Introduction
In higher vertebrates and mammals, the bone is
constantly being remodeled in order to regulate its architecture to
satisfy mechanical needs and to repair damaged tissue; it is a
highly dynamic tissue. Activation of osteoclasts initiates the
remodeling cycle, which resorbs the underlying bone tissue,
followed by the activation of osteoblasts, which are responsible
for the formation of new bone tissue (1,2).
The two processes must be tightly balanced to ensure proper bone
homeostasis and calcium metabolism, and loss of this balance leads
to several skeletal disorders (3,4).
Osteoclasts are multinucleated cells of the monocytic-macrophage
lineage, and are derived from hematopoietic stem cells through a
series of differentiation steps (5). Osteoclasts require cytokines,
especially macrophage colony-stimulating factor (M-CSF), for their
survival (6,7). The receptor activator of nuclear
factor-κB ligand (RANKL), expressed in osteoblasts and synovial
fibroblasts as well as in T cells (5), represents the crucial regulator for
osteoclast differentiation and activation (8). M-CSF and RANKL are essential for
osteoclastogenesis in vitro (9). Simonet et al (10) previously demonstrated that
osteoprotegerin (OPG) is involved in the regulation of bone
density. OPG can prevent the binding of RANKL to its receptor,
RANK, and blocks signaling cascade of osteoclast formation in
vitro and in vivo (11,12). OPG knockout mice displayed
osteoporosis owing to enhanced bone resorption at local sites. The
decreased activity in osteoclasts induced by exogenous OPG may
improve bone mass (13).
It is well known that cathepsin K, MMP-9 and
carbonic anhydrase II (CA II) genes are involved in osteoclastic
bone resorption (14). Wittrant
et al (15) reported that
OPG exerts an overall inhibitory effect on the expression of
cathepsin K and MMP-9 in differentiated RAW264.7 cells in a
dose-dependent manner. Chen et al (16) previously demonstrated that OPG
inhibits the expression of CA II mRNA in mouse osteoclast-like
cells. Therefore, how OPG alters the expression of cathepsin K,
MMP-9 and CA II mRNA in different treatment periods to osteoclasts
becomes a critical question that requires further elucidation. In
this study, the effects of OPG on the bone resorption activity of
osteoclasts were investigated via TRAP staining, F-actin staining,
resorption activity analysis and osteoclastic bone
resorption-related gene expression using RAW264.7 cells. The
purpose of this study was to lay the foundations for the OPG
treatment potential to abnormal osteoclast bone resorption
activity.
Materials and methods
RAW264.7 cell culture
Murine monocyte/macrophage cell line RAW264.7 was
obtained from the American Type Culture Collection (Manassas, VA,
USA). It was cultured in DMEM (Gibco, USA) supplemented with 10%
FBS, 100 U/ml penicillin, 100 μg/ml streptomycin and 2
mmol/l L-glutamine. The cells were suspended in α-MEM (containing
10% FBS) and placed in 96-well plates for TRAP-staining, and were
seeded in Corning Osteo Assay Surface (COAS; Corning Inc., USA) for
F-actin staining and resorption activity analysis, respectively.
After 24 h of incubation, the medium was changed to serum-free
α-MEM with 25 ng/ml M-CSF + 30 ng/ml RANKL (M-CSF and RANKL were
obtained from Peprotech Inc., USA). For 48 h cultivation, 0, 10,
20, 50 and 100 ng/ml OPG (Peprotech Inc.) were added into various
groups in the presence of the two cytokines, and the cells were
cultured for another 24 h.
Cell viability MTT assay
To examine the effect of OPG on cell growth,
RAW264.7 cells were treated with various concentrations of OPG and
cell growth was measured by an MTT assay. In brief, the cells were
plated in 96-well plates in α-MEM containing 10% FBS and incubated
for 24 h. The medium was then changed to α-MEM without FBS and
after 48 h different concentrations of OPG (0, 10, 20, 50 and 100
ng/ml) were added to the culture for another 24 h. At the end of
the incubation, plates were washed with PBS three times, and DMEM
(without FBS) containing 10% MTT solution (0.5 mg/ml in PBS) was
added to each well. After a 4-h incubation period at 37°C, the
insoluble formazan crystals formed were dissolved in 150 μl
of dimethyl sulfoxide (DMSO). The optical density (OD) was
immediately measured at 570 nm using Sunrise microplate reader
(Tecan, Austria).
Osteoclast differentiation from RAW264.7
cells
Osteoclast formation was measured by quantifying
cells positively stained by TRAP (Acid Phosphatase Kit 387-A;
Sigma-Aldrich, USA). TRAP staining was conducted according to the
manufacturer’s protocol. Under a light microscope, TRAP-positive
multinucleated cells having three or more nuclei were considered as
osteoclasts, and their numbers were counted in randomly selected
visual fields in different areas of each well.
Filamentous (F) - actin staining
RAW264.7 cells were seeded in COAS. For
visualization of the actin cytoskeleton, TRITC-conjugated
phalloidin (Invitrogen) was employed, and was used at a
concentration of 20 μmol/l. At the end of the incubation,
cells were fixed, permeabilized and stained following the
manufacturer’s instructions. The stained cells were observed by an
inverted phase contrast fluorescence microscope (DMI 3000B; Leica)
with appropriate filters.
