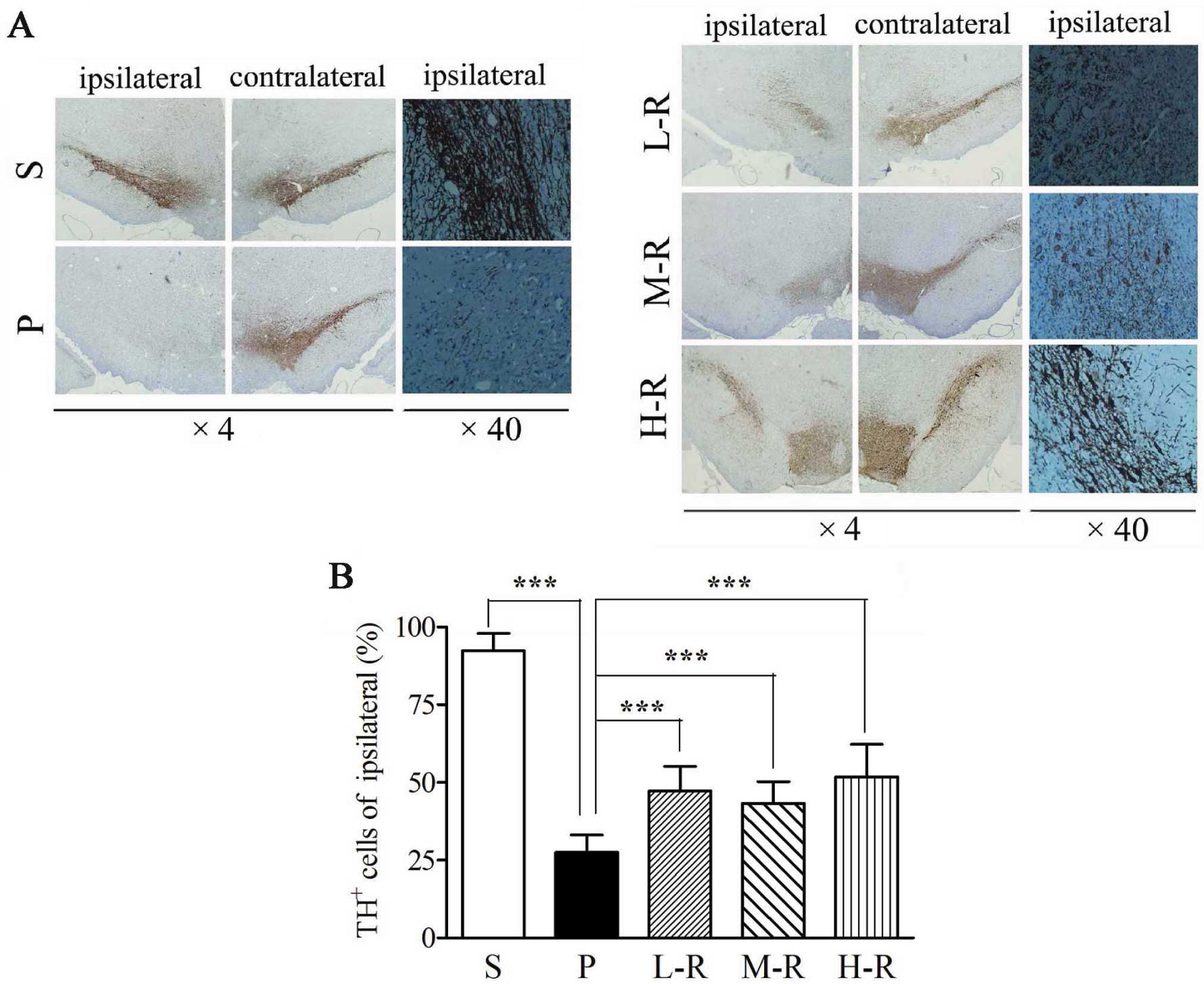
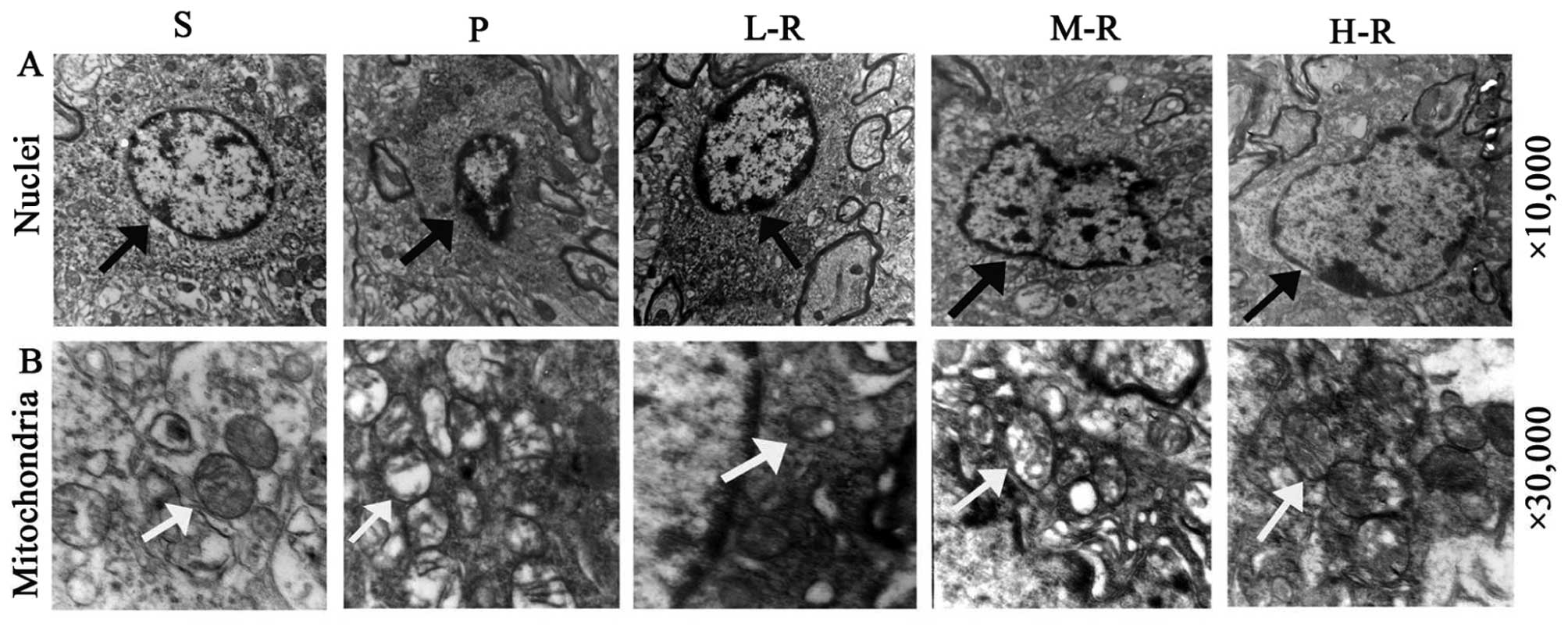
