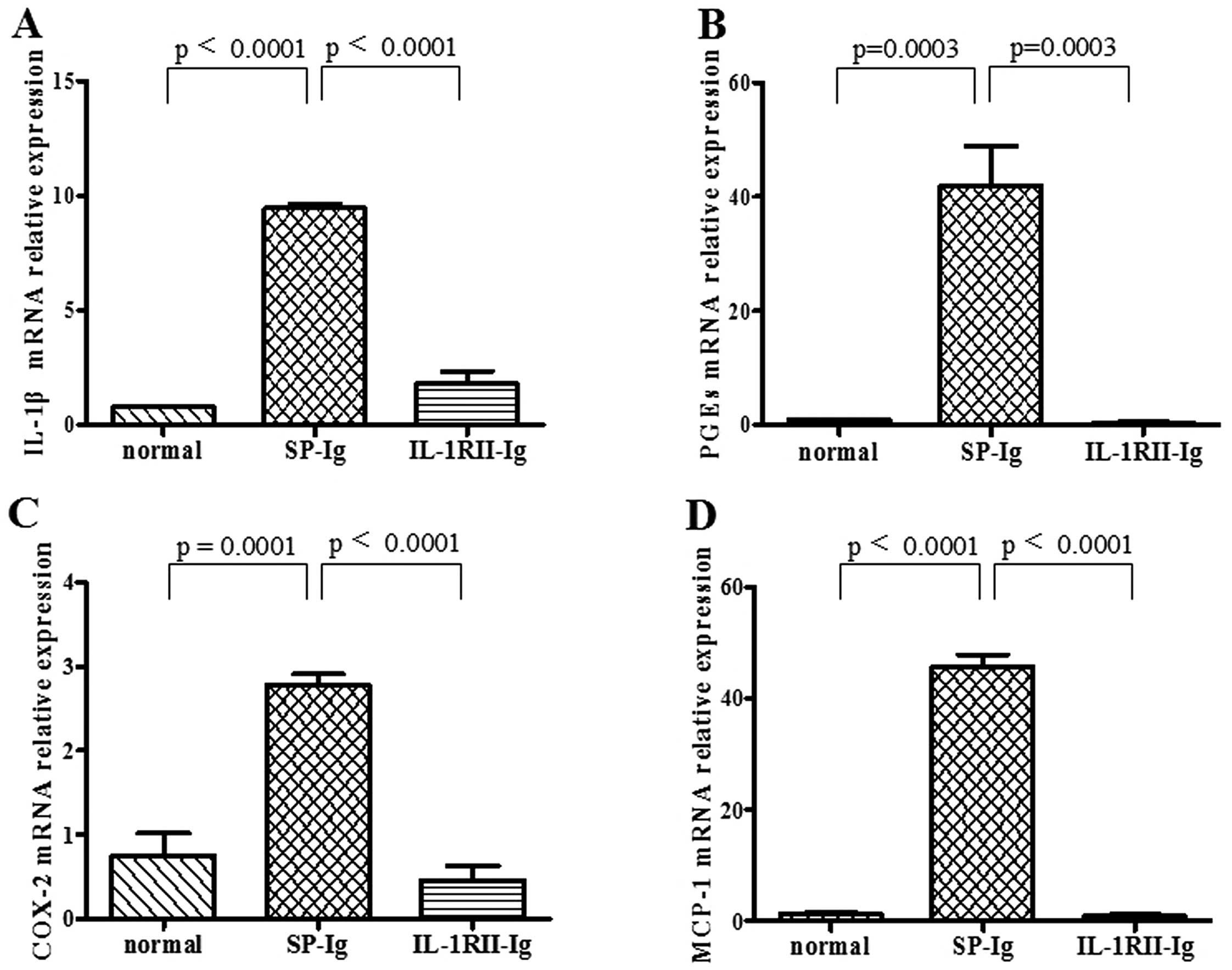
Expression of IL-1-related cytokines in
the EAM hearts
The mRNA expression of IL-1β, PGEs, COX-2 and MCP-1
on day 17 in EAM hearts was detected. The results showed that the
expression of the following cytokines was significantly inhibited
in the IL-1RII-Ig group: IL-1β (0.78±0.05 vs. SP 9.45±0.18,
p<0.0001); PGEs (0.44±0.14 vs. SP 41.97±6.91, p=0.0003); COX-2
(0.46±0.17 vs. SP 2.478±0.13, p<0.0001); MCP-1 (0.94±0.21 vs. SP
45.80±2.10, p<0.0001) (Fig.