Resorption activity analysis
The manipulation followed the COAS product protocol.
In brief, at the end of cultivation, medium was aspirated
completely and 10% bleach solution was added for 5 min of
incubation. Bleach solution was removed and the wells were washed
twice with dH2O. The plates were allowed to air dry
completely for 3–5 h. Pits appeared as individual or multiple
clusters at the bottom of the wells and were visualized via an
inverted phase contrast microscope. The area of resorption lacunae
was measured by image analysis (Version 1.0; JEDA).
Real-time fluorescence quantitative
polymerase chain reaction (PCR) analysis
RAW264.7 cells were suspended in α-MEM (containing
10% FBS) and then seeded in 6-well culture plates. After 24 h of
cultivation, the medium was changed to serum-free α-MEM with 25
ng/ml M-CSF + 30 ng/ml RANKL and was maintained for 48 h. For
concentration gradient study, 0, 10, 20, 50 and 100 ng/ml OPG were
added into various groups in the presence of the two cytokines. The
OPG treatment was continued for 30 min, and cells were treated by
M-CSF + RANKL for another 30 min as control groups. For the time
gradient study, 100 ng/ ml OPG were added into various groups in
the presence of the two cytokines. The OPG treatment was continued
for 15, 30, 60 and 120 min, individually, and cells were treated
with M-CSF + RANKL without OPG for another 15 min as control
groups.
Total RNA was extracted at the indicated
time-points. cDNA synthesis was conducted with 900 ng RNA using
PrimeScript RT reagent Kit with gDNA Eraser (Takara, Japan).
Real-time PCR was performed by the 7500 Real-Time PCR system
(Applied Biosystems). The PCR protocol for cathepsin K, MMP-9 and
CA II consisted of 40 cycles of 95°C for 30 sec and 57°C for 1 min.
The specific sequences of the PCR primers for cathepsin K, MMP-9,
CA II and GAPDH were: cathepsin K, forward,
5′-CGC-CTG-CGG-CAT-TAC-CAA-3′ and reverse,
5′-TAG-CAT-CGC-TGC-GTC-CCT-3′; MMP-9, forward,
