Introduction
The majority of patients with malignant carcinoma
succumb to metastasis (1), and
inhibition of cancer cell metastasis is an effective method to
prolong the survival time of patients. Yet, the mechanisms by which
tumor cells become metastatic are poorly understood (2). Thus, to explore the molecular
mechanisms and molecular targets of metastasis is of vital
importance in the targeted therapy of cancer (3).
Amino-terminal enhancer of split (AES), which is
known as Groucho (Gro) or transducin-like enhancer of split (TLE),
is a member of the transcriptional co-repressor family (4). The Gro/TLE protein family consists
of five members and is highly conserved within different species
(5). These proteins have no
DNA-binding region but can interact with DNA-binding transcription
factors (TFs) and mediate transcription activity of target genes
(6,7). AES, as well as its mouse homolog
GRG5, is the shortest member of the Gro/TLE family (4,8).
It was considered that AES may act as a negative regulator in the
process of transcription. Moreover, a series of previous research
described that AES recruits various TFs such as NF-κB and gp130 to
gene promoters, and functions as a transcription repressor
(9,10). Most of the previous studies
concerning AES, as well as other members of the Gro/TLE family,
have mainly focused on development, including bone growth,
hematopoiesis and eye development (11–14), while there are only a few studies
concerning the function of AES in cancer. AES also forms complexes
with the mitochondrial protein Bit1 and induces cell death with
characteristics of caspase-independent apoptosis (15). Recently, AES was reported as a
metastatic repressor in colorectal cancer (CRC) by inhibiting the
NOTCH signaling pathway, and composite depletion of AES in Apc
intestinal polyposis mice caused marked tumor invasion and
intravasation (16,17). Thus, loss of AES may be an
important factor for inducing tumor invasion. Although AES is known
to regulate the activity of the Notch intercellular domain (NICD),
other pathways involved in AES regulation during the process of
tumor metastasis must be explored.
The Rho GTPase family is a group of small GTPase
proteins that are implicated in several cellular functions such as
actin cytoskeleton organization, microtubule dynamics and cell
cycle progression. Thus, they play an important role in cancer
progression (18). Most Rho
GTPase members function as molecular switches, cycling between an
active GTP-bound and an inactive GDP-bound conformation (19). RND3, also known as RhoE, is an
atypical member of the Rho GTPase family in that it binds, but does
not hydrolyze GTP. The best-characterized function of RND3 is
inhibition of RhoA/ROCK signaling by binding to p190
GTPase-activating protein for Rho (RhoGAP), thus reducing the
RhoA-GTP levels, as well as directly regulating the RhoA effector,
Rho kinase-I (ROCK-I) (20).
Recent studies have indicated that RND3 could acts as a
tumor-suppressor gene in cancer progression (21,22). However, the molecular mechanism
that regulates the expression of RND3 is poorly understood.
Herein, the results revealed that reduced AES
expression downregulates RND3 expression at the mRNA and protein
levels, while enhanced AES expression significantly increases the
activity of the RND3 promoter. Further studies have shown that AES
regulates tumor cell proliferation, cell cycle progression and
invasion, and the process is related to RND3 regulation. For the
first time, we demonstrated that AES regulates the expression of
RND3, and the results further elucidate the mechanisms of AES in
regulating tumor malignancies.
Materials and methods
Cell culture
The human hepatocellular carcinoma cell line HepG2
and the breast cancer cell line MDA-MB-231 were maintained in
RPMI-1640 medium (Invitrogen, Gaithersburg, MD, USA) supplemented
with 10% heat-inactivated fetal bovine serum (FBS) (Gibco,
Gaithersburg, MD, USA), 100 U/ml of penicillin G sodium, and 100
μg/ml streptomycin sulfate (Sigma, St. Louis, MO, USA) in a
humidified atmosphere containing 5% CO2 at 37°C.
Plasmid construct
The human AES gene (NM_001130, 197 amino acids) was
PCR amplified from human HEK293 cDNA. To construct HA-AES, the AES
coding sequence was amplified by sense primer
5′-GCCCCGAATTCCGATTGACATGA-3′ and anti-sense primer
5′-GAACGGTACCCCCTGCTAATC CGACTTCTCGCCAT-3′ and then cloned into the
EcoR1 and Kpn1 sites of the pCMV-HA vector. RND3
promoter was cloned into the pGL3-basic vector to generate the
pGL3-RND3-promoter as previous described (23).
Small interfering RNA transfection
The siRNAs against AES were designed and synthesized
by Ribobio (Guangzhou, China). The sequence of AES siRNA was
5′-CCUACGG CUUGAACAUCGAdTdT-3′ and the sequence of the RND3 siRNA
strand was 5′-AACAGATTGGAGCAGCTACdTdT-3′. siRNAs against AES and
RND3 were transfected into MDA-MA-231 and HepG2 cells respectively
using Lipofectamine 2000 (Invitrogen).