5).
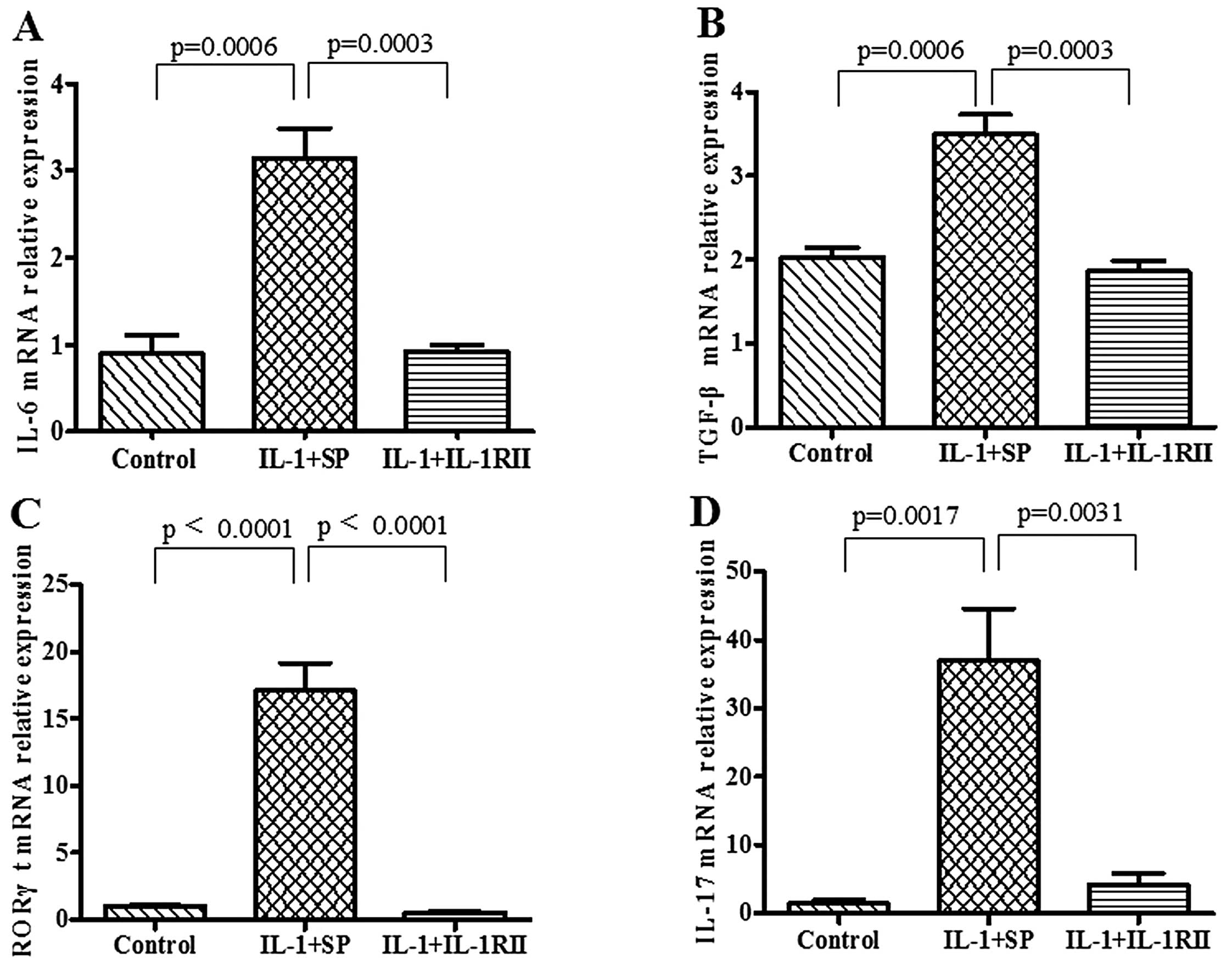
Expression of Th17 cell-derived molecules
in splenocytes cultured with serum containing IL-1RII-Ig
Compared with SP-Ig-containing serum,
IL-1RII-Ig-containing serum had significantly reduced expression of
the Th17 cell-derived molecules including IL-6 (0.92±0.07 vs.
