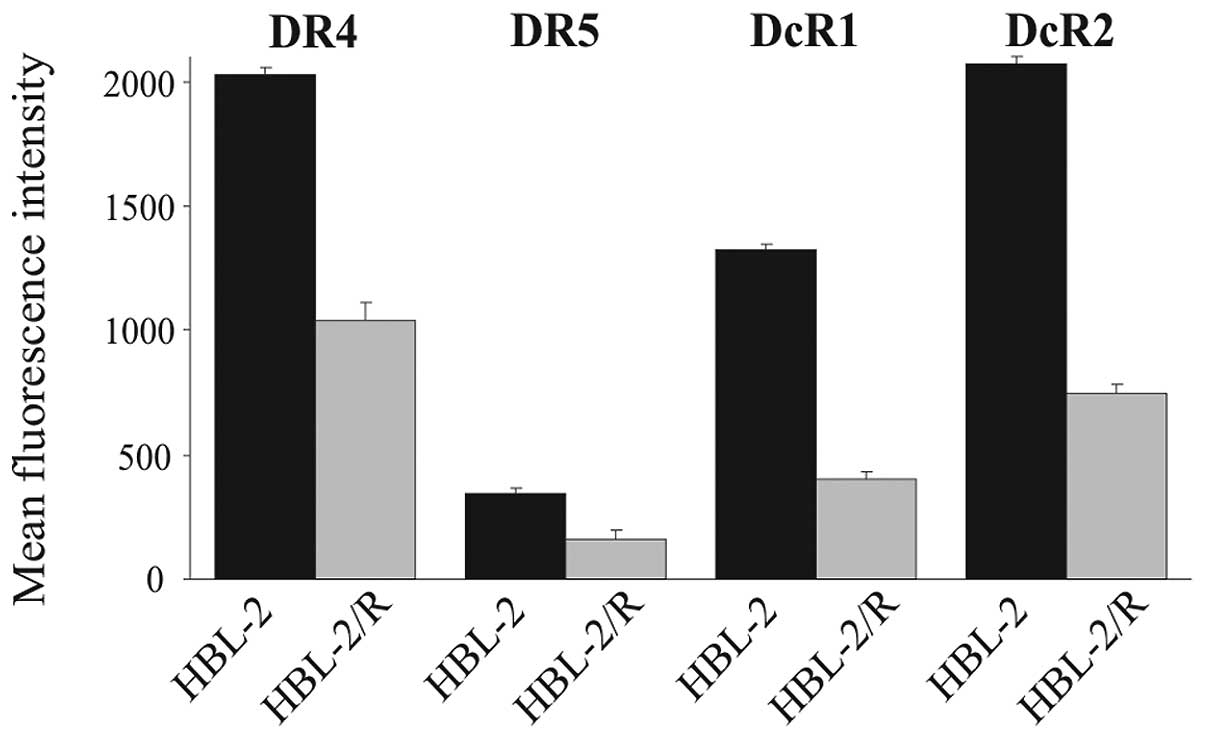
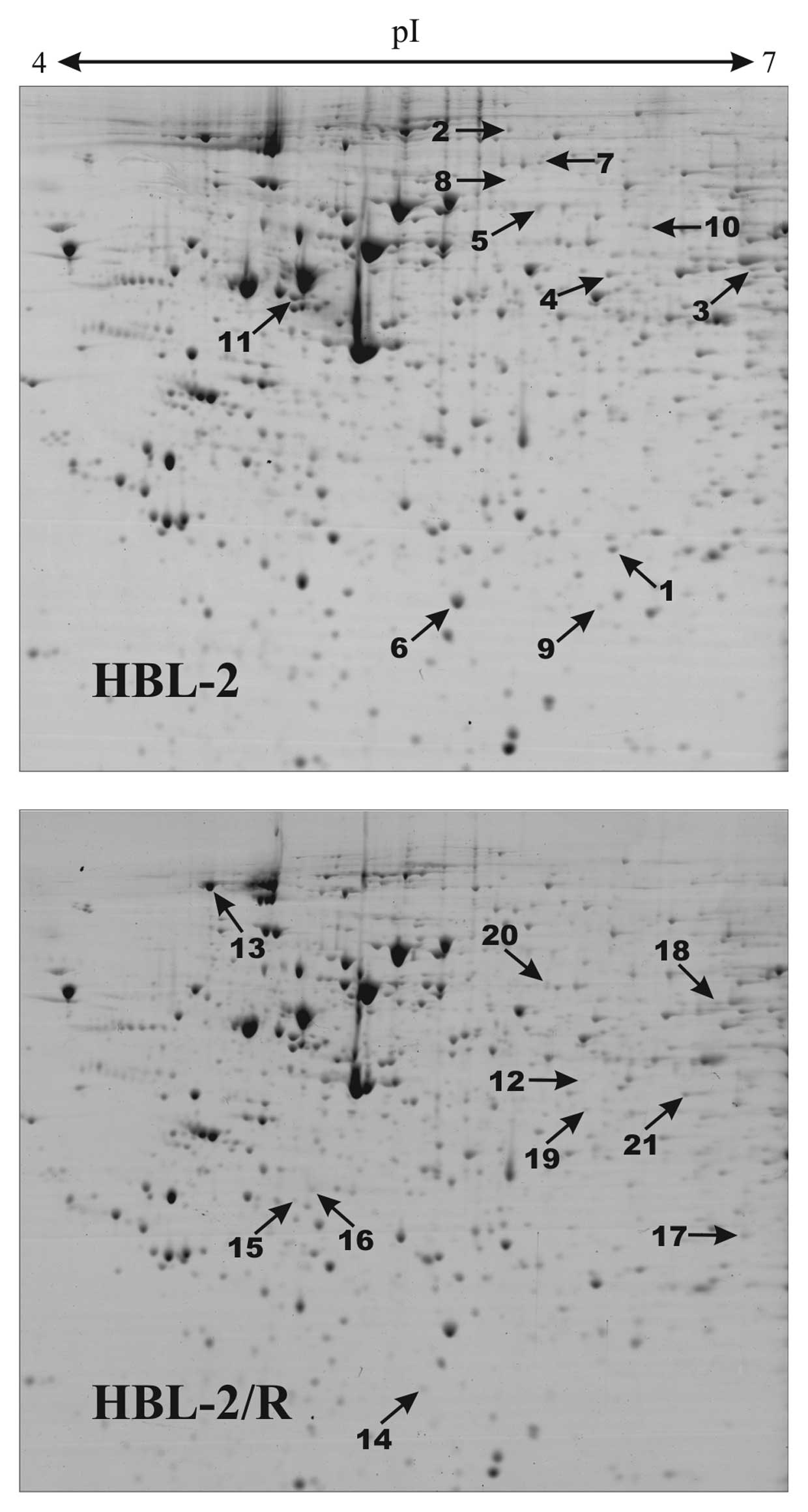
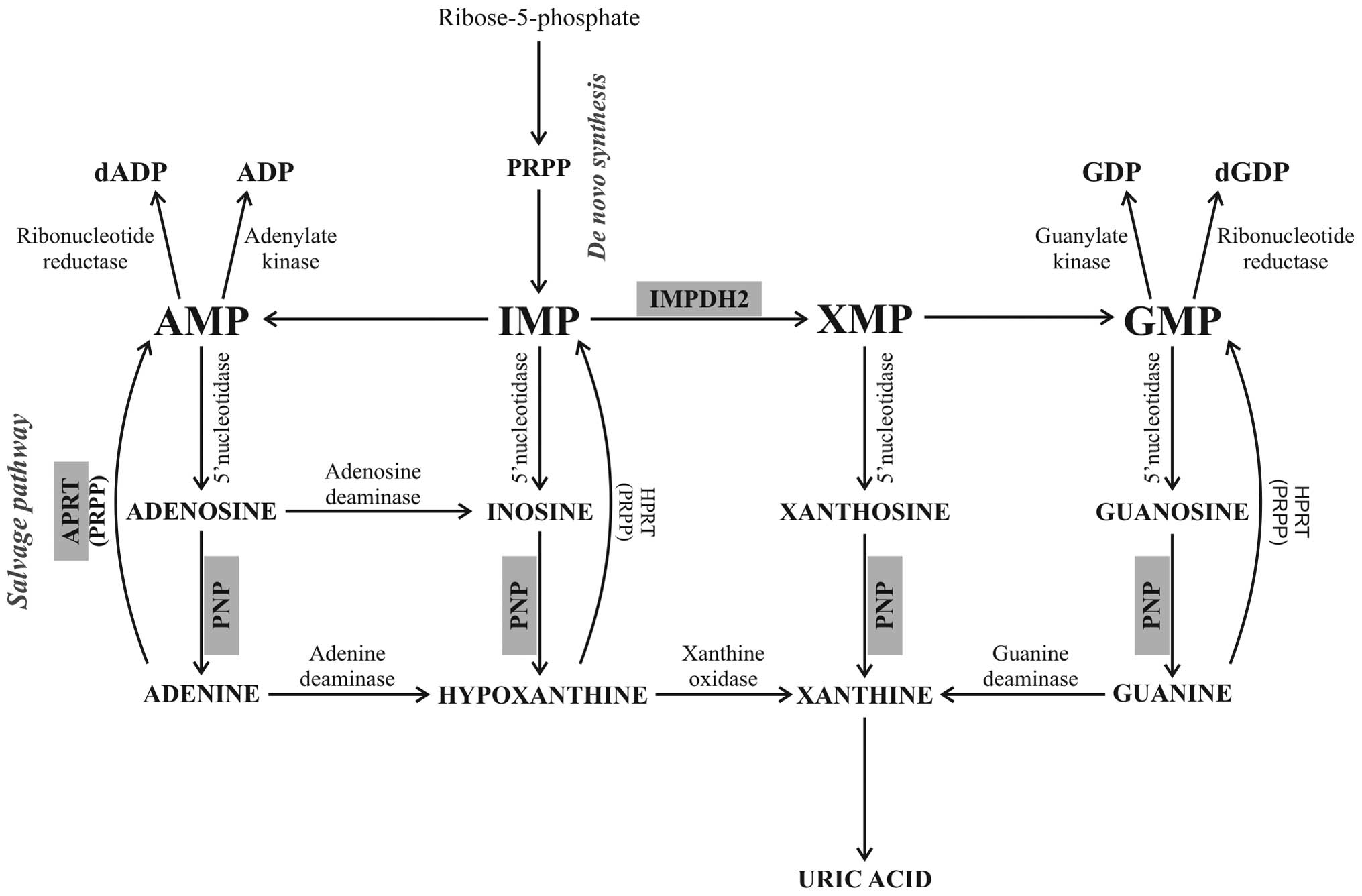
|
1
|
Sant M, Allemani C, Tereanu C, et al:
Incidence of hematologic malignancies in Europe by morphologic
subtype: results of the HAEMACARE project. Blood. 116:3724–3734.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Perez-Galan P, Dreyling M and Wiestner A:
Mantle cell lymphoma: biology, pathogenesis, and the molecular
basis of treatment in the genomic era. Blood. 117:26–38. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsujimoto Y, Yunis J, Onorato-Showe L,
Erikson J, Nowell PC and Croce CM: Molecular cloning of the
chromosomal breakpoint of B-cell lymphomas and leukemias with the
t(11;14) chromosome translocation. Science. 224:1403–1406. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Williams ME, Swerdlow SH, Rosenberg CL and
Arnold A: Characterization of chromosome 11 translocation
breakpoints at the bcl-1 and PRAD1 loci in centrocytic lymphoma.
Cancer Res. 52:5541S–5544S. 1992.PubMed/NCBI
|
|
5
|
Humala K and Younes A: Current and
emerging new treatment strategies for mantle cell lymphoma. Leuk
Lymphoma. Feb 19–2013.(Epub ahead of print).
|
|
6
|
Wiley SR, Schooley K, Smolak PJ, et al:
Identification and characterization of a new member of the TNF
family that induces apoptosis. Immunity. 3:673–682. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pitti RM, Marsters SA, Ruppert S, Donahue
CJ, Moore A and Ashkenazi A: Induction of apoptosis by Apo-2
ligand, a new member of the tumor necrosis factor cytokine family.
