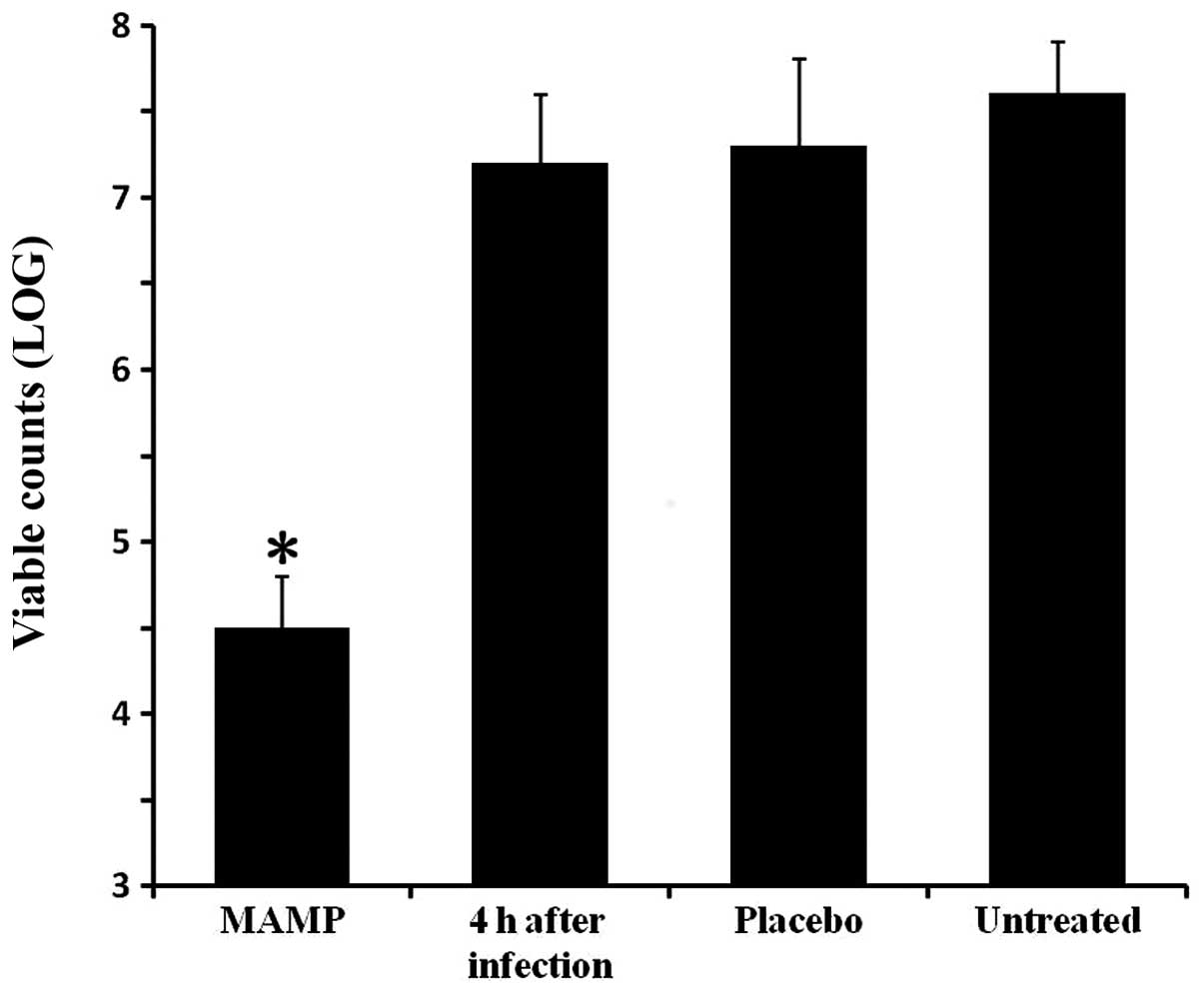
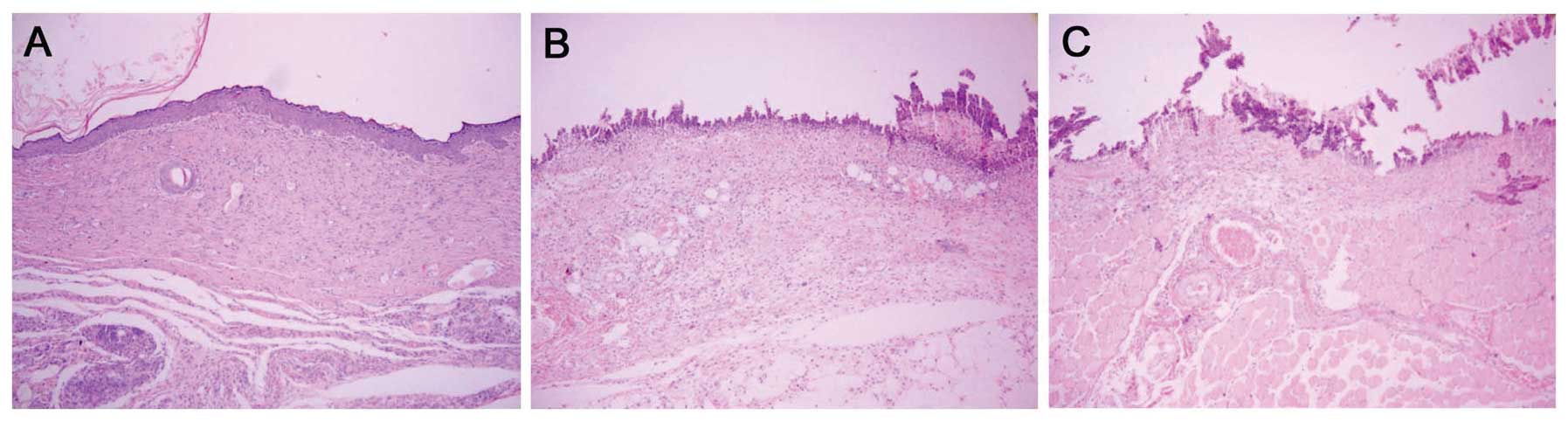
|
1
|
Allen HK, Donato J, Wang HH, et al: Call
of the wild: antibiotic resistance genes in natural environments.
Nat Rev Microbiol. 8:251–259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baer WS: The classic: the treatment of
chronic osteomyelitis with the maggot (larva of the blow fly).
1931. Clin Orthop Relat Res. 469:920–944. 2011. View Article : Google Scholar
|
|
3
|
Sherman RA: Maggot versus conservative
debridement therapy for the treatment of pressure ulcers. Wound
Repair Regen. 10:208–214. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mumcuoglu KY: Clinical applications for
maggots in wound care. Am J Clin Dermatol. 2:219–227. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Courtenay M, Church JC and Ryan TJ: Larva
therapy in wound management. J R Soc Med. 93:72–74. 2000.PubMed/NCBI
|
|
6
|
Davies CE, Turton G, Woolfrey G, et al:
Exploring debridement options for chronic venous leg ulcers. Br J
Nurs. 14:393–397. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Wang S, Zhao G, et al: Treatment
of infected wounds with maggot therapy after replantation. J
Reconstr Microsurg. 22:277–280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang SY, Wang JN, Lv DC, et al: Clinical
research on the bio-debridement effect of maggot therapy for
treatment of chronically infected lesions. Orthop Surg. 2:201–206.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kerridge A, Lappin-Scott H and Stevens JR:
Antibacterial properties of larval secretions of the blowfly,
Lucilia sericata. Med Vet Entomol. 19:333–337. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huberman L, Gollop N, Mumcuoglu KY, et al:
Antibacterial substances of low molecular weight isolated from the
blowfly, Lucilia sericata. Med Vet Entomol. 21:127–131.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bexfield A, Nigam Y, Thomas S, et al:
Detection and partial characterisation of two antibacterial factors
from the excretions/secretions of the medicinal maggot Lucilia
sericata and their activity against methicillin-resistant
Staphylococcus aureus (MRSA). Microbes Infect. 6:1297–1304.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hancock RE and Lehrer R: Cationic
peptides: a new source of antibiotics. Trends Biotechnol. 16:82–88.
