Introduction
Cervical cancer is the second most common malignancy
of the female genital track, with an estimated 12,710 new cases and
4,290 deaths in the United States in 2011 (1). With the introduction of cervical
screening by Pap smear, the testing of cervical specimens for DNA
of oncogenic (high-risk) types of human papilloma virus (HPV) and
the use of HPV vaccination, the incidence and mortality of cervical
cancer have been dramatically reduced, but these methods are only
limited to countries with highly developed health care systems
(2). However ~80% of cervical
cancers occur in developing countries (3). In China, its incidence has increased
in recent years (4), and the
mortality rate has shown an increasing trend in the younger
generation (5). Current
approaches for treating cervical cancer have limited success; the
5-year survival rate of women with cervical cancer is estimated at
71% in the United States (1). To
improve outcomes of patients with cervical cancer, it is important
to investigate the molecular pathways that are critical to the
development of the disease, and to identify novel targets for
therapy. Cervical intraepithelial neoplasia (CIN) is the potential
precursor to cervical cancer (6).
It has been classified as CIN1 (mild dysplasia), CIN2 (moderate
dysplasia) and CIN3 (severe dysplasia and carcinoma in
situ). More recently, CIN2 and CIN3 have been combined into
CIN2/3.
The tumor-suppressor gene, WW domain-containing
oxidoreductase (WWOX), was first identified in 2000, and is also
known as FOR and WOX1 (7). It is
localized to a common fragile site referred to as FRA16D, and is
located at locus 16q23.3-24.1. It encodes a protein containing two
WW domains and a short-chain dehydrogenase/reductase domain (SRD)
(7). The biological role of the
protein is not yet well defined. The highest normal expression of
this gene is detected in hormonally regulated tissues such as the
testis, ovary and prostate (8),
and WWOX-knockout mice show impaired gene expression of key
steroidogenesis enzymes in the testis and ovary. Based on these
findings, it is hypothesized that WWOX plays a role in steroid
metabolism (9). Yet, there is
still no report concerning the physiological function of WWOX in
humans.
Under stress conditions, WWOX may undergo Tyr33
phosphorylation in the first WW domain and then combines to its
partners such as Smad4 (10), p73
(p53 homolog) (11), c-Jun
(12), CREB and NF-κB (13), and then relocates to the
mitochondria and nuclei for inducing apoptosis. With its first
detection as a tumor suppressor in breast cancer, low expression
levels of the WWOX gene have been observed in many types of cancers
(14–17). The low expression of WWOX is
possibly due to the loss of heterozygosity or epigenetic changes,
such as methylation of CpG islands in the promoter region. In 2010,
one study found that WWOX was underexpressed in cervical cancer,
but its role remains undefined (18).
In the present study, we sought to explore the role
of WWOX in the pathogenesis of cervical cancer. The expression of
WWOX in cervical cancer cell lines and tissues was assessed. We
also investigated the effect of WWOX on cervical cancer cell
proliferation, anchorage-dependent and -independent growth and
apoptosis. Moreover, we confirmed this effect in nude mice in
vivo. Our results indicate that WWOX is a tumor suppressor of
cervical cancer.
Materials and methods
Case selection, tissue handling and
pathology
Formalin-fixed, paraffin-embedded surgical specimens
of 75 patients presenting with normal cervix, CIN and invasive
cervical cancer were examined. These tissues were retrieved from
patients at the Department of Pathology at The International Peace
Maternity and Child Health Hospital, affiliated with Shanghai Jiao
Tong University, China, from December 2010 to November 2011. These
patients ranged in age from 22 to 62 years (mean, 42 years). Eleven
cases of normal cervix, 10 cases of CIN1, 22 cases of CIN2/3, 20
cases of International Federation of Gynecology and Obstetrics
(FIGO) stage I invasive squamous cell carcinoma (ISCC) and 12 cases
of FIGO stage II ISCCs were investigated. The diagnosis and
histological classification of cervical cancer were carried out
according to the criteria proposed by FIGO. The clinical
characteristics of the study group are summarized in Table I. Prior written informed consent
was obtained from each patient, and the study was approved by the
Ethics Committee of the Medical Faculty of Shanghai Jiao Tong
University.
 | Table IClinical characteristics of the study
group (N=75). |
Table I
Clinical characteristics of the study
group (N=75).
| Clinical
feature | No. of
patients | WWOX
lost/reduced | P-value |
|---|
| Age (years) | | | 0.295 |
| <40 | 28 | 18 | |
| 40–49 | 29 | 24 | |
| ≥50 | 18 | 13 | |
| Stage | | | 0.000 |
| Normal cervix | 11 | 2 | |
| CIN1 | 10 | 4 | |
| CIN2/3 | 22 | 19 | |
| ISCC grade I | 20 | 17 | |
| ISCC grade
IIa | 12 | 11 | |
| Lymphatic and
vascular invasion in ISCC | | | 0.273 |
| Negative | 12 | 11 | |
| Positive | 20 | 17 | |
| Lymph node
metastasis in ISCC | | | 0.005 |
| Absent | 24 | 20 | |
| Present | 8 | 8 | |
| HPV infection | | | 0.053 |
| Positive | 54 | 44 | |
| Negative | 21 | 11 | |
Immunohistochemical analysis of WWOX
Sections (5-μm) were placed onto
poly-L-lysine-coated glass slides and air dried overnight at room
temperature. Sections were dewaxed in xylene, and then rehydrated
through a series of graded concentrations of ethanol. Endogenous
peroxidase activity was quenched by incubating the slides in 3%
hydrogen peroxide for 10 min.