5′-GCC-CTG-GAA-CTC-ACA-CGA-CA-3′ and reverse,
5′-TTG-GAA-ACT-CAC-ACG-CCA-GAA-G-3′; CA II, forward,
5′-CAT-TAC-TGT-CAG-CAG-CGA-GCA-3′ and reverse,
5′-GAC-GCC-AGT-TGT-CCA-CCA-TC-3′; GAPDH, forward,
5′-AAA-TGG-TGA-AGG-TCG-GTG-TG-3′ and reverse,
5′-TGA-AGG-GGT-CGT-TGA-TGG-3′. All real-time PCR reactions were
carried out at least three times, and the specificity of the PCR
products was verified by melting curve analysis.
Statistical analysis
All experimental data are expressed as the means ±
standard error of the mean and all experiments were repeated in
triplicate. Statistical differences between groups were evaluated
by Tukey’s test using SPSS ver. 17.0 software. P<0.05 was
considered to indicate statistically significant differences.
Results
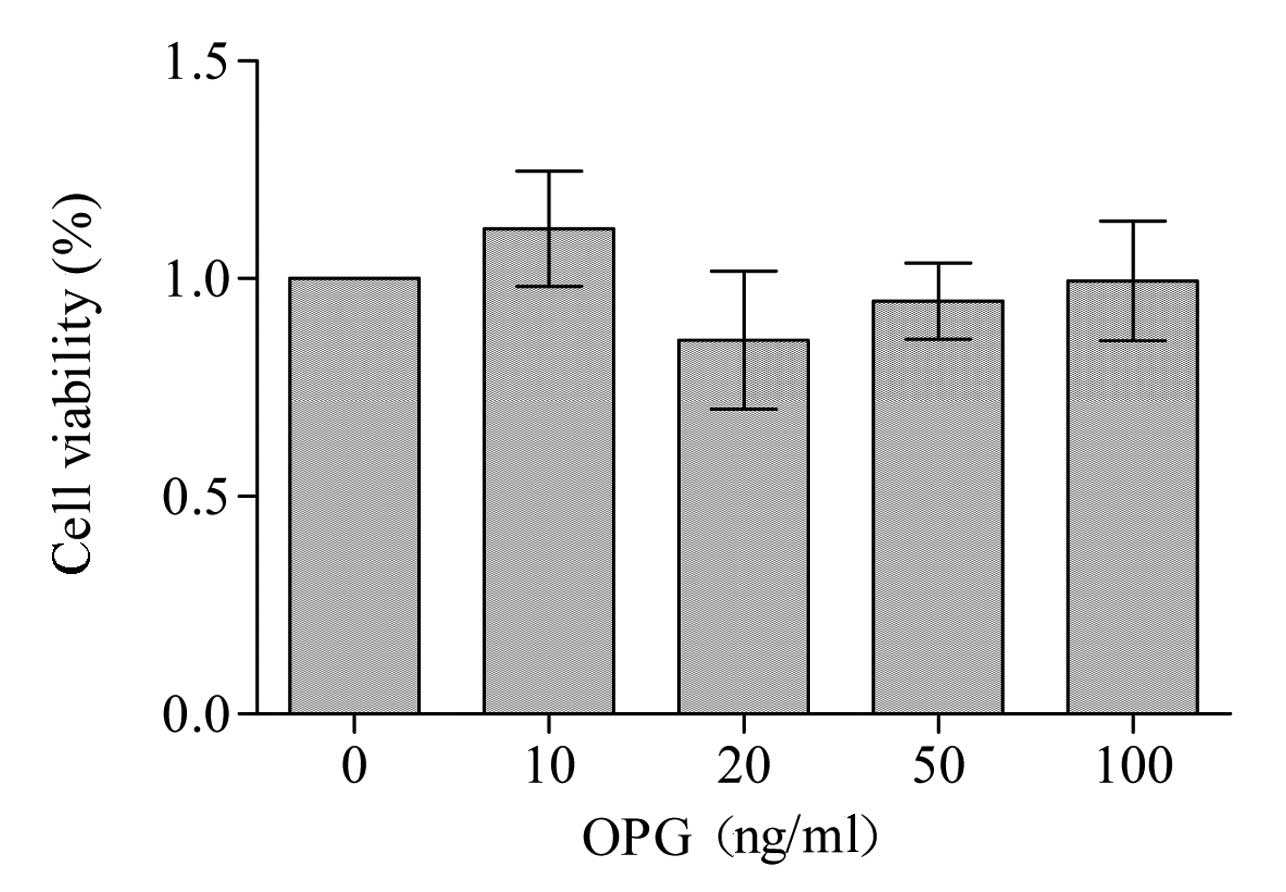
Action of OPG on RAW264.7 cell
viability
OPG did not affect the cell growth rate of RAW264.7
cells (Fig. 1), sustaining
substantial viability even when used at concentrations of 100 ng/ml
OPG (99.45%). This indicated that OPG was not cytotoxic to RAW264.7
cells. Therefore, the concentrations of 0, 10, 20, 50 and 100 ng/ml
OPG were employed in the subsequent experiments.
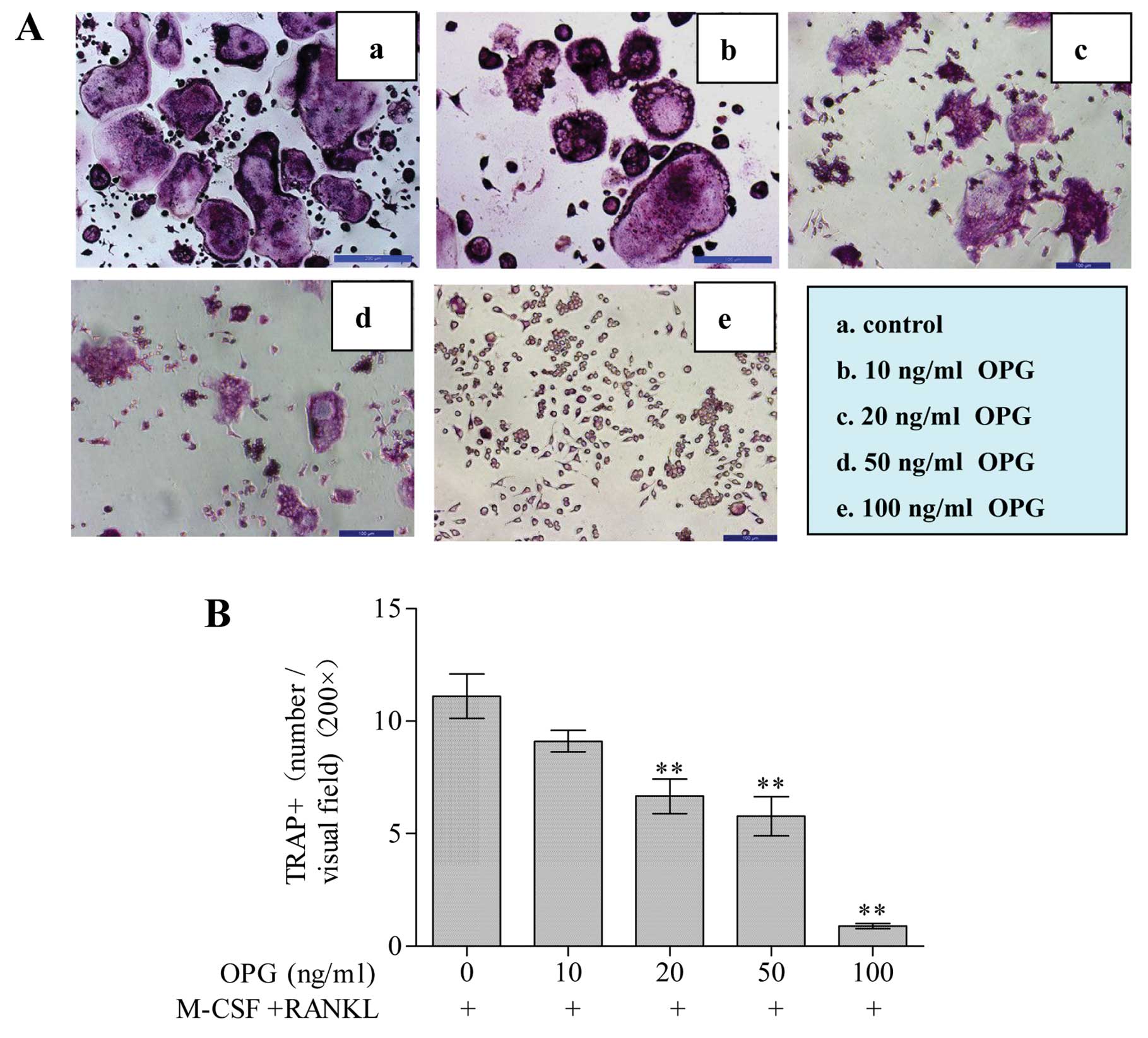
OPG inhibits the formation of
TRAP-positive multi-nucleated cells
RAW264.7 cells cultured in the presence of M-CSF +
RANKL for 4 days formed large mature osteoclasts with multinuclei
characterized by the acquisition of mature phenotypic markers, such
as TRAP (Fig. 2A). At the same
time, different concentrations of OPG were added to RAW264.7 cells
when stimulated with M-CSF + RANKL. The number of TRAP-positive
multinucleated cells decreased with increasing OPG concentrations
(Fig. 2A). OPG reduced the number
of TRAP-positive multinucleated cells generated for 17.28±9.12,
37.91±20.87, 48.12±9.81 and 89.71±3.15% inhibition at 10, 20, 50
and 100 ng/ml concentrations, individually (Fig. 2B). Collectively, these results
indicated that OPG inhibited M-CSF + RANKL-induced
osteoclastogenesis.