Luciferase reporter assay
The pGL3-RND3-promoter (0.4 μg) and pRL-TK (0.005
μg) were co-transfected into HeLa cells in each well of 24-well
plates together with pCMV-HA or pCMV-HA-AES. Firefly and
Renilla luciferase activities were measured 48 h after
transfection using the Dual-Luciferase reporter assay system
(Promega) according to the manufacturer’s protocol. Values are
shown as relative Renilla and Firefly luciferase activity,
and data values are reported as means ± SEM.
RT-PCR
Total RNAs were extracted from transfected cells by
TRIzol (Life Technologies) using the manufacturer’s protocol and
reconstituted in 1.0 μg/μl with nuclease-free water. For
semi-quantitative reverse transcription-PCR, cDNA was synthesized
from total RNA using Olig-dT primer. The primers used for specific
RND3 PCR reactions were: forward 5′-AAGATA GTTGTGGTGGGAGA-3′ and
reverse 5′-CATAGTAAGGAGA ACCCGAA-3′. The specific AES PCR reaction
primers used were: forward 5′-CACCAGGAGGATGATGGCGAG-3′ and reverse
5′-GGCGTGGAGGTGTCTGGAACTA-3′. The primers used for specific GAPDH
PCR reactions were: forward 5′-CAA GGCCAACCGCGAGAA-3′ and reverse
5′-CCCTCGTAGAT GGGCACAGT-3′.
Western blotting
Transfected cells were lysed in RIPA buffer (150 mM
NaCl, 1% NP-40, 50 mM Tris-HCl pH 7.4, 1 mM phenylmethylsulfonyl
fluoride, 1 μg/ml leupeptin, 1 mM deoxycholic acid and 1 mM EDTA)
containing a cocktail of protease inhibitors and phosphatase
inhibitors (Calbiochem, Darmstadt, Germany). Equal amounts of the
protein sample (30–50 μg) were separated by 12% SDS-PAGE and
transferred to PVDF membranes (Millipore, Bedford, MA, USA) using
the Bio-Rad semi-dry transfer system. The following antibodies were
used for western blotting: anti-AES (Sigma), anti-α-tubulin
(Biostar, Wuhan, China), anti-RND3 (Millipore).
Cell proliferation assay
MDA-MB-231 and HepG2 cells were seeded in 96-well
plates, and after 24 h, cells were transfected with two types of
siRNAs or the negative control, respectively. Relative cell growth
was measured using the Cell Counting Kit-8 (Dojingdo, Kumamoto,
Japan).
Matrigel invasion assay
After 24 h of transfection, cells were collected and
suspended in serum-free medium. Cells (4×104) in 0.2 ml
serum-free medium were plated in the top chamber with a
Matrigel-coated membrane (24-well insert; pore size, 8 mm; Becton
Dickinson), with 10% FBS as a attractant. The cells were incubated
for 48 h. The cells that did not invade through the pores were
removed, and the filter was stained with hematoxylin and eosin
(H&E) for visualization and counting.
Cell cycle analysis by flow
cytometry
Cells were transfected with siRNA against AES or
RND3 for 48 h. The cells were then digested by trypsin, collected
by centrifugation, washed with PBS and fixed overnight at 4°C by
70% ethanol. The cells were the washed with PBS and stained by PI
at 4°C for 30 min using the Cell Cycle Detection kit (KeyGen,
Nanjin, China). The cells were then analyzed using a flow cytometer
(BD FACSCalibur).
Statistical analysis
The data are presented as the means ± SEM. The
Student’s t-test was used for comparisons. p<0.05 was considered
to indicate a statistically significant result.