Sections for microwave antigen retrieval
pre-treatment were immersed in sodium citrate buffer (pH 6.0). In
brief, this was carried out by irradiating the sections in a
microwave oven (800 W) at the highest setting for 5 min to permit
the retrieval buffer to be boiled off. This procedure was followed
by microwave irradiation at the low setting for 10 min in order to
maintain the boiling temperature. Sections were then incubated in
5% normal goat serum for 30 min followed by an overnight incubation
with a polyclonal antibody targeted against WWOX (ab33248; Abcam,
Cambridge, MA, USA) at a 1:100 dilution in phosphate-buffered
saline (PBS) at 4°C, followed by 30 min sequential incubations in
biotinylated goat anti-rabbit secondary antibody and an ABC
visualization detection kit (Vector Labs, Burlingame, CA, USA).
Finally, sections were washed in distilled water and weakly
counterstained with hematoxylin. As a negative control, the primary
antibodies were omitted and replaced with preimmune serum. All
slides were evaluated in a blinded manner and quantified for the
percent (P) of positively stained cells and the intensity (I) of
staining. The percent of positively stained cells was arbitrarily
divided into five categories (from 1 to 5): 1, ≤10%; 2, 11–25%; 3,
26–50%; 4, 51–75%; and 5, 76–100%. The intensity of staining was
arbitrarily divided into three categories (from 1 to 3): 1, loss of
staining; 2, moderate staining; and 3, intense staining. The
intensity was assessed by contrasting specimens with the positive
control. From these values, the staining index was calculated
according to the formula: Index of WWOX expression = (P × I).
Tumors with staining scores of 12 or 15 [intensity of staining 3
times the percent of staining score 4 or 5 (>50%)] were
considered to be without loss, and all other values were grouped
together as being representative of lost or reduced staining
(19).
Cell culture and establishment of stable
cell lines
HeLa cells were originally derived from cervical
adenocarcinoma, C-33A cells were originally derived from cervical
cancer, and SiHa cells were originally derived from cervical
squamous cell carcinoma; all cell lines were obtained from the
American Type Culture Collection. All cells were cultured in
Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s F12 (11030; Gibco,
Auckland, New Zealand) supplemented with 10% fetal bovine serum
(FBS) (16000-44; Gibco, Carlsbad, CA, USA) and propagated in a
fully humidified atmosphere of 5% CO2/95% air at 37°C.
The WWOX-overexpressing plasmid was generated by GeneChem
(Shanghai, China); the WWOX shRNA plasmid construct was obtained
from Santa Cruz Biotechnology, Inc. (sc-44193-SH; Santa Cruz, CA,
USA) and the transfection reagent was obtained from Qiagen
(Shanghai, China). Transfection of cells with the
WWOX-overexpressing and WWOX shRNA plasmids was carried out
according to the manufacturer’s instructions. To obtain a stable
cell line, selection pressure was maintained by supplementing the
cultures with either G418 (400 μg/ml) or puromycin (1.5 μg/ml)
(both from Sigma, St. Louis, MO, USA) for a period of 2–8 weeks.
Clonal populations of cells derived from the clonal ancestor, were
selected by isolating single colonies of cells from each well and
transferring them to a 6-well plate. The proliferation of these
cells was permitted to continue in the selection medium for at
least two additional passages.
Real-time RT-PCR
Total RNA was isolated from cells using Tri reagent
(TR118; Molecular Research Center, Cincinnati, OH, USA). The cDNA
was generated by using oligo(dT)18 primers and a Revert Aid First
Strand cDNA Synthesis Kit (K1622; Fermentas Life Science, St.
Leon-Rot, Germany). A 25-μl reaction volume was used for PCR
amplification of single-strand cDNA using reaction conditions of 40
cycles of denaturation at 98°C for 10 sec, annealing at 67°C for 30
sec, and elongation at 72°C for 2 min using the PerfectShot Ex Taq
kit (Loading Dye Mix, DRR05TA; Takara, Dalian, China). The primer
sequences used included: WWOX forward,
5′-GAGCTGCACCGTCGCCTCTCCCCAC-3′ and reverse,
5′-TCCCTGTTGCATGGACTTGGTGAAAGGC-3′; and β-actin forward,
5′-CAGCCATGTACGTTGCTATCCAGG-3′ and reverse,
5′-AGGTCCAGACGCAGGATGGCATG-3′.
Duplicate reactions were performed for each sample,
and the same experiment was repeated three times. The inclusion of
β-actin was used as a reference gene.
Western immunoblot analysis
Cells were grown on 10-cm dishes. After two rinses
in ice-cold PBS, the cells were physically harvested and lysed in
ice-cold HNTG buffer (50 mmol/l HEPES (pH 7.5), 150 mmol/l NaCl,
10% glycerol, 1% Triton X-100, 1.5 mmol/l MgCl2, 1
mmol/l EDTA, 10 mmol/l sodium PPI, 100 μmol/l sodium orthovanadate,
100 mmol/l NaF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1
mmol/l PMSF) on ice for 30 min. Total protein was measured using
the Bio-Rad protein assay kit according to the manufacturer’s
instructions. Protein samples (20 μg) were subsequently separated
on 10% sodium dodecyl sulfate polyacrylamide electrophoresis
(SDS-PAGE) gels and electrotransferred to PVDF membranes. After
blocking in 5% bovine serum albumin in Tris-buffered saline (TBS),
the membranes were incubated with primary antibodies targeted
against WWOX (1:1,000 dilution; Proteintech Group Inc., Chicago,
IL, USA), caspase-3 (1:1,000 dilution; Abcam) and β-actin (1:2,000
dilution; Proteintech Group Inc.) at 4°C overnight. Membranes were
washed three times in TBS containing 0.1% Triton X-100 (TBST) and
incubated with a peroxidase-conjugated secondary antibody (1:1,000
dilution; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. The signals were developed using an ECL kit (Pierce),
scanned, and analyzed using the ImageJ software program (National
Institutes of Health, Bethesda, MD, USA).
