Introduction
ADP-ribosylation plays a role in a number of
physiological and pathophysiological processes, including signal
transduction, transcription, DNA repair, cell cycle regulation and
mitosis, as well as necrosis and apoptosis (1). The classification of
ADP-ribosylation includes poly-ADP-ribosylation and
mono-ADP-ribosylation. According to the acceptor sites of the
different types of mono-ADP ribosylated amino acids, mono-ADP
ribosylation has been subdivided into diphthamide-, arginine-,
asparagine- and cysteine-specific ADP-ribosylation. In
mono-ADP-ribosylation, amino acid ADP-ribosyltransferases (ARTs)
are significant catalyzing enzymes which can transfer the
ADP-ribose moiety of nicotinamide adenine dinucleotide (NAD) to
certain protein amino acids (2,3).
ARTs included ART1-7; however, only ART1, -3, -4, and -5 have been
verified in the human gemone. In the family of ARTs, ART1 and ART5
are arginine-specific transferases. ART5 has only been detected in
humans; however, ART1 has been detected in humans and mice. The
function of ART5 has not yet been fully elucidated, but ART1 has
been reported to be involved in the inhibitory activity of
substrates, including defensin and integrin, and to mediate
ADP-ribosylation in humans; therefore, it may participate in the
innate immune response (4,5).
Poly(ADP-ribose) polymerase-1 (PARP-1) is an
abundant nuclear protein. It belongs to a family of 18 enzymes that
cleave NAD+ to nicotinamide and ADP-ribose, forming
negatively charged long branched (ADP-ribose) polymers on glutamic
acid residues of the PARP-1 protein itself and of other acceptor
proteins (6). In comparison with
ART1, PARP-1 has been investigated in a number of studies on
carcinoma. Previously, we demonstrated that the high expression of
PARP-1 in human colon carcinoma (7) and the inhibition of PARP-1 by
5-aminoisoquinolinone (5-AIQ) decreases the matrix adhesion,
invasion and migration ability of colon carcinoma cells, and
suppresses colon carcinoma liver metastasis in mice (8–10).
Previous studies have demonstrated that the
ADP-ribosylation of cell surface proteins mediated by
arginine-specific mono-ADP-ribosyltransferase suppresses the
proliferation and cytotoxic effector functions of cytotoxic T
lymphocytes in vitro (11,12), and prevents proliferation
(13) and induces apoptosis in
primary T cells (14). As
demonstrated in a previous study, in human gastric adenocarcinoma,
Helicobacter pylori (H. pylori) has enzymatic
mono-ADP-ribosyltransferase activity, which enables it to modify
membranous proteins and H. pylori itself. The authors
hypothesized that mono-ADP-ribosyltransferase may contribute to
human gastric carcinoma progression; however, this requires fruther
clarification (15). In a
previous study, we found that the expression of ART1, which
positively correlates with the expression of integrin αVβ3 and
vascular endothelial growth factor (VEGF), was increased in
colorectal carcinoma. It has been suggested that ART1 has the
ability to promote the formation of blood capillaries in colorectal
carcinoma (16).
Previous studies have reported that inhibitors of
PARP-1 and arginine-dependent mono-ADP-ribosylation, prevent
phenotypic modulation and the proliferation of smooth muscle cells
(SMCs) (17), concluding that
poly- and mono-ADP-ribosylation are both essential for the SMC
response to mitogenic stimulation. High levels of mono- and
poly-ADP-ribosylation activity may be necessary for proliferation
and differentiation (18–20). Certain studies have revealed
opposite effects, according to different cell types, growth
conditions, inhibitors and inhibitor concentrations (21,22). Hence, the correlation between
PARP-1 and ART1 in colon carcinoma has not yet been elucidated.
NF-κB, a transcription factor, has been found to
mediate various processes, including inflammation, immunity,
apoptosis, proliferation, angiogenesis and metastasis (23,24). Of note, certain studies have
indicated that NF-κB may be regulated by PARP (25) and that NF-κB may affect the
expression of PARP through a feedback mechanism (26).
In this study, meta-iodobenzylguanidine (MIBG), a
potent inhibitor of arginine-specific mono-ADP-ribosyltransferases
(27), and 5-aminoisoquinolinone
(5-AIQ), a water-soluble inhibitor of PARP (28), were used to inhibit the activity
of ART1 and PARP, respectively, and the correlation between PARP-1
and ART1 was investigated in colon carcinoma.
Materials and methods
Tissues and cells
A total of 63 samples of colorectal carcinoma were
provided by the Pathology Department of Chongqing Medical
University, Chongqing, China. According to the WHO classification
stystem for colorectal carcinoma, 2 pathologists diagnosed the
tissue samples into grade I (well differentiated), grade II
(moderately differentiated), grade III (poorly differentiated). The
CT26 cell line, a murine colon adenocarcinoma cell line, was a gift
from Professor Yuquan Wei, Sichuan University, Chengdu, China.