Results
siRNA-mediated AES knockdown
downregulates RND3 expression at the mRNA and protein levels
Previous studies reported that AES acted as a
potential tumor-metastasis suppressor (16,17). However, the molecular mechanisms
remain unclear (17). RND3, a
typical member of the Rho family, was demonstrated as a
tumor-suppressor in several studies (14,24). Recent studies indicate that both
AES and RND3 are involved in tumor metastasis. Thus, we
hypothesized that AES could regulate RND3 expression levels in
cancer cells. Breast cancer cell line MDA-MB-231 and hepatocellular
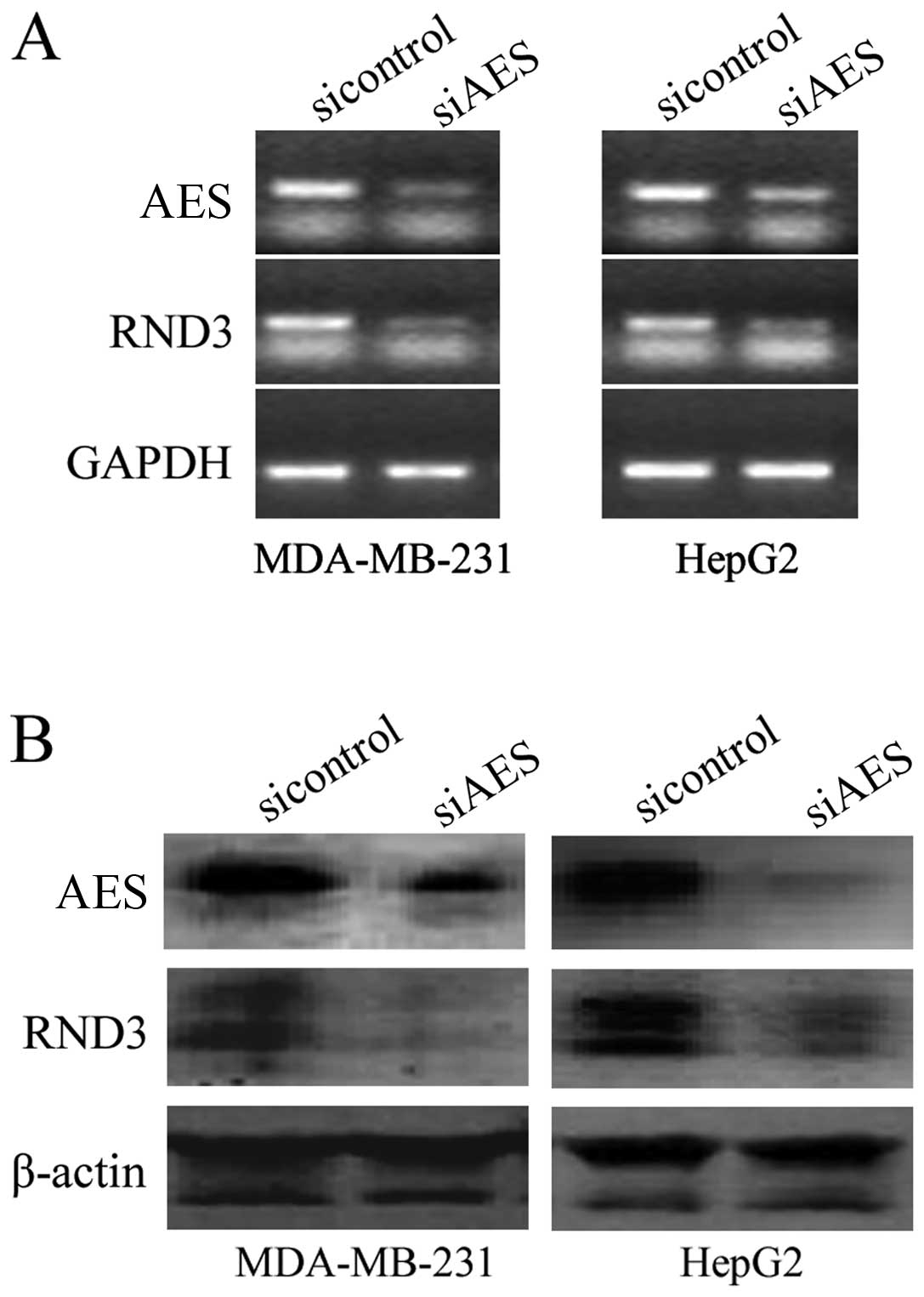
carcinoma cell (HCC) line HepG2 were transfected with AES siRNA.
RT-PCR results revealed that knockdown of AES by RNA interference
(RNAi) downregulated RND3 expression at the mRNA level (Fig. 1A). Western blot analysis showed
that downregulation of AES also inhibited RND3 expression at the
protein level in both the MDA-MB-231 and HepG2 cell lines (Fig. 1B). These results reveal that AES
regulates RND3 at the mRNA and protein levels.