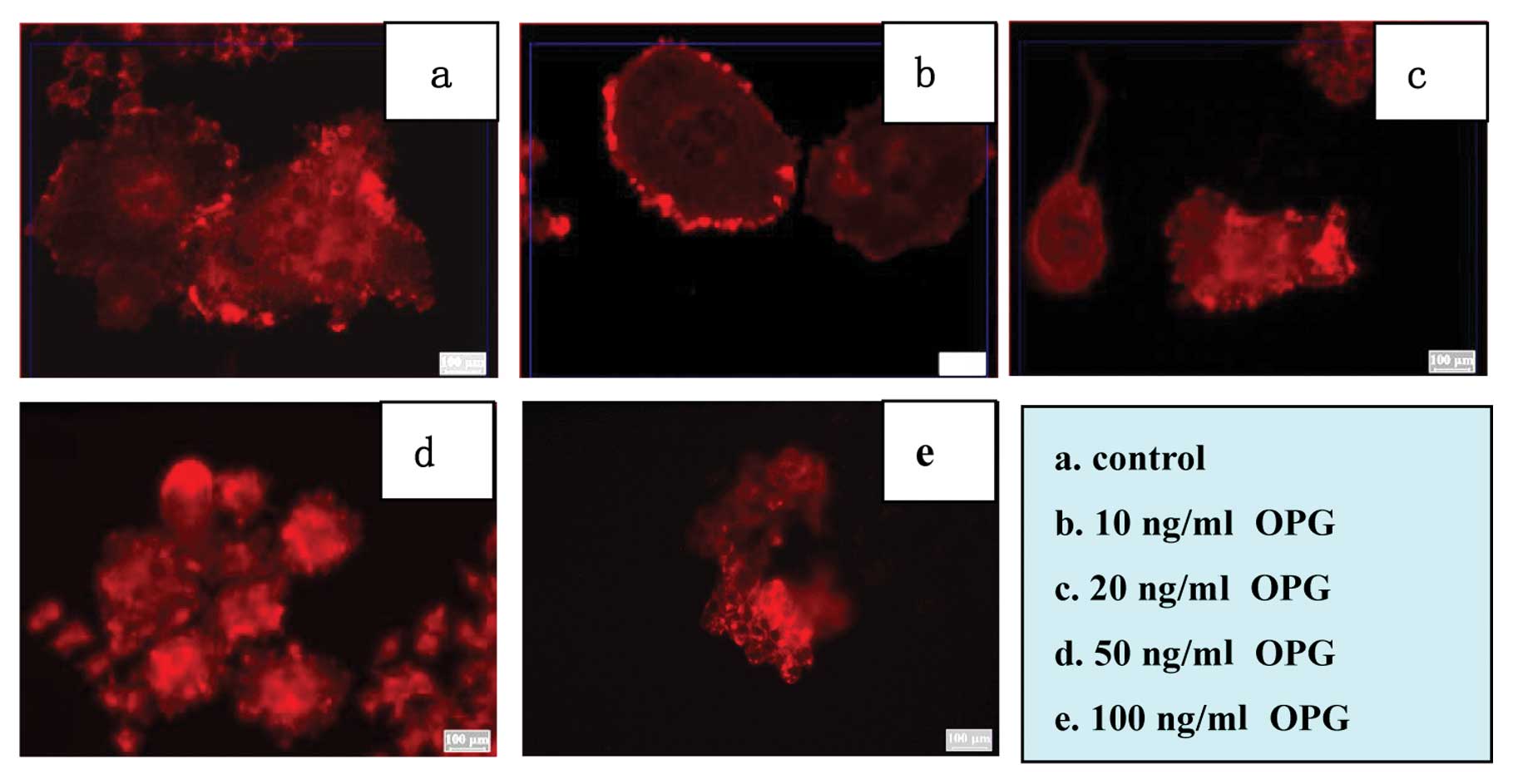
Effect of OPG on the F-actin rings
forming in osteoclasts
M-CSF + RANKL-treated RAW264.7 cells formed large
multinucleated osteoclasts with dense and smooth F-actin rings on
the COAS. However, their development was inhibited by OPG. They
were higher-ordered and evident around osteoclasts in the control
groups and had lower concentrations in the OPG groups.