J Biol Chem. 271:12687–12690. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ashkenazi A and Dixit VM: Death receptors:
signaling and modulation. Science. 281:1305–1308. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sheridan JP, Marsters SA, Pitti RM, et al:
Control of TRAIL-induced apoptosis by a family of signaling and
decoy receptors. Science. 277:818–821. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ashkenazi A: Targeting death and decoy
receptors of the tumour-necrosis factor superfamily. Nat Rev
Cancer. 2:420–430. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Castro Alves C, Terziyska N, Grunert M, et
al: Leukemia-initiating cells of patient-derived acute
lymphoblastic leukemia xenografts are sensitive toward TRAIL.
Blood. 119:4224–4227. 2012.PubMed/NCBI
|
|
12
|
Peter ME and Krammer PH: The
CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 10:26–35. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Spierings DC: Tissue distribution of the
death ligand TRAIL and its receptors. J Histochem Cytochem.
52:821–831. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Petrak J, Toman O, Simonova T, et al:
Identification of molecular targets for selective elimination of
TRAIL-resistant leukemia cells. From spots to in vitro assays using
TOP15 charts. Proteomics. 9:5006–5015. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Molinsky J, Klanova M, Koc M, et al:
Roscovitine sensitizes leukemia and lymphoma cells to tumor
necrosis factor-related apoptosis-inducing ligand-induced
apoptosis. Leuk Lymphoma. 54:372–380. 2013. View Article : Google Scholar
|
|
16
|
Klener P, Leahomschi S, Molinsky J, et al:
TRAIL-induced apoptosis of HL60 leukemia cells: two distinct
phenotypes of acquired TRAIL resistance that are accompanied with
resistance to TNFalpha but not to idarubicin and cytarabine. Blood
Cells Mol Dis. 42:77–84. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leahomschi S, Molinsky J, Klanova M, et
al: Multi-level disruption of the extrinsic apoptotic pathway
mediates resistance of leukemia cells to TNF-related
apoptosis-inducing ligand (TRAIL). Neoplasma. 60:223–231. 2013.
View Article : Google Scholar
|
|
18
|
Wen J, Ramadevi N, Nguyen D, Perkins C,
Worthington E and Bhalla K: Antileukemic drugs increase death
receptor 5 levels and enhance Apo-2L-induced apoptosis of human
acute leukemia cells. Blood. 96:3900–3906. 2000.PubMed/NCBI
|
|
19
|
Plasilova M, Zivny J, Jelinek J, et al:
TRAIL (Apo2L) suppresses growth of primary human leukemia and
myelodysplasia progenitors. Leukemia. 16:67–73. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ashkenazi A, Pai RC, Fong S, et al: Safety
and antitumor activity of recombinant soluble Apo2 ligand. J Clin
Invest. 104:155–162. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Di Pietro R and Zauli G: Emerging
non-apoptotic functions of tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL)/Apo2L. J Cell Physiol.
201:331–340. 2004.
|
|
22
|
Kelley SK, Harris LA, Xie D, et al:
Preclinical studies to predict the disposition of Apo2L/tumor
necrosis factor-related apoptosis-inducing ligand in humans:
characterization of in vivo efficacy, pharmacokinetics, and safety.
J Pharmacol Exp Ther. 299:31–38. 2001.
|
|
23
|
Lawrence D, Shahrokh Z, Marsters S, et al:
Differential hepatocyte toxicity of recombinant Apo2L/TRAIL
versions. Nat Med. 7:383–385. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roth W, Isenmann S, Naumann U, et al:
Locoregional Apo2L/TRAIL eradicates intracranial human malignant
glioma xenografts in athymic mice in the absence of neurotoxicity.