Enhanced AES expression increases RND3
promoter activity
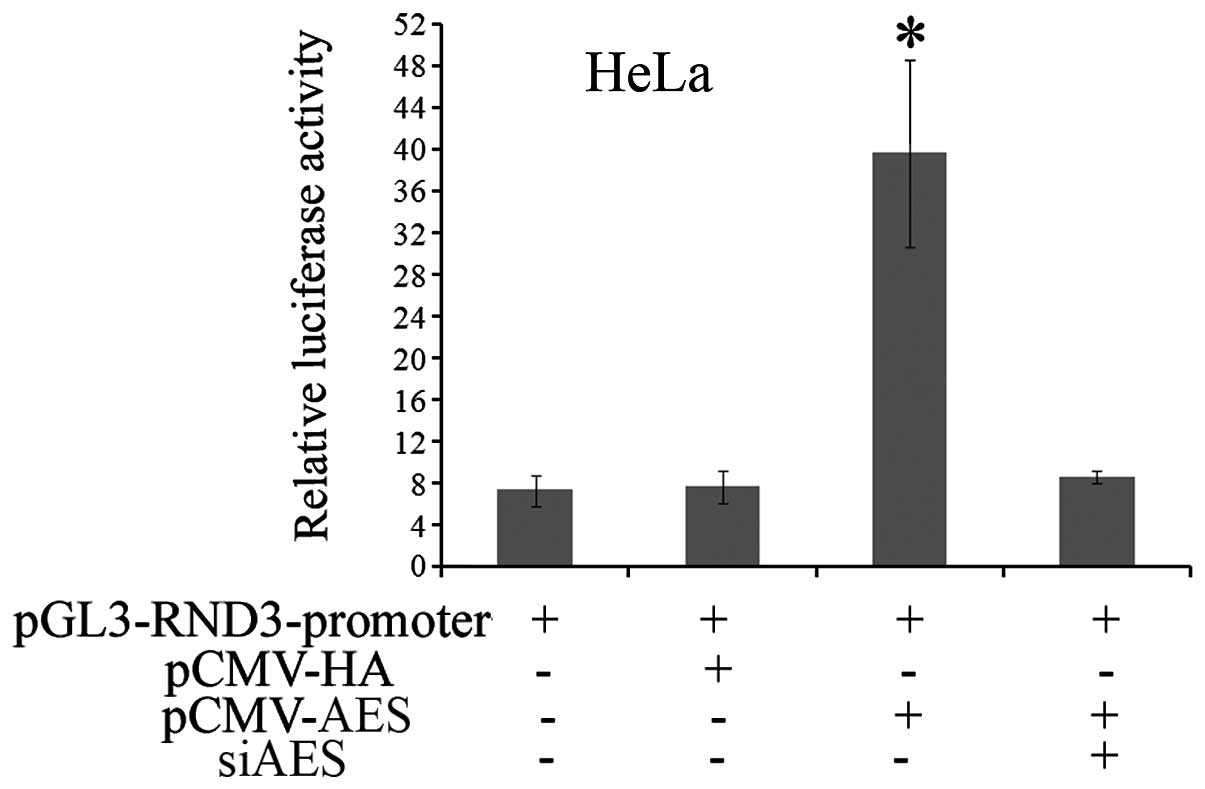
The relationship between AES and RND3 was further
investigated. To investigate whether AES regulates RND3 promoter
activity, the promoter sequence of RND3 was subcloned into the
pGL3-basic vector. Then, the pGL3-RND3-promoter was co-transfected
with pCMV-HA-AES or pCMV-HA, respectively, into HeLa cells.
Dual-luciferase reporter assay revealed that forced AES expression
increased the luciferase activity of the pGL3-RND3-promoter by
5.6-fold compared with the control pCMV-HA group. Meanwhile, AES
siRNA abolished the activation of luciferase activity induced by
enhanced AES expression (Fig. 2).
In summary, these data indicate that AES functions as a positive
regulator of the RND3 promoter.
Downregulation of AES and RND3 expression
enhances the proliferation of cancer cells
Previous studies have shown that RND3 functions as a
tumor-suppressor gene in esophageal squamous cell carcinogenesis,
and is involved in the regulation of tumor cell proliferation,
invasion and cell cycle progression (25–27). Recent research also indicates that
AES may have a similar function with RND3 (16). Our results showed that AES
regulates the expression of RND3 in breast cancer and HCC cells.
Thus, whether AES and RND3 function similarly in these two cancer
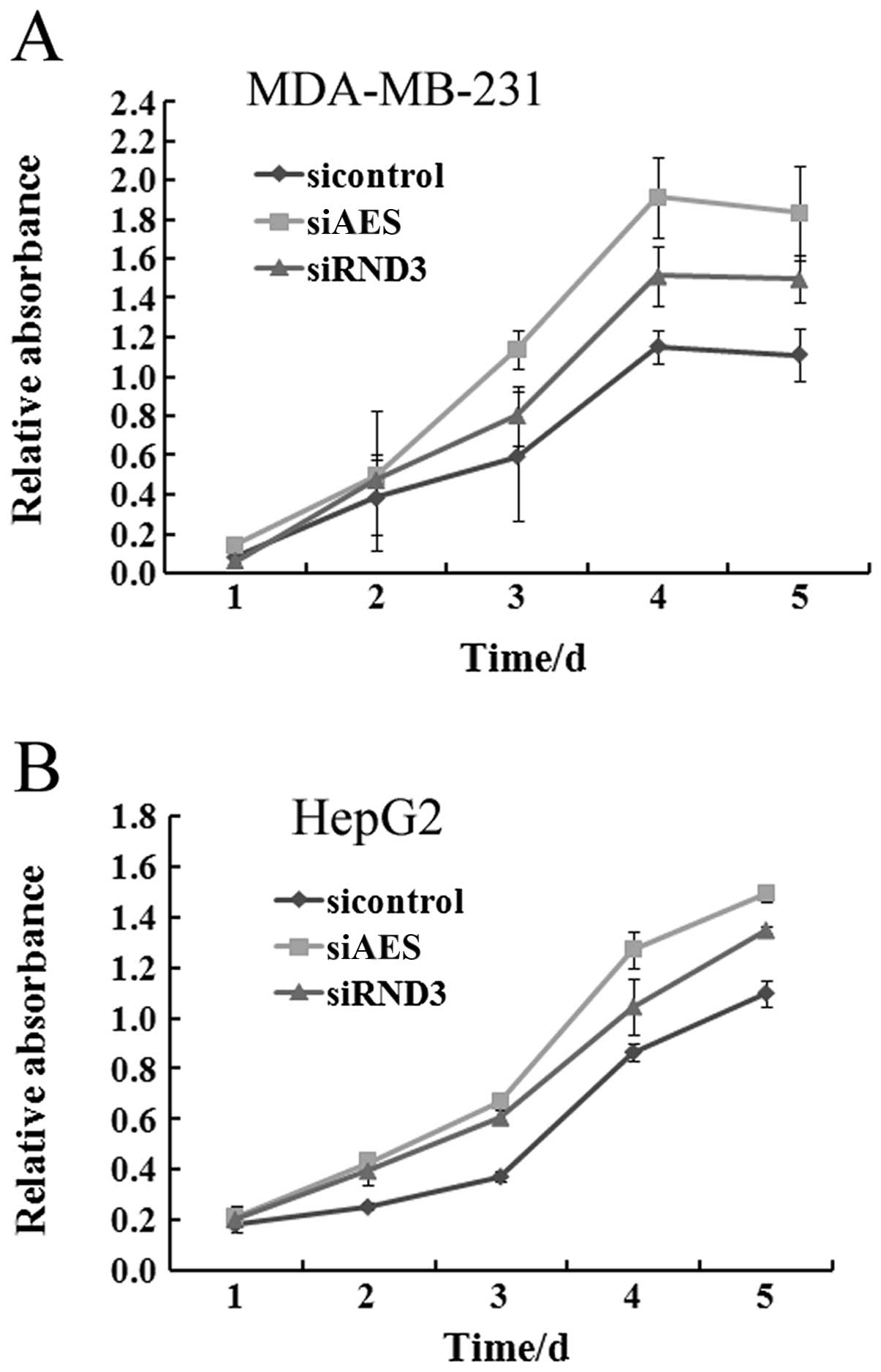
cell lines needs to be further investigated. To determine the
influence of AES and RND3 on tumor proliferation, specific siRNAs
of AES and RND3 were separately transfected into MDA-MB-231 and
HepG2 cells. After transfection, the viability of both HepG2 and
MDA-MB-231 cells was markedly increased at each time point similar
to the effect of RND3 knockdown (Fig.
3). This result demonstrated that downregulation of AES
promotes cancer cell proliferation.
siRNA-mediated AES and RND3
downregulation induces cell cycle progression of cancer cells
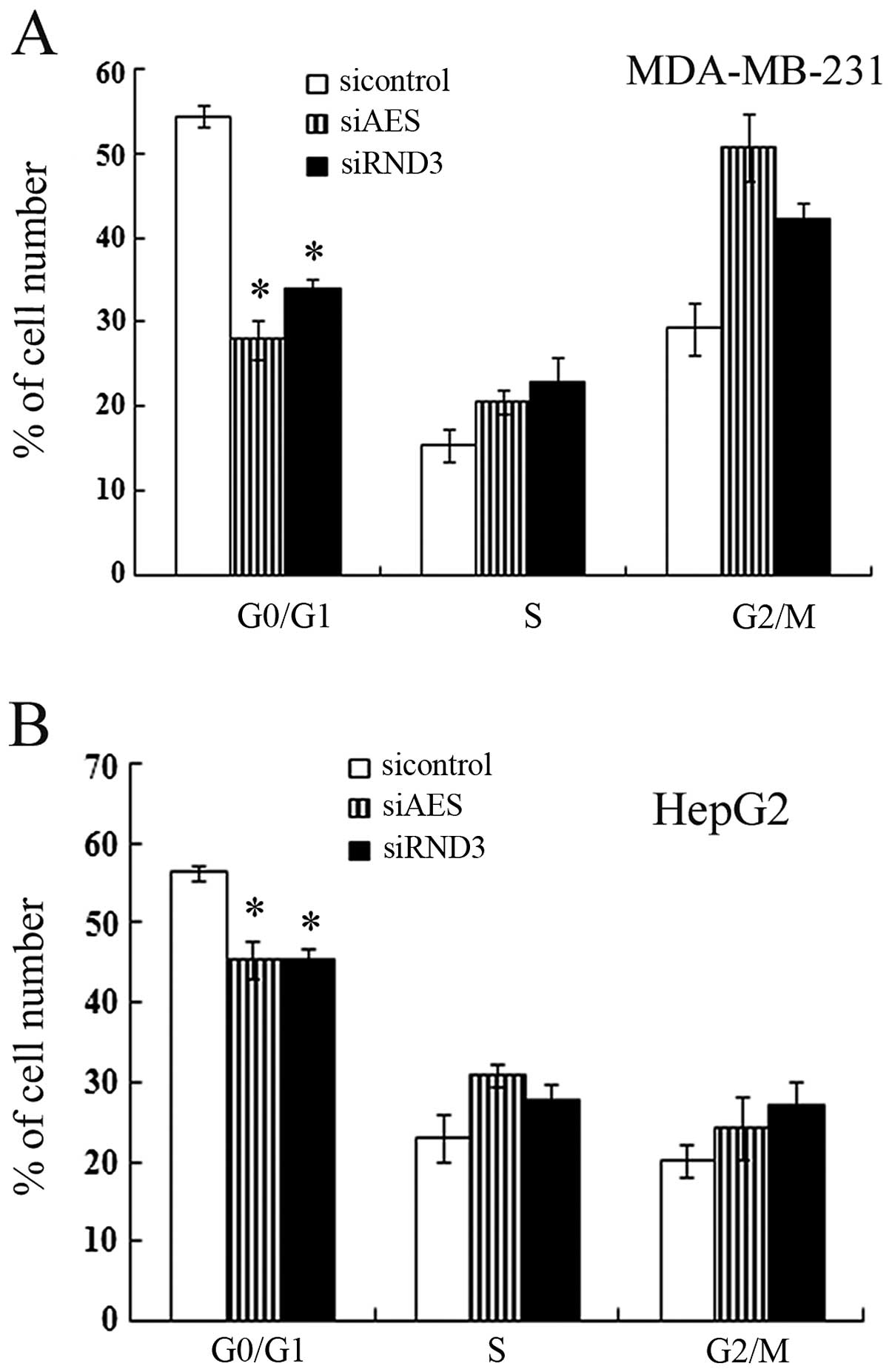
The cell cycle progression of cancer cells with