Nevertheless, only F-actin clusters or belts were observed in 100
ng/ml OPG groups rather than F-actin rings (Fig. 3). These results indicated that the
effect of OPG on bone resorption was due to its negative influence
on the F-actin ring formation.
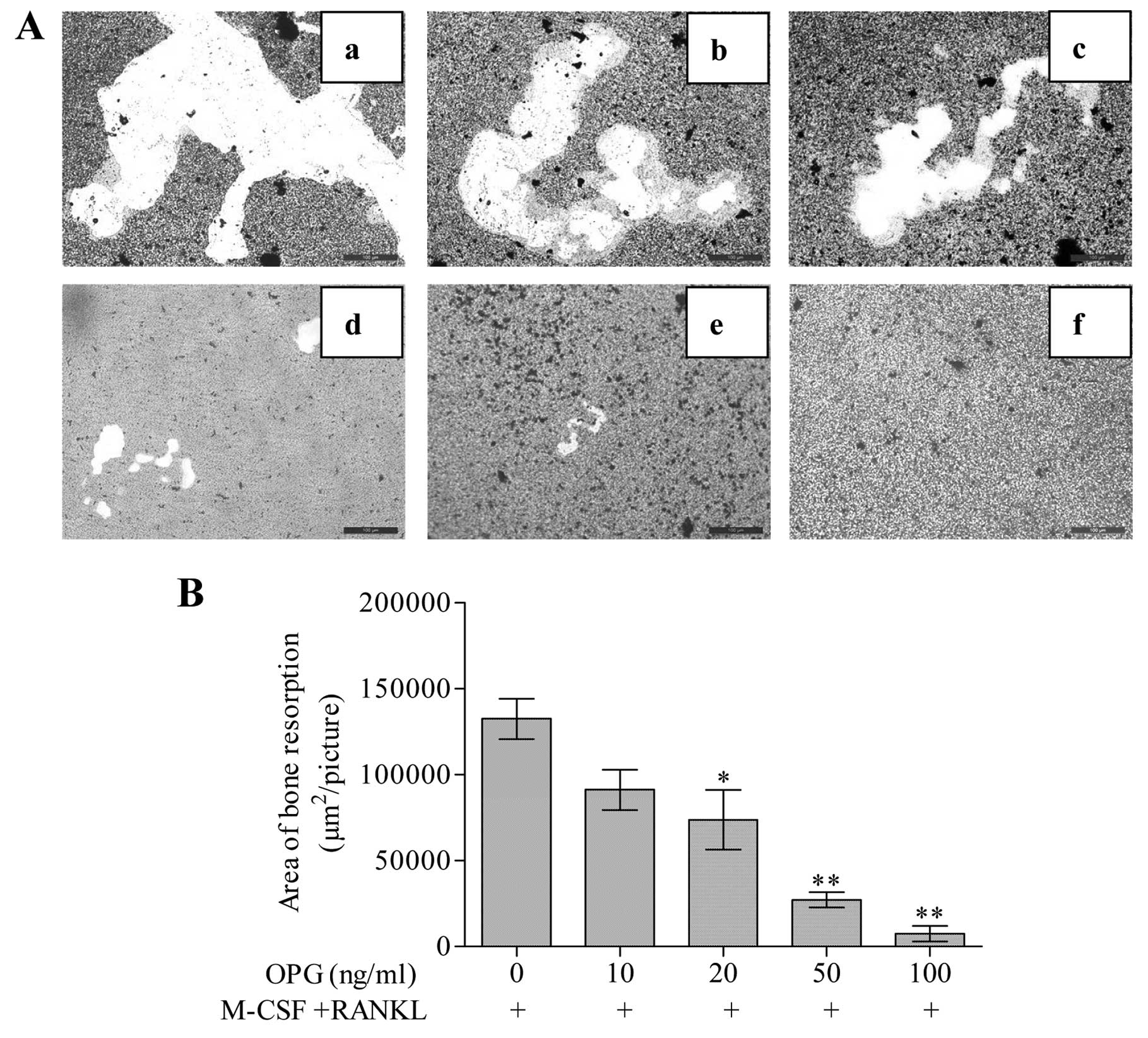
Effect of OPG on the resorption activity
of osteoclasts
RAW264.7 cells were stimulated with M-CSF + RANKL to
induce the differentiation of mature osteoclasts with
bone-resorbing capacity. Cavities appeared in the bottom of COAS
with various shapes. The bottom of COAS was not intact and smooth
in any cell group (Fig. 4A).
However, OPG hindered the resorptive capacity of osteoclasts and in
a dose-dependent manner (Fig.
4B). The volume of resorption lacunae was significantly less in
M-CSF + RANKL + 20, 50 and 100 ng/ml OPG groups than in the control
groups (P<0.05). OPG inhibited the total area of resorption
lacunae with 30.23±16.90, 44.35±21.07, 78.61±9.16 and 94.60±5.88%
at 10, 20, 50 and 100 ng/ml concentrations, individually.