Proliferation assay
Cells were seeded into 96-well plates at
2×105 cells/ml and cultured in DMEM/Ham’s F12 media
supplemented with 10% FBS for 1–5 days. Cell growth was documented
every 24 h via a colorimetric assay using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay (Sigma). Absorbance values were collected at 490 nm using a
SpectraMax 190 microplate reader (Molecular Devices, Sunnyvale, CA,
USA). Control samples were treated with vehicle (0.1% DMSO or
ethanol in DMEM/Ham’s F12 culture media). In each individual
experiment, proliferation was determined in triplicate, and the
overall experiment was repeated at least three times.
Evaluation of apoptosis
Buoyant suspension cells and attached cells were
harvested and subjected to dual staining with Annexin V and
propidium iodide (PI) using an Annexin V-FITC Apoptosis Detection
Kit (BioVision, Palo Alto, CA, USA), according to the
manufacturer’s protocol. The resulting fluorescence intensities
were measured by flow cytometry using a FACS flow cytometer
(Becton-Dickinson, San Jose, CA, USA). Experiments were performed
in triplicate and reproducibility was confirmed in three
independent experiments.
TUNEL assay
DNA fragmentation in WWOX-transfected and
non-transfected (control) HeLa and SiHa cells was assessed by
TdT-mediated dUTP nick end-labeling (TUNEL) assay using the One
Step TUNEL Apoptosis Kit (Beyotime, Jiangsu, China). In this assay,
cell suspensions were placed onto poly-L-lysine-coated glass
slides, fixed, permeabilized, and incubated with the TUNEL reaction
mixture at 4°C overnight according to the manufacturer’s
protocol.
Caspase-3 activity assay
Caspase-3 activity was measured using a commercial
caspase-3 activity assay (Beyotime Institute of Biotechnology,
Shanghai, China). In brief, cells were homogenized in lysis buffer.
The lysate was then centrifuged at 20,000 × g for 10 min at 4°C.
The supernatants were incubated for 1 h at 37°C with 10 μl of 2 mM
caspase-3 substrate (Ac-DEVDpNA). Substrate cleavage was measured
using a spectrofluorometer at a wavelength of 405 nm.
Plate colony formation assay
Approximately 100 cells were seeded into each well
of a 6-well culture plate and incubated for 14 days at 37°C
following which cells were washed twice in PBS and stained with
Giemsa solution. The number of colonies containing ≥50 cells was
counted under a light microscope from which the plate clone
formation efficiency was calculated as follows: Plate clone
formation efficiency (%) = (number of colonies/number of cells
inoculated) × 100. Each experiment was performed in triplicate.
Soft agar colony assays
Cells were seeded in 0.3% top agar in growth medium
over a layer of 0.6% agar in a 6-well plate at a density of
1×104 cells/ml. After 11 days of incubation, colonies of
>50 cells were produced. Only colonies with >50 cells were
counted and photographed with an inverted microscope. All assays
were performed at least three times in triplicate.
Xenograft tumor formation assays
Two HeLa-derived cell lines (HeLa-Blank and
HeLa-WWOX) and two SiHa-derived cell lines (SiHa-Blank and
SiHa-WWOX-SH) were harvested and resuspended at a density of
1×107 cells/200 μl of sterile saline. Mice (4/group and
aged 6 weeks) were injected in the subdermal space subcutaneously
on the medial side of the neck with the transfected or
non-transfected cells. Mice were sacrificed 30 days following the
transfer of cells. Subsequently, tumors were dissected and weighed,
and the tumor volume (mm3) was measured and calculated
using the formula [(a2 × b)/2]; where ‘a’ represents the
smallest diameter, and ‘b’ represents the largest tumor diameter.
The animals were housed under a laminar flow hood in an isolated
room according to a protocol approved by the Animal Care and Use
Committee of Fudan University (Shanghai, China).
Statistical analysis
Statistical analysis was performed using the
Student’s t-test or one way analysis of variance (ANOVA). All tests
were completed using the Statistical Product and Service Solutions
(SPSS) software program, version 16.0 (SPSS Inc., Chicago, IL, USA)
or Prism (GraphPad, San Diego, CA, USA). An α value of P<0.05
was considered to indicate a statistically significant result.