3.14±0.35; p=0.0003), TGF-β (1.86±0.12 vs. 3.49±0.24, p=0.0003),
RORγt (0.55±0.06 vs. 17.08±2.07, p<0.0001) and IL-17 (4.18±1.68
vs. 39.96±7.66, p=0.0031) at the mRNA level in cultivated
splenocytes (Fig. 6).
Discussion
The aim of the present study was to examine the
effect of hydrodynamics-based delivery of a recombinant plasmid
encoding IL-1RII-Ig on EAM. The method used to deliver the naked
plasmid was initially reported by Liu et al (24) and Zhang
et al (25). These studies
showed that extremely high levels of foreign gene expression in
hepatocytes were achieved by hydrodynamics-based delivery; foreign
proteins were expressed in the liver and delivered to other organs
such as the heart and kidney via the circulation (26,27). Furthermore, the fusion of
cytokines with Ig-Fc segments offers advantages over native
cytokines, such as an extended half-life in the circulation,
characteristic of Igs, and a higher avidity for the ligand
(28,29).
In the present study, exogenous IL-1RII (expressed
in vivo) appeared to inhibit IL-1-induced immune responses.
IL-1, particularly IL-1β, plays an important role in the
pathogenesis of EAM which is an animal model for CD4+ T
cell-mediated inflammatory heart disease (2). IL-1 expression is rapidly
upregulated during the early phase of EAM, and can promote the
proliferation and survival of naive T cells. IL-1 binds to IL-1RI
and recruits the IL-1 receptor accessory protein (IL-1RAcp) to form
a heterodimeric receptor, which is required for signal transduction
(30). Therapies that block IL-1
signaling have been proposed over the past decade. Studies on
IL-1RA have shown that it can effectively bind to IL-1RI and
inhibit the activity of either IL-1α or IL-β (16–18). Similar to IL-1RA, IL-1RII serves
as a decoy receptor and can inhibit IL-1 activity by binding to it
without inducing signal transduction. IL-1RII has high affinity for
IL-1, but a much lower affinity for IL-1RA, which allows IL-1RII to
act as an IL-1 inhibitor. In addition, IL-1RAcP can be recruited to
the IL-1RII-IL-1 complex so that the decoy receptor can sequester
the accessory receptor and prevent it from participating in IL-1
signaling mediated by IL-1RI. This sequestration of IL-1RAcp
greatly increases the inhibitory potency of IL-1RII (31). Recent studies showed that
increased IL-1RII levels can neutralize IL-1β and effectively
ameliorate autoimmune disease. Consistent with this, our data
showed that the plasmid encoding IL-1RII-Ig effectively prevents
the progression of LV remodeling and myocardial damage in EAM
rats.
The mechanism underlying the etiology of autoimmune
myocarditis remains unknown, but modulating Th1/Th2 balance appears
to have a beneficial effect on the disease. During the past decade,
studies have shown that treatments can alter the helper T cell
balance: i.e. decrease expression of Th1 cytokines and increase
expression of Th2 cytokines. It has been proven that blocking the
activity of IL-1 may result in a reduction in Th1 cytokines and
IL-1-induced production of PGEs, COX-2 and MCP-1 (18). This, again, is consistent with our
results.
Recent findings indicate that Th17 cells appear to
play a significant role in the progression of autoimmune diseases,
and that IL-1 may induce the polarization of T cells toward a Th17
phenotype; however, the relationship between IL-1 signaling and
Th17 cells in EAM rats has not been elucidated. IL-1, along with
other cytokines, can drive Th17 cell polarization. Chung et
al (32) reported a critical
role of IL-1 in Th17 cell differentiation, and this pathway may
serve as a unique target for Th17 cell-mediated immunopathology.