Immunochemistry assay
Sliced tissues (4-μm-thick) which were fixed
by formalin and embedded in paraffin, were successively immersed in
different levels of ethanol for hydration. Cell membranes were
permeablized with 0.3% Triton X-100 for 20 min at 37°C. After
blocking peroxidase activity with 3%
H2O2/methanol for 10 min, the specimens were
soaked in citrate buffer (ZSGB-Bio, Beijing, China) and heated to
95–98°C in a microwave oven (Gree, Zhuhai, China). After cooling to
room temperature, the specimens were washed 3 times with
phosphate-buffered saline (PBS, ZSGB-Bio) and blocked with
confining liquid (5% bovine serum) (SABC kit; Wuhan Boster
Biological Technology, Ltd., Wuhan, China) for 30 min at 37°C. All
the samples were incubated at 4°C overnight with rabbit polyclonal
antibody to PARP-1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA)
or goat polyclonal antibody to ART1 (Santa Cruz Biotechnology) at
dilutions of 1:100. According to the specifications of the SABC
kit, the samples were incubated with anti-rabbit antibody and
avidin-biotin peroxidase or the anti-goat antibody and
avidin-biotin peroxidase, followed by the addition of SABC for 30
min at 37°C. After being dyed with diaminobenzidine (DAB)
(ZSGB-Bio), the samples were counterstained with hematoxylin in
quick succession. For the negative control group, PBS was used
instead of the primary antibody.
The staining results were assessed according to the
method described in the study by Fromowitz et al (29,30) by 2 independent pathologists as
follows: i) positive degree score: 0, no staining; 1, light yellow;
2, brown; 3, dark brown. ii) Random observations from 5
magnifications (10×20) per field counting 100 tumor cells, the
average percentage of positive cells in each field of vision as the
percentage of positive cells in the slice. Positive range score: 0,
0–5%; 1, 6–25%; 2, 26–50%; 3, 51–75%; 4, >75%. The scores were
judged by positive range score plus positive extent score: <2,
negative (−); 2–3, slight positive (+); 4–5, moderately positive
(++); 6–7, strongly positive (+++).
Double-label immunofluorescence
assay
The CT26 cells were divided into 3 groups: untreated
group, the group treated with 100 μM MIBG (Sigma,
Ronkonkoma, NY, USA) and the group treated with 100 μM 5-AIQ
(a gift from Professor M.D. Threadgill, Bath University, Bath, UK).
After being treated with 0.5% Triton for 15 min and 1% BSA for 30
min in room temperature, the cells in each group were supplemented
with rabbit polyclonal antibody to PARP-1 and goat polyclonal
antibody to ART1 at the dilution of 1:100 at 4°C for overnight. The
cells were then incubated with goat anti-rabbit IgG FITC (ZSGB-Bio)
and rabbit anti-goat IgG RBITC (Wuhan Boster Biological Technology,
Ltd.) for 1 h. The distribution levels of PARP-1 and ART1 were
analyzed by double-label immunofluorescence staining under a laser
scanning confocal microscope (Leica TCS SP2, Leica Microsystems
GmbH, Germany). The mean density of the immunofluorescence images
was analyzed using Image-Pro Plus 6.0 software (IPP; Media
Cybernetics, Bethesda, MD, USA).
CT26 cell transfection
The CT26 cells were transfected with a lentivirus
based short-hairpin RNA (shRNA) vector with the following
ART1-shRNA interference sequence: GCCAACAAAGTATACGCGGAT. CT26 cells
transfected with non-target shRNA (control-shRNA) (interference
sequence: TTCTCCGAACGTGTCACGT) were used as the control group.
Transfection was carried according to the manufacturer’s
instructions (GeneChem, Shanghai, China). When the cells were in
the logarithmic growth phase, they were cultured in 12-well plates
at the concretion of 3×104 cells/well. When the cells
covered 50% of each well, 10 μl lentivirus particles were
added to each well. Transfection efficiency was optimized using
green fluorescent protein and detected under a fluorescence
microscope after 3 days. The CT26 cells transfected with
non-targeted shRNA and the CT26 untransfected cells served as the
controls. The effectiveness of the knockdown of ART1 was determined
by reverse transcriptase (RT)-PCR and western blot analysis.
RT-PCR
RNA was separately extracted from the ART1-shRNA-
and control-shRNA-transfected cells, as well as from the
untransfected CT26 cells according to the manual provided with
TRIzol reagent (Takara, Dalian, China). ART1 (target gene)
and β-actin (internal control gene) expression was detected
using oligonucleotide primers which were designed and produced by
Sangon Biotech Company (Shanghai, China). The primers used were as
follows: ART1, 5′-ACCTTCTTCGG TATCTGGACCT-3′ (F1) and
5′-TAAGTTGCTGGAGA CCTGGATT-3′ (R1); β-actin,
5′-ATATCGCTGCGCTGG TCGTC-3′ (F1) and 5′-AGGATGGCGTGAGGGAG AGC-3′
(R1). Using a one step RT-PCR kit (Takara), reverse transcription
was carried out at 50°C for 30 min, 94°C for 2 min, and extension
was carried out for 30 cycles at 94°C for 30 sec, 60°C for 30 sec
and 72°C for 25 sec. Finally, the amplification products were
electrophoresed on 2% agarose gels (Genview, Tallahasses, FL,
USA).
Western blot analysis
Adherent cells were collected by scraping into EP
tubes after being washed with PBS. The cells were lysed with lysis
buffer (Beyotime, Shanghai, China) for 30 min on ice, followed by
centrifugation at 12,000 rpm for 5 min in centrifuge tubes (Thermo
Fisher Scientific, Waltham, UK). Protein concentration was detected
using a BCA protein assay kit (Beyotime). The proteins were then
loaded in 6 or 10% polyacrylamide gels; separated by
electrophoresis in the range of proper voltage; and transferred
onto polyvinylidene fluoride membranes (Millipore, Billerica, MA,
USA). Skimmed milk (5%) was used to block heterogenetic antigen on
the membranes for 2 h. All blots were respectively incubated in 4°C
overnight with primary antibodies against PARP-1, ART1, NF-κB
(Bioworld Technology, Co., Ltd., St. Louis Park, MN, USA) and
β-actin (Wuhan Boster Biological Technology, Ltd.) at individual
dilutions of 1:1,000, 1:500, 1:500 and 1:500. Secondary antibodies,
peroxidaseconjugated anti-goat or rabbit IgG, were then added,
followed by incubation for 1 h at room temperature. After being
washed 3 times, the blots were dipped into BeyoECL Plus (Beyotime)
for exposure and imaged (Bio-Rad, Hercules, CA, USA). Finally,
Quantity One software (Bio-Rad) was used to for the densitometric
analysis of proteins on each blot.