Results
WWOX is underexpressed in cervical cancer
tissues and cell lines
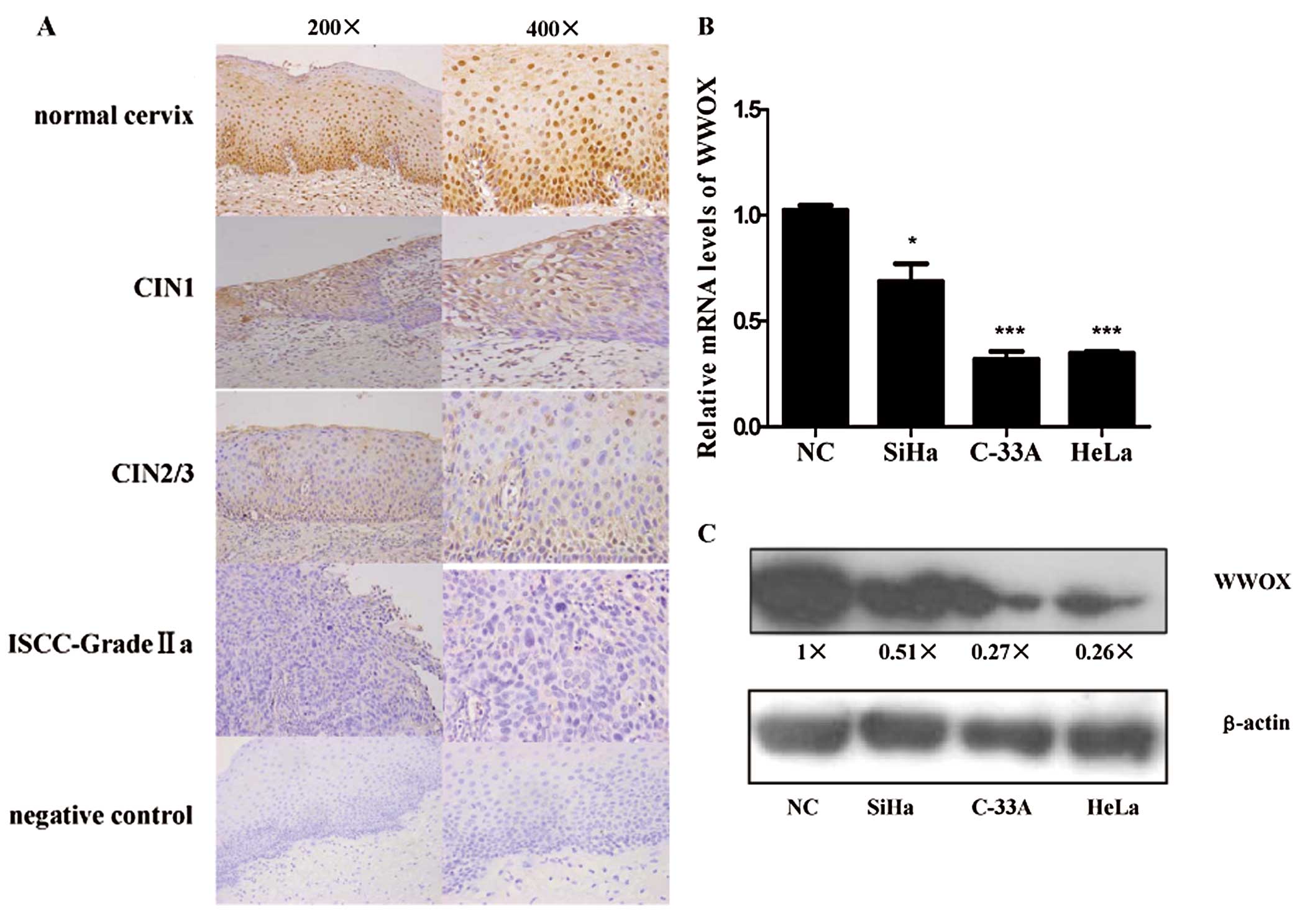
Immunohistochemical staining showed that the WWOX
protein was predominantly localized to the neuclei of cervical
epithelial cells, particularly in the basal layer of the
epithelium. There was strong staining in the normal cervix and
CIN1, whereas lost or reduced immunostaining was noted in CIN2/3
and ISCCs (Fig. 1A).
To account for both staining intensity and the
uniformity of staining, a composite histoscore (percentage of the
epithelium staining x staining intensity) was calculated. Nuclear
WWOX expression (WWOX composite histoscore) was significantly lost
or reduced as the cervical cancer progressed (P<0.05) (Table I). In addition, reduced WWOX
expression was significantly associated with lymph node metastasis
(Table I). These results indicate
a strong association between decreased expression of WWOX and the
development of cervical cancer.
To investigate whether WWOX is also underexpressed
in cervical cancer cell lines, we assessed the expression of WWOX
in three cervical cancer cell lines (HeLa, SiHa and C-33A) by
real-time PCR and western immunoblot analysis. Normal cervical
epithelial tissues were obtained from patients who underwent
hysterectomy due to myoma or adenomyosis. The mRNA (Fig. 1B) and protein (Fig. 1C) expression of WWOX were
decreased in all three cell lines and this was particularly
prominent in both HeLa and C-33A cells.
WWOX inhibits cell growth
To further explore the role of WWOX in cell
proliferation and apoptosis, we sought to overexpress WWOX by
stable transfection of the WWOX plasmid construct into the HeLa
cell line which expresses a low level of the WWOX protein. As shown
in Fig. 2A, the protein
expression level of WWOX was increased significantly. The effect of
WWOX overexpression on cell proliferation was detected by MTT
assay. The MTT assays revealed that stable overexpression of WWOX
significantly decreased HeLa cell growth (Fig. 2C).
To further investigate the effect of WWOX on cell
proliferation, we performed shRNA-mediated stable knockdown of WWOX
in SiHa cells in which WWOX protein expression is relatively high
(Fig. 2B). As shown in Fig. 2D, knockdown of WWOX in SiHa cells
promoted their ability to proliferate. Thus, WWOX is capable of
inhibiting cell growth.