1998.PubMed/NCBI
|
|
13
|
Gauri SS, Mandal SM, Pati BR, et al:
Purification and structural characterization of a novel
antibacterial peptide from Bellamya bengalensis: activity
against ampicillin and chloramphenicol resistant Staphylococcus
epidermidis. Peptides. 32:691–696. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Menzies BE and Kenoyer A:
Staphylococcus aureus infection of epidermal keratinocytes
promotes expression of innate antimicrobial peptides. Infect Immun.
73:5241–5244. 2005. View Article : Google Scholar
|
|
15
|
Knobloch JK, Horstkotte MA, Rohde H, et
al: Evaluation of different detection methods of biofilm formation
in Staphylococcus aureus. Med Microbiol Immunol.
191:101–106. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lowy FD: Staphylococcus aureus
infections. N Engl J Med. 339:520–532. 1998. View Article : Google Scholar
|
|
17
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thomas S, Andrews AM, Hay NP and Bourgoise
S: The anti-microbial activity of maggot secretions: results of a
preliminary study. J Tissue Viability. 9:127–132. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hou Z, Lu J, Fang C, et al: Underlying
mechanism of in vivo and in vitro activity of C-terminal-amidated
thanatin against clinical isolates of extended-spectrum
beta-lactamase-producing Escherichia coli. J Infect Dis.
203:273–282. 2011. View Article : Google Scholar
|
|
20
|
Cao L, Dai C, Li Z, et al: Antibacterial
activity and mechanism of a scorpion venom peptide derivative in
vitro and in vivo. PLoS One. 7:e401352012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marri L, Dallai R and Marchini D: The
novel antibacterial peptide ceratotoxin A alters permeability of
the inner and outer membrane of Escherichia coli K-12. Curr
Microbiol. 33:40–43. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dumville JC, Worthy G, Bland JM, et al:
Larval therapy for leg ulcers (VenUS II): randomised controlled
trial. BMJ. 338:b7732009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Whitaker IS, Twine C, Whitaker MJ, et al:
Larval therapy from antiquity to the present day: mechanisms of
action, clinical applications and future potential. Postgrad Med J.
83:409–413. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Levy O: Antimicrobial proteins and
peptides: anti-infective molecules of mammalian leukocytes. J
Leukoc Biol. 76:909–925. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Davydov L: Maggot therapy in wound
management in modern era and a review of published literature. J
Pharm Pract. 24:89–93. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Opletalová K, Blaizot X, Mourgeon B, et
al: Maggot therapy for wound debridement: a randomized multicenter
trial. Arch Dermatol. 148:432–438. 2012.PubMed/NCBI
|
|
27
|
Steiner H, Hultmark D, Engström A, et al:
Sequence and specificity of two antibacterial proteins involved in
insect immunity. Nature. 292:246–248. 1981. View Article : Google Scholar
|
|
28
|
Cole AM, Weis P and Diamond G: Isolation
and characterization of pleurocidin, an antimicrobial peptide in
the skin secretions of winter flounder. J Biol Chem.
272:12008–12013. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park CB, Lee JH, Park IY, et al: A novel
antimicrobial peptide from the loach, Misgurnus
anguillicaudatus. FEBS Lett. 411:173–178. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nelson A, Hultenby K, Hell E, et al:
Staphylococcus epidermidis isolated from newborn infants
express pilus-like structures and are inhibited by the
cathelicidin-derived antimicrobial peptide LL37. Pediatr Res.
66:174–178. 2009. View Article : Google Scholar
|
|
31
|
Brogden KA: Antimicrobial peptides: pore
formers or metabolic inhibitors in bacteria. Nat Rev Microbiol.
3:238–250. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang L, Weiss TM, Lehrer RI, et al:
Crystallization of antimicrobial pores in membranes: magainin and
protegrin. Biophys J. 79:2002–2009. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Melo MN, Ferre R and Castanho MA:
Antimicrobial peptides: linking partition, activity and high
membrane-bound concentrations. Nat Rev Microbiol. 7:245–250. 2009.
View Article : Google Scholar : PubMed/NCBI
|