different transfection was assayed by flow cytometry. The
percentage of G1 phase cells transfected with AES siRNA was
decreased significantly compared with the controls (Fig. 4). Downregulation of AES in both
MDA-MB-231 and HepG2 cells promoted S-G2-M phase progression, which
was in correspondence with the effect of RND3 knockdown by siRNA
(Fig. 4). These results indicate
that AES also contributed to cell cycle progression and this
function involved RND3 regulation.
Knockdown of AES and RND3 increases the
invasive activity of cancer cells
We performed a Matrigel invasion assay to
investigate the invasive activity of cancer cell lines. MDA-MB-231
and HepG2 cells were transfected with control, AES or RND3 siRNAs
separately. Knockdown of both AES and RND3 expression resulted in a
significant increase in the number of invasive cells compared with
the control groups (Fig. 5). This
result suggests that downregulation of AES enhances breast cancer
and HCC cell invasion by mimicking the inhibition of RND3
expression.
Discussion
AES, a member of the Gro/TLE family, regulates gene
expression at the transcription level by interacting with various
transcriptional factors (TFs) (28–31). In the past few years, studies
concerning AES as well as other members of the TLE/GRG family have
mainly focused on various developmental and pathological processes
(32–34). Recently, Sonoshita et al
reported that AES/GRG5 prevents metastasis of colorectal cancer
cells by inactivating Notch signaling and may function as a
metastasis-suppressor gene, which highlights an innovative therapy
for anti-metastasis (16).
However, the regulatory mechanism of AES in tumorigenesis and
cancer progression remains to be further determined. Our present
study suggests that AES-mediated RND3 regulation plays an important
role in the process of cell proliferation, cell cycle progression
and invasion.
In the present study, small specific siRNA was used
to knockdown AES expression in MDA-MB-231 and HepG2 cells, and the