Regulation of cathepsin K, MMP-9, CA II
mRNA expression in M-CSF + RANKL-induced osteoclast cells by
OPG
The production of cathepsin K, MMP-9 and CA II gene
expression for bone resorption by osteoclasts was monitored by
real-time PCR. For concentration gradient study, expression of the
three genes in cells cultured without M-CSF + RANKL were all
significantly lower than in control groups (P<0.05). Expression
of cathepsin K, MMP-9 mRNA in the OPG groups was statistically
significantly lower than in control groups (P<0.01). OPG
decreased the expression of cathepsin K mRNA 0.28±0. 06, 0.36±0.
06, 0.33±0.06, 0.44±0.11-fold, it decreased the expression of MMP-9
mRNA 0.57±0. 02, 0.60±0.05, 0.54±0.03, 0.44±0.08-fold at 10, 20, 50
and 100 ng/ml concentrations, respectively, and it decreased the
expression of CA II mRNA 0.34±0.07, 0.35±0.16-fold at 10 and 20
ng/ml concentrations, respectively. However, the expression of CA
II mRNA in the 50 and 100 ng/ml concentration groups was not
significantly different to the control groups (Fig. 5A–C). For time gradient study,
after OPG was added, expression of the MMP-9, cathepsin K and CA II
genes in cells cultured without any exogenous cytokine for another
15 min were all significantly lower than in control groups
(P<0.05). Furthermore, expression of the cathepsin K, MMP-9 and
CA II genes in cells cultured with M-CSF + RANKL plus OPG for 15
min were not significantly different compared to their control
groups, respectively. This demonstrated that OPG seldom affected
the expression of MMP-9, cathepsin K and CA II mRNA in RAW264.7
cells in a short period. When treated with OPG for 30 min, all
three significantly decreased (P<0.01), demonstrating that OPG
inhibits the expression of the three genes the most at this
time-point. Expression of MMP-9 and CA II genes was significantly
lower than in the control groups from 30 to 120 min via OPG
treatment (P<0.05). OPG decreased the expression of MMP-9 mRNA
0.82±0.03, 0.55±0.08, 0.18±0.09-fold and it decreased the
expression of CA II mRNA 0.86±0.01, 0.78±0.02, 0.66±0.01-fold, at
30, 60 and 120 min, respectively (Fig. 5D–F). However, the expression of
MMP-9 and CA II mRNA increased as the effect of OPG declined. This
indicated that OPG still exerted inhibitory effects, albeit weaker,
on two of them. The expression of cathepsin K mRNA was
significantly increased with the OPG treatment for 60 to 120 min
(P<0.01) (Fig. 5E).
 | Figure 5Effect of OPG on the expression of the
MMP-9, cathepsin K and CA II genes. RAW264.7 cells were suspended
in α-MEM (containing 10% FBS) and then seeded in 6-well culture
plates. After 24 h of cultivation, the medium was changed to
serum-free α-MEM with 25 ng/ml M-CSF + 30 ng/ml RANKL and sustained
for 48 h. (A–C) For the concentration gradient study, 0, 10, 20, 50
and 100 ng/ml OPG were added into various groups in the presence of
the two cytokines. The OPG treatment was continued for 30 min, and
cells were treated with M-CSF + RANKL for another 30 min as control
groups. The mRNA expression levels of MMP-9, cathepsin K and CA II
genes were determined by real-time PCR and compared with that of
GAPDH. (D–F) For time gradient study, 100 ng/ml OPG were added into
various groups in the presence of the two cytokines. The OPG
treatment was continued for 15, 30, 60 and 120 min, individually,
and cells were treated with M-CSF + RANKL without OPG for another
15 min as control groups. At the indicated time-points, mRNA
expression levels of MMP-9, cathepsin K and CA II genes were
measured by real-time PCR. The results are expressed as the means ±
SEM. **P<0.01, *P<0.05 vs. control
groups (#). |
Discussion
The hallmark of osteoclasts is their unique ability
to resorb mineralized calcium apatite or carbonate substrates such
as bone, dentin or nacre (17).
In the osteoclast resorption process, F-actin ring formation is
critical (18). F-actin is first
organized into podosome (19),
osteoclasts exhibit two different actin cytoskeleton organizations
according to their substratum. They form canonical podosomes on
non-mineralized substrates while they form sealing zones on
mineralized extracellular matrices (17,20). Podosome clusters are built in
early osteoclasts, which evolve into dynamic rings at intermediate
stages and form peripheral podosome belts in mature cells by fusion
of the rings (21). When the
osteoclasts are polarized, they exhibit sealing zones and exert
bone resorption activity (17,20). In sealing zones, the F-actin
filament builds attachment ring structures, thus, in activated
osteoclasts, compact ring structures were usually observed when
F-actin was stained (22). The
RAW264.7 cell line is a recognized pre-osteoclast model and has
been extensively employed in osteoclast studies (2,15).
In the present study, RAW264.7 cells were cultured in the presence
of M-CSF, RANKL established functional F-actin rings and possessed
bone resorption ability, whereas OPG impeded their formation. Only
F-actin clusters or belts were found in high concentration OPG
groups. Accordingly, the resorption activity of osteoclasts was
weakened by OPG.
