Introduction
Recently, it was discovered that seaweed is composed
of certain bioactive compounds, which have antitumor effects.
Seaweed is also used in various functional materials and agents. In
particular, seaweed polysaccharides, which comprise some of its
bioactive compounds, include cellulose and viscous polysaccharides.
Seaweed is also comprised of approximately 30–60% soluble
polysaccharides; these polysaccharides include alginate, laminarin
and fucoidan. Of these, laminarin is composed of β1–3 and
β1–6-glucan, and is a storage glucan for brown seaweed (1). Due to these structural
characteristics, laminarin has bioactivities similar to those of
β-glucan; it has been shown to have immune-enhancing and anticancer
effects, as well as antibacterial activity (2).
In this study, we investigated whether laminarin has
direct anticancer activities in addition to its ability to inhibit
cancer cell proliferation. We demonstrate that laminarin induces
apoptosis in highly proliferative cancer cells. In a previous
study, we showed that activated insulin-like growth factor receptor
(IGF-IR) inhibits apoptosis (3).
We showed that laminarin induces the apoptosis of HT-29 cells
through the Fas and IGF-IR signaling pathways. Many human tumors
express high levels of growth factors and their corresponding
receptors, which contributes to cancer progression (4,5).
Two such growth factors that induce cancer progression are IGF and
epidermal growth factor (6).
In this study, we demonstrate that laminarin
inhibits the activation of the ErbB pathway. The four members of
the ErbB subfamily share a similar structure but have different
functions (7,8). The overexpression of ErbB genes,
particularly ErbB2, has been observed in human cancer (9). Heregulin (HRG) is co-expressed with
ErbB2 proteins in human cancer cells, and heterodimerization with
ErbB3 activates ErbB2 through an autocrine mechanism in colon
cancer cells (10). Therefore,
the HRG/ErbB2/ErbB3 pathway is an important regulator of aberrant
growth in colon cancer (11,12). In our study, we confirmed that
laminarin inhibits the proliferation and survival of colon cancer
cells by regulating the ErbB receptor signaling pathway. Our
results suggest that laminarin is a ligand of the high-affinity
ErbB and IGF-1 receptors, and thereby significantly affects
signaling by the two growth factors. Moreover, our results strongly
suggest that in addition to inhibiting protein expression, specific
mechanisms, such as apoptosis are involved in laminarin-associated
cancer prevention.
Materials and methods
Cell culture
We used HT-29 colon cancer cells (ATCC HTB-38; ATCC,
Manassas, VA, USA) to examine the effects of laminarin. The cells
were maintained in a humidified environment comprised of 5%
CO2 and 95% air at 37°C in RPMI-1640 supplemented with
10% fetal bovine serum (FBS), penicillin/streptomycin (Gibco BRL,
Grand Island, NY, USA). The medium was changed every 2–3 days.
Western blot analysis
To prepare a whole-cell extract, the cells were
washed in PBS and suspended in extraction buffer [20 mM Tris (pH
7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium
pyrophosphate, 1 mM β-glycerophosphate, 1 mM
Na3VO4, 1 μg/ml leupeptin, 1 mM PMSF and 1%
Triton X-100]. Subsequently, 50 μg of boiling sample buffer were
added to the total cell lysate, and the samples were boiled for 10
min at 100°C. Proteins in the extracts were separated by 7.5–15%
SDS-PAGE and transferred onto polyvinylidene fluoride membranes
(Millipore, Billerica, MA, USA). The membranes were blocked for 1 h
at room temperature in blocking buffer [1% bovine serum albumin
(BSA) in TBS-T] then probed with primary antibodies (1:1,000 in 1%
BSA/TBS-T) overnight at 4°C. The membranes were then washed twice
for 15 min each in TBS-T and incubated with peroxidase-conjugated
goat anti-mouse or -rabbit antibodies (1:10,000 in 1%
BSA/TBS-T).
Cell cycle analysis
The cells were cultured in 6-well plates to 60%
confluency then treated with serum-free medium (SFM) for 6 h
followed by various doses of laminarin (0, 1.25, 2.5 and 5 mg/ml)
for 24 h. The cells were then trypsinized, washed with PBS and
treated with 50 μg/ml cold propidium iodide solution containing 0.1
mg/ml RNase A in PBS (pH 7.4) for 30 min in the dark. Flow
cytometric analysis was performed on a FACSCalibur instrument
(Becton-Dickinson, San Jose, CA, USA).