results showed that RND3 expression was decreased accompanied by
AES downregulation. These data indicate that AES regulates RND3
directly or indirectly. A dual-luciferase reporter assay was used
to determine whether AES influences RND3 promoter activity. Forced
AES expression significantly activated RND3 promoter activity in
HeLa cells. However, whether AES interacts with the RND3 promoter
directly still requires further investigation.
At the transcriptional level, RND3 may be directly
regulated by many TFs, including P53, HIF-1α and Foxd3 (35–37). Moreover, DNA damage-inducing
stimuli, including chemotherapeutic agents and ultraviolet (UV)
irradiation, could upregulate the RND3 gene expression at both the
mRNA and protein levels (38).
According to the characteristics of the Gro/TLE family members, we
hypothesized that AES interacts with various TFs and promotes RND3
expression at the transcription level. Therefore, it will be
interesting to explore the accurate molecular mechanisms by which
AES interacts with TFs and affects RND3 transcription.
RND3 is an atypical member of the Rho family, and
studies concerning this molecule are relatively fewer compared with
studies of members of the Rho family (39). Previous studies showed that RND3
regulates a diverse set of biological activities including actin
organization, cell motility, cell-cycle progression and apoptosis
(24,27).
Recent research revealed that elevated RND3
expression markedly increased the expression levels of PTEN and
p27, while decreasing pAkt expression, thus inhibiting cell cycle
progression at the G1 phase (26,40). RND3 also blocks cell cycle
progression at the G2/M phase. A study using a prostate cancer cell
line showed that forced RND3 overexpression inhibits the expression
levels of CDC2 and cyclin B1 which are essential for G2/M
transition, and induces G2/M phase arrest (41). Moreover, downregulation of RND3 in
ESCC cells promoted cell proliferation, cell cycle progression, as
well as cell invasion in vitro (26).