Degradation of mineralized bone matrix by
osteoclasts includes dissolution of crystalline hydroxyapatite and
proteolytic cleavage of the organic matrix. Before proteolytic
enzymes can reach and degrade collagenous bone matrix,
hydroxyapatite crystals must be dissolved (23). CA II is critical not only in bone
resorption but also in osteoclast differentiation (24); it provides the proton source for
extracellular acidification by H+-ATPase and the
HCO3− source for the HCO3−/Cl−
exchanger which is the major transporter for maintenance of normal
intracellular pH (25–28). After the degradation of the
hydroxyapatite crystals, organic matrix is subsequently dissolved.
Two major classes of proteolytic enzymes are involved, i.e., the
cysteine proteinase family (such as cathepsin K) and the MMP family
(29).
In this study, the expression of MMP-9, cathepsin K
and CA II mRNA in RAW264.7 cells was induced by M-CSF + RANKL, and
the results indicated that M-CSF + RANKL may induce bone resorption
by osteoclasts via increased CA II, cathepsin K and MMP-9
production. However, the expression levels of cathepsin K and MMP-9
mRNA were all inhibited by different concentrations of OPG. The OPG
inhibitory effects on the two gene expressions were similar to
those previously reported by Wittrant et al (15). The expression of CA II mRNA was
significantly decreased by 10 and 20 ng/ml OPG, however, it was not
significantly decreased by 50 and 100 ng/ml OPG, contrary to the
report of Chen et al (16). This finding warrants further
study. For the time gradient study, M-CSF + RANKL significantly
enhanced their expression in the short term, whereas OPG markedly
decreased their expression following treatment for 30 min. However,
they all decreased at first but subsequently increased. The study
by Wittrant et al (15)
proved that the RANK-RANKL complex may represent the OPG receptor
at the RAW264.7 cell surface. In the OPG treatment course, its
inhibitory effect on the expression of MMP-9 and CA II mRNA
weakened, as previously mentioned, and the influence of OPG
lessened afterwards. Of note, the expression of cathepsin K mRNA
was significantly raised with the cell cultivation. Cathepsin K is
selectively expressed in osteoclasts and plays a critical role in
the bone resorption. It secretes from the cell as an inactive form
and then converts to its mature active form by proteolytic cleavage
of the prodomain at low pH in vivo (30). The pH value of the cell culture
environment gradually declined in the incubation period. First, a
lot of acidic products were metabolized by cells, the pH value of
medium was dropped. Second, as the initiation of osteoclastic
resorption, the evident production of CA II contributed to the
acidity of cell ambience. Thus, the expression and activity of
cathepsin K markedly increased.
In conclusion, the resorption activity of
osteoclasts was suppressed by high concentrations of OPG.
Furthermore, at the molecular level, OPG decreased the expression
of osteoclastic bone resorption-related genes.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (nos. 30972229 and
31172373) and a project Funded by the Priority Academic Program
Development of Jiangsu Higher Education Institutions and the
Graduate Innovation Project of Jiangsu Province.
References
|
1
|
Li J, Sarosi I, Yan XQ, Morony S,
Capparelli C, Tan HL, McCabe S, Elliott R, Scully S, Van G, Kaufman
S, Juan SC, Sun Y, Tarpley J, Martin L, Christensen K, McCabe J,
Kostenuik P, Hsu H, Fletcher F, Dunstan CR, Lacey DL and Boyle WJ:
RANK is the intrinsic hematopoietic cell surface receptor that
controls osteoclastogenesis and regulation of bone mass and calcium
metabolism. Proc Natl Acad Sci USA. 97:1566–1571. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brinkmann J, Hefti T, Schlottig F, Spencer
ND and Hall H: Response of osteoclasts to titanium surfaces with
increasing surface roughness: an in vitro study. Biointerphases.
7:342012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anderegg F, Geblinger D, Horvath P,
Charnley M, Textor M, Addadi L and Geiger B: Substrate adhesion
regulates sealing zone architecture and dynamics in cultured
osteoclasts. PloS One. 6:e285832011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shibata K, Yoshimura Y, Kikuiri T,
Hasegawa T, Taniguchi Y, Deyama Y, Suzuki Ka and Iida J: Effect of
the release from mechanical stress on osteoclastogenesis in
RAW264.7 cells. Int J Mol Med. 28:73–79. 2011.PubMed/NCBI
|
|
5
|
Shu G, Yamamoto K and Nagashima M:
Differences in osteoclast formation between proximal and distal
tibial osteoporosis in rats with adjuvant arthritis: inhibitory
effects of bisphosphonates on osteoclasts. Mod Rheumatol.
16:343–349. 2006. View Article : Google Scholar
|
|
6
|
Suda T, Udagawa N, Nakamura I, Miyaura C
and Takahashi N: Modulation of osteoclast differentiation by local
factors. Bone. 17(Suppl 2): 87S–91S. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Glantschnig H, Fisher JE, Wesolowski G,
Rodan GA and Reszka AA: M-CSF, TNFalpha and RANK ligand promote
osteoclast survival by signaling through mTOR/S6 kinase. Cell Death
Differ. 10:1165–1177. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nemeth K, Schoppet M, Al-Fakhri N, Helas
S, Jessberger R, Hofbauer LC and Goettsch C: The role of
osteoclast-associated receptor in osteoimmunology. J Immunol.