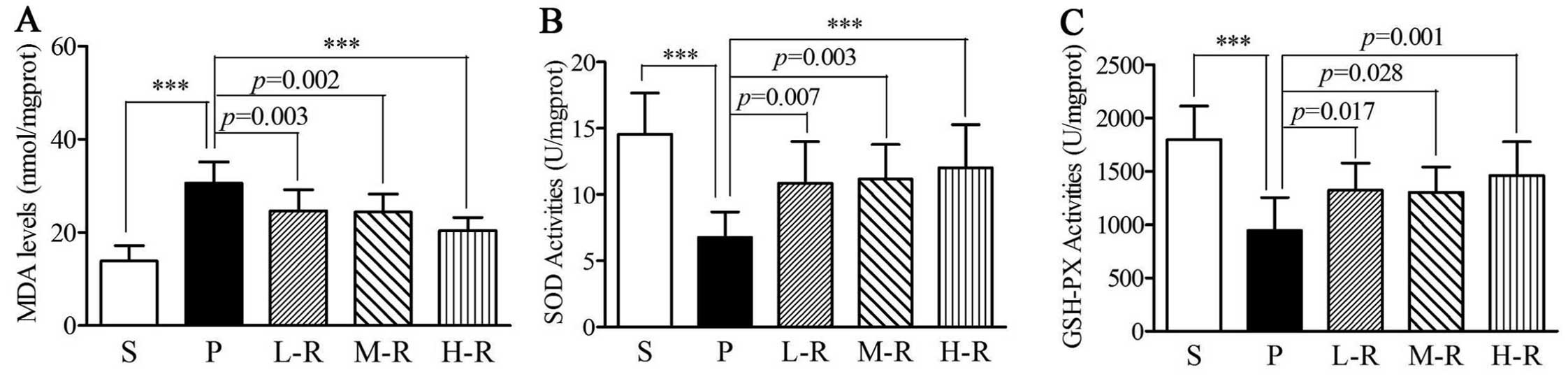
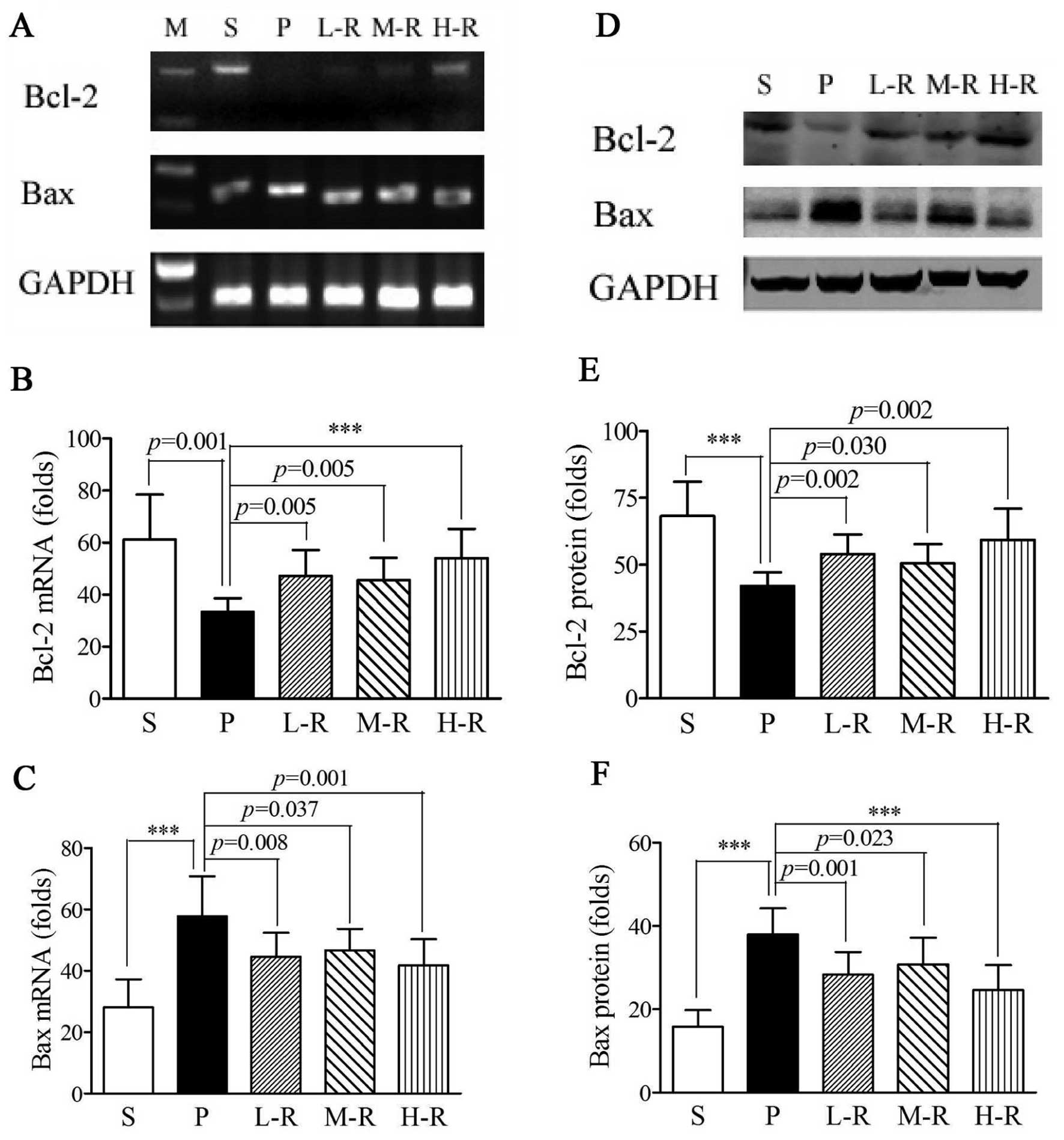
|
1
|
Dauer W and Przedborski S: Parkinson’s
disease: mechanisms and models. Neuron. 39:889–909. 2003.