Our results revealed that siRNA-mediated AES
downregulation decreased the expression of RND3; therefore, we
hypothesized that AES executes its function through RND3. Further
functional experiments were carried out using these two genes.
Rapid proliferation was induced by AES-specific siRNA transfection
in both MDA-MB-231 and HepG2 cells. Knockdown of AES in these two
cell lines also induced cell cycle progression and promoted cell
invasion. These effects were also noted when cells were transfected
with RND3-specific siRNA and were consistent with the effects of
AES-specific siRNA transfection. The above results indicate that
AES regulates RND3 expression and suggest that downregulation of
AES promotes tumor cell proliferation, cell cycle progression and
invasion which involves RND3 expression. We demonstrated, for the
first time, that there is a connection between AES and RND3, a
typical member of the Rho family. Our results also elucidate the
mechanisms of AES regulation and offer new insights into the
molecular mechanisms of how AES executes its tumor repressor
functions.
Acknowledgements
This research was supported by the National Natural
Science Foundation of China (81071640, 30971519), National Basic
Research Program of China (2011CB935800) and International
Cooperation and Communication in Science and Technology Project of
Sichuan Province (2010HH0006).
Abbreviations:
|
AES
|
amino-terminal enhancer of split
|
|
TLE
|
transducin-like enhancer of split
|
|
TFs
|
transcription factors
|
|
RNAi
|
RNA interference
|
|
CRC
|
colorectal cancer
|
|
NICD
|
Notch intercellular domain
|
|
RhoGAP
|
GTPase-activating protein for Rho
|
|
ROCK
|
Rho kinase-I
|
|
HCC
|
hepatocellular carcinoma
|
|
ESCC
|
esophageal squamous cell carcinoma
|
References
|
1
|
Gupta GP and Massague J: Cancer
metastasis: building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oppenheimer SB: Cellular basis of cancer
metastasis: a review of fundamentals and new advances. Acta
Histochem. 108:327–334. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dolgin E: Cancer metastasis scrutinized.
Nature. 461:854–855. 2009. View
Article : Google Scholar
|
|
4
|
Beagle B and Johnson GV: AES/GRG5: more
than just a dominant-negative TLE/GRG family member. Dev Dyn.
239:2795–2805. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bajoghli B: Evolution of the Groucho/Tle
gene family: gene organization and duplication events. Dev Genes
Evol. 217:613–618. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen G and Courey AJ: Groucho/TLE family
proteins and transcriptional repression. Gene. 249:1–16. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fisher AL and Caudy M: Groucho proteins:
transcriptional corepressors for specific subsets of DNA-binding
transcription factors in vertebrates and invertebrates. Gene Dev.
12:1931–1940. 1998. View Article : Google Scholar
|
|
8
|
Jennings BH and Ish-Horowicz D: The
Groucho/TLE/Grg family of transcriptional co-repressors. Genome
Biol. 9:2052008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu F, Liu Y, Li D, et al: The
transcription co-repressor TLE1 interacted with the intracellular
region of gpl30 through its Q domain. Mol Cell Biochem.
232:163–167. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ghosh HS, Spencer JV, Ng B, McBurney MW
and Robbins PD: Sirt1 interacts with transducin-like enhancer of
split-1 to inhibit nuclear factor kappaB-mediated transcription.
Biochem J. 408:105–111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Swingler TE, Bess KL, Yao J, Stifani S and
Jayaraman PS: The proline-rich homeodomain protein recruits members
of the Groucho/Transducin-like enhancer of split protein family to
co-repress transcription in hematopoietic cells. J Biol Chem.
279:34938–34947. 2004. View Article : Google Scholar
|
|
12
|
Wang WF, Wang YG, Reginato AM, Plotkina S,
Gridley T and Olsen BR: Growth defect in Grg5 null mice is
associated with reduced Ihh signaling in growth plates. Dev Dyn.
224:79–89. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aghaallaei N, Bajoghli B, Walter I and
Czerny T: Duplicated members of the Groucho/Tle gene family in
fish. Dev Dyn. 234:143–150. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Steffen B, Knop M, Bergholz U, et al:
AML1/ETO induces self-renewal in hematopoietic progenitor cells via
the Groucho-related amino-terminal AES protein. Blood.