186:13–18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mancino AT, Klimberg VS, Yamamoto M,
Manolagas SC and Abe E: Breast cancer increases osteoclastogenesis
by secreting M-CSF and upregulating RANKL in stromal cells. J Surg
Res. 100:18–24. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Simonet WS, Lacey DL, Dunstan CR, Kelley
M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T,
Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G,
Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D,
Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R
and Boyle WJ: Osteoprotegerin: a novel secreted protein involved in
the regulation of bone density. Cell. 89:309–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roodman GD: Cell biology of the
osteoclast. Exp Hematol. 27:1229–1241. 1999. View Article : Google Scholar
|
|
12
|
Hsu H, Lacey DL, Dunstan CR, Solovyev I,
Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, Wang
L, Xia XZ, Elliott R, Chiu L, Black T, Scully S, Capparelli C,
Morony S, Shimamoto G, Bass MB and Boyle WJ: Tumor necrosis factor
receptor family member RANK mediates osteoclast differentiation and
activation induced by osteoprotegerin ligand. Proc Nat Acad Sci
USA. 96:3540–3545. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Makihira S, Mine Y, Nikawa H, Shuto T,
Kosaka E, Sugiyama M and Hosokawa R: Immobilized-OPG-Fc on a
titanium surface inhibits RANKL-dependent osteoclast
differentiation in vitro. J Mater Sci Mater Med. 21:647–653. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vaananen HK, Liu YK, Lehenkari P and
Uemara T: How do osteoclasts resorb bone? Mater Sci Eng C.
6:205–209. 1998. View Article : Google Scholar
|
|
15
|
Wittrant Y, Theoleyre S, Couillaud S,
Dunstan C, Heymann D and Rédini F: Relevance of an in vitro
osteoclastogenesis system to study receptor activator of NF-κB
ligand and osteoprotegerin biological activities. Exp Cell Res.
293:292–301. 2004.PubMed/NCBI
|
|
16
|
Chen J, He JQ, Zhen SY and Huang LQ: OPG
inhibits gene expression of RANK and CAII in mouse osteoclast-like
cell. Rheumatol Int. 32:3993–3998. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saltel F, Chabadel A, Bonnelye E and
Jurdic P: Actin cytoskeletal organisation in osteoclasts: a model
to decipher transmigration and matrix degradation. Eur J Cell Biol.
87:459–468. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee JW, Mase N, Yonezawa T, Seo HJ, Jeon
WB, Cha BY, Nagai K and Woo JT: Palmatine attenuates osteoclast
differentiation and function through inhibition of receptor
activator of nuclear factor-κb ligand expression in osteoblast
cells. Biol Pharm Bull. 33:1733–1739. 2010.PubMed/NCBI
|
|
19
|
Ye S, Fowler TW, Pavlos NJ, Ng PY, Liang
K, Feng Y, Zheng M, Kurten R, Manolagas SC and Zhao H: LIS1
regulates osteoclast formation and function through its
interactions with dynein/dynactin and Plekhm1. PloS One.
6:e272852011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jurdic P, Saltel F, Chabadel A and
Destaing O: Podosome and sealing zone: specificity of the
osteoclast model. Eur J Cell Biol. 85:195–202. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Destaing O, Saltel F, Geminard JC, Jurdic
P and Bard F: Podosomes display actin turnover and dynamic
self-organization in osteoclasts expressing actin-green fluorescent
protein. Mol Biol Cell. 14:407–416. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meng B, Yang X, Chen Y, Zhai J and Liang
X: Effect of titanium particles on osteoclast activity in
vitro. Mol Med Rep. 3:1065–1069. 2010.PubMed/NCBI
|
|
23
|
Vaananen HK, Zhao H, Mulari M and Halleen
JM: The cell biology of osteoclast function. J Cell Sci.
113:377–381. 2000.
|
|
24
|
Lehenkari P, Hentunen TA, Laitala-Leinonen
T, Tuukkanen J and Vaananen HK: Carbonic anhydrase II plays a major
role in osteoclast differentiation and bone resorption by effecting
the steady state intracellular pH and Ca2+. Exp Cell
Res. 242:128–137. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gu JH, Liu JD, Shen Y and Liu ZP: Effects
of RANKL, osteoprotegerin, calcium and phosphorus on survival and
activation of Muscovy duck osteoclasts in vitro. Vet J.
181:321–325. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Teitelbaum SL, Tondravi MM and Ross FP:
Osteoclasts, macrophages, and the molecular mechanisms of bone
resorption. J Leukoc Biol. 61:381–388. 1997.PubMed/NCBI
|
|
27
|
Rousselle AV and Heymann D: Osteoclastic
acidification pathways during bone resorption. Bone. 30:533–540.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Riihonen R, Supuran CT, Parkkila S,
Pastorekova S, Vaananen HK and Laitala-Leinonen T: Membrane-bound
carbonic anhydrases in osteoclasts. Bone. 40:1021–1031. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kusano K, Miyaura C, Inada M, Tamura T,
Ito A, Nagase H, Kamoi K and Suda T: Regulation of matrix
metalloproteinases (MMP-2, -3, -9, and -13) by interleukin-1 and
interleukin-6 in mouse calvaria: association of MMP induction with
bone resorption. Endocrinology. 139:1338–1345. 1998.PubMed/NCBI
|
|
30
|
Yang J, Shang GD and Zhang YQ: Study of a
novel antiosteoporosis screening model targeted on cathepsin K.
Biomed Environ Sci. 17:273–280. 2004.PubMed/NCBI
|