Immunoprecipitation and western blot
analysis
The cells were incubated in SFM for 24 h and
stimulated with 100 ng/ml HRG. To prepare a whole-cell extract,
cells were washed in PBS and suspended in extraction buffer [20 mM
HEPES (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 100 mM NaF, 10
mM sodium pyrophosphate, 1 mM Na3VO4, 20
μg/ml aprotinin, 10 μg/ml antipain, 10 μg/ml leupeptin, 80 μg/ml
benzamidine HCl, 0.2 mM PMSF and 1% Triton X-100]. For
immunoprecipitation, cell lysates (750 μg) were incubated at 10°C
with anti-ErbB2 antibodies. After 12 h, protein A-Sepharose beads
were added to the cell lysates. The beads were collected by
centrifugation for 2 min at 10,000 × g and washed 3 times with
lysis buffer. The beads were then boiled with the immunocomplex in
1X sample buffer. The eluted proteins were analyzed by SDS-PAGE and
western blot analysis.
Statistical analysis
All variables were compared with an analysis of
variance using SPSS software version 10.0 (SPSS Inc., Chicago, IL,
USA). All values are presented as the means ± SD. A P-value
<0.05 was considered to indicate a statistically significant
difference.
Results
Laminarin induces a loss of mitochondrial
membrane potential
The mitochondrial pathway is a critical apoptotic
pathway that involves signaling by Bcl-2 family proteins. The
mitochondrial pathway of apoptosis also involves changes in
mitochondrial potential and the mitochondrial release of cytochrome
c into the cytosol.
Thus, we monitored the expression of Bcl-2 family
proteins. To determine whether laminarin triggers the release of
cytochrome c, we examined the cytosolic and mitochondrial
levels of cytochrome c. As shown in Fig. 1A, Bcl-2 expression decreased
following treatment with laminarin, whereas Bad and Bax expression
increased. Simultaneously, the levels of cytochrome c in the
mitochondrial fraction decreased, whereas the levels in the
cytosolic fraction increased and the expression of cytosolic
apoptotic protease activating factor-1 (Apaf-1) also increased
(Fig. 1B). This suggests a role
for the mitochondria in laminarin-induced apoptosis.
Effect of laminarin on cell cycle
progression
Laminarin-induced apoptosis was assessed by cell
cycle analysis (Fig. 2). The cell
cycle response was examined in the cells treated with various
concentrations of laminarin. We observed an increase in the
percentage of cells in the sub-G1 and G2-M phase, while the
percentage of cells in the other phases decreased. Treatment with
laminarin markedly increased the proportion of cells in the sub-G1
and G2-M phase, suggesting that laminarin interferes with cell
cycle progression.
Effect of laminarin on the expression of
cell cycle-related proteins
To investigate the apoptotic mechanisms through
which laminarin interferes with cell cycle progression, we
confirmed the cell cycle-related protein content. The levels of
p27, c-myc, pRb, Cdk2 and Cdk6 were measured by western blot
analysis using specific antibodies against these proteins. The
HT-29 cell cycle response was examined following treatment with
laminarin at various concentrations. As shown in Fig. 3, the levels of Cdk2, Cdk6, pRb and
c-myc decreased, whereas the p27 level in the nuclear fraction
increased.