Statistical analysis
The results from RT-PCR and western blot analysis
are presented as the means ± standard deviation (SD). Using SPSS
18.0 software (SPSS, Chicago, IL, USA), differences between
different groups were analyzed using one-way ANOVA and the Wilcoxon
test and correlation analysis was carried out using Spearman’s
correlation analysis. A P-value <0.05 was considered to indicate
a statistically significant difference.
Results
Expression of PARP-1 and ART1 in
colorectal carcinoma
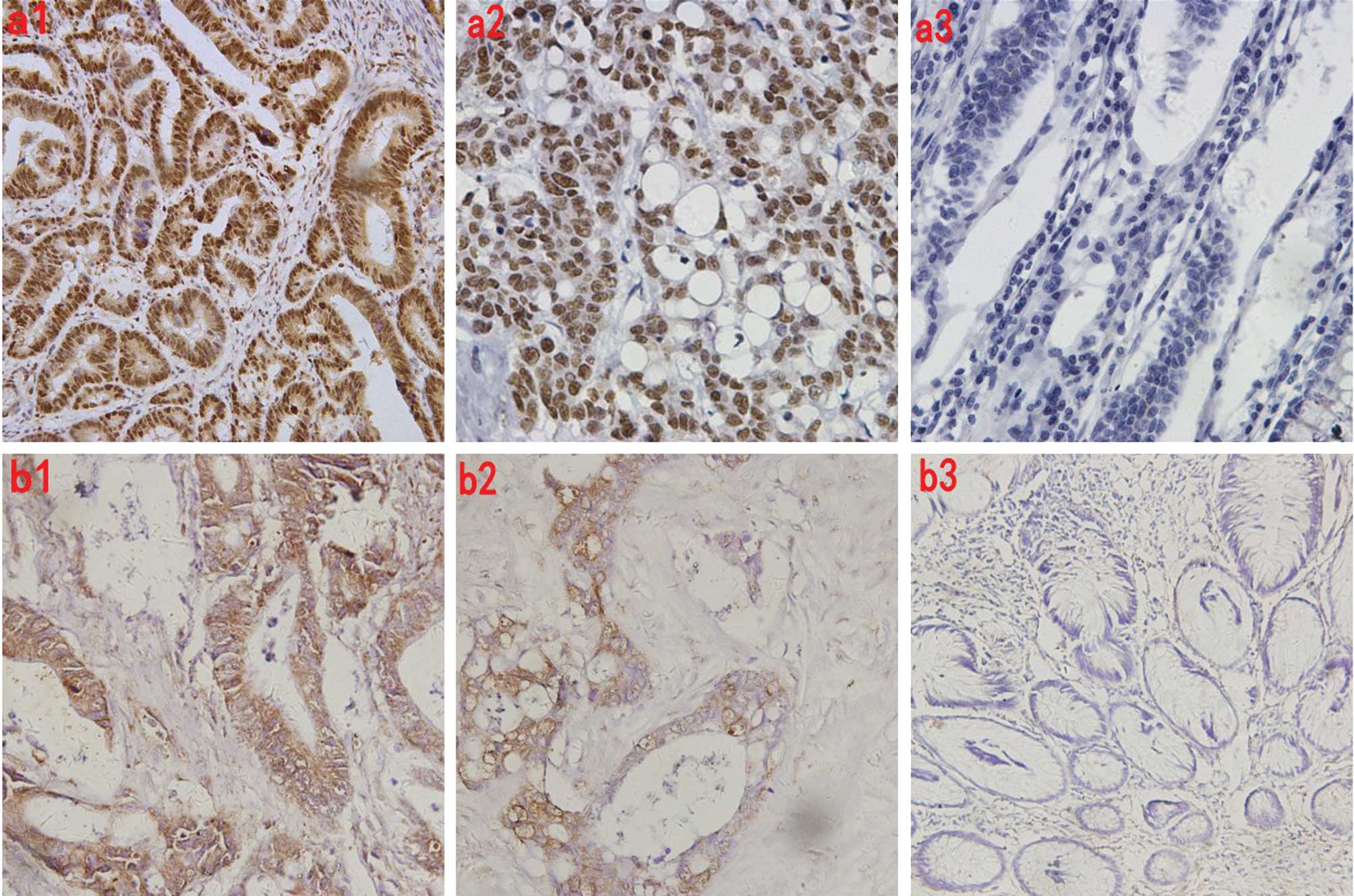
PARP-1 positive staining was observed in the tumor
cell nuclei, closer to the nucleus, and ART1 staining was observed
in the cytomembrane and cytoplasm of the carcinoma cells (Fig. 1). In the 63 cases of colorectal
carcinoma, the positive ratio of PARP-1 and ART1 was 95.24% (60/63)
and 63.49% (40/63), respectively, which was significantly higher
than the positive ratio of PARP-1 (4.76%) and ART1 (36.51%) in the
control colonic mucosa samples (P<0.05) (Table I). A positive correlation was
observed in the expression of PARP-1 and ART1 in the colorectal
carcinoma tissues (r=0.65) (Table
II) (P<0.05).
 | Table I.Expression of PARP-1 and ART1 in
colorectal carcinoma and control colonic mucosa. |
Table I.