Veldhoen and colleagues indicated that IL-1 can increase the number
of Th17 cells generated in vitro in the presence of IL-6
plus TGF-β (33). RORγt is the
key transcription factor that orchestrates the differentiation of
Th17 cells. RORγt-deficient CD4+ T cells do not produce
IL-17 in response to TGF-β and IL-6 (34). Our results showed that, in
vitro, serum containing IL-1RII-Ig inhibited the expression of
IL-6, TGF-β and RORγt during Th17 cell polarization, which may
suppress the secretion of IL-17. However, the association between
blocking IL-1 signaling and a reduction in the level of Th17
cell-related cytokines remains elusive and further studies are
needed.
In conclusion, the results of the present study
suggest that hydrodynamics-based delivery of a recombinant plasmid
encoding IL-1RII-Ig ameliorates EAM in rats. The possible mechanism
may be through blocking IL-1 and inhibiting production of the
cytokines critical for the polarization of T cells toward a Th17
phenotype. Our future studies will focus on identifying therapy
targets based on this mechanism, which will aid in the prevention
of autoimmune myocarditis.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81270294) and the Natural
Science Foundation of Fujian Province (grant no. 2012J01415).
References
|
1
|
Blauwet LA and Cooper LT: Myocarditis.
Prog Cardiovasc Dis. 52:274–288. 2010. View Article : Google Scholar
|
|
2
|
Leuschner F, Katus HA and Kaya Z:
Autoimmune myocarditis: past, present and future. J Autoimmun.
33:282–289. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kodama M, Matsumoto Y, Fujiwara M, Masani
F, Izumi T and Shibata A: A novel experimental model of giant cell
myocarditis induced in rats by immunization with cardiac myosin
fraction. Clin Immunol Immunopathol. 57:250–262. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arend WP and Dayer JM: Inhibition of the
production and effects of interleukin-1 and tumor necrosis factor
alpha in rheumatoid arthritis. Arthritis Rheum. 38:151–160. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vicenová B, Vopálenský V, Burýsek L and
Pospísek M: Emerging role of Interleukin-1 in cardiovascular
diseases. Physiol Res. 58:481–498. 2009.
|
|
6
|
Scala G, Allavena P, Djeu JY, Kasahara T,
Ortaldo JR, Herberman RB and Oppenheim JJ: Human large granular
lymphocytes are potent producers of interleukin-1. Nature.
309:56–59. 1984. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
March CJ, Mosley B, Larsen A, et al:
Cloning sequence and expression of two distinct human interleukin-1
complementary DNAs. Nature. 315:641–647. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dinarello CA, Donath MY and
Mandrup-Poulsen T: Role of IL-1beta in type 2 diabetes. Curr Opin
Endocrinol Diabetes Obes. 17:314–321. 2010.PubMed/NCBI
|
|
9
|
Bujak M and Frangogiannis NG: The role of
IL-1 in the pathogenesis of heart disease. Arch Immunol Ther Exp.
57:165–176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dinarello CA: IL-1: discoveries,
controversies and future directions. Eur J Immunol. 40:599–606.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Colotta F, Re F, Muzio M, et al:
Interleukin-1 type II receptor: a decoy target for IL-1 that is
regulated by IL-4. Science. 261:472–475. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Greenfeder SA, Nunes P, Kwee L, Labow M,
Chizzonite RA and Ju G: Molecular cloning and characterization of a
second subunit of the interleukin 1 receptor complex. J Biol Chem.
270:13757–13765. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Smith DE, Hanna R, Friend Della, et al:
The soluble form of IL-1 receptor accessory protein enhances the
ability of soluble type II IL-1 receptor to inhibit IL-1 action.