Effect of laminarin on the expression of
ErbB signaling pathway-related proteins
ErbB receptor pathway-related proteins play
important roles in normal cells, as well as in cancer cells. ErbB
receptors control key pathways that govern cellular processes, such
as proliferation, metabolism and survival (13,14). In tumor cells, ErbB2 activates
ErbB3, which stimulates several intracellular signaling proteins
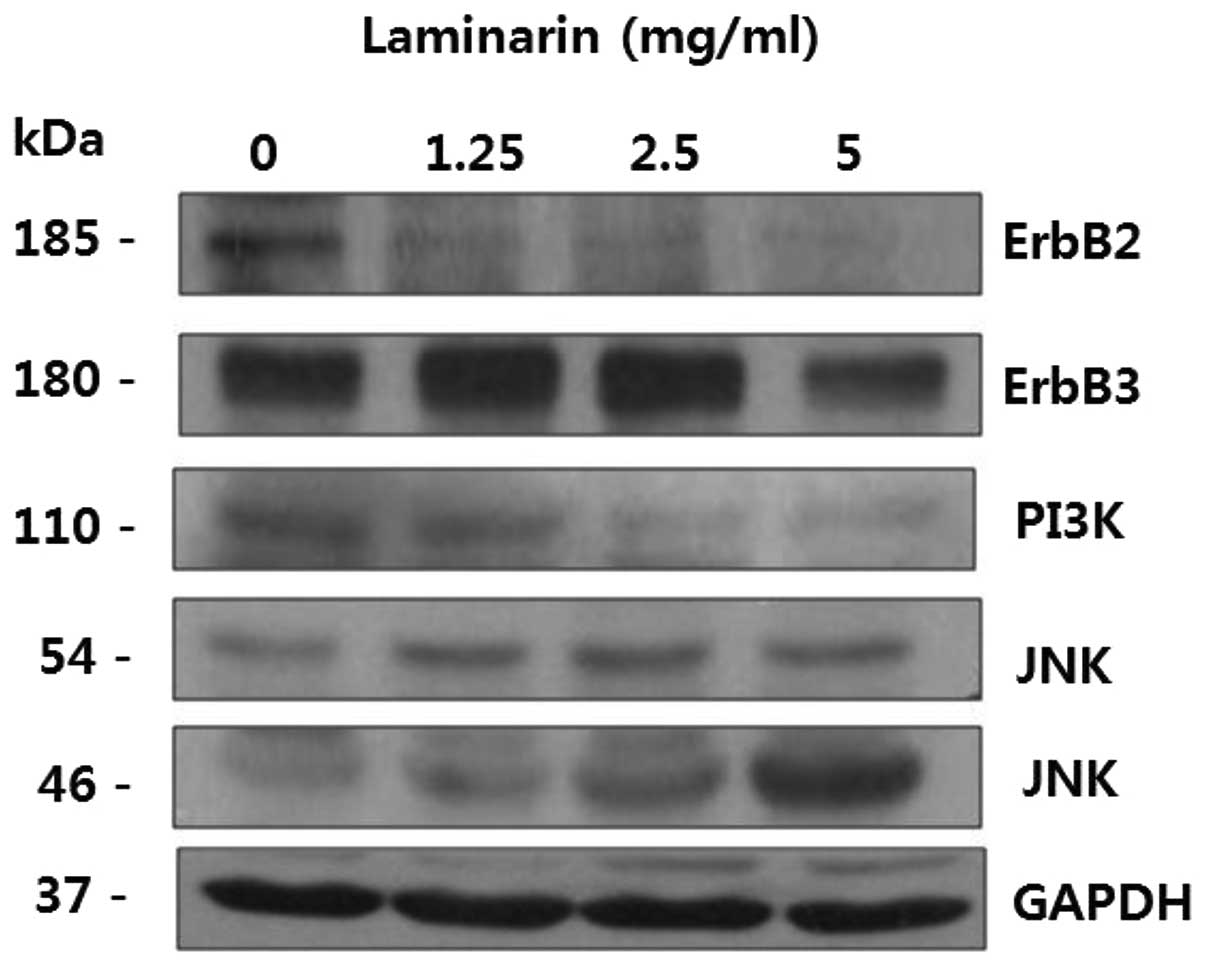
and pathways, including MAPK, PI3K/Akt and Src kinase (13,15,16). As shown in Fig. 4, ErbB2, ErbB3 and PI3K expression
decreased following treatment with laminarin, whereas that of JNK
increased. These results suggest that laminarin alters the
expression of ErbB signaling pathway-related proteins.
Inhibition of HRG-induced p-Akt
activation and ErbB2 phosphorylation by laminarin
The expression of p-Akt in the HT-29 cells treated
with increasing levels of laminarin was examined by western blot
analysis. As shown in Fig. 5, the
recruitment of p-Akt, ErbB2 and PY99 was observed, which lasted 60
min in the control group. By contrast, protein expression was
inhibited for up to 60 min following treatment with laminarin. The
effect of laminarin on the HRG-induced association of ErbB2 and
p-Akt was examined by immunoprecipitation using anti-ErbB
antibodies followed by western blot analysis using anti-p-Akt
antibodies. Additionally, we found that laminarin inhibited the
HRG-induced increase in ErbB2 phosphorylation in HT-29 cells.
Discussion
We have previously shown that treatment with
laminarin inhibits the proliferation of colon cancer cells through
the Fas and IGF-IR signaling pathways (3). In this study, we demonstrate that
laminarin inhibits HT-29 cell growth through the intrinsic
apoptotic and ErbB pathways.