Expression of PARP-1 and ART1 in
colorectal carcinoma and control colonic mucosa.
| ART1
| P-value | PARP-1
| P-value |
|---|
| − | + | − | + |
|---|
| Carcinoma | 7 | 56 | <0.001 | 3 | 60 | <0.001 |
| Control group | 8 | 2 | | 9 | 1 | |
 | Table II.Immunohistochemical expression of
PARP-1 and ART1 in colorectal cancer. |
Table II.
Immunohistochemical expression of
PARP-1 and ART1 in colorectal cancer.
| ART1 | PARP-1
| r |
|---|
| − | + |
|---|
| − | 2 | 1 | 0.65a |
| + | 1 | 60 | |
Effects of MIBG or 5-AIQ on the
expression of ART1 and PARP-1 in CT26 cells
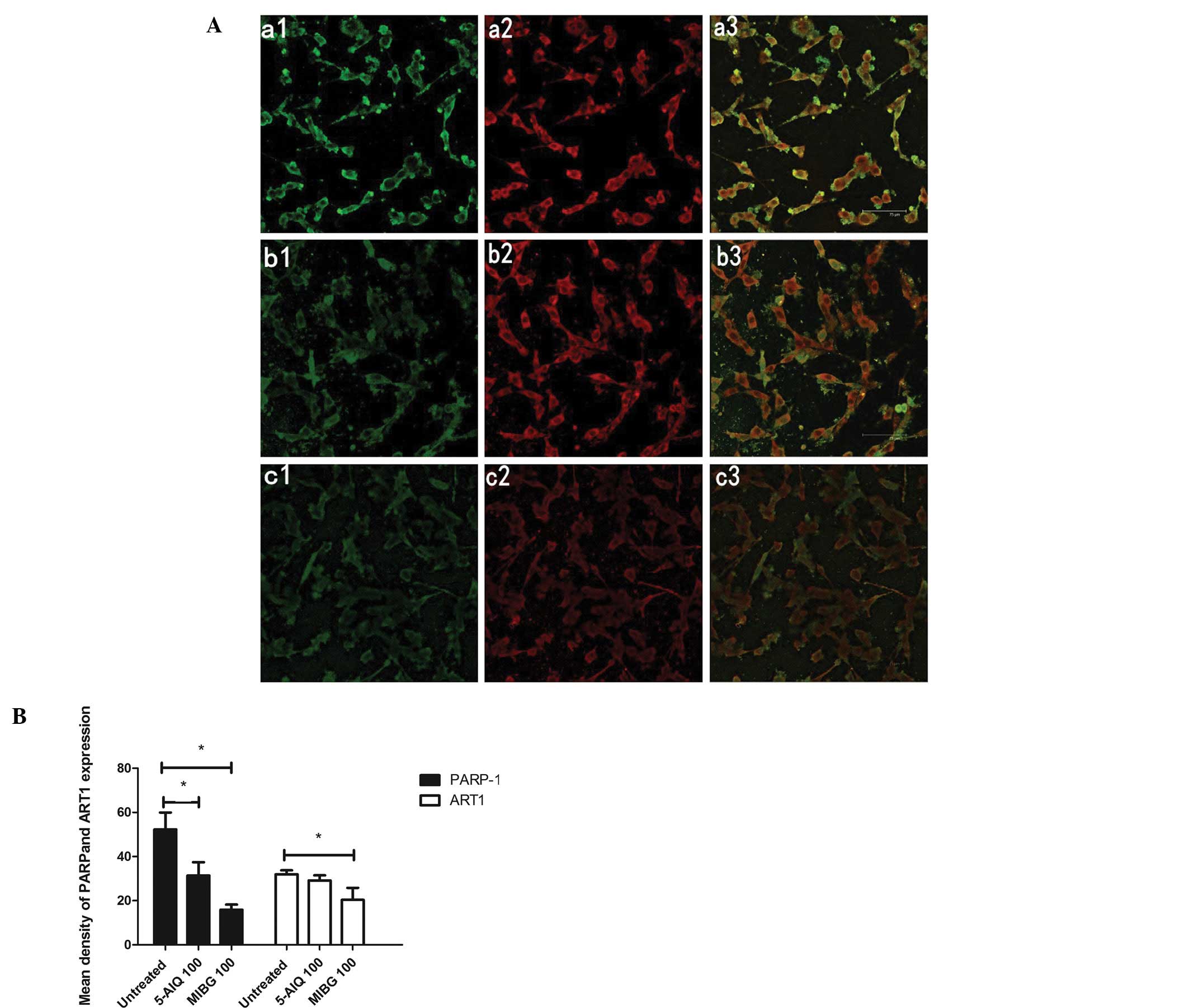
PARP-1 and ART1 double-label immunofluoresence
staining revealed that PARP-1 and ART1 were located in the
cytoplasm and nucleus separately (Fig. 2A). A significant decrease in PARP
and ART1 expression was observed in the group treated with MIBG
compared to the untreated group (P<0.05). However, in the group
treated with 5-AIQ, the expression of PARP was lower than that in
untreated group (P<0.05); however, the expression of ART1 did
not differ between the 2 groups (P>0.05) (Fig. 2B).