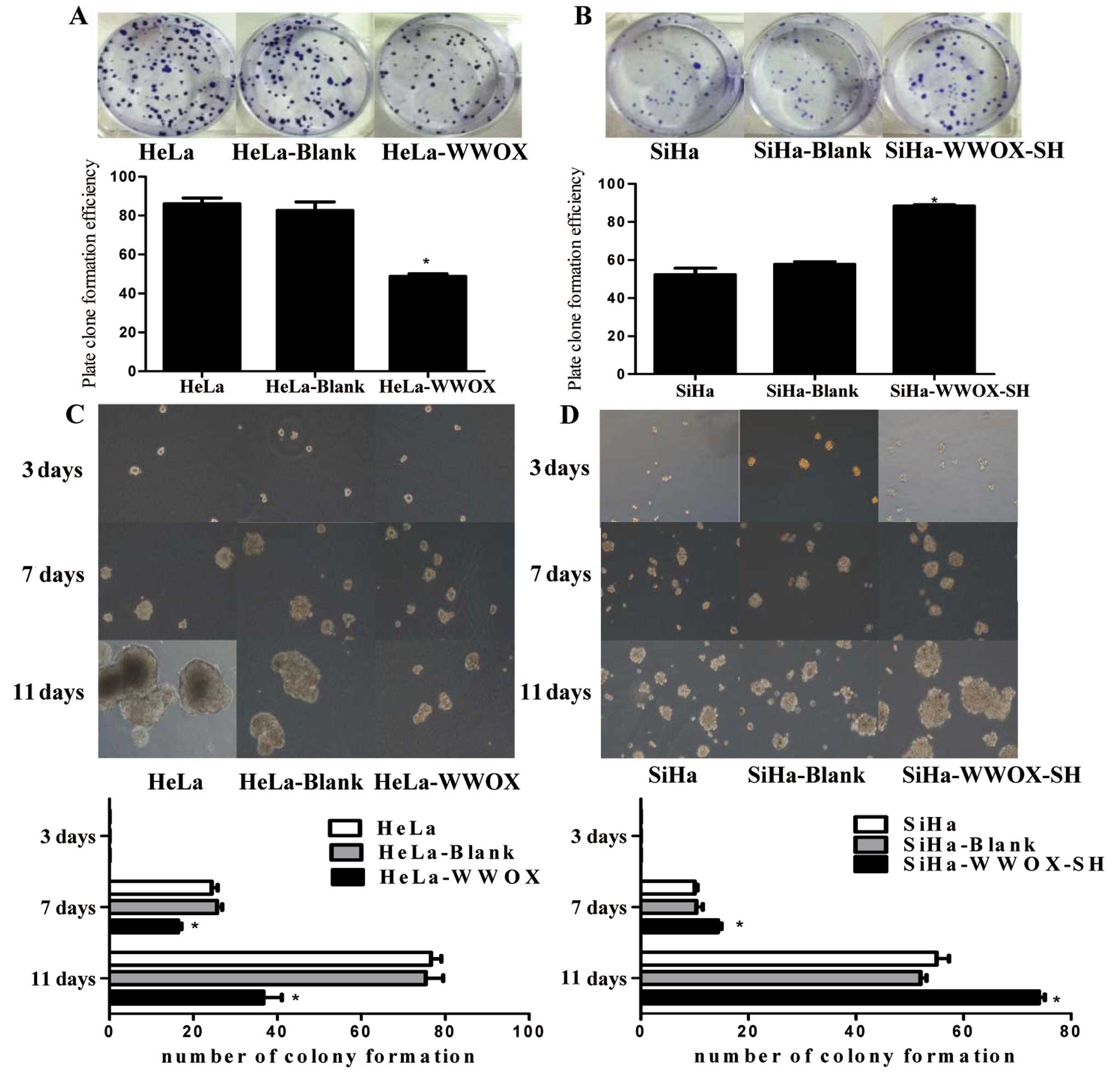
We next investigated whether WWOX is required in
anchorage-dependent and -independent growth, a hallmark of
oncogenic transformation (20).
In HeLa cells transfected with the WWOX vector, the plate colony
formation (Fig. 3A) and soft agar
assays (Fig. 3C) showed that
there was a significant decrease in the number and size of colonies
when compared to the cells transfected with a control vector or
parental wild-type cells. In contrast, stable knockdown of WWOX
promoted plate colony formation (Fig.
3B) and proliferation in soft agar assays (Fig. 3D) in SiHa cells. These findings
indicate that WWOX inhibits cervical cancer cell proliferation.
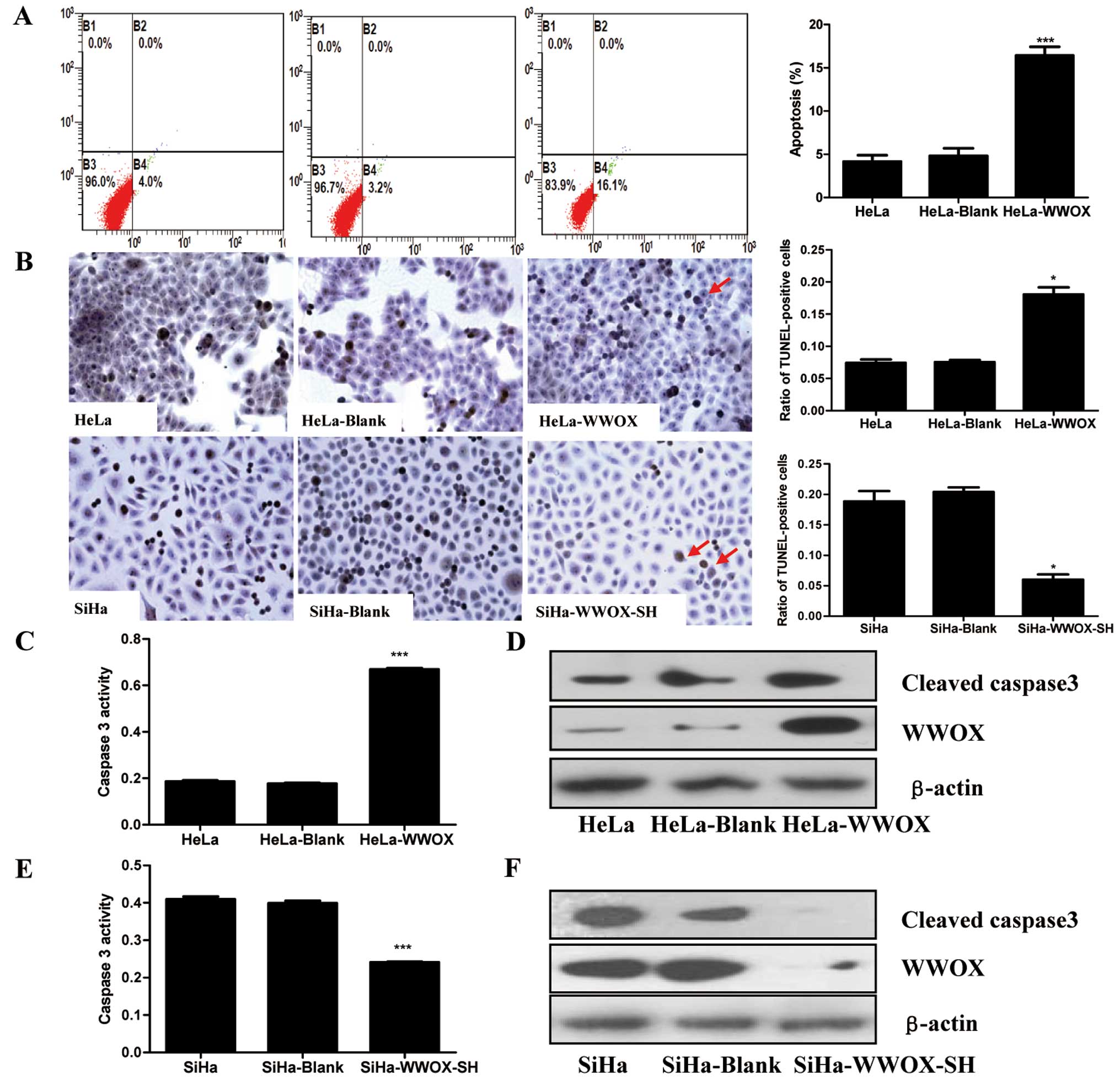
WWOX promotes apoptosis
The apoptotic ratio was determined by flow cytometry
(Fig. 4A) and TUNEL assay
(Fig. 4B). HeLa-WWOX cells
displayed enhanced apoptosis when compared to their non-transfected
counterparts (HeLa and HeLa-Blank control groups stably transfected
with the p-EGFP-N1 control plasmid). In contrast, in SiHa cells
transfected with the WWOX shRNA, the apoptotic ratio was
significantly reduced (Fig.
4B).
To further elucidate the mechanism by which WWOX
functions, we tested the activation and protein expression of
caspase-3, a crucial mediator of programmed cell death (21). We found that overexpression of
WWOX in HeLa cells markedly induced caspase-3 activation (Fig. 4C) and protein expression (Fig. 4D). Conversely, knockdown of WWOX
by shRNA in SiHa cells decresed the caspase-3 activity (Fig. 4E) and protein expression (Fig. 4F). Our results indicate that WWOX
plays an essential role in promoting apoptosis in cervical cancer
cells.
WWOX inhibits xenograft formation in
vivo
To further confirm the role of WWOX in cell
proliferation, a human tumor xenograft model was established in a
nude mouse model (Fig. 5). At the
conclusion of this assay, it was found that the WWOX-overexpressing
xenografts displayed reduced average tumor size and weight as
compared with the HeLa control group (Fig. 5A). In contrast, the average size
and weight of the tumors were significantly increased after mice
were transfected with WWOX-knockdown cells as compared with the
SiHa control group (Fig. 5B).