In our previous study, we showed that laminarin
induces apoptosis (3). Therefore,
in this study, we examined the effects of laminarin on other
apoptotic pathways. To our knowledge, this study provides the first
evidence that laminarin decreases Bcl-2 family protein expression
and inhibits cell cycle progression by regulating the ErbB
signaling pathway.
Bcl-2 family proteins regulate apoptosis and the
release of pro-apoptotic factors (17–19). Bcl-2 family protein and cytochrome
c expression in the cytosol and mitochondria was detected.
As shown in Fig. 1, laminarin
increased the expression of apoptotic molecules, such as Bax and
Bad, which belong to the Bcl-2 family. By contrast, the expression
of anti-apoptotic molecules, such as Bcl-2 was decreased following
treatment with laminarin. The anti-apoptotic factors, Bcl-2 and
Bcl-xL, function by heterodimerizing with multidomain Bax
effectors.
A loss of mitochondrial membrane potential is
associated with apoptosis following the release of cytochrome
c(20). The induction of
Bax is associated with the release of cytochrome c from the
mitochondria to the cytosol and the cleavage of poly(ADP-ribose)
polymerase (21). As indicated in
Fig. 1B, the release of
cytochrome c from the mitochondria to the cytosol was
induced following treatment with laminarin.
Apaf-1 plays a role in the activation of apoptosis
in the intrinsic mitochondrial pathway. Apaf-1 induces the
formation of apoptosomes and activates caspase-9. Activated
caspase-9 then cleaves and activates downstream caspases, such as
caspase-3, -6 and -7, leading to apoptosis (22). In this study, cytosolic Apaf-1
levels increased as the laminarin concentrations increased. As
shown in Fig. 1, laminarin
increased the expression of the apoptotic molecules, Bax and Bad,
members of the Bcl-2 family, thus inducing apoptosis and
mitochondrial dysfunction by the release of apoptotic factors, such
as cytosolic cytochrome c and Apaf-1. Therefore, laminarin
increased the protein levels of cytosolic cytochrome c and
Apaf-1, suggesting that laminarin induced apoptosis through a
mitochondrial-dependent pathway.
Laminarin-induced apoptosis was examined by cell
cycle analysis (Fig. 2). The
results revealed an increase in the percentage of cells in the
sub-G1 and G2-M phase, while the percentage of cells in the other
phases decreased following treatment with laminarin. Treatment with
laminarin markedly increased the proportion of cells in the
sub-G1-phase from 8.28 to 63.48%, those in the G2-phase from 9.41
to 18.30%, and those in the M-phase from 8.50 to 12.92%, suggesting
that laminarin inhibits cell cycle progression.
As shown in Fig.
2, we found that laminarin induced a dose-dependent sub-G1 and
G2-M phase cell cycle arrest; this was followed by apoptosis, which
was associated with the expression of cell cycle-related proteins,
such as Cdk and cyclin. The levels of Cdk2, Cdk6, pRb, p27 and
c-myc were measured by western blot analysis. As shown in Fig. 3, the levels of Cdk2, Cdk6, pRb and
c-myc decreased, whereas the level of p27 increased. Our results
also indicated that laminarin-induced apoptosis was not associated
with an alteration in p53 protein expression (data not shown). An
increase in the levels of cyclin B1 and A regulates Cdk2 kinase
expression at the G2-M phase (23). We demonstrated that laminarin
induced cell cycle arrest, followed by cell death in highly
proliferative colon cancer cells.
The dysregulation of the ErbB receptor family signal
transduction pathway is observed in several types of cancer,
including lung, breast, prostate, colon and duodenal cancer. The
abnormal activation of the ErbB receptor family signal transduction
pathway is considered one of the main causes of cancer (24).
ErbB family receptor tyrosine kinases are considered
to play crucial roles in the incidence of cancer. These kinases
include epidermal growth factor receptor (EGFR or ErbB1), ErbB2,
ErbB3 and ErbB4. In addition, HRG is co-expressed with ErbB2 in
colon cancer, and the autocrine activation of ErbB2 occurs through
dimerization with ErbB3 (10).