Knockdown of ART1 expression by
transfection with shRNA lentivirus in CT26 cells
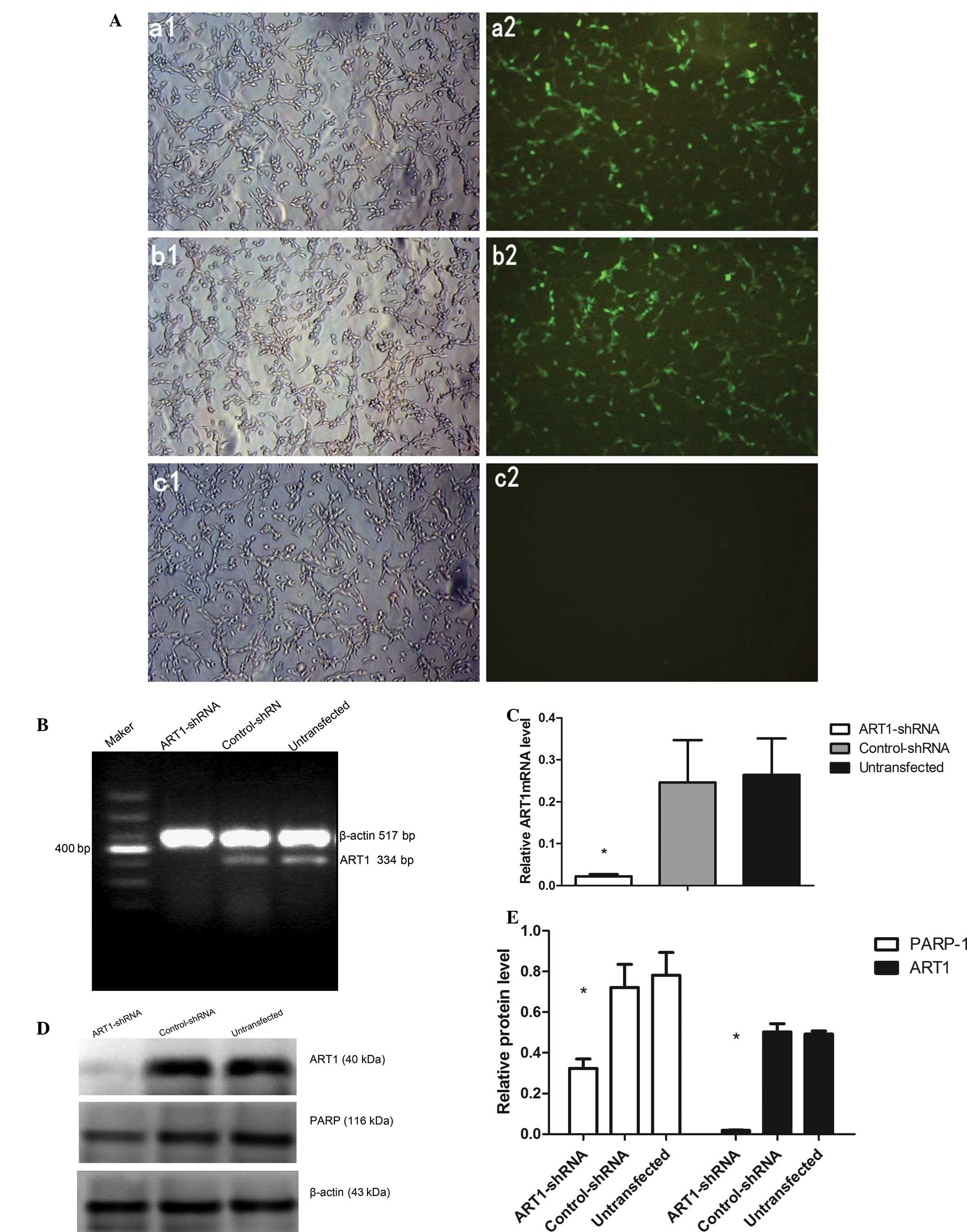
Immunofluorescence images of the transfected cells
revealed that the transfection efficiency reached 80% following
transfection with lentivirus particles (Fig. 3A). Compared to the
control-shRNA-transfected and untransfected cells, a significant
decrease in ART1 mRNA and protein expression was observed in the
ART1-shRNA-transfected cells by RT-PCR and western blot analysis
(Fig. 3B–E). However, the
difference in ART1 mRNA and protein expression between the
control-shRNA-transfected and untransfected cells was not
significant (p>0.05).