Furthermore, the difference in tumor size between the HeLa and SiHa
control groups may partially suggest the importance of WWOX in
tumor formation. Collectively, these data indicate that genetic
disruption of WWOX expression increases xenograft proliferation,
while overexpression of WWOX inhibits xenograft tumor formation.
These observations indicate a critical role for WWOX in tumor
formation in vivo.
Discussion
WWOX is a tumor suppressor in several human tumor
types (14–17). However, its role in cervical
cancer is still unknown. In the present study, WWOX protein
expression was significantly reduced as the cervical cancer
progressed. And the decreased WWOX expression is related to lymph
node metastasis. To further explore the possible role of WWOX in
cervical cancer, we tested three cell lines derived from cervical
cancer. These cell lines included HeLa (adenocarcinoma,
HPV+), SiHa (squamous carcinoma, HPV+) and
C-33A (HPV−). Due to the lack of a normal cervical
epithelium cell line, we used normal cervical tissue lysate as a
normal control (16). As shown in
Fig. 1C, the protein expression
in cervical cancer cell lines was significantly reduced,
particularly in the HeLa and C-33A cell lines. This observation was
inconsistent with a previously published study (18), in which it was demonstrated that
the protein expression of WWOX was absent or underexpressed in all
cell lines chosen with the notable exception of Caski cells. This
may be due to the different antibody and control used. In the
future, the primary cultured normal cervical epithelial cells will
be included to confirm this effect.
The immunohistochemical assay suggests that WWOX
expression is decreased with cervical cancer progression. We
believe that underexpression of the WWOX protein promotes tumor
progression in cervical cancer cells. Therefore, we established
models of WWOX overexpression by stable transfection of HeLa cells
with the WWOX plasmid (Fig. 2A).
In addition, cells with knockdown expression of WWOX were
established by stable transfection of WWOX shRNA into SiHa cells
(Fig. 2B). We noted that by
altering the expression of WWOX, cells showed highly variable
proliferative characteristics (Figs.
2C and D and 3).