The extracellular domain of the ErbB receptor is responsible for
ligand binding, inducing the formation of receptor dimers and the
phosphorylation of tyrosine residues in the cytoplasmic domain of
the receptor occurs through the activation of intrinsic tyrosine
kinase. The phosphorylated tyrosine residues play a role in
intracellular signaling. Phosphatidylinositol-3 kinase (PI3K) is
activated through the binding of the SH domain in the p85 subunit
to autophosphorylated tyrosine kinase receptors, and activated PI3K
produces phosphatidylinositol-3,4,5-triphosphate, thus promoting
the phosphorylation of Akt (25–27). Activated Akt is inactivated by the
phosphorylation of the apoptosis-related protein; activated Akt is
known to promote cell survival and suppress apoptosis (28).
ErbB receptor pathway-related proteins play
important roles in normal cells; ErbB receptors regulate cell
proliferation, metabolism and survival (13,14). In tumor cells, ErbB2 activates
ErbB3 by stimulating several intracellular signaling proteins, such
as MAPK, PI3K/Akt and Src kinase (13,15,16). As shown in Fig. 4, ErbB2, ErbB3 and PI3K expression
levels decreased following treatment with laminarin, whereas the
JNK expression level increased. These results suggest that
laminarin inhibits the expression of ErbB signaling pathway-related
proteins.
The HT-29 cells were incubated in SFM with the
addition of 5 mg/ml of laminarin for 24 h; the cells were then
stimulated with 100 ng/ml of HRG for 0, 1, 5 and 60 min. The effect
of laminarin on the HRG-induced association of ErbB2 and p-Akt was
examined by immunoprecipitation. As HRG binds with ErbB2,
immunoprecipitation was carried out by the addition of ErbB2
antibodies to the cell lysates. The immunoprecipitated cell lysates
were analyzed by western blot analysis. As shown in Fig. 5, the recruitment of p-Akt, PY-99
and ErbB2 was observed, which lasted 60 min in the laminarin-free
treatment group (control group). By contrast, the protein
expression of p-Akt, PY-99 and ErbB2 was inhibited for up to 60 min
following treatment with laminarin. The HRG-induced ErbB2 protein
expression levels were examined following treatment of the HT-29
cells with 5 mg/ml laminarin. Treatment of the HT-29 cells with
laminarin inhibited phosphorylation and ErbB2 expression, as well
as the phosphorylation of Akt; therefore, these results suggest
that treatment with laminarin inhibits the proliferation of HT-29
cells.
In this study, we demonstrate that laminarin induces
apoptosis through an apoptotic pathway involving growth factors and
also demonstrate the effects of laminarin on the ErbB signaling
pathway in HT-29 colon cancer cells. These findings suggest the
important role of EGFR in colon cancer tumorigenesis, as well as
the potential value of laminarin as a bio-functional food with
anticancer effects on human colon cancer.
Acknowledgements
This study was supported by iPET (Korea Institute of
Planning and Evaluation for Technology in Food, Agriculture,
Forestry and Fisheries), Ministry for Food, Agriculture, Forestry
and Fisheries, Republic of Korea.
References
|
1
|
Painter TJ: Algal polysaccharides. The
Polysaccharides. Aspinall GO: 2. Academic press; New York, NY: pp.
195–285. 1983
|
|
2
|
Zvyagintseva TN, Shevchenko NM, Nazarova
IV, Scobum AS, Luk’yanov PA and Elyakova LA: Inhibition of
complement activation by water-soluble polysaccharides of some
far-eastern brown seaweeds. Comp Biochem Physiol C Toxicol
Pharmacol. 126:209–215. 2000.PubMed/NCBI
|
|
3
|
Park HK, Kim IH, Kim J and Nam TJ:
Induction of apoptosis by laminarin, regulating the insulin-like
growth factor-IR signaling pathways in HT-29 human colon cells. Int
J Mol Med. 30:734–738. 2012.PubMed/NCBI
|
|
4
|
Kumar CC: Signaling by integrin receptors.
Oncogene. 17:1365–1373. 1998. View Article : Google Scholar
|
|
5
|
Hung MC and Lau YK: Basic science of
HER-2/neu: a review. Semin Oncol. 26(4 Suppl 12): 51–59.