|
|
2
|
Marras C and Lang A: Invited article:
changing concepts in Parkinson disease: moving beyond the decade of
the brain. Neurology. 70:1996–2003. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Levy OA, Malagelada C and Greene LA: Cell
death pathways in Parkinson’s disease: proximal triggers, distal
effectors, and final steps. Apoptosis. 14:478–500. 2009.
|
|
4
|
Smith MP and Cass WA: Oxidative stress and
dopamine depletion in an intrastriatal 6-hydroxydopamine model of
Parkinson’s disease. Neuroscience. 144:1057–1066. 2007.PubMed/NCBI
|
|
5
|
Exner N, Lutz AK, Haass C and Winklhofer
KF: Mitochondrial dysfunction in Parkinson’s disease: molecular
mechanisms and pathophysiological consequences. EMBO J.
31:3038–3062. 2012.
|
|
6
|
Yamato M, Kudo W, Shiba T, Yamada KI,
Watanabe T and Utsumi H: Determination of reactive oxygen species
associated with the degeneration of dopaminergic neurons during
dopamine metabolism. Free Radic Res. 44:249–257. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee JE, Park JH, Shin IC and Koh HC:
Reactive oxygen species regulated mitochondria-mediated apoptosis
in PC12 cells exposed to chlorpyrifos. Toxicol Appl Pharmacol.
263:148–162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hori A, Yoshida M, Shibata T and Ling F:
Reactive oxygen species regulate DNA copy number in isolated yeast
mitochondria by triggering recombination-mediated replication.
Nucleic Acids Res. 37:749–761. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Noda T and Ohsumi Y: Tor, a
phosphatidylinositol kinase homologue, controls autophagy in yeast.
J Biol Chem. 273:3963–3966. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sudarsanam S and Johnson DE: Functional
consequences of mTOR inhibition. Curr Opin Drug Discov Devel.
13:31–40. 2010.PubMed/NCBI
|
|
11
|
Ravikumar B, Duden R and Rubinsztein DC:
Aggregate-prone proteins with polyglutamine and polyalanine
expansions are degraded by autophagy. Hum Mol Genet. 11:1107–1117.
2002. View Article : Google Scholar
|
|
12
|
Ravikumar B, Vacher C, Berger Z, Davies
JE, Luo S, Oroz LG, et al: Inhibition of mTOR induces autophagy and
reduces toxicity of polyglutamine expansions in fly and mouse
models of Huntington disease. Nat Genet. 36:585–595. 2004.
View Article : Google Scholar
|
|
13
|
Pan T, Kondo S, Zhu W, Xie W, Jankovic J
and Le W: Neuroprotection of rapamycin in lactacystin-induced
neurodegeneration via autophagy enhancement. Neurobiol Dis.