Apoptosis plays a central role in tumor development,
and a lack or failure of apoptosis leads to the development of many
types of tumors, including cervical cancer (22,23). This suggests that induction of
apoptosis in tumor cells may be an effective approach for delaying
tumor progression. In this study, we found that overexpression of
WWOX induced apoptosis in the cervical cancer cell line HeLa
(Fig. 4A and B).
We further investigated the possible pathway through
which WWOX induces apoptosis. Previous research has established
that caspase-3 is a principle effector caspase of apoptosis and
that the intrinsic pathway of apoptosis is initiated by
mitochondrial damage which in turn promotes activation of caspase-3
(24). In the cytosol or on the
surface of the mitochondria, cytochrome c is bound to the
protein Apaf-1 (apoptotic protease activating factor), which
activates the initiating caspase, which then activates caspase-3
(25,26). In western immunoblot analyses and
caspase-3 activity assays, both HeLa and SiHa cells underwent
apoptosis by a WWOX-dependent caspase-3-mediated signaling pathway.
This observation was consistent with studies conducted in hepatoma
and pulmonary cell lines (27,28) and by contrast, was inconsistent
with research carried out in a glioblastoma cell line model
(29). We suspect that such
differences were partly due to the different cells used in the
respective research models.
Next, the notion that disruption of the normal
expression of WWOX may promote tumor formation, and that, by
contrast, overexpression of WWOX may inhibit tumor formation was
demonstrated in an in vivo model using nude mice (Fig. 5). Overexpression of WWOX caused
tumors to display smaller sizes and weight, while silencing of WWOX
expression promoted the reverse effect by enhancing tumor size and
weight. Collectively, these observations imply an important role
for WWOX as a determinant of tumor proliferation.
The WWOX gene is located on chromosome 16q, and the
common mutated form of this gene is due to the loss of
heterozygosity (LOH), which has been confirmed by several studies
using various cancers including breast cancer (30), esophageal squamous cell carcinoma
(14), gastric cancer (15), pancreatic cancer (16) and lung cancer (17). However, to our knowledge, there is
no evidence showing WWOX LOH in cervical cancer. It was previously
shown that there is loss of 16q in 20% of cervical squamous cell
carcinomas (31) and 38% of
cervical adenocarcinomas (32).
Therefore, we speculated that LOH in 16q in the context of cervical
cancer may be due, at least in part, to the loss in functional
expression of WWOX. This requires further direct study.
The integration of HPV into the host genome is
regarded as a key step in the progression from cervical
intraepithelial CIN to invasive carcinoma (33,34). However, there is still a
proportion of cervical cancers that show no evidence of infection
by HPV (35). In contrast with
these findings, we found that WWOX was reduced in C-33A cells
(which is a HPV-negative cell line). This suggests that WWOX may be
another important factor in tumor formation, irrespective of HPV
infection. However, the possibility that HPV integration may
produce a zone of fragility prone to breakage, loss and gene
rearrangements (including WWOX), cannot be excluded. Thus, it is
necessary to further explore the relationship between these
factors.
Acknowledgements
We thank Dr Huijuan Zhang and Mrs Yuan Liu for their
help in sample collection. This study was supported by grants from
the National Natural Science Funds of China (nos. 81072139,
81172476 and 81272885) and Research Fund for the Doctoral Program
of Higher Education of China (20120073110090).
Abbreviations:
|
WWOX
|
WW domain-containing
oxidoreductase
|
|
LOH
|
loss of heterozygosity
|
|
IHC
|
immunohistochemistry
|
|
MTT
|
methyl-thiazolyl tetrazolium
|
|
TUNEL
|
TdT-mediated dUTP nick
end-labeling
|
|
HPV
|
human papilloma virus
|
|
CIN
|
cervical intraepithelial neoplasia
|
|
ISCC
|
invasive squamous cell carcinoma
|
References
|
1
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: the impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mayrand MH, Duarte-Franco E, Rodrigues I,
et al: Human papillomavirus DNA versus Papanicolaou screening tests
for cervical cancer. N Engl J Med. 357:1579–1588. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kent A: HPV vaccination and testing. Rev
Obstet Gynecol. 3:33–34. 2010.
|
|
4
|
Wang Y, Chen J, Zhang W, Hong W and Yu F:
Study of the prevalence of human Papillomavirus infection in
Chinese women with cervical cancer. Afr J Microbiol Res.
6:1048–1053. 2012.
|
|
5
|
Aleyamma M and Preethi SG: Trends in
incidence and mortality rates of squamous cell carcinoma and
adenocarcinoma of cervix - worldwide. Asian Pac J Cancer Prev.
10:645–650. 2009.PubMed/NCBI
|
|
6
|
Misson DR, Abdalla DR, Borges AM, et al:
Cytokine serum levels in patients with cervical intraepithelial
neoplasia grade II-III treated with intralesional interferon-α 2b.
Tumori. 97:578–584. 2011.PubMed/NCBI
|
|
7
|
Bednarek AK, Laflin KJ, Daniel RL, Liao Q,
Hawkins KA and Aldaz CM: WWOX, a novel WW domain-containing protein
mapping to human chromosome 16q23.3-24.1, a region frequently
affected in breast cancer. Cancer Res. 60:2140–2145. 2000.
|
|
8
|
Del Mare S, Salah Z and Aqeilan RI: WWOX:
its genomics, partners, and functions. J Cell Biochem. 108:737–745.
2009.PubMed/NCBI
|
|
9
|
Aqeilan RI, Hagan JP, de Bruin A, et al:
Targeted ablation of the WW domain-containing oxidoreductase tumor
suppressor leads to impaired steroidogenesis. Endocrinology.