1999.PubMed/NCBI
|
|
6
|
Fürstenberger G and Senn HJ: Insulin-like
growth factors and cancer. Lancet Oncol. 3:298–302. 2002.
|
|
7
|
Peles E and Yarden Y: Neu and its ligands:
from an oncogene to neural factors. Bioessays. 15:815–824. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carraway KL III and Cantley LC: A neu
acquaintance for erbB3 and erbB4: a role for receptor
heterodimerization in growth signaling. Cell. 78:5–8. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hamdy FC and Thomas BG: New therapeutic
concepts in prostate cancer. BJU Int. 88(Suppl 2): 43–48. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Venkateswarlu S, Dawson DM, St Clair P,
Gupta A, Willson JK and Brattain MG: Autocrine heregulin generates
growth factor independence and blocks apoptosis in colon cancer
cells. Oncogene. 21:78–86. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kapitanović S, Radosević S, Kapitanović M,
Andelinović S, Ferencić Z, Tavassoli M, Primorać D, Sonicki Z,
Spaventi S, Pavelic K and Spaventi R: The expression of p185
(HER-2/neu) correlates with the stage of disease and survival in
colorectal cancer. Gastroenterology. 112:1103–1113. 1997.PubMed/NCBI
|
|
12
|
Safran H, Steinhoff M, Mangray S, Rathore
R, King TC, Chai L, Berzein K, Moore T, Iannitti D, Reiss P,
Pasquariello T, Akerman P, Quirk D, Mass R, Goldstein L and
Tantravahi U: Over expression of the HER-2/neu oncogene in
pancreatic adenocarcinoma. Am J Clin Oncol. 24:496–499. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hynes NE and Lane HA: ERBB receptors and
cancer: the complexity of targeted inhibitors. Nat Rev Cancer.
5:341–354. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Citri A and Yarden Y: EGF-ERBB signalling:
towards the systems level. Nat Rev Mol Cell Biol. 7:505–516. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sharma SV and Settleman J: ErbBs in lung
cancer. Exp Cell Res. 315:557–571. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yarden Y and Sliwkowski MX: Untangling the
ErbB signaling network. Nat Rev Mol Cell Biol. 2:127–137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reed JC: Double identity for proteins of
the Bcl-2 family. Nature. 387:773–776. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chao DT and Korsmeyer SJ: BCL-2 family:
regulators of cell death. Annu Rev Immunol. 16:395–419. 1998.
View Article : Google Scholar
|
|
19
|
Kluck RM, Bossy-Wetzel E, Green DR and
Newmeyer DD: The release of cytochrome c from mitochondria: a
primary site for Bcl-2 regulation of apoptosis. Science.
275:1132–1136. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shimizu S, Narita M and Tsujimoto Y: Bcl-2
family proteins regulate the release of apoptogenic cytochrome c by
the mitochondrial channel VDAC. Nature. 399:483–487. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bossy-Wetzel E, Newmeyer DD and Green DR:
Mitochondrial cytochrome c release in apoptosis occurs upstream of
DEVD-specific caspase activation and independently of mitochondrial
transmembrane depolarization. EMBO J. 17:37–49. 1998. View Article : Google Scholar
|
|
22
|
Tang YJ, Yang JS, Lin CF, Shyu WC, Tsuzuki
M, Lu CC, Chen YF and Lai KC: Houttuynia cordata Thunb
extract induces apoptosis through mitochondrial-dependent pathway
in HT-29 human colon adenocarcinoma cells. Oncol Rep. 22:1051–1056.
2009.
|
|
23
|
Graña X and Reddy EP: Cell cycle control
in mammalian cells: role of cyclins, cyclin dependent kinases
(CDKs), growth suppressor genes and cyclin-dependent kinase
inhibitors (CKIs). Oncogene. 11:211–219. 1995.PubMed/NCBI
|
|
24
|
Salomon DS, Brandt R, Ciardiello F and
Normanno NP: Epidermal growth factor-related peptides and their
receptors in human malignancies. Crit Rev Oncol Hematol.
19:183–232. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Varticovski L, Harrison-Findik D, Keeler
ML and Susa M: Role of PI 3-kinase in mitogenesis. Biochim Biophys
Acta. 1226:1–11. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Toker A and Cantley LC: Signalling through
the lipid products of phosphoinositide-3-OH kinase. Nature.
387:673–676. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Klippel A, Kavanaugh WM, Pot D and
Williams LT: A specific product of phosphatidylinositol 3-kinase
directly activates the protein kinase Akt through its pleckstrin
homology domain. Mol Cell Biol. 17:338–344. 1997.PubMed/NCBI
|
|
28
|
Datta SR, Brunet A and Greenberg ME:
Cellular survival: a play in three Akts. Genes Dev. 13:2905–2927.
1999. View Article : Google Scholar : PubMed/NCBI
|