Effect of silencing ART1 on the
expression of PARP-1 and NF-κB in CT26 cells
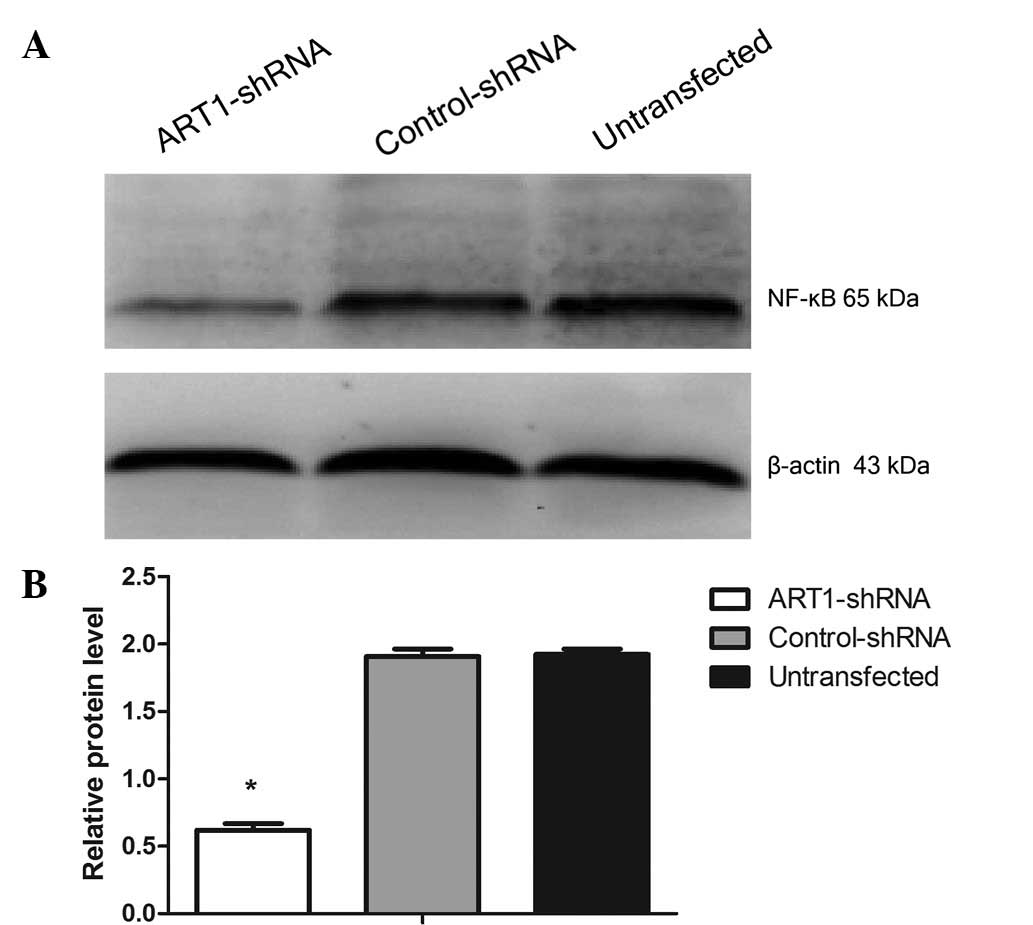
The results from western blot analsyis revealed that
the expression of PARP-1 and NF-κB was lower in the
ART1-shRNA-transfected cells compared to the untransfected cells or
the control-shRNA-transfected cells (p<0.05) (Figs. 3D and E, 4A and B). However, the difference in
PARP-1 and NF-κB expression between the control-shRNA-transfected
and untransfected cells was not significant (p>0.05).
Discussion
In a recent study, Kato et al reported that
the absence of ADP-ribosylarginine hydrolase (ARH1),
arginine-specific mono-ADP-ribosylation hydrolase, may increase the
frequency and extent of tumorigenesis in mice, including lung
adnocarcinoma, hepatocellular carcinoma and lymphoma (31). They suggested that the levels of
protein ADP-ribosylation controlled by ARH1 may suppress carcinoma.
ARH1 and ART1 collectively participate in the reversible
modification of proteins in the cycle of mono-ADP-ribosylation, to
control the levels of arginine ADP-ribosylation on substrate
proteins. ARH1, which has an opposite function to ART1, cleaves
α-ADP-ribose-arginine to regenerate the arginine-guanidino group
(1,32). The knockdown of ARH1 in cells has
been shown to increase the ADP-ribose-arginine content (31). Hence, the suppression of
carcinogenesis by ARH1 suggests that ART1 has a positive effect on
carcinogenesis. In the present study, our results revealed a higher
expression of ART1 in human colon carcinoma tissues compared to
normal colonic mucosa. This suggests that ART1 plays a role in
colon carcinoma.
Thyberg et al previously demonstrated that
inhibitors of poly-ADP-ribosylation (hexamethylenebisacetamide,
HMBA) and mono-ADP-ribosylation (MIBG) suppress the phenotypic
modulation and proliferation of SMCs. Both inhibitors of
ADP-ribosylation inhibited SMC proliferation and suppressed DNA
synthesis. However, compared to the inhibition of
mono-ADP-ribosylation, the inhibition of poly-ADP-ribosylation
significantly reduced the expression of transcripts for
differentiation markers and matrix metalloproteinases, which are
involved in SMC migration and proliferation (17). However, to our knowledge, to date,
there are no studies available on the correlation between
poly-ADP-ribosylation and mono-ADP-ribosylation in colon carcinoma.
Thus, in this study, we examined the activity of PARP-1 and ART1 to
elucidate the correlation between poly-ADP-ribosylation and
mono-ADP-ribosylation in colon carcinoma. The results revealed a
positive correlation between the expression of ART1 and PARP-1 in
colon carcinoma. To further confirm the correlation between these 2
enzymes, we used an inhibitor of PARP-1 and ART1 and observed the
changes in the expression of PARP-1 and ART1. MIBG (an inhibitor of
ART1) simultaneously inhibited the expression of PARP-1 and ART1,
but 5-AIQ (an inhibitor of PARP-1) only inhibited the expression of
PARP-1. Furthermore, after silencing the ART1 gene, the
levels of PARP-1 and ART1 in the CT26 cells decreased. However, the
mechanisms involved require further clarification.
Yau et al indicated that MIBG inhibits the
phosphorylation of the Rho effector, PRK1/2, a downstream effector
of Rho, suggesting that mono-ADP-ribosylation participates in a
Rho-dependent signaling pathway (33). Rho proteins are members of the Ras
superfamily of GTPases, which includes 9 members, RhoA, RhoB, RhoC,
RhoG, RhoE, CDC42, Rac-1, Rac-2 and TC10 (34). Our data demonstrated that the
expression of RhoA was reduced in the ART1-shRNA-transfected CT26
cells compared with the untransfected CT26 cells, (data not shown).