32:16–25. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Malagelada C, Jin ZH, Jackson-Lewis V,
Przedborski S and Greene LA: Rapamycin protects against neuron
death in in vitro and in vivo models of Parkinson’s disease. J
Neurosci. 30:1166–1175. 2010.PubMed/NCBI
|
|
15
|
Ravikumar B, Berger Z, Vacher C, O’Kane CJ
and Rubinsztein DC: Rapamycin pre-treatment protects against
apoptosis. Hum Mol Genet. 15:1209–1216. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marobbio CM, Pisano I, Porcelli V, Lasorsa
FM and Palmieri L: Rapamycin reduces oxidative stress in
frataxin-deficient yeast cells. Mitochondrion. 12:156–161. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Siddiqui A, Hanson I and Andersen JK:
Mao-B elevation decreases parkin’s ability to efficiently clear
damaged mitochondria: protective effects of rapamycin. Free Radic
Res. 46:1011–1018. 2012.PubMed/NCBI
|
|
18
|
Kofman AE, McGraw MR and Payne CJ:
Rapamycin increases oxidative stress response gene expression in
adult stem cells. Aging (Albany NY). 4:279–289. 2012.PubMed/NCBI
|
|
19
|
Rieker C, Engblom D, Kreiner G, Domanskyi
A, Schober A, Stotz S, et al: Nucleolar disruption in dopaminergic
neurons leads to oxidative damage and parkinsonism through
repression of mammalian target of rapamycin signaling. J Neurosci.
31:453–460. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Paxinos G and Watson C: The Rat Brain in
Stereotaxic Coordinates. 6th edition. Academic Press; London:
2007
|
|
21
|
Schieke SM, Phillips D, McCoy JP Jr,
Aponte AM, Shen RF, Balaban RS, et al: The mammalian target of
rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption
and oxidative capacity. J Biol Chem. 281:27643–27652. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wullschleger S, Loewith R and Hall MN: TOR
signaling in growth and metabolism. Cell. 124:471–484. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pan T, Rawal P, Wu Y, Xie W, Jankovic J
and Le W: Rapamycin protects against rotenone-induced apoptosis
through autophagy induction. Neuroscience. 164:541–551. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hall ED, Detloff MR, Johnson K and Kupina
NC: Peroxynitrite-mediated protein nitration and lipid peroxidation
in a mouse model of traumatic brain injury. J Neurotrauma. 21:9–20.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Smith MP and Cass WA: GDNF reduces
oxidative stress in a 6-hydroxydopamine model of Parkinson’s
disease. Neurosci Lett. 412:259–263. 2007.PubMed/NCBI
|
|
26
|
Long J, Liu C, Sun L, Gao H and Liu J:
Neuronal mitochondrial toxicity of malondialdehyde: inhibitory
effects on respiratory function and enzyme activities in rat brain
mitochondria. Neurochem Res. 34:786–794. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Onyango IG: Mitochondrial dysfunction and
oxidative stress in Parkinson’s disease. Neurochem Res. 33:589–597.
2008.
|
|
28
|
Martinou JC and Youle RJ: Mitochondria in
apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev
Cell. 21:92–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kirkland RA and Franklin JL: Bax, reactive
oxygen, and cytochrome c release in neuronal apoptosis. Antioxid
Redox Signal. 5:589–596. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bhagwat SV and Crew AP: Novel inhibitors
of mTORC1 and mTORC2. Curr Opin Investig Drugs. 11:638–645.
2010.PubMed/NCBI
|
|
31
|
Santini E, Heiman M, Greengard P, Valjent
E and Fisone G: Inhibition of mTOR signaling in Parkinson’s disease
prevents L-DOPA-induced dyskinesia. Sci Signal. 2:ra362009.
|
|
32
|
Lin MT and Beal MF: Mitochondrial
dysfunction and oxidative stress in neurodegenerative diseases.
Nature. 443:787–795. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee J, Giordano S and Zhang J: Autophagy,
mitochondria and oxidative stress: cross-talk and redox signalling.
Biochem J. 441:523–540. 2012. View Article : Google Scholar : PubMed/NCBI
|