150:1530–1535. 2009. View Article : Google Scholar
|
|
10
|
Hsu LJ, Schultz L, Hong Q, et al:
Transforming growth factor beta1 signaling via interaction with
cell surface Hyal-2 and recruitment of WWOX/WOX1. J Biol Chem.
284:16049–16059. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aqeilan RI, Pekarsky Y, Herrero JJ, et al:
Functional association between Wwox tumor suppressor protein and
p73, a p53 homolog. Proc Natl Acad Sci USA. 101:4401–4406. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gaudio E, Palamarchuk A, Palumbo T, et al:
Physical association with WWOX suppresses c-Jun transcriptional
activity. Cancer Res. 66:11585–11589. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li MY, Lai FJ, Hsu LJ, et al: Dramatic
co-activation of WWOX/WOX1 with CREB and NF-kappaB in delayed loss
of small dorsal root ganglion neurons upon sciatic nerve
transection in rats. PLoS One. 4:e78202009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo W, Wang G, Dong Y, Guo Y, Kuang G and
Dong Z: Decreased expression of WWOX in the development of
esophageal squamous cell carcinoma. Mol Carcinog. Dec 27–2011.(Epub
ahead of print). View
Article : Google Scholar
|
|
15
|
Maeda N, Semba S, Nakayama S, Yanagihara K
and Yokozaki H: Loss of WW domain-containing oxidoreductase
expression in the progression and development of gastric carcinoma:
clinical and histopathologic correlations. Virchows Arch.
457:423–432. 2010. View Article : Google Scholar
|
|
16
|
Kuroki T, Yendamuri S, Trapasso F, et al:
The tumor suppressor gene WWOX at FRA16D is involved in pancreatic
carcinogenesis. Clin Cancer Res. 10:2459–2465. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Donati V, Fontanini G, Dell’Omodarme M, et
al: WWOX expression in different histologic types and subtypes of
non-small cell lung cancer. Clin Cancer Res. 13:884–891. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Giarnieri E, Zanesi N, Bottoni A, et al:
Oncosuppressor proteins of fragile sites are reduced in cervical
cancer. Cancer Lett. 289:40–45. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guler G, Uner A, Guler N, et al: The
fragile genes FHIT and WWOX are inactivated coordinately in
invasive breast carcinoma. Cancer. 100:1605–1614. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kurek KC, Del Mare S, Salah Z, et al:
Frequent attenuation of the WWOX tumor suppressor in osteosarcoma
is associated with increased tumorigenicity and aberrant RUNX2
expression. Cancer Res. 70:5577–5586. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: a basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Evan G and Littlewood T: A matter of life
and cell death. Science. 281:1317–1322. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arnoult D, Gaume B, Karbowski M, Sharpe
JC, Cecconi F and Youle RJ: Mitochondrial release of AIF and EndoG
requires caspase activation downstream of Bax/Bak-mediated
permeabilization. EMBO J. 22:4385–4399. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu X, Kim CN, Yang J, Jemmerson R and
Wang X: Induction of apoptotic program in cell-free extracts:
requirement for dATP and cytochrome c. Cell. 86:147–157. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zou H, Henzel WJ, Liu X, Lutschg A and
Wang X: Apaf-1, a human protein homologous to C. elegans
CED-4, participates in cytochrome c-dependent activation of
caspase-3. Cell. 90:405–413. 1997.PubMed/NCBI
|
|
27
|
Hu BS, Tan JW, Zhu GH, Wang DF, Zhou X and
Sun ZQ: WWOX induces apoptosis and inhibits proliferation of human
hepatoma cell line SMMC-7721. World J Gastroenterol. 18:3020–3026.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang P, Jia R, Ying L, et al:
WWOX-mediated apoptosis in A549 cells mainly involves the
mitochondrial pathway. Mol Med Rep. 6:121–124. 2012.PubMed/NCBI
|
|
29
|
Chiang MF, Yeh ST, Liao HF, Chang NS and
Chen YJ: Overexpression of WW domain-containing oxidoreductase WOX1
preferentially induces apoptosis in human glioblastoma cells
harboring mutant p53. Biomed Pharmacother. 66:433–438. 2012.
View Article : Google Scholar
|
|
30
|
Finnis M, Dayan S, Hobson L, et al: Common
chromosomal fragile site FRA16D mutation in cancer cells. Hum Mol
Genet. 14:1341–1349. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jee KJ, Kim YT, Kim KR, Aalto Y and
Knuutila S: Amplification at 9p in cervical carcinoma by
comparative genomic hybridization. Anal Cell Pathol. 22:159–163.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tsuda H, Takarabe T, Okada S, et al:
Different pattern of loss of heterozygosity among endocervical-type
adenocarcinoma, endometrioid-type adenocarcinoma and adenoma
malignum of the uterine cervix. Int J Cancer. 98:713–717. 2002.
View Article : Google Scholar
|
|
33
|
Cullen AP, Reid R, Campion M and Lörincz
AT: Analysis of the physical state of different human
papillomavirus DNAs in intraepithelial and invasive cervical
neoplasm. J Virol. 65:606–612. 1991.PubMed/NCBI
|
|
34
|
Hopman AH, Smedts F, Dignef W, et al:
Transition of high-grade cervical intraepithelial neoplasia to
micro-invasive carcinoma is characterized by integration of HPV
16/18 and numerical chromosome abnormalities. J Pathol. 202:23–33.
2004. View Article : Google Scholar
|
|
35
|
Crook T, Wrede D, Tidy JA, Mason WP, Evans
DJ and Vousden KH: Clonal p53 mutation in primary cervical cancer:
association with human-papillomavirus-negative tumours. Lancet.
339:1070–1073. 1992. View Article : Google Scholar : PubMed/NCBI
|