Accordingly, RhoA lies downstream of mono-ADP-ribosylation mediated
by ART1. Studies have demonstrated that the inhibition of RhoA has
a negative effect on NF-κB activity by decreasing phospho-IκBα
levels in prostate carcinoma cells (35); and may also suppress TNFα-induced
NF-κB activation, decreasing the invasive and metastatic ability of
breast carcinoma cells (36). Su
et al revealed a positive correlation between NF-κB and RhoA
in gastric carcinoma (37). It is
also known that RhoA is involved in the regulation of
NF-κB-dependent transcription (38). In a previous study, we
demonstrated that the inhibition of NF-κB, using the inhibitor,
pyrrolidinedithiocarbamic acid (PDTC) decreased the expressoin of
PARP-1 in human colon carcinoma Lovo cells compared to untreated
Lovo cells. Therefore, the inhibtion of NF-κB may inhibit the
expression of PARP-1 through a feedback mechanism (26). We hypothesized that the
ART1-mediated activity of RhoA may affect NF-κB transcription,
regulating the expression of PARP-1 through the feedback mechanism
of NF-κB to PARP-1. In this study, confirming our hypothesis, we
detected the reduced expression of NF-κB following the transfection
of CT26 cells with ART1-shRNA. Therefore, it can be concluded that
ART1 mediates the expression of PARP-1 by altering the expression
of NF-κB. However, the mechanisms behind the effects of the
ART1-mediated activity of RhoA require further clarification.
In brief, our data demonstrate that PARP-1
expression is affected by ART1 in colon carcinoma. The proposed
mechanism behind this effect may involve arginine
mono-ADP-ribosylation mediated by ART1 which negatively affects the
expression of RhoA and NF-κB activity, and sequentially decreases
PARP-1 expression. However, further studies are required to
elucidate the precise mechanisms involved. Further studies
investigating the mechanisms behind the correlation between PARP-1
and ART1 may help to clarify the role of ADP-ribosylation in the
development of colon carcinoma.
Acknowledgements
This study was supported by the
Ministry of Education Specialized Research Fund for the Doctoral
Program of Higher Education (Grant no. 20105503110009) and the
Science and Technology Project of the Education Commission of
Chongqing (Grant no. KJ110322).
References
|
1.
|
Hassa PO, Haenni SS, Elser M and Hottiger
MO: Nuclear ADP-ribosylation reactions in mammalian cells: where
are we today and where are we going? Microbiol Mol Biol Rev.
70:789–829. 2006. View Article : Google Scholar
|
|
2.
|
Moss J, Balducci E, Cavanaugh E, et al:
Characterization of NAD: arginine ADP-ribosyltransferases. Mol Cell
Biochem. 193:109–113. 1999. View Article : Google Scholar
|
|
3.
|
Zolkiewska A, Nightingale MS and Moss J:
Molecular characterization of NAD: arginine ADP-ribosyltransferase
from rabbit skeletal muscle. Proc Natl Acad Sci USA.
89:11352–11356. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Corda D and Di Girolamo M: Functional
aspects of protein mono-ADP-ribosylation. EMBO J. 22:1953–1958.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Corda D and Di Girolamo M:
Mono-ADP-ribosylation: a tool for modulating immune response and
cell signaling. Sci STKE 2002. pe532002.PubMed/NCBI
|
|
6.
|
Helleday T, Bryant HE and Schultz N:
Poly(ADP-ribose) polymerase (PARP-1) in homologous recombination
and as a target for cancer therapy. Cell Cycle. 4:1176–1178. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Hao LX, Wang YL and LI YY: Correlation of
PARP expression with P-selectin and ICAM-1 expression in colorectal
carcinoma. Basic Clin Med. 26:882–887. 2006.
|
|
8.
|
Li M, Cai L and Wang Y: Effect of
poly(ADP-ribose) polymerase inhibition on liver metastasis of mouse
colorectal carcinoma CR26 cell line in vivo. Acta Acad Med Mil
Tert. 30:237–240. 2008.
|
|
9.
|
Wang YL and Hao LX: Effect of
5-aminoisoquinolinone on the adhesion of colon carcinoma. Chin J
Cancer Res. 19:119–123. 2007. View Article : Google Scholar
|
|
10.
|
Effect of poly(ADP-ribose) polymerase
inhibition on liver metastasis of mouse colorectal carcinoma CT26
cell line in vivo. Acta Acad Med Mil Tert. 29:1621–1624. 2008.
|
|
11.
|
Wang J, Nemoto E, Kots AY, Kaslow HR and
Dennert G: Regulation of cytotoxic T cells by ecto-nicotinamide
adenine dinucleotide (NAD) correlates with cell surface
GPI-anchored/arginine ADP-ribosyltransferase. J Immunol.
153:4048–4058. 1994.PubMed/NCBI
|
|
12.
|
Wang J, Nemoto E and Dennert G: Regulation
of CTL by ecto-nictinamide adenine dinucleotide (NAD) involves
ADP-ribosylation of a p56lck-associated protein. J Immunol.
156:2819–2827. 1996.PubMed/NCBI
|
|
13.
|
Okamoto S, Azhipa O, Yu Y, Russo E and
Dennert G: Expression of ADP-ribosyltransferase on normal T
lymphocytes and effects of nicotinamide adenine dinucleotide on
their function. J Immunol. 160:4190–4198. 1998.PubMed/NCBI
|
|
14.
|
Adriouch S, Ohlrogge W, Haag F, Koch-Nolte
F and Seman M: Rapid induction of naive T cell apoptosis by
ecto-nicotinamide adenine dinucleotide: requirement for
mono(ADP-ribosyl) transferase 2 and a downstream effector. J
Immunol. 167:196–203. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Akai T, Nabeya Y, Yahiro K, et al:
Helicobacter pylori induces mono-(adenosine
5′-diphosphate)-ribosylation in human gastric adenocarcinoma. Int J
Oncol. 29:965–972. 2006.
|
|
16.
|
Yang L, Wang Y, Sheng Y, Xiong W, Xu J,
Tang Y and Li X: The correlation of ART1 expression with
angiogenesis in colorectal carcinoma and it relationship with VEGF
and integrin αVβ3 expressions. Basic Clin Med. 9:1064–1069.