117:4328–4337. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jan Y, Matter M, Pai JT, et al: A
mitochondrial protein, Bit1, mediates apoptosis regulated by
integrins and Groucho/TLE corepressors. Cell. 116:751–762. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sonoshita M, Aoki M, Fuwa H, et al:
Suppression of colon cancer metastasis by Aes through inhibition of
Notch signaling. Cancer Cell. 19:125–137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Christofori G: Metastatic colon cancer
cells negotiate the intravasation Notch. Cancer Cell. 19:6–8. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vega FM and Ridley AJ: Rho GTPases in
cancer cell biology. FEBS Lett. 582:2093–2101. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Villalonga P and Ridley AJ: Rho GTPases
and cell cycle control. Growth Factors. 24:159–164. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wennerberg K, Forget MA, Ellerbroek SM, et
al: Rnd proteins function as RhoA antagonists by activating p190
RhoGAP. Curr Biol. 13:1106–1115. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fortier M, Comunale F, Kucharczak J,
Blangy A, Charrasse S and Gauthier-Rouviere C: RhoE controls
myoblast alignment prior fusion through RhoA and ROCK. Cell Death
Differ. 15:1221–1231. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Garg R, Riento K, Keep N, Morris JD and
Ridley AJ: N-terminus-mediated dimerization of ROCK-I is required
for RhoE binding and actin reorganization. Biochem J. 411:407–414.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen J, Zhou H, Li Q, et al: Epigenetic
modification of RhoE expression in gastric cancer cells. Oncol Rep.
25:173–180. 2011.PubMed/NCBI
|
|
24
|
Chardin P: Function and regulation of Rnd
proteins. Nat Rev Mol Cell Biol. 7:54–62. 2006. View Article : Google Scholar
|
|
25
|
Grise F, Sena S, Bidaud-Meynard A, et al:
Rnd3/RhoE is down-regulated in hepatocellular carcinoma and
controls cellular invasion. Hepatology. 55:1766–1775. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao H, Yang J, Fan T, Li S and Ren X:
RhoE functions as a tumor suppressor in esophageal squamous cell
carcinoma and modulates the PTEN/PI3K/Akt signaling pathway. Tumour
Biol. 33:1363–1374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Klein RM and Aplin AE: Rnd3 regulation of
the actin cytoskeleton promotes melanoma migration and invasive
outgrowth in three dimensions. Cancer Res. 69:2224–2233. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rave-Harel N, Miller NL, Givens ML and
Mellon PL: The Groucho-related gene family regulates the
gonadotropin-releasing hormone gene through interaction with the
homeodomain proteins MSX1 and OCT1. J Biol Chem. 280:30975–30983.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang W, Wang YG, Reginato AM, et al:
Groucho homologue Grg5 interacts with the transcription factor
Runx2-Cbfa1 and modulates its activity during postnatal growth in
mice. Dev Biol. 270:364–381. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu X, Li P, Roeder RG and Wang Z:
Inhibition of androgen receptor-mediated transcription by
amino-terminal enhancer of split. Mol Cell Biol. 21:4614–4625.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Turki-Judeh W and Courey AJ: Groucho: a
corepressor with instructive roles in development. Curr Top Dev
Biol. 98:65–96. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brinkmeier ML, Potok MA, Cha KB, et al:
TCF and Groucho-related genes influence pituitary growth and
development. Mol Endocrinol. 17:2152–2161. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Metzger DE, Gasperowicz M, Otto F, Cross
JC, Gradwohl G and Zaret KS: The transcriptional co-repressor
Grg3/Tle3 promotes pancreatic endocrine progenitor delamination and
β-cell differentiation. Development. 139:1447–1456. 2012.PubMed/NCBI
|
|
34
|
Orian A, Delrow JJ, Rosales Nieves AE, et
al: A Myc-Groucho complex integrates EGF and Notch signaling to
regulate neural development. Proc Natl Acad Sci USA.
104:15771–15776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ongusaha PP, Kim HG, Boswell SA, et al:
RhoE is a pro-survival p53 target gene that inhibits ROCK
I-mediated apoptosis in response to genotoxic stress. Curr Biol.
16:2466–2472. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou J, Li K, Gu Y, et al: Transcriptional
up-regulation of RhoE by hypoxia-inducible factor (HIF)-1 promotes
epithelial to mesenchymal transition of gastric cancer cells during
hypoxia. Biochem Biophys Res Commun. 415:348–354. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Katiyar P and Aplin AE: FOXD3 regulates
migration properties and Rnd3 expression in melanoma cells. Mol
Cancer Res. 9:545–552. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Boswell SA, Ongusaha PP, Nghiem P and Lee
SW: The protective role of a small GTPase RhoE against UVB-induced
DNA damage in keratinocytes. J Biol Chem. 282:4850–4858. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ellenbroek SI and Collard JG: Rho GTPases:
functions and association with cancer. Clin Exp Metastasis.
24:657–672. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Klein RM and Higgins PJ: A switch in
RND3-RHOA signaling is critical for melanoma cell invasion
following mutant-BRAF inhibition. Mol Cancer. 10:1142011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bektic J, Pfeil K, Berger AP, et al: Small
G-protein RhoE is underexpressed in prostate cancer and induces
cell cycle arrest and apoptosis. Prostate. 64:332–340. 2005.
View Article : Google Scholar : PubMed/NCBI
|