Biochem Biophys Res Commun. 265:479–483. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Walczak H, Miller RE, Ariail K, et al:
Tumoricidal activity of tumor necrosis factor-related
apoptosis-inducing ligand in vivo. Nat Med. 5:157–163. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hylander BL, Pitoniak R, Penetrante RB, et
al: The anti-tumor effect of Apo2L/TRAIL on patient pancreatic
adenocarcinomas grown as xenografts in SCID mice. J Transl Med.
3:222005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dimberg LY, Anderson CK, Camidge R,
Behbakht K, Thorburn A and Ford HL: On the TRAIL to successful
cancer therapy? Predicting and counteracting resistance against
TRAIL-based therapeutics. Oncogene. 14–May;2012.(Epub ahead of
print). View
Article : Google Scholar : 1642012.PubMed/NCBI
|
|
28
|
Zhang L and Fang B: Mechanisms of
resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther.
12:228–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maksimovic-Ivanic D, Stosic-Grujicic S,
Nicoletti F and Mijatovic S: Resistance to TRAIL and how to
surmount it. Immunol Res. 52:157–168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Scavennec J, Maraninchi D, Gastaut JA,
Carcassonne Y and Cailla HL: Purine and pyrimidine ribonucleoside
monophosphate patterns of peripheral blood and bone marrow cells in
human acute leukemias. Cancer Res. 42:1326–1330. 1982.PubMed/NCBI
|
|
31
|
Natsumeda Y, Prajda N, Donohue JP, Glover
JL and Weber G: Enzymic capacities of purine de novo and salvage
pathways for nucleotide synthesis in normal and neoplastic tissues.
Cancer Res. 44:2475–2479. 1984.PubMed/NCBI
|
|
32
|
Bollee G, Harambat J, Bensman A,
Knebelmann B, Daudon M and Ceballos-Picot I: Adenine
phosphoribosyltransferase deficiency. Clin J Am Soc Nephrol.
7:1521–1527. 2012. View Article : Google Scholar
|
|
33
|
Bantia S, Miller PJ, Parker CD, et al:
Purine nucleoside phosphorylase inhibitor BCX-1777 (Immucillin-H) -
a novel potent and orally active immunosuppressive agent. Int
Immunopharmacol. 1:1199–1210. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bantia S, Montgomery JA, Johnson HG and
Walsh GM: In vivo and in vitro pharmacologic activity of the purine
nucleoside phosphorylase inhibitor BCX-34: the role of GTP and
dGTP. Immunopharmacology. 35:53–63. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Galmarini CM, Popowycz F and Joseph B:
Cytotoxic nucleoside analogues: different strategies to improve
their clinical efficacy. Curr Med Chem. 15:1072–1082. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Allison AC and Eugui EM: Mycophenolate
mofetil and its mechanisms of action. Immunopharmacology.
47:85–118. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hedstrom L: IMP dehydrogenase: structure,
mechanism, and inhibition. Chem Rev. 109:2903–2928. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fairbanks LD, Ruckemann K, Qiu Y, et al:
Methotrexate inhibits the first committed step of purine
biosynthesis in mitogen-stimulated human T-lymphocytes: a metabolic
basis for efficacy in rheumatoid arthritis? Biochem J. 342:143–152.
1999. View Article : Google Scholar
|
|
39
|
Allison AC and Eugui EM: The design and
development of an immunosuppressive drug, mycophenolate mofetil.
Springer Semin Immunopathol. 14:353–380. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhou S, Liu R, Baroudy BM, Malcolm BA and
Reyes GR: The effect of ribavirin and IMPDH inhibitors on hepatitis
C virus subgenomic replicon RNA. Virology. 310:333–342. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gandhi V, Kilpatrick JM, Plunkett W, et
al: A proof-of-principle pharmacokinetic, pharmacodynamic, and
clinical study with purine nucleoside phosphorylase inhibitor
immucillin-H (BCX-1777, forodesine). Blood. 106:4253–4260. 2005.
View Article : Google Scholar
|
|
42
|
Miles RW, Tyler PC, Furneaux RH,
Bagdassarian CK and Schramm VL: One-third-the-sites
transition-state inhibitors for purine nucleoside phosphorylase.
Biochemistry. 37:8615–8621. 1998. View Article : Google Scholar : PubMed/NCBI
|