2012.
|
|
17.
|
Thyberg J, Hultgårdh-Nilsson A and Kallin
B: Inhibitors of ADP-ribosylation suppress phenotypic modulation
and proliferation of smooth muscle cells cultured from rat aorta.
Differentiation. 59:243–252. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Brac T and Ebisuzaki K: PolyADP
ribosylation and Friend erythroleukemic-cell differentiation:
action of poly(ADP-ribose) polymerase inhibitors. Differentiation.
34:139–143. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Colon-Otero G, Sando JJ, Sims JL, McGrath
E, Jensen DE and Quesenberry PJ: Inhibition of hemopoietic growth
factor-induced proliferation by adenosine diphosphate-ribosylation
inhibitors. Blood. 70:686–693. 1987.
|
|
20.
|
Francis G, Gray D, Berney J, Wing M,
Guimaraes J and Hoffbrand A: Role of ADP-ribosyltransferase in
differentiation of human granulocyte-macrophage progenitors to the
macrophage lineage. Blood. 62:1055–1062. 1983.PubMed/NCBI
|
|
21.
|
Kuo ML, Chau YP, Wang JH and Shiah SG:
Inhibitors of poly(ADP-ribose) polymerase block nitric
oxide-induced apoptosis but not differentiation in human leukemia
HL-60 cells. Biochem Biophys Res Commun. 219:502–508. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Terada M, Fujiki H, Marks PA and Sugimura
T: Induction of erythroid differentiation of murine erythroleukemia
cells by nicotinamide and related compounds. Proc Natl Acad Sci
USA. 76:6411–6414. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Luo J, Kamata H and Karin M: IKK/NF-kappaB
signaling: balancing life and death - a new approach to cancer
therapy. J Clin Invest. 115:2625–2632. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Karin M and Ben-Neriah Y: Phosphorylation
meets ubiquitination: the control of NF-κB activity. Annu Rev
Immunol. 18:621–663. 2000.PubMed/NCBI
|
|
25.
|
Virág L and Szabó C: The therapeutic
potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev.
54:375–429. 2002.
|
|
26.
|
Pan J, Fauzee NJ, Wang YL, et al: Effect
of silencing PARG in human colon carcinoma LoVo cells on the
ability of HUVEC migration and proliferation. Cancer Gene Ther.
19:715–722. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Loesberg C, van Rooij H and Smets LA:
Meta-iodobenzylguanidine (MIBG), a novel high-affinity substrate
for cholera toxin that interferes with cellular
mono(ADP-ribosylation). Biochim Biophys Acta. 1037:92–99. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Mota-Filipe H, Sepodes B, McDonald M,
Cuzzocrea S, Pinto R and Thiemermann C: The novel PARP inhibitor
5-aminoisoquinolinone reduces the liver injury caused by ischemia
and reperfusion in the rat. Med Sci Monit. 8:BR444–BR453.
2002.PubMed/NCBI
|
|
29.
|
Fromowitz FB, Viola MV, Chao S, et al: Ras
p21 expression in the progression of breast cancer. Hum Pathol.
18:1268–1275. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Qin JM, Fu XY, Li SJ, et al: Gene and
protein expressions of p28GANK in rat with liver regeneration.
World J Gastroenterol. 9:2523–2527. 2003.PubMed/NCBI
|
|
31.
|
Kato J, Zhu J, Liu C, et al:
ADP-ribosylarginine hydrolase regulates cell proliferation and
tumorigenesis. Cancer Res. 71:5327–5335. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Moss J, Stanley S, Nightingale M, et al:
Molecular and immunological characterization of ADP-ribosylarginine
hydrolases. J Biol Chem. 267:10481–10488. 1992.PubMed/NCBI
|
|
33.
|
Yau L, Litchie B, Thomas S, Storie B,
Yurkova N and Zahradka P: Endogenous mono-ADP-ribosylation mediates
smooth muscle cell proliferation and migration via protein kinase
N-dependent induction of c-fos expression. Eur J Biochem.
270:101–110. 2003. View Article : Google Scholar
|
|
34.
|
Bourne HR, Sanders DA and McCormick F: The
GTPase superfamily: conserved structure and molecular mechanism.
Nature. 349:117–127. 1991. View
Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Hwang YS, Hodge JC, Sivapurapu N and
Lindholm PF: Lysophosphatidic acid stimulates PC-3 prostate cancer
cell matrigel invasion through activation of RhoA and NF-κB
activity. Mol Carcinog. 45:518–529. 2006.PubMed/NCBI
|
|
36.
|
Cho SG, Li D, Stafford LJ, et al: KiSS1
suppresses TNFα-induced breast cancer cell invasion via an
inhibition of RhoA-mediated NF-κB activation. J Cell Biochem.
107:1139–1149. 2009.
|
|
37.
|
Su X, Tang Z, Li Q, et al: Expression and
significance of RhoA and NF-κB in human gastric carcinoma. Zhonghua
Zhong Liu Za Zhi. 33:276–279. 2011.(In Chinese).
|
|
38.
|
Perona R, Montaner S, Saniger L,
Sanchez-Perez I, Bravo R and Lacal JC: Activation of the nuclear
factor-kappaB by Rho, CDC42, and Rac-1 proteins. Genes Dev.
11:463–475. 1997. View Article : Google Scholar : PubMed/NCBI
|