Immunity. 18:87–96. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sims JE and Smith DE: The IL-1 family:
regulators of immunity. Nat Rev Immunol. 10:89–102. 2010.PubMed/NCBI
|
|
15
|
Larsen CM, Faulenbach M, Vaag A, et al:
Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N
Engl J Med. 356:1517–1526. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
El-Osta H, Janku F and Kurzrock R:
Successful treatment of Castle-man's disease with interleukin-1
receptor antagonist (Anakinra). Mol Cancer Ther. 9:1485–1488.
2010.
|
|
17
|
Mertens M and Singh JA: Anakinra for
rheumatoid arthritis: a systematic review. J Rheumatol.
36:1118–1125. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu H, Hanawa H, Yoshida T, et al: Effect
of hydrodynamics-based gene delivery of plasmid DNA encoding
interleukin-1 receptor antagonist-Ig for treatment of rat
autoimmune myocarditis: possible mechanism for lymphocytes and
noncardiac cells. Circulation. 111:1593–1600. 2005. View Article : Google Scholar
|
|
19
|
Maruyama H, Higuchi N, Nishikawa Y, et al:
High-level expression of naked DNA delivered to rat liver via tail
vein injection. J Gene Med. 4:333–341. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abe S, Hanawa H, Hayashi M, et al:
Prevention of experimental autoimmune myocarditis by
hydrodynamics-based naked plasmid DNA encoding CTLA4-Ig gene
delivery. J Card Fail. 11:557–564. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang H, Hanawa H, Yoshida T, et al:
Alteration of IL-17 related protein expressions in experimental
autoimmune myocarditis and inhibition of IL-17 by IL-10-Ig fusion
gene transfer. Circ J. 72:813–819. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Attur MG, Dave M, Cipolletta C, et al:
Reversal of autocrine and paracrine effects of interleukin 1 (IL-1)
in human arthritis by type II IL-1 decoy receptor. Potential for
pharmacological intervention. J Biol Chem. 275:40307–40315. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu F, Song Y and Liu D:
Hydrodynamics-based transfection in animals by systemic
administration of plasmid DNA. Gene Ther. 6:1258–1266. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang G, Budker V and Wolff JA: High
levels of foreign gene expression in hepatocytes after tail vein
injections of naked plasmid DNA. Hum Gene Ther. 10:1735–1737. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chang H, Hanawa H, Liu H, et al:
Hydrodynamic-based delivery of an interleukin-22-Ig fusion gene
ameliorates experimental autoimmune myocarditis in rats. J Immunol.
177:3635–3643. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Higuchi N, Maruyama H, Kuroda T, et al:
Hydrodynamics-based delivery of the viral interleukin-10 gene
suppresses experimental crescentic glomerulonephritis in
Wistar-Kyoto rats. Gene Ther. 10:1297–1310. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang J, Yamato E and Miyazaki J:
Sustained expression of Fc-fusion cytokine following in vivo
electroporation and mouse strain differences in expression levels.
J Biochem. 133:423–427. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Elnaggar R, Hanawa H, Liu H, et al: The
effect of hydrodynamics-based delivery of an IL-13-Ig fusion gene
for experimental autoimmune myocarditis in rats and its possible
mechanism. Eur J Immunol. 35:1995–2005. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dinarello CA: Immunological and
inflammatory functions of the interleukin 1 family. Annu Rev
Immunol. 27:519–550. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Boraschi D and Tagliabue A: The
interleukin-1 receptor family. Vitam Horm. 74:229–254. 2006.
View Article : Google Scholar
|
|
32
|
Chung Y, Chang SH, Martinez GJ, et al:
Critical regulation of early Th17 cell differentiation by
interleukin-1 signaling. Immunity. 30:576–587. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Veldhoen M, Hocking RJ, Atkins CJ,
Locksley RM and Stockinger B: TGFbeta in the context of an
inflammatory cytokine milieu supports de novo differentiation of
IL-17-producing T cells. Immunity. 24:179–189. 2006. View Article : Google Scholar
|
|
34
|
Aranami T and Yamamura T: Th17 cells and
autoimmune encephalomyelitis (EAE/MS). Allergol Int. 57:115–120.
2008. View Article : Google Scholar : PubMed/NCBI
|